The Safe Campus Project— Resilience of Academic Institutions during the COVID-19 Crisis
Abstract
1. Introduction
2. Theory and Background
2.1. The Green Zone Concept
2.2. Creating a Baseline of Safety by Vaccination and Clean Air
2.3. Testing Strategies
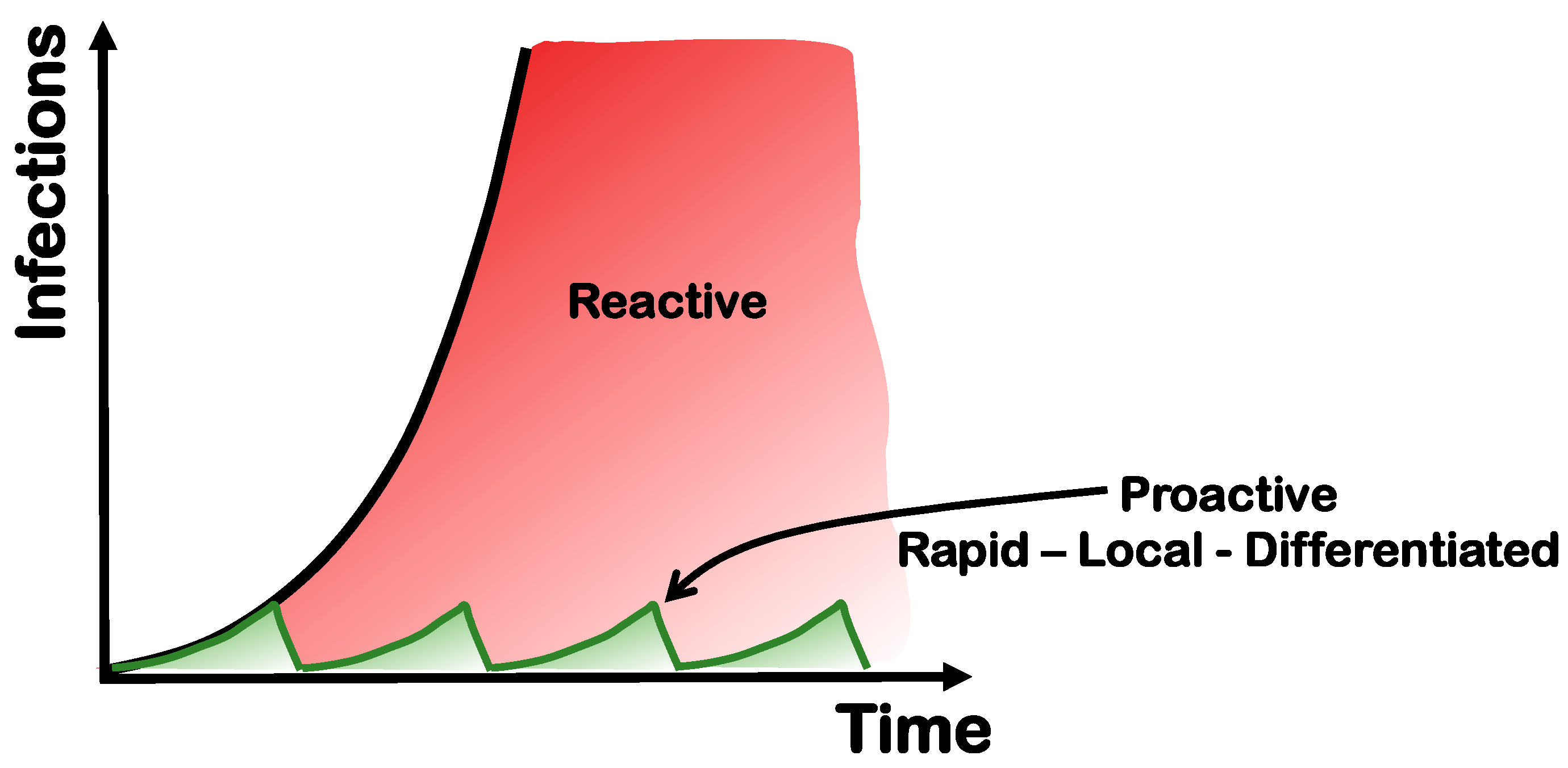
2.4. Massive Testing Instead of Lockdowns
2.5. Local Efforts in a Global Environment
3. Testing Procedures
3.1. Test Strategy 1.0—PCR Plus Antigen
3.2. Why a New Testing Strategy?
3.3. Test Strategy 2.0
3.4. Procedure
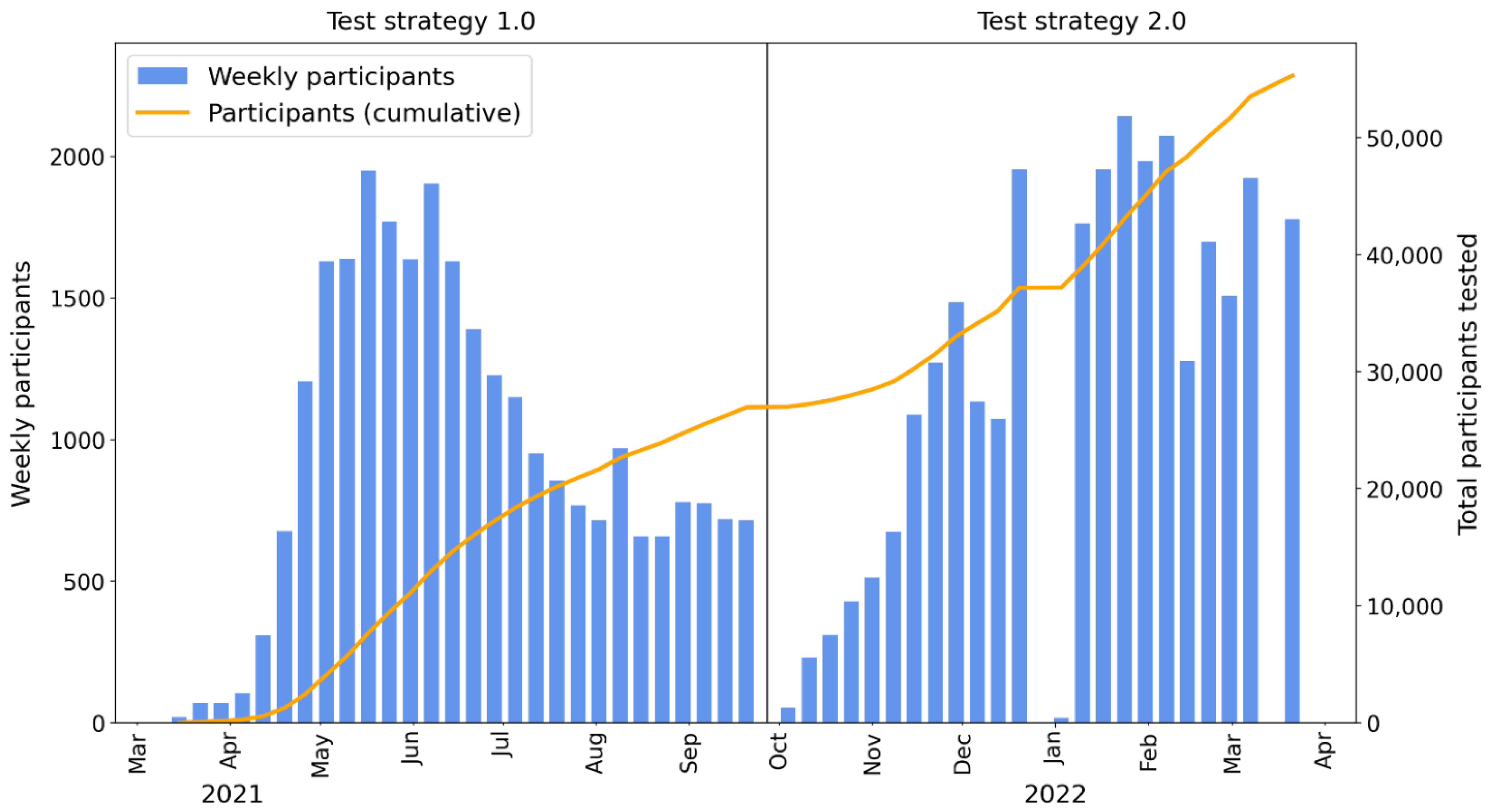
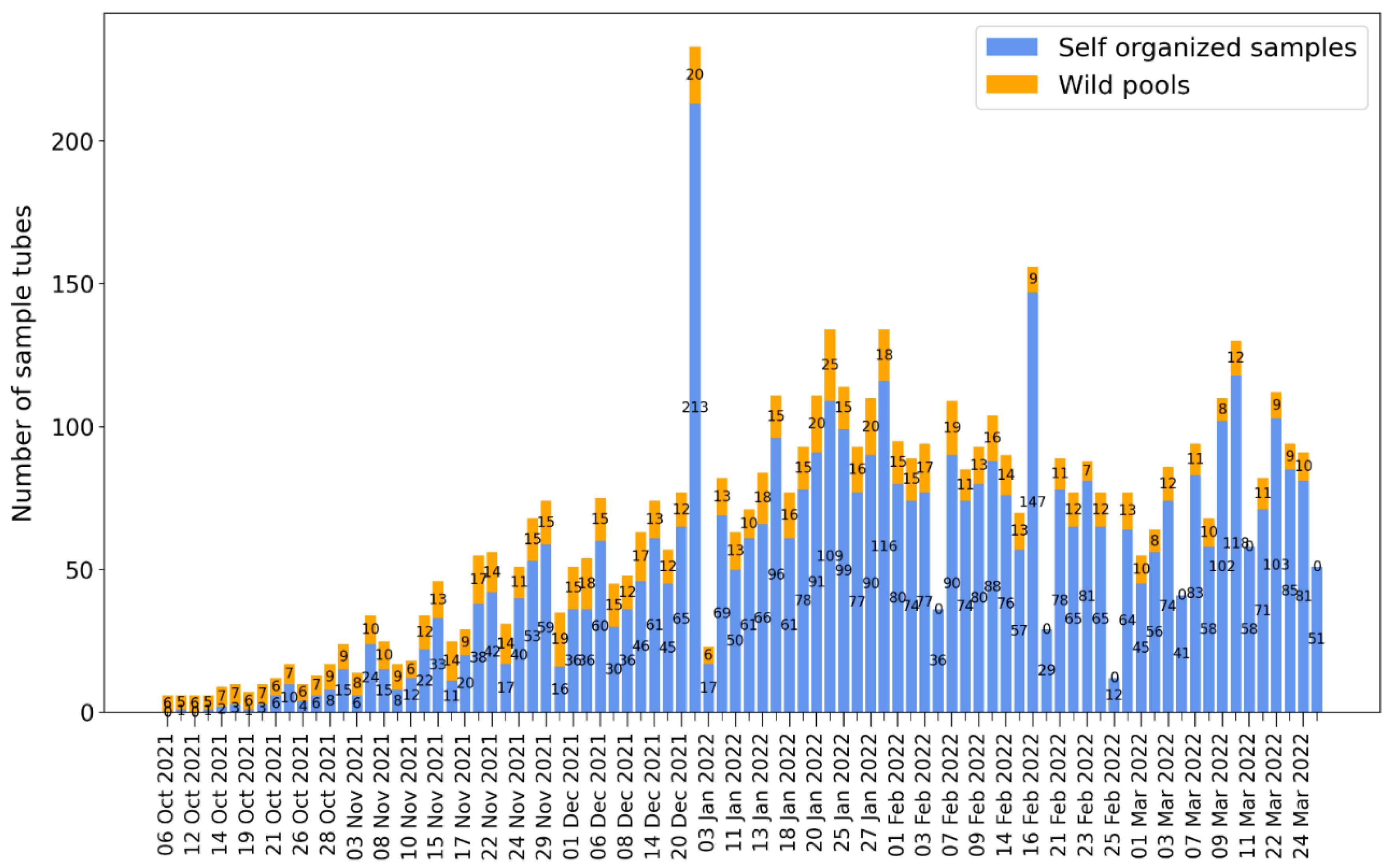
4. Results
5. Discussion
Avoiding Outbreaks
6. Conclusions
7. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philippe, C.; Marques, N. The Zero Covid Strategyprotects People, Economies and Freedoms More Effectively. 2021. Available online: https://www.institutmolinari.org/2021/08/19/the-zero-covid-strategy-protects-people-economies-and-freedoms-more-effectively/ (accessed on 10 June 2022).
- Marshall, M. Zero-Covid Strategies Are Being Ditched, but They Were the Best Option. New Scientist. 30 March 2022. Available online: https://www.newscientist.com/article/mg25433800-100-zero-covid-strategies-are-being-ditched-but-they-were-the-best-option/ (accessed on 10 January 2022).
- Taleb, N.N.; Read, R.; Douady, R.; Norman, J.; Bar-Yam, Y. The Precautionary Principle (with Application to the Genetic Modification of Organisms). arXiv 2014, arXiv:1410.5787. [Google Scholar]
- Bar-Yam, Y. New England Complex Science Insitute. Available online: https://necsi.edu/ending-pandemics (accessed on 10 January 2022).
- Oliu-Barton, M.; Pradelski, B.S.R. Green zoning: An effective policy tool to tackle the COVID-19 pandemic. Health Policy 2021, 125, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Kühn, M.J.; Abele, D.; Mitra, T.; Koslow, W.; Abedi, M.; Rack, K.; Siggel, M.; Khailaie, S.; Klitz, M.; Binder, S.; et al. Assessment of effective mitigation and prediction of the spread of SARS-CoV-2 in Germany using demographic information and spatial resolution. Math. Biosci. 2021, 339, 108648. [Google Scholar] [CrossRef]
- Tindale, L.C.; Stockdale, J.E.; Coombe, M.; Garlock, E.S.; Lau, W.Y.V.; Saraswat, M.; Zhang, L.; Chen, D.; Wallinga, J.; Colijn, C. Evidence for transmission of COVID-19 prior to symptom onset. eLife 2020, 9, 1–34. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 Transmission from People without COVID-19 Symptoms. JAMA Netw. Open 2021, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID-19 vaccines and decreased transmission of SARS-CoV-2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Prather, K.A.; Marr, L.C.; Schooley, R.T.; McDiarmid, M.A.; Wilsonand, M.E.; Milton, D.K. Airborne transmission of SARS-CoV-2. Science 2020, 370, 303–304. [Google Scholar]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef]
- Dal Porto, R.; Kunz, M.N.; Pistochini, T.; Corsi, R.L.; Cappa, C.D. Characterizing the performance of a do-it-yourself (DIY) box fan air filter. Aerosol Sci. Technol. 2022, 56, 1–9. [Google Scholar] [CrossRef]
- Arnaout, R.; Lee, R.A.; Lee, G.R.; Callahan, C.; Cheng, A.; Yen, C.F.; Smith, K.P.; Arora, R.; Kirby, J.E. The Limit of Detection Matters: The Case for Benchmarking Severe Acute Respiratory Syndrome Coronavirus 2 Testing. Clin. Infect. Dis. 2021, 73, E3042–E3046. [Google Scholar] [CrossRef]
- Mina, M.J.; Andersen, K.G. COVID-19 testing: One size does not fit all. Science 2021, 371, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.J.; Peto, T.E.; García-Fiñana, M.; Semple, M.G.; Buchan, I.E. Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19. Lancet 2021, 397, 1425–1427. [Google Scholar] [CrossRef]
- Scheiblauer, H.; Filomena, A.; Nitsche, A.; Puyskens, A.; Corman, V.M.; Drosten, C.; Zwirglmaier, K.; Lange, C.; Emmerich, P.; Muller, M.; et al. Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV-2 antigen, Germany, September 2020 to April 2021. Eurosurveillance 2021, 26, 1–13. [Google Scholar] [CrossRef]
- Esteve, C.; Catherine, F.X.; Chavanet, P.; Blot, M.; Piroth, L. How should a positive PCR test result for COVID-19 in an asymptomatic individual be interpreted and managed? Med. Mal. Infect. 2020, 50, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montero, A.; Argemi, J.; Rodríguez, J.A.; Ariño, A.H.; Moreno-Galarraga, L. Validation of a rapid antigen test as a screening tool for SARS-CoV-2 infection in asymptomatic populations. Sensitivity, specificity and predictive values. eClinicalMedicine 2021, 37, 100954. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.J.; Matl, M.; Ross, J.J.; Schnabl, J.; Handler, D.; Heinen, R.; Schaeffer, J.; Hufnagl, P.; Indra, A.; Dekens, M.P.S.; et al. Head-to-head comparison of direct-input RT-PCR and RT-LAMP against RT-qPCR on extracted RNA for rapid SARS-CoV-2 diagnostics. medRxiv 2021. [Google Scholar] [CrossRef]
- Kühn, M.J.; Abele, D.; Binder, S.; Rack, K.; Klitz, M.; Kleinert, J.; Gilg, J.; Spataro, L.; Koslow, W.; Siggel, M.; et al. Regional opening strategies with commuter testing and containment of new SARS-CoV-2 variants in Germany. BMC Infect. Dis. 2022, 22, 333. [Google Scholar] [CrossRef] [PubMed]
- Larremore, D.B.; Wilder, B.; Lester, E.; Shehata, S.; Burke, J.M.; Hay, J.A.; Tambe, M.; Mina, M.J.; Parker, R. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci. Adv. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Paltiel, A.D.; Zheng, A.; Walensky, R.P. Assessment of SARS-CoV-2 Screening Strategies to Permit the Safe Reopening of College Campuses in the United States. JAMA Netw. Open 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Paul, S.; Lorin, E. Distribution of incubation periods of COVID-19 in the Canadian context. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Baumann, M.; Brinkmann, M.; Beier, M.; Brockmann, D.; Bude, H.; Fuest, C.; Feldner, D.; Hallek, M.; Kickbusch, I.; Mayer, M.; et al. NO-COVID. Part 1 & 2. 2021. Available online: https://nocovid-europe.eu/ (accessed on 10 January 2021).
- Horton, R. Offline: The case for No-COVID. Lancet 2021, 397, 359. [Google Scholar] [CrossRef]
- Dewald, F.; Suárez, I.; Johnen, R.; Grossbach, J.; Tovar, R.M.; Steger, G.; Joachim, A.; Rubio, G.H.; Fries, M.; Behr, F.; et al. Effective high-throughput RT-qPCR screening for SARS-CoV-2 infections in children. Nat. Commun. 2022. [Google Scholar] [CrossRef]
- Joachim, A.; Dewald, F.; Suárez, I.; Zemlin, M.; Lang, I.; Stutz, R.; Marthaler, A.; Bosse, H.M.; Lübke, N.; Münch, J.; et al. Pooled RT-qPCR testing for SARS-CoV-2 surveillance in schools—A cluster randomised trial. eClinicalMedicine 2021, 39, 1–10. [Google Scholar] [CrossRef]
- Katzourakis, A. COVID-19: Endemic doesn’t mean harmless. Nature 2022, 601, 485. [Google Scholar] [CrossRef]
- Kheiroddin, P.; Schöberl, P.; Althammer, M.; Cibali, E.; Würfel, T.; Wein, H.; Kulawik, B.; Buntrock-Döpke, H.; Weigl, E.; Gran, S.; et al. Results of WICOVIR Gargle Pool PCR Testing in German Schools Based on the First 100,000 Tests. Front. Pediatr. 2021, 9, 1–12. [Google Scholar] [CrossRef]



| Temperature | Ramp Rate | Time | Number of Cycles |
|---|---|---|---|
| 53 °C | 3.2 °C per second | 5 min | 1 |
| 85 °C | 3.2 °C per second | 5 min | 1 |
| 95 °C | 3.2 °C per second | 2 min | 1 |
| 95 °C | 3.2 °C per second | 1 s | 40 |
| 62 °C | 2.5 °C per second | 30 s | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, M.F.; Dohmen, L.; Hanisch, D.T.; Haider, G.; Gruhn, A. The Safe Campus Project— Resilience of Academic Institutions during the COVID-19 Crisis. COVID 2022, 2, 1435-1448. https://doi.org/10.3390/covid2100103
Schneider MF, Dohmen L, Hanisch DT, Haider G, Gruhn A. The Safe Campus Project— Resilience of Academic Institutions during the COVID-19 Crisis. COVID. 2022; 2(10):1435-1448. https://doi.org/10.3390/covid2100103
Chicago/Turabian StyleSchneider, Matthias F., Lukas Dohmen, Daniel T. Hanisch, Gregor Haider, and Andreas Gruhn. 2022. "The Safe Campus Project— Resilience of Academic Institutions during the COVID-19 Crisis" COVID 2, no. 10: 1435-1448. https://doi.org/10.3390/covid2100103
APA StyleSchneider, M. F., Dohmen, L., Hanisch, D. T., Haider, G., & Gruhn, A. (2022). The Safe Campus Project— Resilience of Academic Institutions during the COVID-19 Crisis. COVID, 2(10), 1435-1448. https://doi.org/10.3390/covid2100103





