Anti-SARS-CoV-2 Activity of Adamantanes In Vitro and in Animal Models of Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Antiviral Compounds
2.3. Antiviral Assays
2.4. Immunostaining Assay
2.5. SARS-CoV-2 Plaque Assays
2.6. Viability Assay
2.7. Animal Antiviral Studies
2.8. Statistical Analysis
3. Results
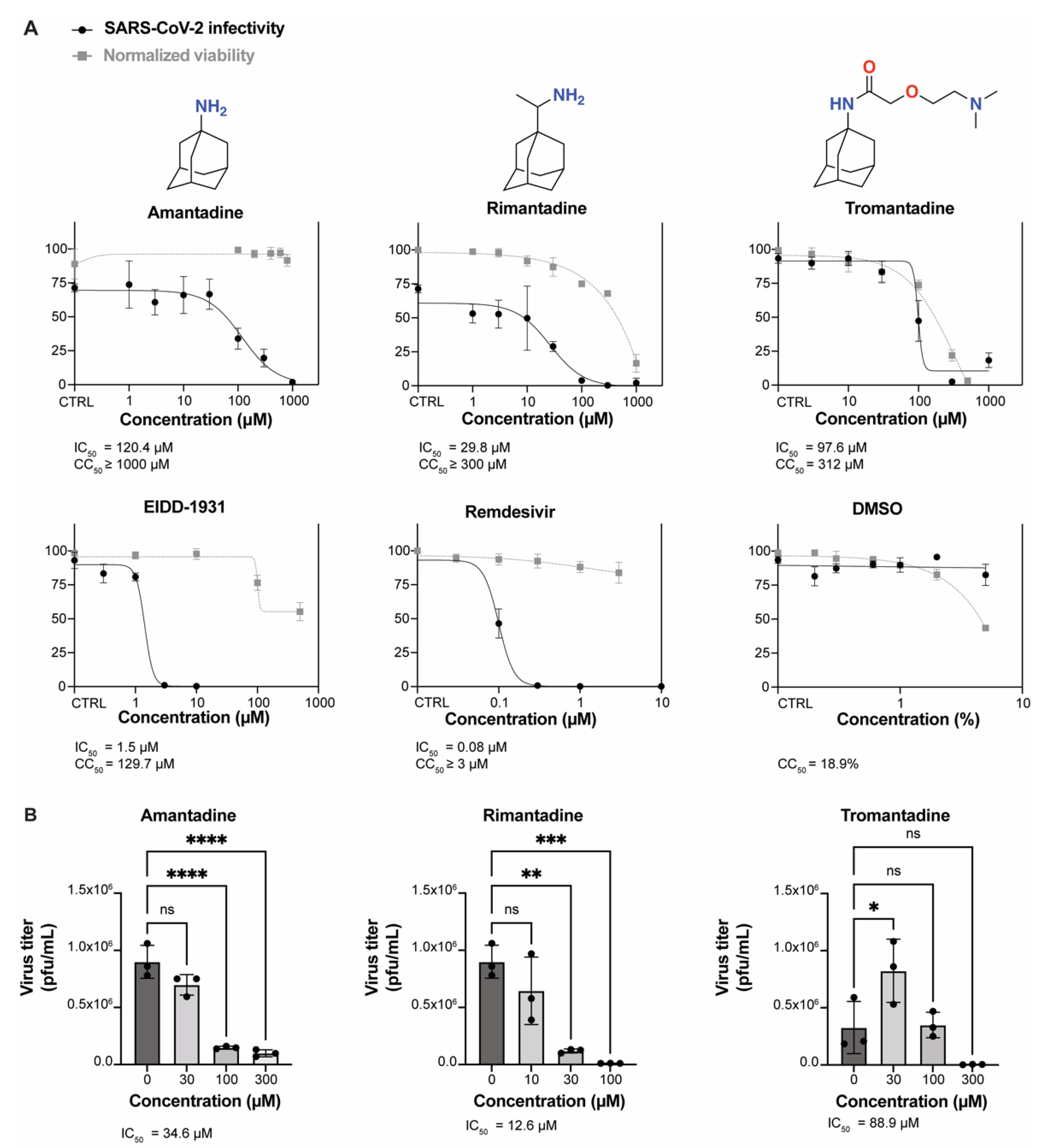
3.1. Amantadine, Rimantadine, and Tromantadine Inhibit SARS-CoV-2 In Vitro in Human Lung Epithelial Cells
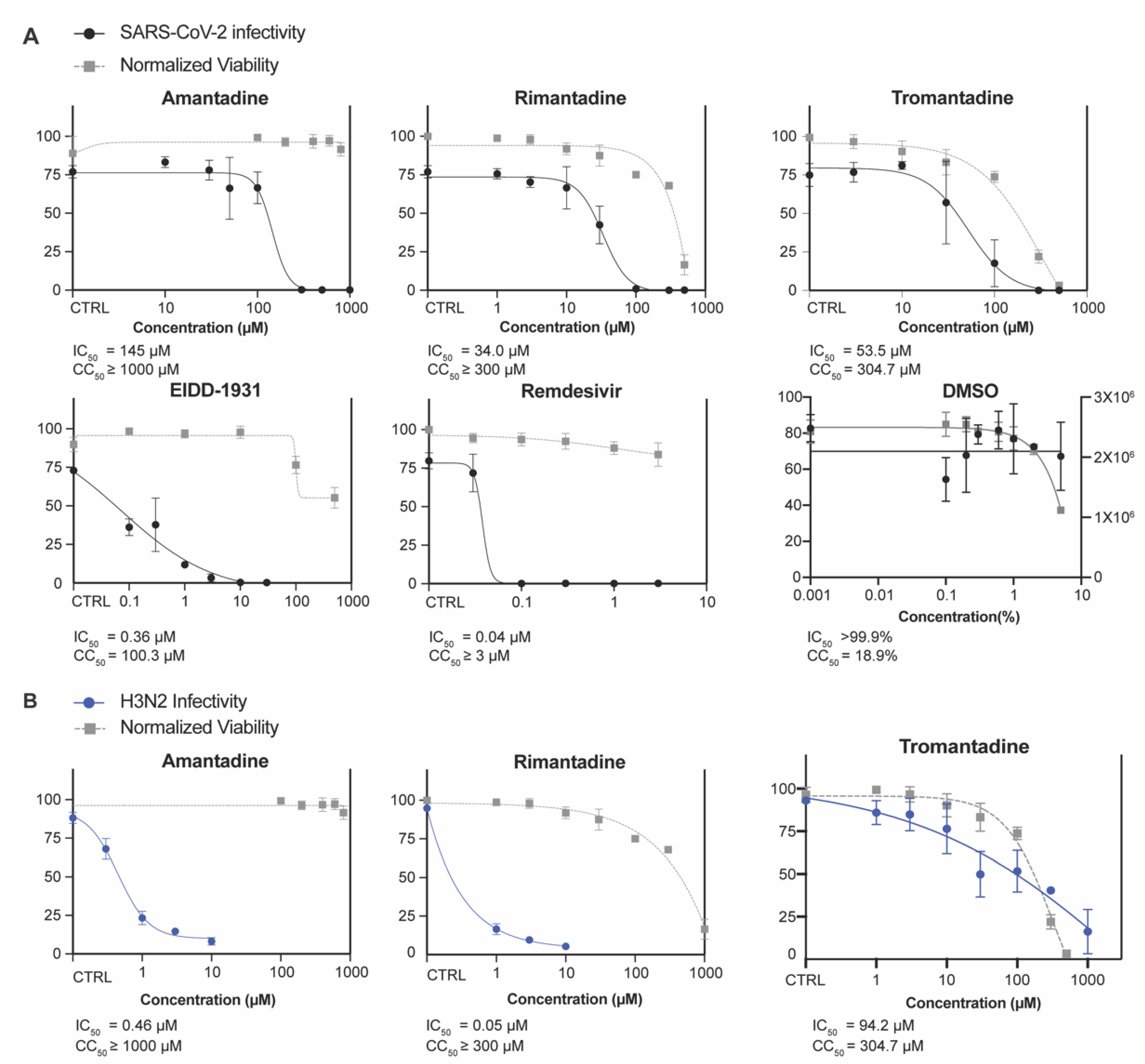
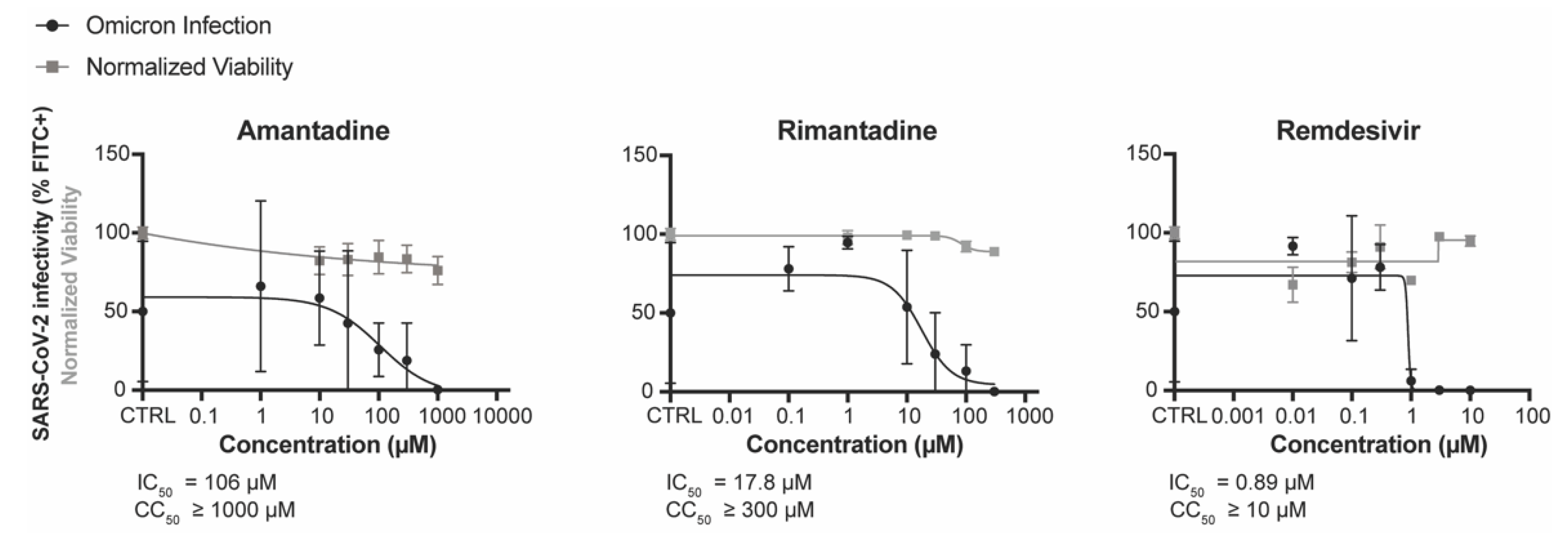
3.2. Amantadine and Rimantadine Inhibit SARS-CoV-2 Omicron Variant B.1.1.529 In Vitro in Vero E6-TMPRSS2-T2A-ACE2 (Vero E6 T/A) Cells
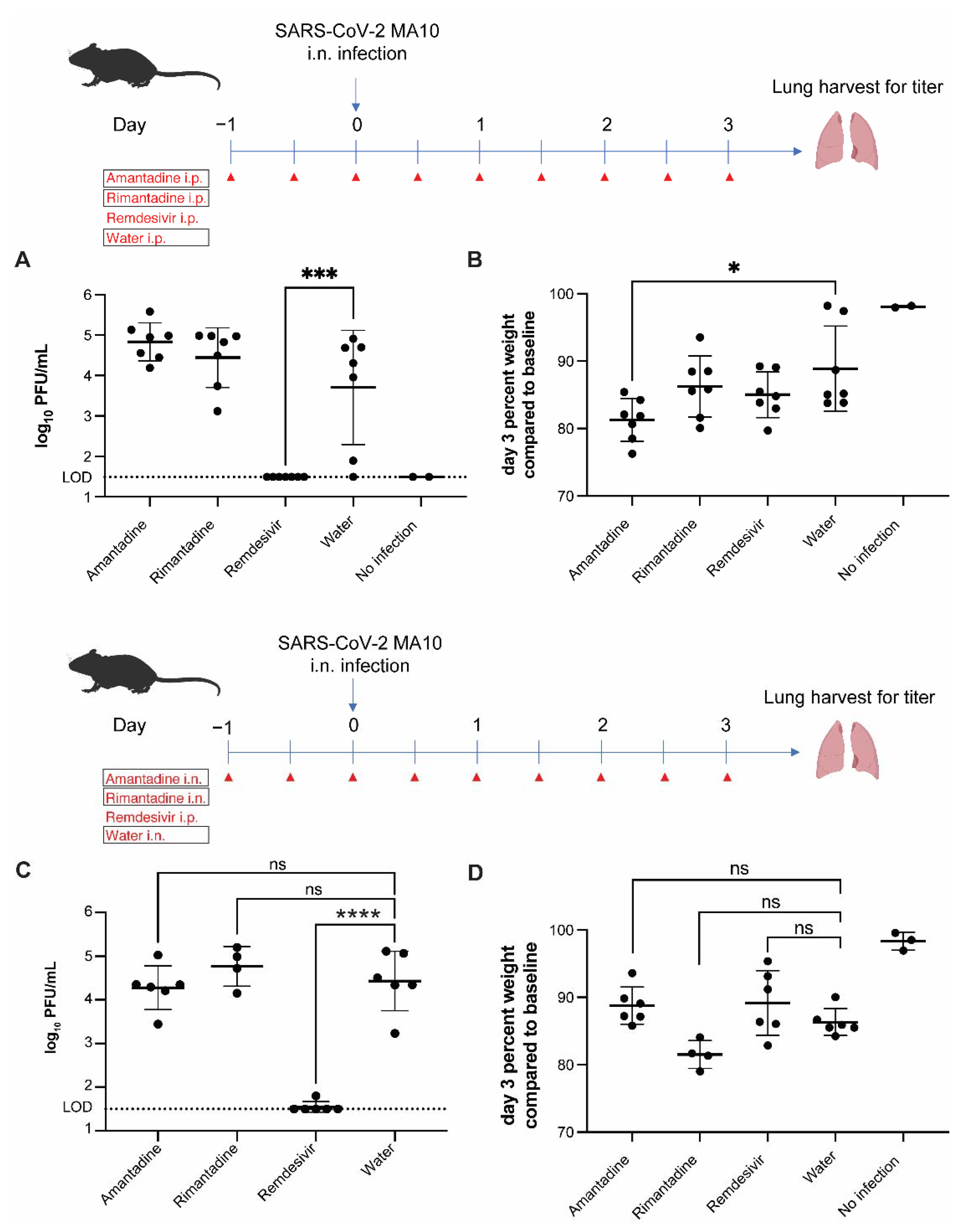
3.3. Amantadine and Rimantadine Do Not Significantly Inhibit SARS-CoV-2 Growth in Lungs of Mice When Administered by Intraperitoneal or Intranasal Route
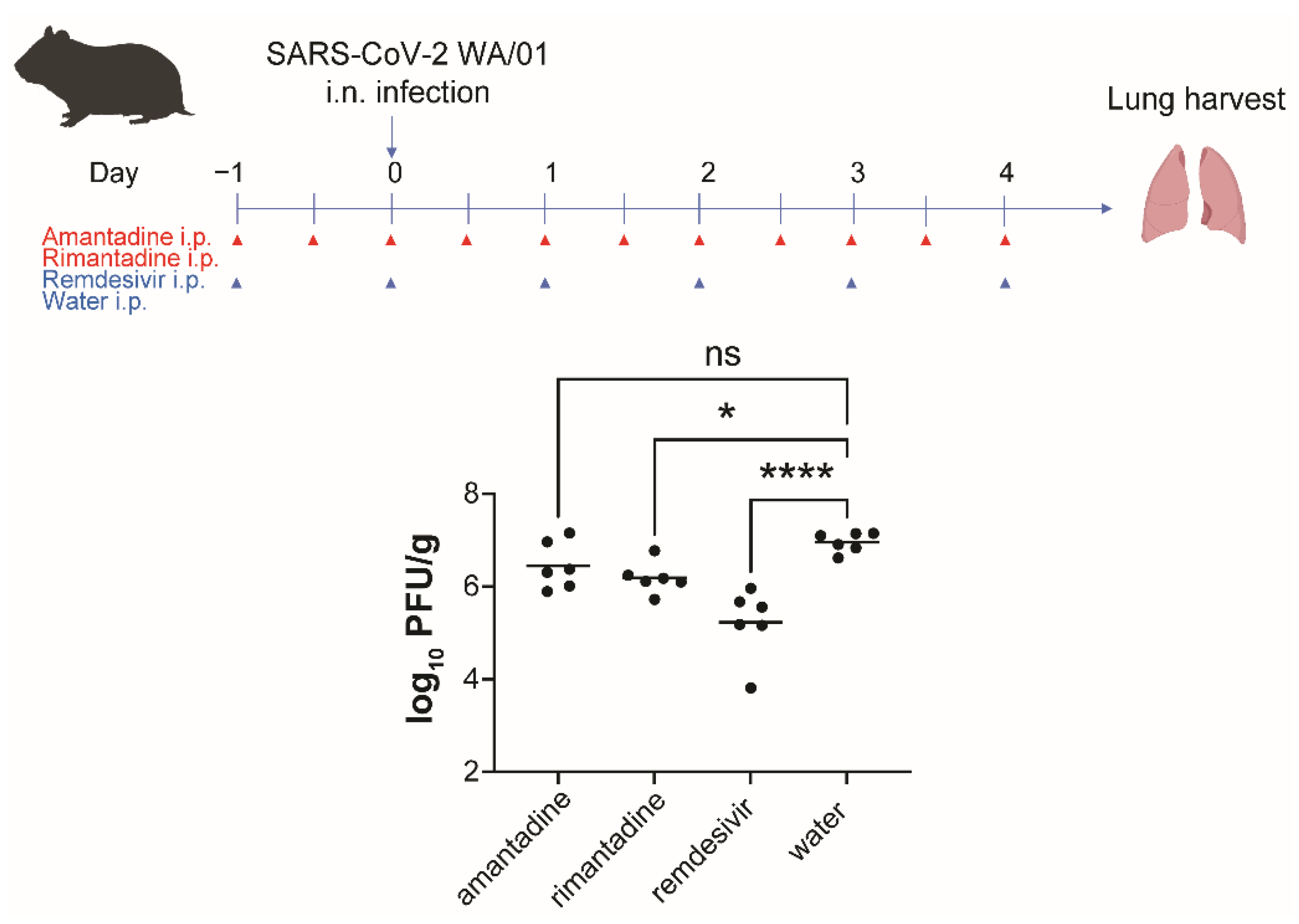
3.4. Rimantadine Inhibits SARS-CoV-2 Growth in Lungs of Hamsters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenza A. Clin. Infect Dis. 2019, 68, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Abreu, G.E.; Aranda-Martinez, J.D.; Araujo, R. Use of amantadine in a patient with SARS-CoV-2. J. Med. Virol. 2020, 93, 110–111. [Google Scholar] [CrossRef]
- Cortes Borra, A. Does amantadine have a protective effect against COVID-19? Pol. J. Neurol. Neurosurg. 2020, 54, 284–285. [Google Scholar] [CrossRef]
- Rejdak, K.; Grieb, P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: Multiple sclerosis, parkinsonism and cognitive impairment. Mult. Scler. Relat. Disord. 2020, 42, 102163. [Google Scholar] [CrossRef] [PubMed]
- Kamel, W.A.; Kamel, M.I.; Alhasawi, A.; Elmasry, S.; AlHamdan, F.; Al-Hashel, J.Y. Effect of Pre-exposure Use of Amantadine on COVID-19 Infection: A Hospital-Based Cohort Study in Patients with Parkinson’s Disease or Multiple Sclerosis. Front. Neurol. 2021, 12, 704186. [Google Scholar] [CrossRef]
- Mathur, A.; Beare, A.S.; Reed, S.E. In vitro antiviral activity and preliminary clinical trials of a new adamantane compound. Antimicrob. Agents Chemother. 1973, 4, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Brison, E.; Jacomy, H.; Desforges, M.; Talbot, P.J. Novel treatment with neuroprotective and antiviral properties against a neuroinvasive human respiratory virus. J. Virol. 2014, 88, 1548–1563. [Google Scholar] [CrossRef]
- Chen, F.; Chan, K.H.; Jiang, Y.; Kao, R.Y.; Lu, H.T.; Fan, K.W.; Cheng, V.C.; Tsui, W.H.; Hung, I.F.; Lee, T.S.; et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004, 31, 69–75. [Google Scholar] [CrossRef]
- Tanner, J.A.; Zheng, B.J.; Zhou, J.; Watt, R.M.; Jiang, J.Q.; Wong, K.L.; Lin, Y.P.; Lu, L.Y.; He, M.L.; Kung, H.F.; et al. The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem. Biol. 2005, 12, 303–311. [Google Scholar] [CrossRef]
- Daniloski, Z.; Jordan, T.X.; Wessels, H.H.; Hoagland, D.A.; Kasela, S.; Legut, M.; Maniatis, S.; Mimitou, E.P.; Lu, L.; Geller, E.; et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell 2021, 184, 92–105.e116. [Google Scholar] [CrossRef]
- Xie, X.; Lokugamage, K.G.; Zhang, X.; Vu, M.N.; Muruato, A.E.; Menachery, V.D.; Shi, P.Y. Engineering SARS-CoV-2 using a reverse genetic system. Nat. Protoc. 2021, 16, 1761–1784. [Google Scholar] [CrossRef]
- ter Meulen, J.; van den Brink, E.N.; Poon, L.L.; Marissen, W.E.; Leung, C.S.; Cox, F.; Cheung, C.Y.; Bakker, A.Q.; Bogaards, J.A.; van Deventer, E.; et al. Human monoclonal antibody combination against SARS coronavirus: Synergy and coverage of escape mutants. PLoS Med. 2006, 3, e237. [Google Scholar] [CrossRef]
- Rejdak, K.; Fiedor, P.; Bonek, R.; Goch, A.; Gala-Bladzinska, A.; Chelstowski, W.; Lukasiak, J.; Kiciak, S.; Dabrowski, P.; Dec, M.; et al. The use of amantadine in the prevention of progression and treatment of COVID-19 symptoms in patients infected with the SARS-CoV-2 virus (COV-PREVENT): Study rationale and design. Contemp. Clin. Trials 2022, 116, 106755. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Nitsche, A.; Neumann, M.; Grossegesse, M.; Eisele, K.H.; Danysz, W. Amantadine Inhibits SARS-CoV-2 In Vitro. Viruses 2021, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gammeltoft, K.A.; Galli, A.; Offersgaard, A.; Fahnoe, U.; Ramirez, S.; Bukh, J.; Gottwein, J.M. Efficacy of Ion-Channel Inhibitors Amantadine, Memantine and Rimantadine for the Treatment of SARS-CoV-2 In Vitro. Viruses 2021, 13, 2082. [Google Scholar] [CrossRef] [PubMed]
- Leist, S.R.; Dinnon, K.H., 3rd; Schafer, A.; Tse, L.V.; Okuda, K.; Hou, Y.J.; West, A.; Edwards, C.E.; Sanders, W.; Fritch, E.J.; et al. A Mouse-Adapted SARS-CoV-2 Induces Acute Lung Injury and Mortality in Standard Laboratory Mice. Cell 2020, 183, 1070–1085.e1012. [Google Scholar] [CrossRef]
- Vernier, V.G.; Harmon, J.B.; Stump, J.M.; Lynes, T.E.; Marvel, J.P.; Smith, D.H. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol. Appl. Pharmacol. 1969, 15, 642–665. [Google Scholar] [CrossRef]
- Chan, J.F.; Zhang, A.J.; Yuan, S.; Poon, V.K.; Chan, C.C.; Lee, A.C.; Chan, W.M.; Fan, Z.; Tsoi, H.W.; Wen, L.; et al. Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in a Golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility. Clin. Infect. Dis. 2020, 71, 2428–2446. [Google Scholar] [CrossRef]
- Rosenke, K.; Meade-White, K.; Letko, M.; Clancy, C.; Hansen, F.; Liu, Y.; Okumura, A.; Tang-Huau, T.L.; Li, R.; Saturday, G.; et al. Defining the Syrian hamster as a highly susceptible preclinical model for SARS-CoV-2 infection. Emerg. Microbes Infect. 2020, 9, 2673–2684. [Google Scholar] [CrossRef]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef]
- Sia, S.F.; Yan, L.M.; Chin, A.W.H.; Fung, K.; Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Perera, R.; Poon, L.L.M.; Nicholls, J.M.; et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 2020, 583, 834–838. [Google Scholar] [CrossRef]
- Brigham, E.F.; Johnston, T.H.; Brown, C.; Holt, J.D.S.; Fox, S.H.; Hill, M.P.; Howson, P.A.; Brotchie, J.M.; Nguyen, J.T. Pharmacokinetic/Pharmacodynamic Correlation Analysis of Amantadine for Levodopa-Induced Dyskinesia. J. Pharmacol. Exp. Ther. 2018, 367, 373–381. [Google Scholar] [CrossRef]
- Hoffman, H.E.; Gaylord, J.C.; Blasecki, J.W.; Shalaby, L.M.; Whitney, C.C., Jr. Pharmacokinetics and metabolism of rimantadine hydrochloride in mice and dogs. Antimicrob. Agents Chemother. 1988, 32, 1699–1704. [Google Scholar] [CrossRef]
- Toft-Bertelsen, T.L.; Jeppesen, M.G.; Tzortzini, E.; Xue, K.; Giller, K.; Becker, S.; Mujezinovic, A.; Bentzen, B.H.; Andreas, L.B.; Kolocouris, A.; et al. Author Correction: Amantadine inhibits known and novel ion channels encoded by SARS-CoV-2 in vitro. Commun. Biol. 2021, 4, 1402. [Google Scholar] [CrossRef] [PubMed]
- Mandala, V.S.; McKay, M.J.; Shcherbakov, A.A.; Dregni, A.J.; Kolocouris, A.; Hong, M. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020, 27, 1202–1208. [Google Scholar] [CrossRef]
- Harrison, N.L.; Abbott, G.W.; Gentzsch, M.; Aleksandrov, A.; Moroni, A.; Thiel, G.; Grant, S.; Nichols, C.G.; Lester, H.A.; Hartel, A.; et al. How many SARS-CoV-2 “viroporins” are really ion channels? Commun. Biol. 2022, 5, 859. [Google Scholar] [CrossRef]
- deVries, T.; Dentiste, A.; Handiwala, L.; Jacobs, D. Bioavailability and Pharmacokinetics of Once-Daily Amantadine Extended-Release Tablets in Healthy Volunteers: Results from Three Randomized, Crossover, Open-Label, Phase 1 Studies. Neurol. Ther. 2019, 8, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Hoffman, H.E.; Spyker, D.A. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrob. Agents Chemother. 1983, 23, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Sperber, S.J.; Hayden, F.G. Antiviral Chemotherapyand Prophylaxis of Viral Respiratory Disease. Clin. Lab. Med. 1987, 7, 869–896. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ (accessed on 3 October 2022).
- Keyser, L.A.; Karl, M.; Nafziger, A.N.; Bertino, J.S., Jr. Comparison of central nervous system adverse effects of amantadine and rimantadine used as sequential prophylaxis of influenza A in elderly nursing home patients. Arch. Intern. Med. 2000, 160, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Ilyushina, N.A.; Hoffmann, E.; Salomon, R.; Webster, R.G.; Govorkova, E.A. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir. Ther. 2007, 12, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Hurst, B.L.; Wong, M.H.; Bailey, K.W.; Morrey, J.D. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob. Agents Chemother. 2009, 53, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.-Y.; Guo, Z.; Liu, P.; McKay, L.G.A.; Storm, N.; Griffiths, A.; Qu, M.D.; Finberg, R.W.; Somasundaran, M.; Wang, J.P. Anti-SARS-CoV-2 Activity of Adamantanes In Vitro and in Animal Models of Infection. COVID 2022, 2, 1551-1563. https://doi.org/10.3390/covid2110111
Lim S-Y, Guo Z, Liu P, McKay LGA, Storm N, Griffiths A, Qu MD, Finberg RW, Somasundaran M, Wang JP. Anti-SARS-CoV-2 Activity of Adamantanes In Vitro and in Animal Models of Infection. COVID. 2022; 2(11):1551-1563. https://doi.org/10.3390/covid2110111
Chicago/Turabian StyleLim, Sun-Young, Zhiru Guo, Ping Liu, Lindsay G. A. McKay, Nadia Storm, Anthony Griffiths, Ming Da Qu, Robert W. Finberg, Mohan Somasundaran, and Jennifer P. Wang. 2022. "Anti-SARS-CoV-2 Activity of Adamantanes In Vitro and in Animal Models of Infection" COVID 2, no. 11: 1551-1563. https://doi.org/10.3390/covid2110111
APA StyleLim, S.-Y., Guo, Z., Liu, P., McKay, L. G. A., Storm, N., Griffiths, A., Qu, M. D., Finberg, R. W., Somasundaran, M., & Wang, J. P. (2022). Anti-SARS-CoV-2 Activity of Adamantanes In Vitro and in Animal Models of Infection. COVID, 2(11), 1551-1563. https://doi.org/10.3390/covid2110111





