Survival by Mediastinal Chest Drain Due to Pneumomediastinum Resulting from COVID-19
Abstract
:1. Introduction
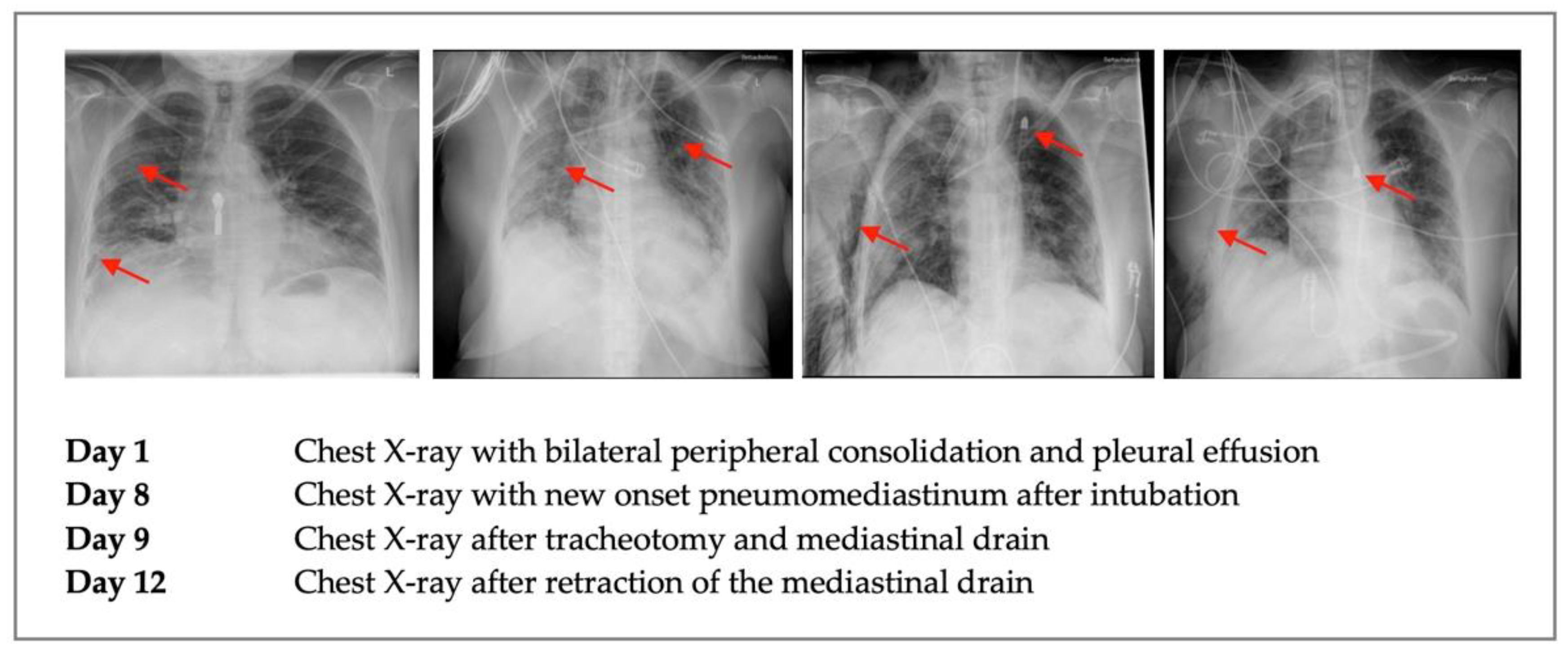
2. Case Description
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinelli, A.W.; Ingle, T.; Newman, J.; Nadeem, I.; Jackson, K.; Lane, N.D.; Melhorn, J.; Davies, H.E.; Rostron, A.J.; Adeni, A.; et al. COVID-19 and pneumothorax: A multicentre retrospective case series. Eur. Respir. J. 2020, 56, 2002697. [Google Scholar] [CrossRef]
- Volpi, S.; Ali, J.M.; Suleman, A.; Ahmed, R.N. Pneumomediastinum in COVID-19 patients: A case series of a rare complication. Eur. J. Cardiothorac. Surg. 2020, 58, 646–647. [Google Scholar] [CrossRef]
- Damous, S.H.B.; dos Santos Junior, J.P.; Pezzano, Á.V.A.; Chams, M.A.M.; Haritov, N.; Waksman, R.; Lima, H.V.G.; Miranda, J.D.S.; Rasslan, R.; Utiyama, E.M. Pneumomediastinum complicating COVID-19: A case series. Eur. J. Med Res. 2021, 26, 114. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Douedi, S.; Alshami, A.; Al-Saoudi, G.; Mikhail, J. Spontaneous Subcutaneous Emphysema and Pneumomediastinum in COVID-19 Patients: An Indicator of Poor Prognosis? Am. J. Case Rep. 2020, 21, e925557. [Google Scholar] [CrossRef] [PubMed]
- Aljehani, Y.; Alkhunaizi, A.A.; Othman, S.A.; Alqumber, H.A.; Almubarak, Y.; Al-Musawi, T.; Al Bazroun, M.I.; Alshaikhmohamed, K. Surgical and mediastinal emphysema in critically ill COVID-19 patients: A multicentric experience. Ann. Thorac. Med. 2022, 17, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Macklin, M.T.; Macklin, C.C. Malignant interstital emphysema of the lungs and mediastinum as an important occult complication in many respiratory diseases and other conditions. Medicine 1944, 23, 281–358. [Google Scholar] [CrossRef]
- Kouritas, V.K.; Papagiannopoulos, K.; Lazaridis, G.; Baka, S.; Mpoukovinas, I.; Karavasilis, V.; Lampaki, S.; Kioumis, I.; Pitsiou, G.; Papaiwannou, A.; et al. Pneumomediastinum. J. Thorac. Dis. 2015, 7, S44–S49. [Google Scholar] [PubMed]
- Reis, A.E.; Emami, N.; Chand, S.; Ogundipe, F.; Belkin, D.L.; Ye, K.; Keene, A.B.; Levsky, J.M. Epidemiology, Risk Factors and Outcomes of Pneumomediastinum in Patients with Coronavirus Disease 2019: A Case-Control Study. J. Intensive Care Med. 2022, 37, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, R.; Zheng, Y.; Jiang, L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J. Travel Med. 2020, 27, taaa062. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Patel, D.; Sayedy, N.; Anjum, F. COVID-19 and Spontaneous Pneumomediastinum: A case series. Heart Lung J. Crit. Care 2021, 50, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Hazariwala, V.; Hadid, H.; Kirsch, D.; Big, C. Spontaneous pneumomediastinum, pneumopericardium, pneumothorax and subcutaneous emphysema in patients with COVID-19 pneumonia, a case report. J. Cardiothorac. Surg. 2020, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.P.; Stefaniak, C.; Bunch, C.M.; March, R.; Zamlut, M.; Raza, S.; Osorio, W.; Korzan, J.; Show, J.; Mjaess, N.; et al. Tension pneumomediastinum and diffuse subcutaneous emphysema with severe acute respiratory syndrome coronavirus 2 infection requiring operative management for impending airway collapse: A case report. Clin. Case Rep. 2021, 9, e04656. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lingens, J.E.; Lingens, J.B.; Gutersohn, A.; Hönemann, C. Survival by Mediastinal Chest Drain Due to Pneumomediastinum Resulting from COVID-19. COVID 2022, 2, 1710-1714. https://doi.org/10.3390/covid2120122
Lingens JE, Lingens JB, Gutersohn A, Hönemann C. Survival by Mediastinal Chest Drain Due to Pneumomediastinum Resulting from COVID-19. COVID. 2022; 2(12):1710-1714. https://doi.org/10.3390/covid2120122
Chicago/Turabian StyleLingens, Johanna Elisabeth, Jan Berend Lingens, Achim Gutersohn, and Christian Hönemann. 2022. "Survival by Mediastinal Chest Drain Due to Pneumomediastinum Resulting from COVID-19" COVID 2, no. 12: 1710-1714. https://doi.org/10.3390/covid2120122
APA StyleLingens, J. E., Lingens, J. B., Gutersohn, A., & Hönemann, C. (2022). Survival by Mediastinal Chest Drain Due to Pneumomediastinum Resulting from COVID-19. COVID, 2(12), 1710-1714. https://doi.org/10.3390/covid2120122




