Characterization of CCP: Can We Use Past Convalescent Plasma from COVID-19 Patients for Treatment of New Emerging Variants?
Abstract
Highlights
- Not all past CCP could be used to treat patients with a new SARS-CoV-2 delta and omicron infection due to the lack of specific neutralizing antibodies (Nt-Abs);
- For the moment, the neutralization test remains the gold standard to select potential CCP donors;
- We did not find a statistical difference between Nt-Abs responses in North and South Italy CCP donors.
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. SARS-CoV-2 Variants Isolation and Characterization
2.3. Microneutralization Assay
2.4. Quantitative SARS-CoV-2 TrimericS IgG Measurement
3. Data Analysis
4. Ethical Statement
5. Results
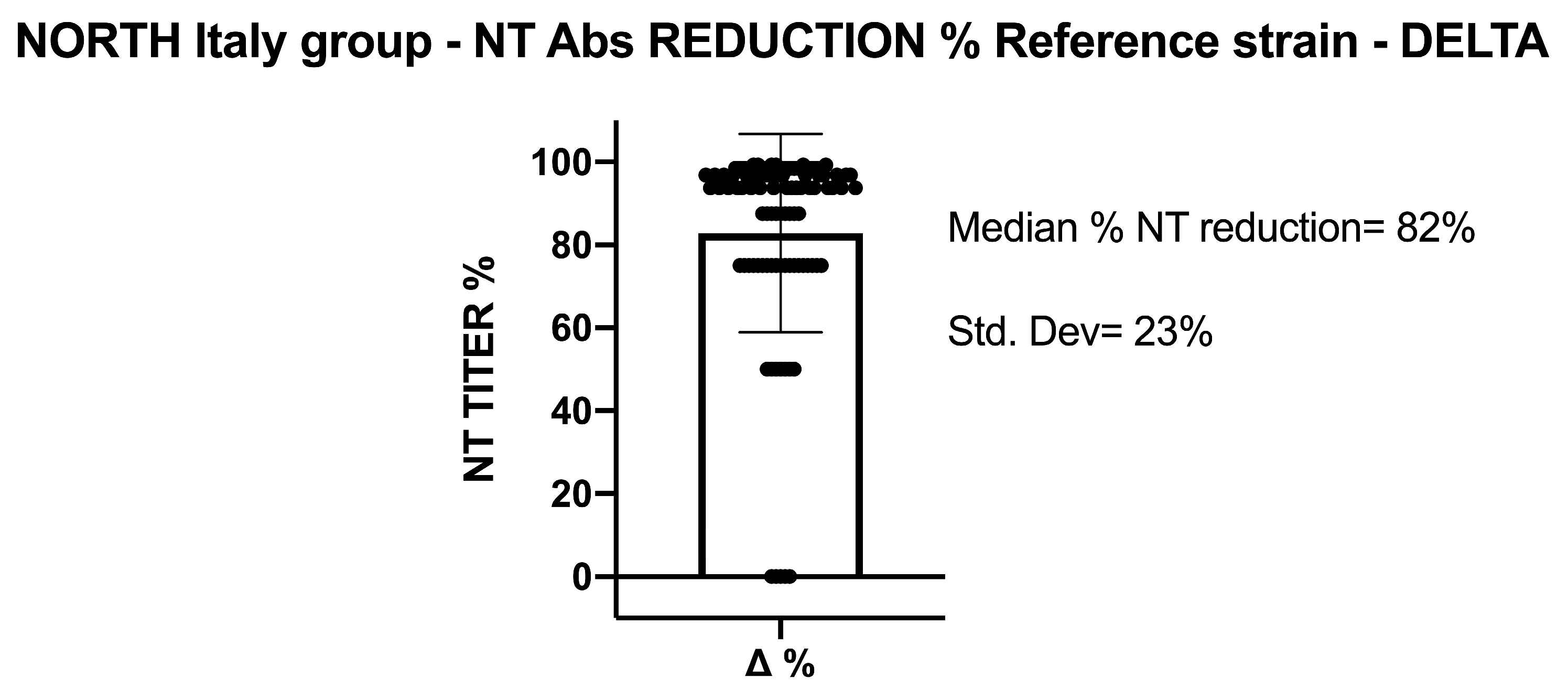
5.1. SARS-CoV-2 NT-Abs in Sera Collected from Northern Italy
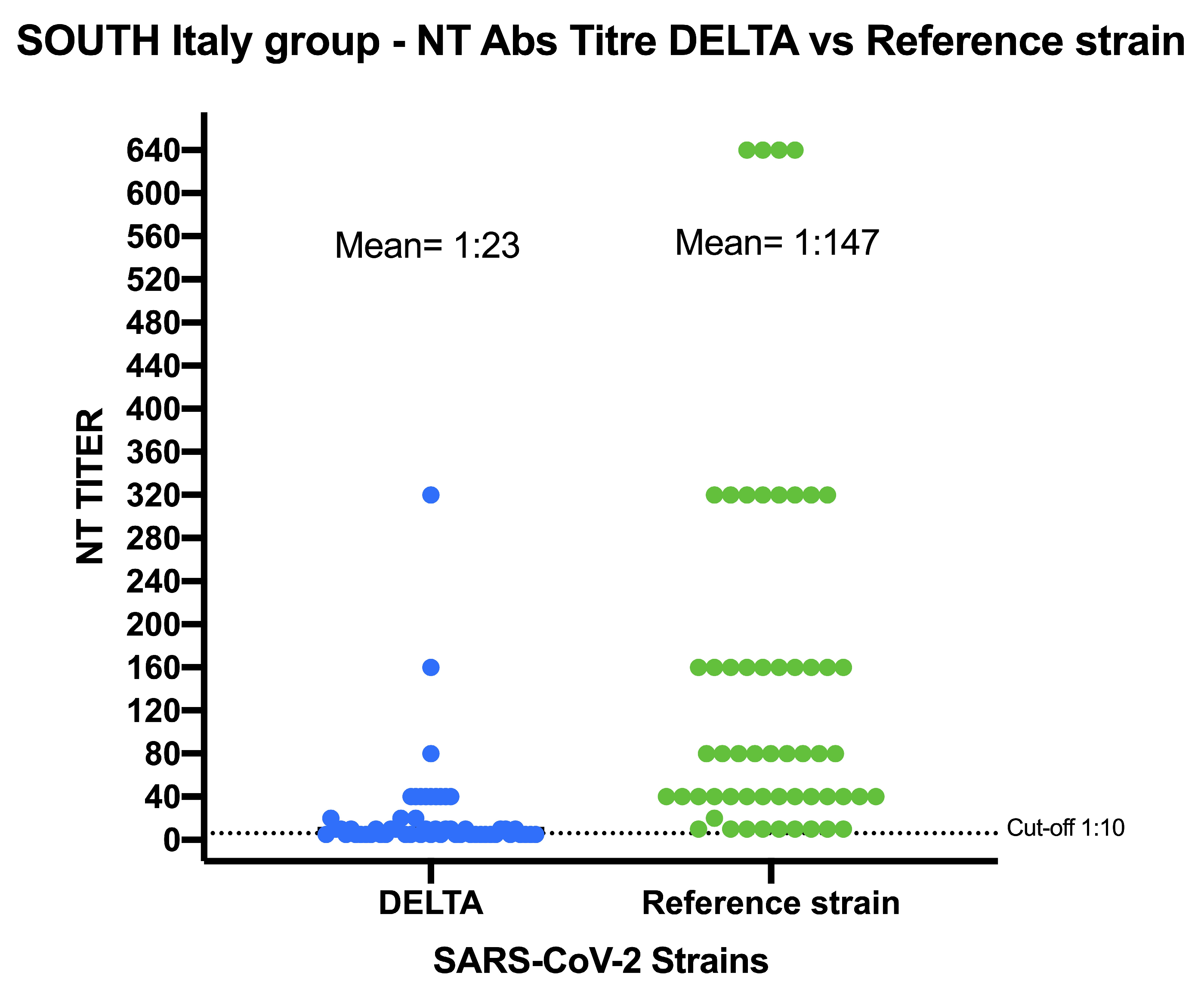
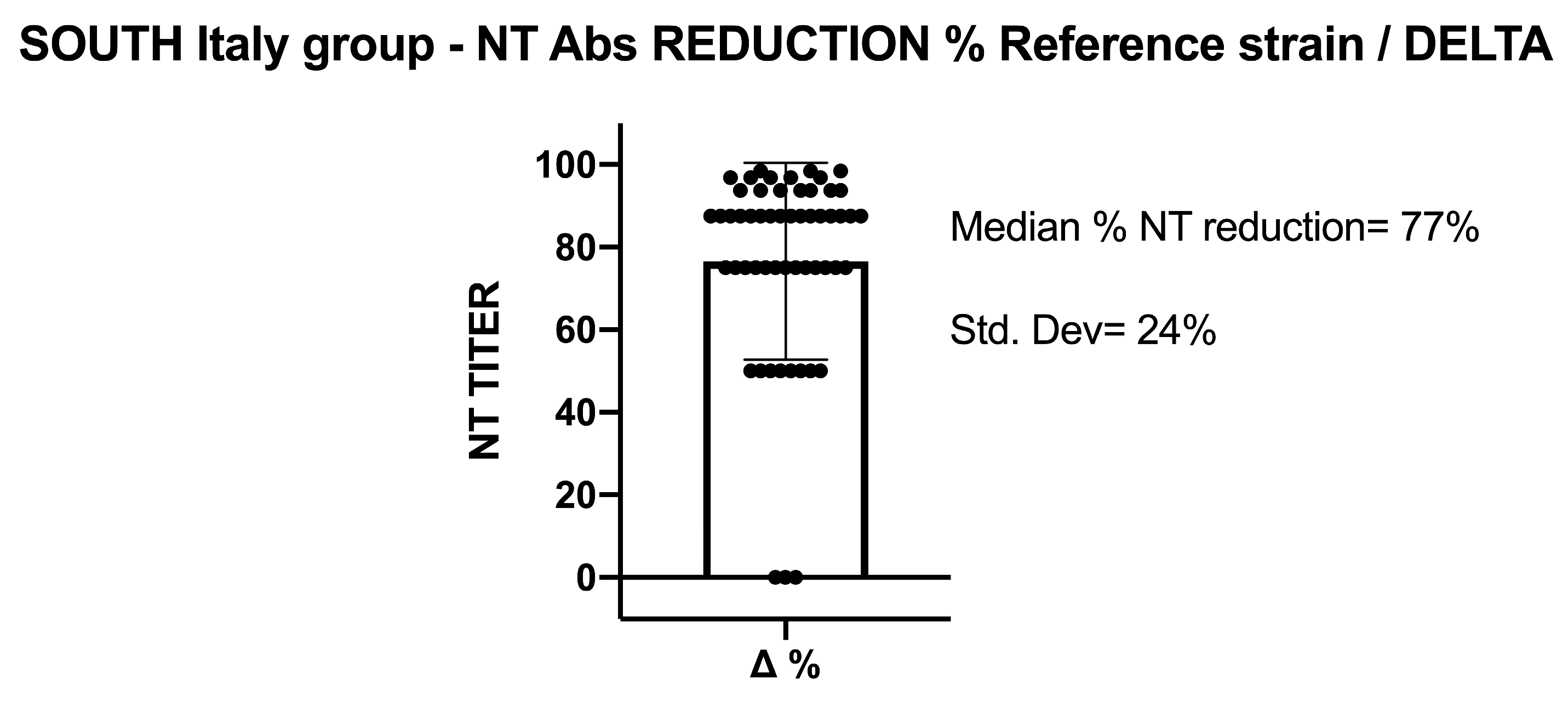
5.2. SARS-CoV-2 NT-Abs in Sera Collected from Southern Italy
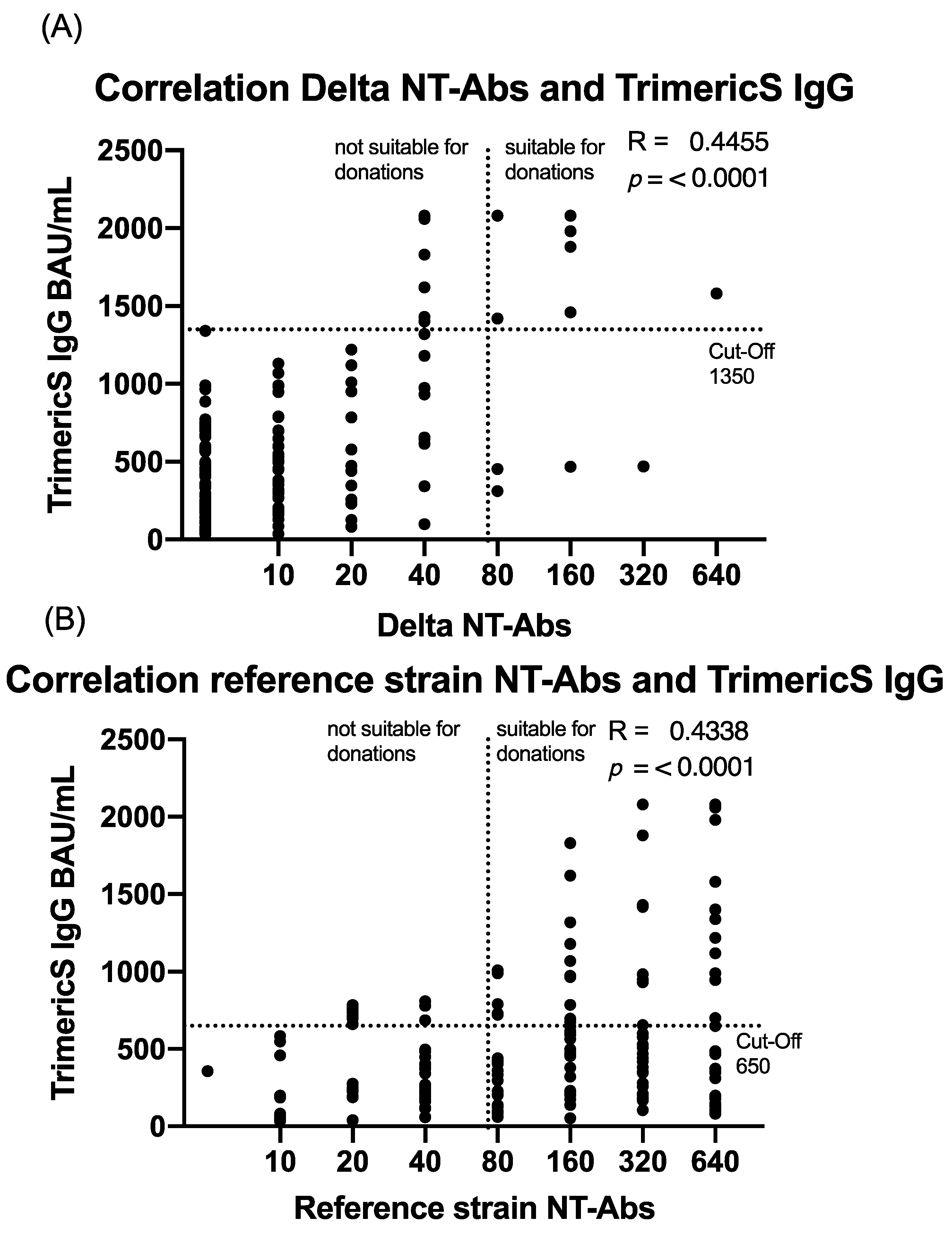
5.3. Characterization of Total IgG Level
5.4. SARS-CoV-2 NT-Abs in Sera Collected from Northern and Southern Italy against Omicron Variant and Characterization of Total IgG Level
6. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- The COVID-19 Vaccine Race|Gavi, the Vaccine Alliance. Available online: https://www.gavi.org/vaccineswork/all (accessed on 3 January 2021).
- Wu, K.; Werner, A.P.; Koch, M.; Choi, A.; Narayanan, E.; Stewart-Jones, G.B.E.; Boyoglu-Barnum, S.; Carfi, A.; Corbett, K.S.; Edwards, D.K. Serum Neutralizing Activity Elicited by mRNA-1273 Vaccine. N. Engl. J. Med. 2021, 384, 1468–1470. [Google Scholar] [CrossRef]
- Franchini, M.; Del Fante, C.; Klersy, C.; Glingani, C.; Percivalle, E.; Baldanti, F.; Perotti, C. Challenges in the Production of Convalescent Hyperimmune Plasma in the Age of COVID-19. Semin. Thromb. Hemost. 2020, 46, 804–806. [Google Scholar] [CrossRef]
- Perotti, C.; Baldanti, F.; Bruno, R.; Del Fante, C.; Seminari, E.; Casari, S.; Percivalle, E.; Glingani, C.; Musella, V.; Belliato, M.; et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica 2020, 105, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Perotti, C.; Del Fante, C.; Baldanti, F.; Franchini, M.; Percivalle, E.; Nepita, E.V.; Seminari, E.; De Silvestri, A.; Bruno, R.; Klersy, C. Plasma from donors recovered from the new Coronavirus 2019 as therapy for critical patients with COVID-19 (COVID-19 plasma study): A multicentre study protocol. Intern. Emerg. Med. 2020, 15, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Garraud, O.; Heshmati, F.; Pozzetto, B.; Lefrere, F.; Girot, R.; Saillol, A.; Laperche, S. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus. Clin. Biol. 2016, 23, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Libster, R.; Marc, G.P.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Perez, L.G.; Sanchez, M.d.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2021, 384, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Gharbharan, A.; Jordans, C.C.E.; GeurtsvanKessel, C.; den Hollander, J.G.; Karim, F.; Mollema, F.P.N.; Dofferhoff, A.; Ludwig, I.; Koster, A.; Hassing, R.-J.; et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat. Commun. 2021, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. The convalescent sera option for containing COVID-19. J. Clin. Investig. 2020, 130, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Alteri, C.; Cento, V.; Piralla, A.; Costabile, V.; Tallarita, M.; Colagrossi, L.; Renica, S.; Giardina, F.; Novazzi, F.; Gaiarsa, S.; et al. Genomic epidemiology of SARS-CoV-2 reveals multiple lineages and early spread of SARS-CoV-2 infections in Lombardy, Italy. Nat. Commun. 2021, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Edwing, H.; Lennette, N.; Schmidt, J. Diagnostic Procedures for Viral and Rickettsial Infections, 4th ed.; American Public Health Association, Inc.: New York, NY, USA, 1969. [Google Scholar]
- Percivalle, E.; Cambiè, G.; Cassaniti, I.; Nepita, E.V.; Maserati, R.; Ferrari, A.; Di Martino, R.; Isernia, P.; Mojoli, F.; Bruno, R.; et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 6 April 2020. Euro Surveill. 2020, 25, 2001031. [Google Scholar] [CrossRef] [PubMed]
- Stanworth, S.J.; New, H.V.; O Apelseth, T.; Brunskill, S.; Cardigan, R.; Doree, C.; Germain, M.; Goldman, M.; Massey, E.; Prati, D.; et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020, 7, e756–e764. [Google Scholar] [CrossRef]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Beck, C.R.; Mateus, A.L.P.; et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Klassen, S.A.; Senefeld, J.W.; Senese, K.A.; Johnson, P.W.; Wiggins, C.C.; Baker, S.E.; van Helmond, N.; Bruno, K.A.; Pirofski, L.-A.; Shoham, S.; et al. Convalescent Plasma Therapy for COVID-19: A Graphical Mosaic of the Worldwide Evidence. Front. Med. 2021, 8, 684151. [Google Scholar] [CrossRef] [PubMed]
- Hagla, Z. In adults, the Oxford/AstraZeneca vaccine had 70% efficacy against COVID-19 >14 d after the 2nd dose. Ann. Intern. Med. 2021, 174, JC29. [Google Scholar]
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, 372, n579. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Kunze, K.L.; Johnson, P.W.; van Helmond, N.; Senefeld, J.W.; Petersen, M.M.; Klassen, S.A.; Wiggins, C.C.; Klompas, A.M.; Bruno, K.A.; Mills, J.R.; et al. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat. Commun. 2021, 12, 4864. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, A.; Cassaniti, I.; Sarasini, A.; Lilleri, D.; Sammartino, J.C.; Del Fante, C.; Baldanti, F.; Percivalle, E.; Perotti, C. Characterization of CCP: Can We Use Past Convalescent Plasma from COVID-19 Patients for Treatment of New Emerging Variants? COVID 2022, 2, 1564-1574. https://doi.org/10.3390/covid2110112
Ferrari A, Cassaniti I, Sarasini A, Lilleri D, Sammartino JC, Del Fante C, Baldanti F, Percivalle E, Perotti C. Characterization of CCP: Can We Use Past Convalescent Plasma from COVID-19 Patients for Treatment of New Emerging Variants? COVID. 2022; 2(11):1564-1574. https://doi.org/10.3390/covid2110112
Chicago/Turabian StyleFerrari, Alessandro, Irene Cassaniti, Antonella Sarasini, Daniele Lilleri, Josè Camilla Sammartino, Claudia Del Fante, Fausto Baldanti, Elena Percivalle, and Cesare Perotti. 2022. "Characterization of CCP: Can We Use Past Convalescent Plasma from COVID-19 Patients for Treatment of New Emerging Variants?" COVID 2, no. 11: 1564-1574. https://doi.org/10.3390/covid2110112
APA StyleFerrari, A., Cassaniti, I., Sarasini, A., Lilleri, D., Sammartino, J. C., Del Fante, C., Baldanti, F., Percivalle, E., & Perotti, C. (2022). Characterization of CCP: Can We Use Past Convalescent Plasma from COVID-19 Patients for Treatment of New Emerging Variants? COVID, 2(11), 1564-1574. https://doi.org/10.3390/covid2110112






