Abstract
Background: The presence and level of anti-SARS-CoV-2 antibodies in PLWH from the Lower Silesia region in Poland. Material and Methods: A total of 216 serum samples of both sexes, aged 21–77, and treated with TDF or TAF together with FTC and INSTI at two points of time. Anyone who did not experience COVID-19 symptoms. Samples were checked for the presence and levels of anti-SARS-CoV-2 antibodies regarding CD4 + T and CD8 + T cells counts, the ratio of these cells, age, sex, VL, and type of tenofovir used. Results: The average level and prevalence of anti-SARS-CoV-2 antibodies during the first wave were 65.81 IU/mL and 4.17%, while during the second wave, they were 125.98 IU/mL and 14.29%, respectively. There was a significant correlation between the number and type of lymphocytes and the presence of anti-SARS-CoV-2 antibodies. We did not find the same correlation regarding anti-SARS-CoV-2 levels. The average level of antibodies was higher during the second wave. There was no difference between the type of tenofovir used and the humoral response, as well as no correlation of anti-SARS-CoV-2 levels with age, gender, or VL. Conclusion: PLWH can have asymptomatic SARS-CoV-2 infection, which can influence the presence, but not levels, of anti-SARS-CoV-2 Ab. No correlation with type of tenofovir was observed.
1. Introduction
COVID-19 is a new nosological entity that we still know very little about. On 11 March 2020, the World Health Organization (WHO) announced the COVID-19 pandemic, caused by the SARS-CoV-2 virus. However, the situation in which SARS-CoV-2 is co-infected with the human immunodeficiency virus (HIV) still seems not to be obvious. As both HIV and SARS-CoV-2 are immunocompromised, it was expected that HIV-infected individuals would be at high risk of developing severe COVID-19. Meanwhile, it turned out that the risk of developing severe COVID-19 disease affected only those people infected with HIV with extreme immunodeficiency [1]. The answer to the question of why the HIV infected do not develop COVID-19 more often than the population of uninfected people may be the young age of this group of patients [2]. It is known that young people are more likely to suffer from mild illness or be asymptomatic [3]. Perhaps the asymptomatic course of coronavirus infection is influenced by the so-called broadly neutralizing antibodies. There is a concept that these antibodies have the ability to recognize the glycan envelope of the SARS-CoV-2 protein and block it [4]. In addition, it has been shown that in HIV-infected people treated with an effective antiretroviral therapy, a higher number and activity of CD8 + T cells correlated with a milder course of COVID-19. A lack of this activity in HIV-infected patients correlated with a more severe course of COVID-19 [5]. The impact of antiretrovirals in reducing the risk of SARS-CoV-2 infection cannot be excluded. It has been suggested that lopinavir and ritonavir may have antiviral activity with respect to SARS-CoV-2. Ultimately, such an action was not confirmed, based on the results of the RECOVERY study, among others [6]. For nucleoside reverse transcriptase inhibitors (NRTIs), it has been suggested that both tenofovir, emtricitabine, and abacavir may inhibit SARS-CoV-2 replication, by affecting the viral RdRp protein. In addition, tenofovir disoproxil has been shown to have immunomodulatory effects. In vitro, a decrease in the secretion of IL-8, IL-10, and MCP-1 in the monocytes and mononuclear cells of peripheral blood was demonstrated, as well as an increase in the synthesis of IL-12 (Th1 dependent response) [7,8,9,10]. As COVID-19 is associated with the occurrence of an abnormal, excessive inflammatory reaction, the improvement of the specific immune response to a specific pathogen may be of significant importance in patients with COVID-19 with an increased inflammatory reaction [11,12].
Objective of the Work
The aim of this study was to assess the occurrence and the levels of anti-SARS-CoV-2 IgG antibodies in a group of HIV-infected patients treated with antiretroviral drugs.
2. Materials and Methods
2.1. Material
The material consisted of archival plasma samples collected from 216 HIV-infected patients from the Prevention and Treatment Clinic of the Wrocław Health Center in Wrocław, who received antiretroviral drugs: tenofovir disoproxil with emtricitabine (TDF/FTC) or tenofovir alafenamide with emtricitabine (TAF/FTC) in combination with integrase inhibitors for antiretroviral therapy. The first samples were collected from 4 February 2020 to 29 September 2020 during standard visits to the clinic. The second samples were tested from June 2020 to 22 February 2021 after the second visit of recruited patients. Previously negative people were qualified for the second determination, and this group excluded patients who did not report to the consultation point in the test interval. The two ranges corresponded to the first and second “waves” of SARS-CoV-2 infections, respectively.
The samples originated from patients without symptoms of COVID-19 on the day of their first visit to the clinic. Nobody was vaccinated against COVID-19. The patients did not report any symptoms of COVID-19 and were not aware of this disease. They came from the Lower Silesia region, Poland.
The characteristics of the study group are presented in Table 1.

Table 1.
General characteristics of the participants.
The regimens containing tenofovir are presented in Table 2.

Table 2.
Regimens containing tenofovir (BIC: bictegravir, COBI: cobicistat, EVG: elvitegravir, FTC: emtricitabine, TAF: tenofovir alefenamide, TDF: Tenofovir disoproxil).
2.2. Methods
Plasma samples were collected using EDTA, centrifuged, and stored in aliquots at −70 °C for later use. The presence of anti-SARS-CoV-2 IgG antibodies was determined using an Anti-SARS-CoV-2 ELISA (IgG) test (EUROIMMUN MedicinischeLabordiagnostica AG, Luebeck, Germany). Samples positive in Anti-SARS-CoV-2 ELISA (IgG) were then tested in a quantitative assay. The Anti-SARS-CoV-2 QuantiVac ELISA (IgG) (EUROIMMUN MedicinischeLabordiagnostica AG, Luebeck, Germany) was used for quantitative evaluation of anti-SARS-CoV-2 antibodies by means of a six-point calibration curve. The assay was standardized against “First WHO International Standard for anti-SARS-CoV-2 immunoglobin” (NIBSC 20/136), so the quantitative results are given in standardized units: IU/mL (IU—international units), which are identical to BAU/mL (BAU—binding antibody units).
In both enzyme-linked immunoabsorbant assays, the S1 domain of the spike protein of SARS-CoV-2 including the receptor binding domain (RBC) was used as an antigen. This antigen is characterized by the lowest homology with analogous regions of other human pathogenic coronaviruses.
ELISA assays were performed and the results were evaluated as recommended by the manufacturer. The percentages and absolute counts (cells/µL) of CD3+, CD4+, and CD8+ T lymphocytes were tested. CYTO-STAT tri-CHROME CD8-FITC (fluorescein isothiocyanate)/CD4-RD1 (phycoerythrin)/ CD3-PC5 (phycoerythrin cyanine 5) reagents (Beckman Coulter Inc., Brea, CA, USA) were used. This reagent is a combination of three murine monoclonal antibodies and allows simultaneous identification and enumeration of total CD3+, total CD4+, total CD8+, dual CD3+/CD4+, and dual CD3+/CD8+ lymphocytes in whole blood. Samples were analyzed using a Navios EX flow cytometer (Beckman Coulter). Peripheral blood samples were collected in EDTA anticoagulant tubes.
A cobas® HIV-1 quantitative real-time nucleic acid test (Roche Diagnostics GmbH, Mannheim, Germany) performed on a cobas® 4800 System was used for the determination of the HIV-1 RNA viral load.
2.3. Statistical Methods
A chi-square test was used to calculate the relationship between the presence of anti-SARS-CoV-2 antibodies and gender or treatment. The relationships between age, levels of CD4 + T cells, CD8 + T cells, ratio of CD4 + T cells to CD8 + T cells, viremia and the presence and level of antibodies were calculated using the Kendall tau test and Spearman’s rank correlation. A Mann-Whitney U test was used to calculate the relationship between sex and antibody levels. A p < 0.05 was considered a significant value.
The study was approved by the bioethics committee, No. KB-714/2020.
3. Results
The study group consisted of 216 HIV-infected people aged 21 to 77 years. The average age was 41 years. The study group included 174 men (80.56%) and 42 (19.44%) women at the first sampling. Previously anti-SARS-CoV-2-negative people were tested for the second time. The percentage of people with a positive test for antibodies was 4.17% in the first sampling) and 14.29% in the second sampling. They were mostly men, reflecting the characteristics of the whole group. The age of the two groups was similar. The measurement results are presented in Table 3. The values of the level of anti-SARS-CoV-2 antibodies depending on the wave of infections are presented in Figure 1.

Table 3.
Characterization of the group of patients with positive anti-SARS-CoV-2 IgG antibody results.
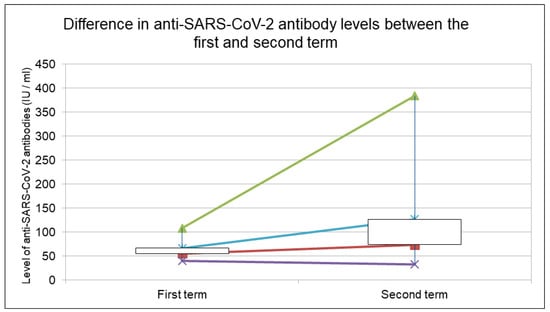
Figure 1.
Value of the level of anti-SARS-CoV-2 antibodies, depending on the wave of infections. The chart shows the minimum and maximum values. The opening value represents the median, the closing value represents the mean value of the antibody level. White color of the body of chart—the difference between the opening and closing value is greater than 0; black color—less than 0.
There was no statistically significant difference between the presence of anti-SARS-CoV-2 antibodies and gender, age, HIV viral load, or type of tenofovir used. However, CD4 + T cell count, CD8 + T cell count, and CD4+ to CD8+ ratio had a statistically significant effect on the presence of anti-SARS-CoV-2 antibodies, see Table 4.

Table 4.
Correlation of the presence or level of anti-SARS-CoV-2 antibodies with the number of CD4+ cells, CD8 + T cells, the ratio of CD4+/CD8 + T cells to age, calculated Spearman’s rank order, and Kendall tau correlation (the p values). CD4 + T cells, CD8 + T cell count, and CD4 + to CD8 + ratio had a statistically significant effect on the presence of anti-SARS-CoV-2 antibodies.
Despite the correlation between the presence of anti-SARS-CoV-2 antibodies and the CD4 + T cells, CD8 + T cell count, and CD4+ to CD8+ ratio, we did not observe such a correlation regarding the levels of anti-SARS-CoV-2 antibodies, see Table 4.
In the course of our study, special attention was paid to one patient who on 10 March 2020 had IgG anti-SARS-CoV-2 antibodies in serum. This 39 year old man under the care of the clinic was treated with ARV preparations containing TDF/FTC and did not show any symptoms during this period. In his interview, he did not mention any trips outside Poland in the months preceding the first study (the last trip abroad was 15 years ago). At the same time, he did not travel outside Wrocław and did not contact people going abroad. An interesting piece of information is that he worked in a city cleaning company, where he could potentially have come into contact with infectious material. This suggests that the SARS-CoV-2 virus may have been in the Polish environment much earlier, especially since the first media case was reported on 4 March 2020 with a patient traveling from abroad. Taking into account that antibodies in the IgG class appear about a week/one and a half after infection, the patient included in the study could have become infected earlier than the Patient “0” in the media.
4. Discussion
The main goal of our study was to assess the prevalence of anti-SARS-CoV-2 antibodies in the group of HIV-infected people in the period from 4 February 2020 to 29 September 2020, who were patients of the Consulting and Diagnostic Center in Wrocław, Poland and received antiretroviral regimens containing tenofovir with emtricitabine or tenofovir alafenamide with emtricitabine in combination with integrase inhibitors. After 6 months, another serological test for the presence of anti-SARS-CoV-2 antibodies was performed in those negative in the first test. The seroprevalence calculated from the first time point in our study, at the first determination, was 4.17%. Comparing with the data obtained by researchers in Poznań, the seroprevalence in the general health population of the city as of 24 September 2020 was low and amounted to 0.93% [13]. The percentage of people with positive anti-SARS-CoV-2 antibodies in a similar period in other large cities included Madrid 13.6% [14], Geneva 10.8% [15], Warsaw 17.3 % [16], and Gdańsk 10.4% [17]. It can be concluded that the seroprevalence of our research group was much lower than that of the general population.
The percentage of people infected with the SARS-CoV-2 virus in the general population of Lower Silesia as of 22 February 2021 was 3.71% [18]; however, this result applies only to positive PCR swabs. In our study group, this percentage was 14.37% and concerned the presence of antibodies. However, it should be noted that in our study, patients did not report SARS-CoV-2 infection or flu-like symptoms during the observed period. At the same time, in the Republic of Poland, swabs for SARS-CoV-2 infection were performed only from people with symptoms of COVID-19. In this case, it can be assumed with high probability that the percentage of 3.7% in the general population of Lower Silesia was underestimated and did not include people with mild symptoms and asymptomatic infections. At the same time, due to the lack of research and determination of the prevalence of anti-SARS-CoV-2 antibodies in the population of Lower Silesia during this period, it is technically not possible to compare our results, which is why we compare our results with cities with a population of similar size, at a similar time. There is an interesting observation of higher antibody levels at the second test term compared to the first term test in a population that was previously negative. This can be explained by the difference in the types of virus that dominated the successive waves. The second wave was related to the alpha variant, which spread more efficiently, and those infected with it were at a higher risk of severe disease and death than the original variant of SARS-CoV-2. At the same time, more severe courses of SARS-CoV-2 infection were associated with higher levels of antibodies [19,20]. Of course, there is the possibility that the higher level of anti-SARS-CoV-2 antibodies could also have been due to the time interval of blood sampling. A certain group of patients may have been asymptomatically infected at the first visit and not yet producing antibodies, which gave the immune system more time to produce higher levels of Ab. There is also the possibility of multiple exposures to the virus, which would naturally also result in higher levels of anti-SARS-CoV-2.
It is also possible that the lower prevalence of anti-SARS-CoV-2 antibodies in the first wave was due to impaired humoral immunity in PLWH, which could have resulted in a lack of specific antibody production, which in turn could have led to negative results in the test used in this study. At the same time, there is the possibility that the decreased humoral and/or cellular responses may have been associated with a less intense inflammatory response and a reduction in the frequency of symptoms observed.
Comparing our results to the general population, they seem to be consistent with the observations of other researchers. The “second wave” of the COVID-19 epidemic in Poland began in September 2020. The results of the study for the general population in Katowice, Upper Silesia region, Poland showed that the frequency of positive IgG tests was 5.6% in October 2020 and increased to 15.0% in November 2020 [21].
For an additional comparison, the prevalence of IgG antibodies against SARS-CoV-2 in the general health population of Upper Silesia as of 20 November 2020, calculated in a group of 5479 subjects, was 22.9% [17].
A seroprevalence study in the PLWH population in Rome indicated that, after the “first wave” of COVID-19, PLWH were characterized by a lower prevalence of antibodies [22].
Seroprevalence may differ significantly between the populations of individual regions of Poland. Unfortunately, there are still very few studies describing the prevalence of anti-SARS-CoV-2 antibodies, especially in the Polish community.
The patients included into this study were treated either with tenofovir disoproxil/emtricitabine or tenofovir alafenamide/emtricitabine together with integrase inhibitors, based on the reports from Spain indicating that tenofovir disoproxil, in particular, can decrease the risk of SARS-CoV-2 infection [23,24]. In addition, tenofovir disoproxil has been shown to have immunomodulatory effects. In vitro, a decrease in the secretion of IL-8, IL-10, and MCP-1 in monocytes and mononuclear cells of peripheral blood, as well as an increase in the synthesis of IL-12 (stimulation of the Th1-dependent reaction), was observed [7,8,9,10]. As COVID-19 is associated with the occurrence of an abnormal, excessive inflammatory reaction, the improvement of the specific immune response to a specific pathogen may be of significant importance in patients with COVID-19 with an increased inflammatory reaction [11,12]. At the same time, it should be mentioned that antiretroviral drugs perfuse into the lungs and other tissues, as has been shown for tenofovir in animals [25,26] and in humans [27]. There are also indications that TDF/FTC intake may reduce the viral load in the nasopharynx, while accelerating the elimination of the virus [28]. Our results do not confirm those obtained by others. There was no difference between the ratio of SARS-CoV-2 infection in the groups receiving TDF/FTC or TAF/FTC, although the percentage of positive results obtained at the two time points was lower than in general population.
It cannot be ruled out that there were patients whose antibody levels, despite infection, did not exceed the detection level on the day of the test [29,30,31,32,33].
Another probable explanation of the lower anti-SARS-CoV-2 antibody levels in HIV patients compared with the general population are the broadly neutralizing antibodies (bnAbs) that are produced in a part of individuals during chronic HIV-1 infection. A cross-neutralizing activity against SARS-CoV-2 was observed. The SARS-CoV-2 S protein, similarly to the type I fusion proteins of viruses such as HIV-1 envelope (Env), is highly glycosylated and may theoretically react with all neutralizing antibodies. Several types of HIV-1 bnAb exhibited cross-reactivity with SARS-CoV-2, while one HIV-1 bnAb CD4 binding site, N6, neutralized SARS-CoV-2 in in vitro samples [34].
The cellular immune response also plays an important role in SARS-CoV-2 infection. It has been shown that, in HIV-infected people treated with an effective antiretroviral therapy, a higher number and activity of CD8 + T cells correlated with a milder course of COVID-19. At the same time, the lack of this activity in infected patients correlated with a more severe course of COVID-19. It was also observed that after undergoing COVID-19 in laboratory tests, patients participating in the study showed an increase in the expansion (activity) of CD8 + T cells [35].
Finally, the younger age of the study population compared with the general population may be another explanation for the lower rate of SARS-CoV-2 infection. During the first and the second wave, mainly older people were suffering from COVID-19.
In the course of this study, we noticed correlations between presence of anti- SARS-CoV-2 antibodies and the number of CD4 + T cells, CD8 + T cells, and the ratio of CD4 + T cells to CD8 + T cells. Other studies have also suggested a correlation between the number of CD4 + T cells and the severity of COVID-19 and mortality [36,37]. At the same time, we showed no effect of the number of CD4 + T cells, CD8 + T cells, and the ratio of CD4 + T cells to CD8 + T cells on the level of anti-SARS-CoV-2 IgG antibodies.
HIV viral, age, sex, or type of antiretroviral therapy did not affect the frequency of infections in the study group. Our observations are consistent with the results of similar studies carried out around the world [20,38]. However, other researchers noticed the influence of the type of tenofovir on the course of the infection [27,39,40]. This difference can be explained by the low number anti-SARS-CoV-2-positive patients. However, on the other hand, a French study found a similar prevalence of SARS-CoV-2 IgG in PrEP users and in a matched cohort in the Paris region after the COVID-19 lockdown, suggesting that TDF/FTC has no role in reducing SARS-CoV-2 acquisition [41].
5. Conclusions
HIV-1-positive patients are not a group at high risk for SARS-CoV-2 infection, as was suspected at the beginning of pandemic. In many asymptomatic cases, it is impossible to establish a diagnosis but the coronaviruses can be transmitted to other persons. Further observations are required to establish the reasons behind the low prevalence of SARS-CoV-2 infection among HIV-infected populations without advanced immune deficiency.
The research group was small, and large-scale studies with larger groups of patients are needed to obtain better results. There are still very few studies describing the prevalence of anti-SARS-CoV-2 antibodies, especially in the Polish community.
The very small number of similar studies assessing the presence of anti-SARS-CoV-2 antibodies and their serum levels among the Polish population during this period did not allow us to compare our results with the situation in the general population of our country and region.
Author Contributions
Conceptualization, H.D.C. and B.S.; methodology, M.Z. and K.K.; statistical analysis, K.K.; laboratory testing, M.Z.; writing—original draft preparation, H.D.C.; writing—review and editing, B.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Wroclaw Medical University (protocol code No. KB-714/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are given within the manuscript and its Supporting Information files. Sensitive personal data of patients participating in the study are not available. The Patient Base is located in the Prevention and Treatment Clinic of the Wrocław Health Center in Wrocław.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Prevalence of HIV in patients hospitalized for COVID-19 and associated outcomes: A systematic review and meta-analysis. MedRxiv 2020. [Google Scholar] [CrossRef]
- COVID-19 Stats: COVID-19 Incidence, by Age Group—United States, 1 March–14 November 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 69, 1664. [CrossRef] [PubMed]
- Snider, B.; Patel, B.; McBean, E. Asymptomatic Cases, the Hidden Challenge in Predicting COVID-19 Caseload Increases. Infect. Dis. Rep. 2021, 13, 340–347. [Google Scholar] [CrossRef] [PubMed Central]
- Mannar, D.; Leopold, K.; Subramaniam, S. Glycan reactive anti-HIV-1 antibodies bind the SARS-CoV-2 spike protein but do not block viral entry. Sci. Rep. 2021, 11, 12448. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomized, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]
- Melchjorsen, J.; Risør, M.W.; Søgaard, O.S.; O’Loughlin, K.L.; Chow, S.; Paludan, S.R.; Ellermann-Eriksen, S.; Hedley, D.W.; Minderman, H.; Østergaard, L.; et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J. Acquir. Immune. Defic. Syndr. 2011, 57, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zídek, Z.; Holý, A.; Franková, D. Antiretroviral agent (R)-9-(2-phosphonomethoxypropyl) adenine stimulates cytokine and nitric oxide production. Eur. J. Pharmacol. 1997, 331, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zídek, Z.; Holý, A.; Franková, D. Immunomodulatory properties of antiviral acyclic nucleotide analogues: Cytokine stimulatory and nitric oxide costimulatory effects. Int. J. Immunopharmacol. 1997, 19, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Zídek, Z.; Franková, D.; Holý, A. Activation by 9-(R)-[2-(phosphonomethoxy) propyl] adenine of chemokine (RANTES, macrophage inflammatory protein 1alpha) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1beta) production. Antimicrob. Agents Chemother. 2001, 45, 3381–3386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Catanzaro, M.; Fagiani, F.; Racchi, M.; Corsini, E.; Govoni, S.; Lanni, C. Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Sig. Transduct. Target. Ther. 2020, 5, 84. [Google Scholar] [CrossRef]
- Lorent, D.; Nowak, R.; Roxo, C.; Lenartowicz, E.; Makarewicz, A.; Zaremba, B.; Nowak, S.; Kuszel, L.; Stefaniak, J.; Kierzek, R.; et al. Prevalence of Anti-SARS-CoV-2 Antibodies in Poznań, Poland, after the First Wave of the COVID-19 Pandemic. Vaccines 2021, 9, 541. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartin, J.L.; Fernández-García, A.; Cruz, I.; Fernández de Larrea, N.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Stringhini, S.; Wisniak, A.; Piumatti, G.; Azman, A.S.; Lauer, S.A.; Baysson, H.; Ridder, D.D.; Petrovis, D.; Schrempft, S.; Marcus, K.; et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): A population-based study. Lancet 2020, 396, 313–319. [Google Scholar] [CrossRef]
- Available online: https://www.pzh.gov.pl/wp-content/uploads/2021/02/Suplement-do-Rozdzialu-7-seroprewalencja.pdf (accessed on 3 November 2022).
- Kowalska, M.; Niewiadomska, E.; Barański, K.; Kaleta-Pilarska, A.; Brożek, G.; Zejda, J.E. Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland. Vaccines 2021, 9, 415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://koronawirusunas.pl/wojewodztwo-dolnoslaskie (accessed on 3 November 2022).
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Grint, D.J.; Wing, K.; Houlihan, C.; Gibbs, H.P.; Evans, S.J.W.; Williamson, E.; I McDonald, H.; Bhaskaran, K.; Evans, D.; Walker, A.J.; et al. Severity of SARS-CoV-2 alpha variant (B.1.1.7) in England. Clin Infect Dis. 2021, 6, ciab754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zejda, J.; Brożek, G.; Kowalska, M.; Barański, K.; Kaleta-Pilarska, A.; Nowakowski, A.; Xia, Y.; Buszman, P. Seroprevalence of Anti-SARS-CoV-2 Antibodies in a Random Sample of Inhabitants of the Katowice Region, Poland. Int. J. Environ. Res. Public Health 2021, 18, 3188. [Google Scholar] [CrossRef]
- Lombardi, F.; Ricci, R.; Belmonti, S.; Fabbiani, M.; Borghetti, A.; Baldin, G.; Ciccullo, A.; Tamburrini, E.; Visconti, E.; Sanguinetti, M.; et al. Seroprevalence of SARS-CoV-2 Antibodies in HIV-Infected Patients in Rome, Italy during the COVID-19 Outbreak. Diagnostics 2021, 11, 1154. [Google Scholar] [CrossRef]
- del Amo, J.; Polo, R.; Moreno, S.; Díaz, A.; Martínez, E.; Arribas, J.R.; Jarrín, I.; Hernán, M.A. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy: A Cohort Study. Ann. Intern. Med. 2020, 173, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, andTenofovir against SARS-CoV-2 RNA dependent RNApolymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, M.; Srinivasula, S.; Bhattacharjee, A.; Cheng, L.; Martiniova, L.; Herscovitch, P.; Lertora, J.; Kiesewetter, D. Antiretroviral tissue kinetics: In vivo imaging using positron emission tomography. Antimicrob. Agents Chemother. 2009, 53, 4086–4095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, S.; Yu, K.-M.; Kim, Y.-I.; Kim, S.-M.; Kim, E.-H.; Kim, S.-G.; Kim, E.J.; Casel, M.A.B.; Rollon, R.; Jang, S.-G.; et al. Antiviral efficacies ofFDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio 2020, 11, e01114-20. [Google Scholar] [CrossRef] [PubMed]
- Twigg, H.L.; Schnizlein-Bick, C.T.; Weiden, M.; Valentine, F.; Wheat, J.; Day, R.B.; Rominger, H.; Zheng, L.; Collman, R.G.; Coombs, R.W.; et al. Measurement of antiretroviral drugs in the lungs of HIV-infected patients. HIV Ther. 2010, 4, 247–251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parienti, J.J.; Prazuck, T.; Peyro-Saint-Paul, L.; Fournier, A.; Valentin, C.; Brucato, S.; Verdon, R.; Sève, A.; Colin, M.; Lesne, F.; et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. eClinicalMedicine 2021, 38, 100993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; Li, P.; Ding, Y.; Liu, M.; Liu, L.; Yi, B.; Wu, T.; Dong, H.; Lao, X.; Ding, K.; et al. Epidemiological feature, viral shedding, and antibody seroconversion among asymptomatic SARS-CoV-2 carriers and symptomatic/presymptomatic COVID-19 patients. J. Infect. Public Health 2021, 14, 845–851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marchi, S.; Viviani, S.; Remarque, E.J.; Ruello, A.; Bombardieri, E.; Bollati, V.; Milani, G.P.; Manenti, A.; Lapini, G.; Rebuffat, A.; et al. Characterization of antibody response in asymptomatic and symptomatic SARS-CoV-2 infection. PLoS ONE 2021, 16, e0253977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Boyton, R.J.; Altmann, D.M. The immunology of asymptomatic SARS-CoV-2 infection: What are the key questions? Nat. Rev. Immunol. 2021, 21, 762–768. [Google Scholar] [CrossRef]
- Leister, I.; Ponocny-Seliger, E.; Kollaritsch, H.; Dungel, P.; Holzer, B.; Grillari, J.; Redl, H.; Ponocny, I.; Wilfing, C.; Aigner, L.; et al. Antibody seroprevalence and rate of asymptomatic infections with SARS-CoV-2 in Austrian hospital personnel. BMC Infect. Dis. 2021, 21, 915. [Google Scholar] [CrossRef]
- Mishra, N.; Kumar, S.; Singh, S.; Bansal, T.; Jain, N.; Saluja, S.; Kumar, R.; Bhattacharyya, S.; Palanichamy, J.K.; Mir, R.A.; et al. Cross-neutralization of SARS-CoV-2 by HIV-1 specific broadly neutralizing antibodies and polyclonal plasma. LoS Pathog. 2021, 17, e1009958. [Google Scholar] [CrossRef]
- Karim, F.; Gazy, I.; Cele, S.; Zungu, Y.; Krause, R.; Bernstein, M.; Ganga, Y.; Rodel, H.; Mthabela, N.; Mazibuko, M.; et al. Alex Sigal HIV status alters disease severity and immune cell responses in β variant SARS-CoV-2 infection wave. medRxiv 2021. [Google Scholar] [CrossRef]
- Wen, X.-S.; Jiang, D.; Gao, L.; Zhou, J.-Z.; Xiao, J.; Cheng, X.-C.; Bin He, B.; Chen, Y.; Lei, P.; Tan, X.-W.; et al. Clinical characteristics and predictive value of lower CD4+T cell level in patients with moderate and severe COVID-19: A multicenter retrospective study. BMC Infect. Dis. 2021, 21, 57. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.; Xu, L.; Lin, C. T cell response in patients with COVID-19. Blood Sci. 2020, 2, 76–78. [Google Scholar] [CrossRef]
- del Amo, J.; Polo, R.; Moreno, S.; Diaz, A.; Martínez, E.; Arribas, J.R.; Jarrín, I.; Hernán, M.A. Antiretrovirals and Risk of COVID-19 Diagnosis and Hospitalization in HIV-Positive Persons. Epidemiology 2020, 31, e49–e51. [Google Scholar] [CrossRef]
- Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa. Erratum to: Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2022, 74, 1321, Erratum for: Clin Infect Dis. 2021, 73, e2005–e2015. [CrossRef] [PubMed] [PubMed Central]
- Charre, C.; Icard, V.; Pradat, P.; Brochier, C.; Lina, B.; Chidiac, C.; Cotte, L. Coronavirus disease 2019 attack rate in HIV-infected patients and in preexposure prophylaxis users. AIDS 2020, 34, 1765–1770. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).