Early Insights into AI and Machine Learning Applications in Hydrogel Microneedles: A Short Review
Abstract
1. Introduction
2. Methodology
2.1. Article Selection and Inclusion Criteria
2.2. Thematic Grouping Developmental Pathways
2.3. Analytical Dimensions
- Thematic Group Description—A synthesized definition of the core AI/ML application within the domain.
- Author-Year Attribution—Each contributing author was assigned to only one group to maintain exclusivity and balance.
- Data Structure—Parameters for analytical comparison that enable systematic evaluation across groups while preserving domain-specific depth.
- Future Work—Suggestions from the authors for advancing the field, harmonized across thematic groups.
- Author(s) and Year—Identifies researchers and publication date, placing studies in chronological context.
- AI/ML—Indicates the use of AI, ML, or both.
- HMN—Specifies the type of material or platform, such as microneedles or hydrogels.
- AI/ML Techniques & Algorithms—Details computational models and data-driven approaches (e.g., CNNs, SVM, PCA, RL).
- Key AI/ML Role/Purpose/Application—Defines primary functions of AI/ML, including predictive modeling, formulation optimization, biosignal analysis, or drug release profiling.
- AI/ML Integration in HMN—Explains how AI/ML is embedded in microneedle systems to enhance functionality.
- AI-Enhanced HMN Features—Highlights functional improvements, such as optimized material selection, microneedle design, enhanced drug release, predictive alerts, or adaptive performance.
- Materials Used—Lists polymers, nanomaterials, or conductive substances employed (e.g., PVA, chitosan, graphene, CNTs).
- AI-Enhanced Targeted Design Features—Describes AI-enabled customization of performance metrics, such as hydrogel porosity, degradability, drug release kinetics, or mechanical-electrical synergy.
- Dataset/Data Type/Size—Provides the nature and scale of data used, including experimental, simulated, field-collected, or real-time streaming data.
- Targeted Application—Defines practical use, including drug delivery, biosensing, wound healing, drug discovery, or precision agriculture.
- Target Drug Parameter—Lists compounds or physiological markers studied (e.g., glucose, doxorubicin, environmental toxins).
- Targeted Parameter/Sickness—Specifies targeted diseases or conditions (e.g., diabetes, cancer, crop stress, environmental contamination).
- Results, Limitations, and Future Work—Summarizes performance indicators (accuracy, sensitivity), acknowledged limitations (data availability, computational cost, biocompatibility), and anticipated research directions (closed-loop optimization, clinical testing, AI-health integration).
2.4. Methodological Strengths and Limitations
3. Developmental Pathway of Five Thematic Groupings
3.1. Thematic Group 1: Material and Microneedle Design
| (A) Thematic Group 1: Material and Microneedle Design | |||||||||
| Author (Year) | AI/ML Both | Hydrogel Microneedle HMN | AI/ML Techniques& Algorithms | Key AI/ML Role/ Purpose Application | AI/MLIntegration Innovation in HMN | AI-Enhanced HMN Features | AI-Enhanced Targeted Design Features | ||
| 1 | He W et al. [21] | ML | HMN | XGBoost, SVM, RF, PCA, K-means, CNN, RNN, GNN, GAT, BO. | Material screening, drug release, pain prediction, stress analysis | Pain minimization via FEA-COMSOL, BO.; CNN for defect detection | Stability, defect detection, optimized length, reduced pain, improved flow & geometry | Design, QC, material selection, geometry, stress, flow, comfort | |
| 2 | Teodoro et al. [22] | Both | HMN | Predictive modeling, generative design, supervised ML, FEM | Defect prediction, design optimization, signal tuning | Predictive modeling and performance | Geometry, signal response | Geometry, drug release kinetics, Insertion force, signal mapping | |
| 3 | Negut & Bita [10] | Both | Hydrogel | DOE, PCA ANN, SVM, RF, CNN, GAN, DNN | Modeling, formulation optimization, release kinetics | Virtual screening, ML-guided synthesis, gelation, print QC | Mechanical strength, precise release, swelling, degradation | Porosity, biodegradability, swelling, printability, delivery tuning | |
| 4 | Finster et al. [23] | Both | Hydrogel | ANN, DNN, XGBoost, PCA, DDPG, DFT, FEA, MD, CFD, SVM, RF, NN, GA | Optimize formulations, predict properties, automate bioprinting | Conductive hydrogel design, real-time sensing, ML-guided tuning | Mechanical strength, precise release, electrical, adhesive properties | Porosity, conductivity, mech–electrical synergy, biocompatibility | |
| 5 | Ji Wang et al. [24] | Both | HMN | AlphaFold, ML design tool | mRNA sequence design and structure prediction | AlphaFold for LNP-mRNA structure optimization | Enhanced wound healing via mRNA design | Predictive targeting for pro-healing proteins | |
| (B) Thematic Group 1: Material and Microneedle Design | |||||||||
| Author (Year) | Materials Used | Dataset/Data Type/Size | Targeted Application | Target Drug/Biosensing Parameter | Targeted Parameter/ Sickness | Results | Limitations | Future Work | |
| 1 | He W et al. [21] | Hydrogel, sugar, Silicon, glass, metals, polymers, sugar, ceramics | Simulated (30 k), empirical (2.4 k) | Drug delivery, biosensing, wound healing, dermatology | Triamcinolone, glucose, Minoxidil, TA, acyclovir, AZA, MTX, VEGF, PBNs, SD-208, EGCG, quercetin, etc. | Diabetes, Alopecia, acne, herpes, lupus, psoriasis, wounds, cancer | CNN = 96% accuracy, GAT = 8.3 × 10−5 MPa MSE, optimized flow & pain scores | Limited clinical trials, High cost (MEMS, TPP), complexity, data volume | IoT integration, personalized models, real-time ML feedback, explainable AI |
| 2 | Teodoro et al. [22] | PVA, Ag NPs, methacrylated polymers, Thermoplastics, UV polymers | Simulated + empirical | Biosensing, Diagnostics | Glucose, Lactate | Biosensing, drug delivery, Diabetes, Metabolic disorder | Thiram LOD: 10-710-7 M; 95% Cu release, Enhanced sensitivity and wearable design | Scalability, data gaps, biocompatibility, regulatory hurdles | AI-closed-loop design, smart integration |
| 3 | Negut & Bita [10] | Alginate, PNIPAAm, PEG NIPAAm, AAc, PEG, PLGA, PVA, Chitosan | Experimental (small), simulated (large) | Tissue engineering Drug delivery, biosensing | Antibiotic permeability Doxorubicin, glucose, stress | Bacterial infections Cancer, chronic wounds | Accurate modeling (93.5% CNN accuracy) | Data scarcity, model opacity, limited interpretability | DL for personalization, domain-integrated ML, scalable data |
| 4 | Finster et al. [23] | Chitosan, PNIPAm, carbon nanotubes, PEDOT:PSS, PVA, polyaniline, alginate | Experimental/Simulated | Drug delivery, wound healing, Bioelectronics Biosensing, Neuromodulation | Doxorubicin, PTEN inhibitors, Glucose, dopamine, electrical signals | Diabetic ulcers, infections, Neurodegeneration | 95% gesture recognition, 40% fewer trials, Higher accuracy, faster optimization | Small datasets, high computational cost, Data scarcity, transfer-ability | Multi-objective RL, clinical trials, real-time ML, feedback loop |
| 5 | Ji Wang et al. [24] | MXene hydrogel MNs + HA + LNPs | AlphaFold mRNA design | Wound healing therapy | Triplet mRNA: PDGF, FGF-7, VEGF | Diabetic wound healing | 92.33% wound closure in 12 days | Scalability, human trial lacking | Anti-inflammatory mRNA, real-time monitoring, clinical trials |
3.2. Thematic Group 2: Microneedles for Diagnostics and Therapy
| (A) Thematic Group 2: Microneedles for Diagnostics and Therapy | |||||||||
| Author (Year) | AI/ML Both | Hydrogel Microneedle HMN | AI/ML Techniques &Algorithms | Key AI/ML Role/Purpose Application | AI/ML Integration Innovation in HMN | AI-Enhanced HMN Features | AI-Enhanced Targeted Design Features | ||
| 1 | Ashraf et al. [25] | Both | MN | ML, DL, supervised/unsupervised, signal denoising | Noise reduction, biomarker prediction, sensor calibration, signal filtering, diagnostics | Integrated into wearable MNs for real-time diagnostics | Predictive sensing, personalized biosensing, improved accuracy, adaptive calibration | Signal calibration, multi-biomarker detection | |
| 2 | S. Wang et al. [26] | Both | HMN | AI, ANN, Logic Encoding, IoT | Logic encoding, real-time monitoring, smart tracking, adaptive therapy | Programmable MNs, AI-driven control, therapeutic pathways | Adaptive release, biosensing, multi-therapy MNs, remote control | Multi-disease targeting, drug timing control | |
| 3 | Merzougui et al. [27] | AI (proposed) | HMN | Not specified (general AI/ML for data analysis) | Enhance diagnostic accuracy, real-time POC analysis | AI for biomarker signal interpretation | Signal filtering, noise reduction, automated quantification | Data processing efficiency | |
| 4 | Liu et al. [28] | Both | HMN | XGBoost, LSTM | Closed-loop inflammation monitoring | Real-time biosensing with integrated feedback | Theranostic system for RA | Prediction of cytokine levels & treatment response | |
| 5 | Xiao et al. [29] | Both | HMN | MobileNet, PCA | Smartphone-assisted pH detection | AI-visualization via smartphone fluorescence | Real-time wound pH monitoring | Dynamic healing status identification | |
| (B) Thematic Group 2: Microneedles for Diagnostics and Therapy | |||||||||
| Author (Year) | Materials Used | Data Type/Size | Targeted Application | Biosensing Parameter | Targeted Parameter/ Sickness | Results | Limitations | Future Work | |
| 1 | Ashraf et al. [25] | Metals, semiconductors, polymers, colorimetric/electrochemical MN | Simulated/empirical (small data), High-dimensional time-series biosensor data | Biosensing, therapy, Non-invasive diagnostics, AI-guided monitoring | Glucose, lactate, cortisol, nucleic acids, pH, electrolytes | Glucose, Diabetes, cancer, inflammation, autoimmune disease | AI-enabled real-time monitoring with high-fidelity, adaptive biosensing | High accuracy, limited scalability; ISF variability, signal drift, costly integration | AI-driven closed-loop systems, novel materials, Standardized datasets, clinical trials, cost savings, regulatory compliance |
| 2 | S. Wang et al. [26] | GelMA, PLGA, PVP, metals MeHA, PEGDA, PDA, GO, AuNR, BP, MOFs | Experimental (in vivo/in vitro) | Drug delivery, biosensing Wound healing, cancer, diabetes, obesity, alopecia | Insulin, chemotherapeutics, ROS, TNF-α, VEGF, NO | Cancer, Tumor, wounds, diabetes | 12-day wound healing, tumor suppression Strong therapeutic outcomes | Preclinical stage; no AI; mechanical design; complex data handling | AI-driven MN chips, clinical trials, Wireless AI control, full wearable systems |
| 3 | Merzougui et al. [27] | Polymers, hydrogels, metals | Simulated/empirical (small datasets) | Biosensing (cancer biomarkers) | Tyrosinase, CA15-3, GPC1 exosomes | Breast cancer, melanoma, colorectal cancer | Detection limits: 0.52 μM–1.1 copies/μL | Low sample volume, no clinical AI validation | AI-driven portable readers, multiplex detection |
| 4 | Liu et al. [28] | PPy-based HMNs(PEGDA + dopants) | Impedance, temp, clinical score (RA rats) | RA drug delivery + inflammation monitoring | Methotrexate (MTX), Rhodamine B | Rheumatoid Arthritis | Paw swelling ↓40%, cytokines ↓, ML AUC = 0.787 | ML accuracy (76.74%), rat-only study | Closed-loop system, multiplexing, human trials |
| 5 | Xiao et al. [29] | MOF-HMNs, fluorescent reagents | Smartphone image dataset (fluorescence pH data) | Wound pH monitoring | No drug (self-sterilization + pH monitoring) | Chronic wounds, sepsis prevention | Reliable pH detection via ML, self-sterilization confirmed | No clinical data, device scalability | Clinical trials, biomarker expansion, IoT integration |
3.3. Thematic Group 3: Microneedles for Drug Delivery
| (A) Thematic Group 3: Microneedles for Drug Delivery | |||||||||
| Author (Year) | AI/ML Both | Hydrogel Microneedle HMN | AI/ML Techniques & Algorithms | Key AI/ML Role/Purpose Application | AI/ML Integration Innovation in HMN | AI-Enhanced HMN Features | AI-Enhanced Targeted Design Features | ||
| 1 | Albayati et al. [30] | Both | HMN | DNN, ANN, BioSIM, COMSOL, KNN, SVM | Personalized delivery, predictive skin permeability, diffusion & release modeling | Optimized MN geometry, ML-integrated skin data, AI-driven real-time delivery | Permeability prediction, real-time dosing, personalized delivery, spatial MN design, kinetic control | Drug release precision, Microneedle geometry, diffusion control | |
| 2 | Aghajani et al. [31] | Proposed | HMN | No specific technique applied | Future optimization of delivery systems | Briefly mentioned (AI for future optimization) | None (theoretical potential) | Sustained release, drug penetration, Riluzole CNS targeting | |
| 3 | Suriyaamporn et al. [32] | Both | HMN | RF, PCA, SVM, ANN | Optimization of formulation & release prediction | AI-guided optimization of PEGylated formulations | Improved drug permeation and cancer cell apoptosis | Feature extraction for enhanced therapeutic targeting | |
| 4 | Bagde et al. [33] | Both | Hydrogel | CNN | Quality control and drug diffusion modeling | CNN-based image inspection of MN prints | Controlled ibuprofen release | Biphasic delivery modeling with CNN | |
| 5 | Yuan et al. [34] | Both | HMN | XGBoost, Ridge, Lasso, SVR | Drug flux prediction | XGBoost outperforming traditional Fick models | Data-driven simulation of MN performance | Multi-drug transport modeling | |
| 6 | Zong et al. [35] | Both | HMN | Real-time sensor monitoring, adaptive ML algorithms | Optimize gas production, drug penetration, safety control | Dynamic feedback for microbial metabolism and gas/drug control | Real-time monitoring, 94% dermal retention, controlled gas propulsion | Spatiotemporal drug release, minimized systemic exposure | |
| (B) Thematic Group 3: Microneedles for Drug Delivery | |||||||||
| Author (Year) | Materials Used | Dataset/Data Type/Size | Targeted Application | Target Drug/Biosensing Parameter | Targeted Parameter/Sickness | Results | Limitations | Future Work | |
| 1 | Albayati et al. [30] | Chitosan, ethosomes, liposomes, PLA, hydrogels, metallic/biodegradable MNs (referenced) | Clinical trials, simulated & empirical wearable biosensor data | Personalized transdermal drug delivery and biosensing | Acyclovir, SARS-CoV-2 vaccine, insulin, lidocaine, fentanyl, biologics; pH, temp, hydration | HSV, COVID-19, Alzheimer’s, Diabetes, pain, hormone therapy, neurological diseases | Fewer side effects; improved dose prediction, adaptive delivery, enhanced bioavailability | Data bias, dataset standardization, skin variability, wearable integration complexity | AI-nano integration, real-time AI patches, IoT feedback, smart skin models, AI-guided fabrication |
| 2 | Aghajani et al. [31] | Polymers, lipids (nano-particles); phospho-lipids (liposomes), micelles | Preclinical (rodents), small trials, review-based analysis | Drug delivery | Riluzole | ALS (Amyotrophic Lateral Sclerosis) | Improved CNS riluzole delivery in animal models, better compliance | Regulatory barriers, scalability, cost, BBB penetration, patient variability, no AI | AI-driven optimization, MN refinement, AI/ML for personalization & scale-up |
| 3 | Suriyaamporn et al. [32] | Crosslinked hydrogel MNs (PEGylated liposomes, HPβCD, limonene) | 75 formulations (experimental dataset) | Transdermal chemotherapy | 5-FU (fluorouracil) | Non-melanoma skin cancer (BCC, SCC) | 91.5% apoptosis, 41.78% permeation, >90% recovery | Limited to in vitro/ex vivo, scalability issues | Clinical trials, other drugs, real-time tracking, long-term stability |
| 4 | Bagde et al. [33] | PEGDAMA-based resin MNs | CNN-analyzed print images + in vivo drug data | Transdermal drug delivery | Ibuprofen (IBU) | Pain/inflammation | Sustained release (AUC = 62,812 ng/mL*h), biphasic delivery | Single drug, small-scale testing | Biologic delivery, clinical trials, IoT integration |
| 5 | Yuan et al. [34] | Hydrogel & plastic MNs | 191 points (6 drugs) for ML prediction | Transdermal drug delivery simulation | Caffeine, Lidocaine, Rhodamine B, GHK, BSA, Copper | Generic delivery modeling | XGBoost: R2 = 0.98; better than Fick’s law | Small, unbalanced dataset | Expand data, model validation, clinical integration |
| 6 | Zong et al. [35] | Gas-permeable hydrogels, thermosensitive polymers | Experimental, porcine & murine models | Transdermal drug delivery | Calcipotriol, H2, NO, H2S | Psoriasis, tumors, chronic wounds | 94% dermal drug retention, 1000 μm depth, effective symptom reduction | Microbial safety, gas control precision, clinical scalability | Use safer microbes, smart hydrogels, regulatory advancement |
3.4. Thematic Group 4: Drug Development for Microneedles
| (A) Thematic Group 4: Drug Development for Microneedles | |||||||||
| Author (Year) | AI/ML Both | Hydrogel Microneedle HMN | AI/ML Techniques & Algorithms | Key AI/ML Role/ Purpose Application | AI/ML Integration Innovation in HMN | AI-Enhanced HMN Features | AI-Enhanced Targeted Design Features | ||
| 1 | Biswas et al. [15] | Both | HMN | Voting Regressor, Ensemble ML | Prediction of transdermal drug delivery | Model ensemble for flux prediction | Improved RMSE in simulated release | Framework validation across various drugs | |
| 2 | Reddy et al. [36] | Both (conceptual) | HMN | Predictive modeling, deep learning (conceptual) | Design optimization, personalized dosing, feedback systems | Conceptual integration with biosensors and smart release | Proposed: Real-time feedback, adaptive dosing | Responsive release, closed-loop regulation (future) | |
| (B) Thematic Group 4: Drug Development for Microneedles | |||||||||
| Author (Year) | Materials Used | Dataset/Data Type/Size | Targeted Application | Target Drug/ Biosensing Parameter | Targeted Parameter/Sickness | Results | Limitations | Future Work | |
| 1 | Biswas et al. [15] | Hydrogel MNs and solid MNs | Dataset from Yuan et al., ML predictions | Drug permeation prediction | Unspecified | General drug delivery | Voting regressor RMSE: 3.24 (percentage), 654.94 (amount) | No experimental validation | Advanced models, expanded datasets, clinical validation |
| 2 | Reddy et al. [36] | Chitosan, HA, gelatin, PEG, PVA, smart hydrogels | Review, multiple cited works | Smart drug delivery | Insulin, doxorubicin, 5-FU, curcumin, antibiotics | Diabetes, cancer, infection, pain, inflammation | Improved compliance, targeted release, reduced toxicity | Toxicity, clinical translation, regulation, cost | AI integration, feedback DDS, better targeting and biocompatibility |
3.5. Thematic Group 5: Microneedles Sensors for Health and Agriculture
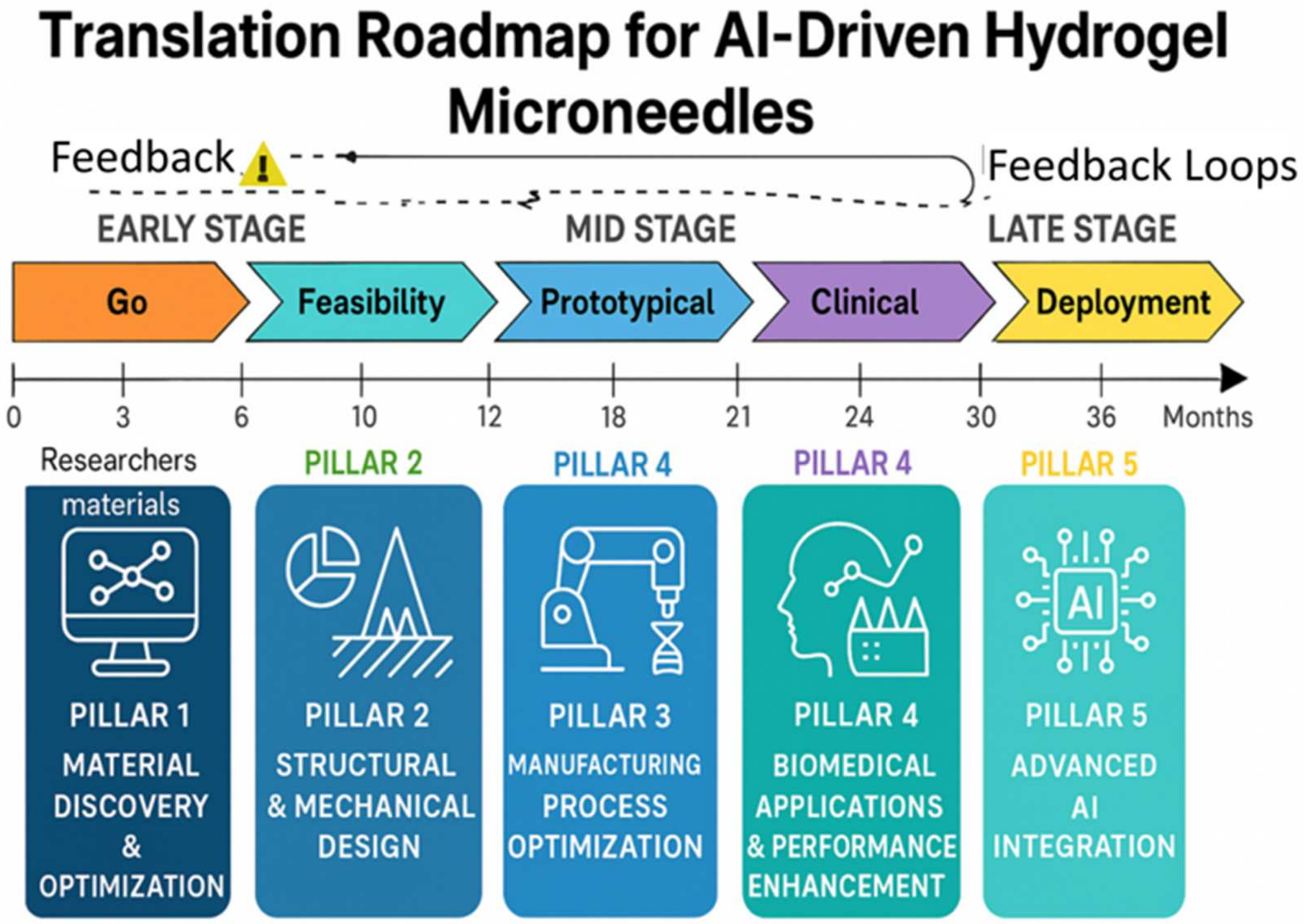
4. Development Pathways for AI/ML Applications in Hydrogel Microneedles
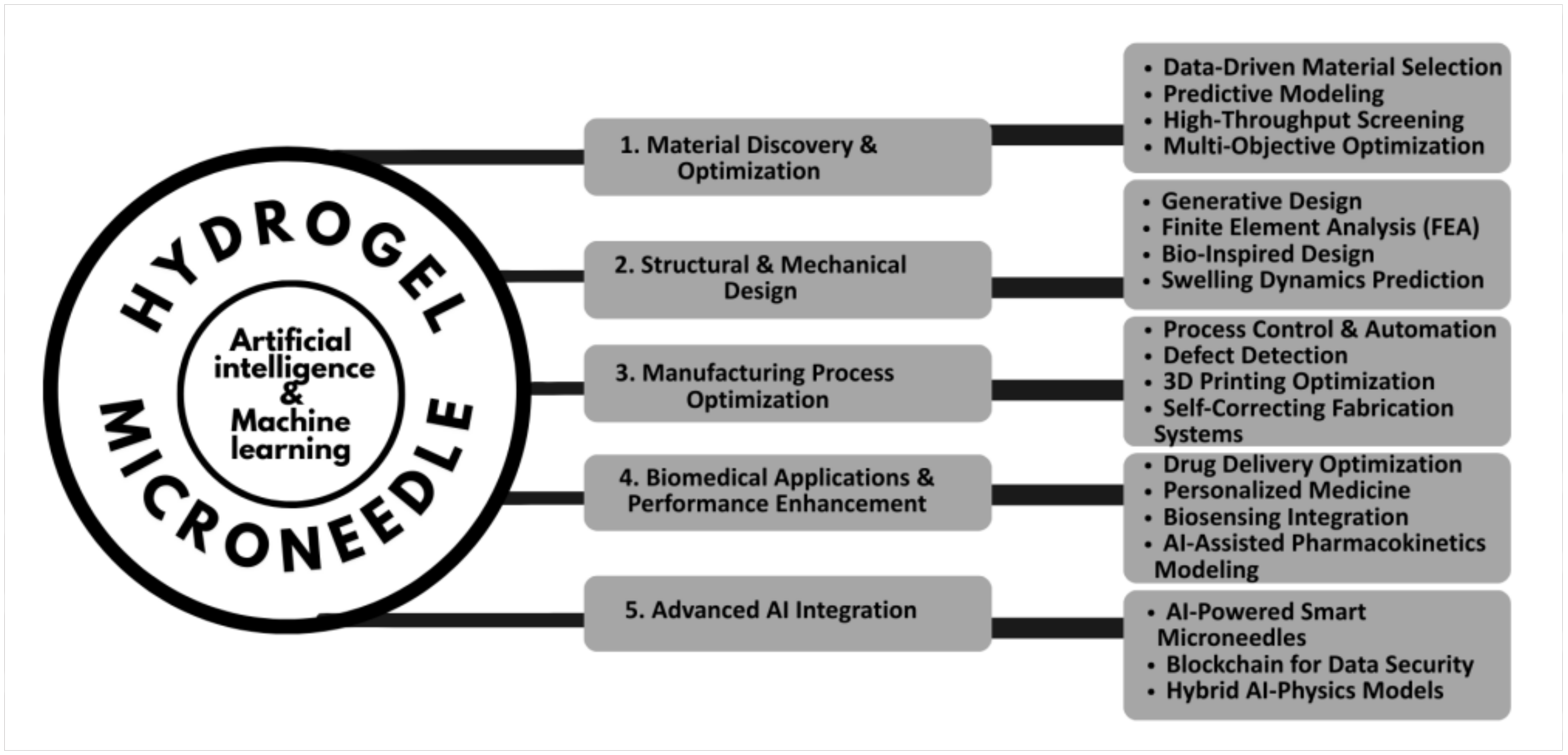
4.1. Pillar 1: Material Discovery & Optimization
4.2. Pillar 2: Structural & Mechanical Design
4.3. Pillar 3: Manufacturing Process Optimization
4.4. Pillar 4: Biomedical Applications & Performance Enhancement
4.5. Pillar 5: Advanced AI Integration
4.6. Synergistic Impact and Future Directions
5. Challenges & Future Work
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
| HMN | Hydrogel Microneedle |
| MN | Microneedle |
| AIM-DO | AI-driven Microneedle Design Optimization |
| AI | Artificial Intelligence |
| ML | Machine Learning |
| CNN | Convolutional Neural Network |
| RNN | Recurrent Neural Network |
| GAN | Generative Adversarial Network |
| RL | Reinforcement Learning |
| SVM | Support Vector Machine |
| RF | Random Forest |
| PCA | Principal Component Analysis |
| FEA/FEM | Finite Element Analysis/Method |
| CFD | Computational Fluid Dynamics |
| COMSOL | Multiphysics Simulation Software |
| PVA | Polyvinyl Alcohol |
| PEG/PEGDA | Poly(ethylene glycol)/PEG Diacrylate |
| HA | Hyaluronic Acid |
| MOFs | Metal–Organic Frameworks |
| LNP | Lipid Nanoparticle |
| QC | Quality Control |
| PK/PD | Pharmacokinetics/Pharmacodynamics |
| IoT | Internet of Things |
| LOD | Limit of Detection |
| MARD | Mean Absolute Relative Difference |
References
- Omidian, H.; Dey Chowdhury, S. Multifunctional Hydrogel Microneedles (HMNs) in Drug Delivery and Diagnostics. Gels 2025, 11, 206. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, H.; Liao, Z.; Gao, B.; He, B. Bridging the gap between invasive and noninvasive medical care: Emerging microneedle approaches. Anal. Chem. 2023, 95, 515–534. [Google Scholar] [CrossRef]
- Ahmad, N.N.; Ghazali, N.N.N.; Wong, Y.H. Concept design of transdermal microneedles for diagnosis and drug delivery: A review. Adv. Eng. Mater. 2021, 23, 2100503. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Abu Sofian, A.D.A.B.; Razak, S.I.A.; Shamsuddin, S.A.A. Advancing 3D printing through integration of machine learning with algae-based biopolymers. Mater. Today Bio 2024, 25, 100987. [Google Scholar] [CrossRef]
- Wang, K.; Lin, Y.; Cheng, X. Smart wearable sensor fuels noninvasive body fluid analysis. ACS Appl. Mater. Interfaces 2025, 17, 12345–12356. [Google Scholar] [CrossRef]
- Boppana, V.R. Machine Learning and AI Learning: Understanding the Revolution. J. Innov. Technol. 2022. [Google Scholar] [CrossRef]
- Abdullah, A.C.; Ahmadinejad, E.; Tasoglu, S. Optimizing Solid Microneedle Design: A Comprehensive ML-Augmented DOE Approach. ACS Meas. Sci. Au 2024, 4, 504–514. [Google Scholar] [CrossRef]
- Akhtar, Z.B. Exploring biomedical engineering (BME): Advances within accelerated computing and regenerative medicine for a computational and medical science perspective exploration analysis. J. Emerg. Med. OA 2024, 2, 1–23. [Google Scholar]
- Negut, I.; Bita, B. Exploring the potential of artificial intelligence for hydrogel development—A short review. Gels 2023, 9, 123–135. [Google Scholar] [CrossRef]
- Parvin, B.G.; Parvin, L.G. Enhancing mechanical properties of materials using artificial intelligence. Mater. Today 2023, 58, 45–58. [Google Scholar]
- Shriky, B.; Naji, L.; Aljabali, A.; Alzahrani, K. Dissolving and swelling hydrogel-based microneedles: An overview. Drug Deliv. Transl. Res. 2023, 13, 1890–1905. [Google Scholar]
- Mohite, P.; Shah, D.; Patel, D. Hydrogel-forming microneedles in the management of dermal disorders through a non-invasive process. Int. J. Pharm. 2024, 650, 123456. [Google Scholar] [CrossRef] [PubMed]
- Qallaf, B.; Das, D.B. Transdermal drug delivery by coated microneedles: Geometry effects on effective skin thickness and drug permeability. Chem. Eng. Res. Des. 2008, 86, 1196–1206. [Google Scholar] [CrossRef]
- Biswas, A.A.; Dhondale, M.R.; Singh, M.; Agrawal, A.K.; Muthudoss, P.; Mishra, B.; Kumar, D. Development and comparison of machine learning models for in-vitro drug permeation prediction from microneedle patch. Eur. J. Pharm. Biopharm. 2024, 199, 114311. [Google Scholar] [CrossRef]
- Luciano, A.; Reale, P.; Cutaia, L.; Carletti, R.; Pentassuglia, R.; Elmo, G.; Mancini, G. Resources optimization and sustainable waste management in construction chain in Italy: Toward a resource efficiency plan. Waste Biomass Valorization 2020, 11, 5405–5417. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Xiao, M.; Li, Z.; Zhu, Z. Advances in biomedical systems based on microneedles: Design, fabrication, and application. Biomater. Sci. 2024, 12, 530–563. [Google Scholar] [CrossRef]
- Da Silva, R.G.L. The advancement of artificial intelligence in biomedical research and health innovation: Challenges and opportunities in emerging economies. Glob. Health 2024, 20, 44. [Google Scholar]
- Vila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- He, W.; Chen, J.; Wang, M. Machine learning assists in the design and application of microneedles. Micromachines 2024, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, K.B.R.; Pereira, T.S.; Alves, A.L.M.; dos Santos, F.V.; dos Santos, F.A.; Correa, D.S. 3D-printed microneedles for sensing applications: Emerging topics and future trends. Adv. Sens. Energy Mater. 2025, 4, 100139. [Google Scholar] [CrossRef]
- Finster, R.; Keller, T.; Maier, L. Computational and AI-driven design of hydrogels for bioelectronic applications. Adv. Intell. Syst. 2025, 7, 2300456. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Lu, M.; Cao, X.; Xia, M.; Zhao, M.; Zhao, Y. Biomimetic triplet messenger RNA formulation in MXene hydrogel microneedles for wound healing. Aggregate 2024, 6, e700. [Google Scholar] [CrossRef]
- Ashraf, G.; Farooq, U.; Khan, M. Microneedle wearables in advanced microsystems: Unlocking next-generation biosensing with AI. Sens. Actuators B Chem. 2025, 345, 135678. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhao, X. Flexible monitoring, diagnosis, and therapy by microneedles with versatile materials. Adv. Healthc. Mater. 2023, 12, 2203018. [Google Scholar]
- Merzougui, R.; Yahiaoui, M.; Zohra, A. Microneedle array-based dermal interstitial fluid biopsy for cancer diagnosis: Advances and challenges. Cancers 2025, 17, 789–801. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, W.; Tang, Z.; Tan, Z.; He, Y.; Luo, J.; Wang, X. A reconfigurable integrated smart device for real-time monitoring and synergistic treatment of rheumatoid arthritis. Sci. Adv. 2024, 10, eadj0604. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, Z.; Zhong, G.; Xu, T.; Zhang, X. Self-sterilizing microneedle sensing patches for machine learning-enabled wound pH visual monitoring. Adv. Funct. Mater. 2024, 34, 2315067. [Google Scholar] [CrossRef]
- Albayati, R.; Al-Rikabi, A.; Salman, H. AI-driven innovation in skin kinetics for transdermal drug delivery: Overcoming barriers and enhancing precision. Pharmaceutics 2025, 17, 567. [Google Scholar] [CrossRef]
- Aghajani, M.; Ghassemi, M.; Mohammadi, R. Drug delivery options for riluzole in the treatment of amyotrophic lateral sclerosis. Expert Opin. Drug Deliv. 2025, 22, 321–335. [Google Scholar] [CrossRef]
- Suriyaamporn, P.; Pornpitchanarong, C.; Charoenying, T.; Dechsri, K.; Ngawhirunpat, T.; Opanasopit, P.; Pamornpathomkul, B. Artificial intelligence-driven hydrogel microneedle patches integrating 5-fluorouracil inclusion complex-loaded flexible pegylated liposomes for enhanced non-melanoma skin cancer treatment. Int. J. Pharm. 2025, 669, 125072. [Google Scholar] [CrossRef] [PubMed]
- Bagde, A.; Dev, S.; Madhavi, K.; Sriram, L.; Spencer, S.D.; Kalvala, A.; Nathani, A.; Salau, O.; Mosley-Kellum, K.; Dalvaigari, H.; et al. Biphasic burst and sustained transdermal delivery in vivo using an AI-optimized 3D-printed MN patch. Int. J. Pharm. 2023, 636, 122647. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Han, Y.; Yap, C.W.; Kochhar, J.S.; Li, H.; Xiang, X.; Kang, L. Prediction of drug permeation through microneedled skin by machine learning. Bioeng. Transl. Med. 2023, 8, e10512. [Google Scholar] [CrossRef]
- Zong, C.; Wang, F.; Cui, W. In situ: Microbial aerodynamic microneedles for targeted drug delivery systems. Research 2025, 8, 0775. [Google Scholar] [CrossRef]
- Reddy, S.S.; Shahid, S.; Sowmya, G.; Reddy, L.T.K.; Kumar, B.R.; Mahammed, N. Smart drug delivery systems: The integration of wearable and implantable technologies for precision medicine. Int. J. Sci. Res. Arch. 2025, 15, 1181–1200. [Google Scholar] [CrossRef]
- Kharb, S. Future directions: What lies ahead for smart biochemical wearables in health monitoring. Sensors 2025, 25, 789. [Google Scholar] [CrossRef]
- Ermis, S.A.; Guler, C.; Kilic, V. Advanced wearable sensors for body fluid monitoring in sports and health: Technologies, applications and future prospects. ACS Sens. 2025, 10, 2100–2115. [Google Scholar]
- Sen, A. Polymer nanocomposites in diabetes management: Advances in biosensors and drug delivery systems. Diabetes Technol. Ther. 2025, 27, 45–60. [Google Scholar] [CrossRef]
- Ataei Kachouei, M.; Kaushik, A.; Ali, M.A. Internet of Things-Enabled Food and Plant Sensors to Empower Sustainability. Adv. Intell. Syst. 2023, 5, 2300321. [Google Scholar] [CrossRef]
- Zhang, F.; Lee, Y.H.; Yuan, Q. New horizons in smart plant sensors: Key technologies, applications, and prospects. Biosens. Bioelectron. 2025, 220, 114567. [Google Scholar] [CrossRef] [PubMed]
- Ausri, I.R.; Sadeghzadeh, S.; Biswas, S.; Zheng, H.; GhavamiNejad, P.; Huynh, M.D.T.; Keyvani, F.; Shirzadi, E.; Rahman, F.A.; Quadrilatero, J.; et al. Multifunctional dopamine-based hydrogel microneedle electrode for continuous ketone sensing. Adv. Mater. 2024, 36, 2402009. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Liu, Y.; Chen, L. Integrating machine learning for the optimization of polyacrylamide-alginate hydrogel. Polymers 2024, 16, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Seifermann, M.; Meckel, T.; Groll, J. High-throughput synthesis and machine learning assisted design of photodegradable hydrogels. Adv. Mater. 2023, 35, 2300123. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.-C.; Cheng, Y.-H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting Nanoparticle Delivery to Tumors Using Machine Learning and Artificial Intelligence Approaches. Int. J. Nanomed. 2022, 17, 1365–1379. [Google Scholar] [CrossRef]
- Vora, L.; Anwar, E.; Patadia, R. Artificial intelligence in pharmaceutical technology and drug delivery design. J. Pharm. Sci. 2023, 112, 2345–2358. [Google Scholar] [CrossRef]
- Li, Z.; Sun, W.; Zhan, D.; Kang, Y.; Chen, L.; Bozzon, A.; Hai, R. Amalur: The Convergence of Data Integration and Machine Learning. IEEE Trans. Knowl. Data Eng. 2024, 36, 7353–7367. [Google Scholar] [CrossRef]
- Kuziemsky, C.E.; Chrimes, D.; Minshall, S.; Mannerow, M.; Lau, F. AI quality standards in health care: Rapid umbrella review. J. Med. Internet Res. 2024, 26, e54705. [Google Scholar] [CrossRef]
- Bucholc, M.; James, C.; Khleifat, A.A.; Badhwar, A.; Clarke, N.; Dehsarvi, A.; Madan, C.R.; Marzi, S.J.; Shand, C.; Schilder, B.M.; et al. Artificial intelligence for dementia research methods optimization. Alzheimer’s Dement. 2023, 19, 5934–5951. [Google Scholar] [CrossRef]
- Morley, J.; Machado, C.C.V.; Burr, C.; Cowls, J.; Joshi, I.; Taddeo, M.; Floridi, L. The ethics of AI in health care: A mapping review. Soc. Sci. Med. 2020, 260, 113172. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.M.; Prybutok, V. Balancing privacy and progress: A review of privacy challenges, systemic oversight, and patient perceptions in AI-driven healthcare. Appl. Sci. 2024, 14, 675. [Google Scholar] [CrossRef]
- Umeyor, C.E.; Kenechukwu, F.C.; Attama, A.A. Biomimetic microneedles for precision delivery. J. Control. Release 2023, 361, 123–135. [Google Scholar]
- Shivaswamy, R.H.; Krishnappa, H.; Murthy, S. Microneedles as a promising technology for disease monitoring and drug delivery: A review. Biomed. Eng. Adv. 2024, 6, 100098. [Google Scholar]
- Tyagi, S.; Sharma, V.; Rajput, R. AI-assisted formulation design for improved drug delivery and bioavailability. Pharm. Res. 2023, 40, 789–802. [Google Scholar]
- Ali, S.; Gohri, S.; Ali, S.A. Nanotechnology for diabetes management: Transforming anti-diabetic drug delivery systems. Curr. Pharm. Res. 2025, 1, 45–59. [Google Scholar] [CrossRef]
- Priya, S.; Jain, K.K.; Daryani, J. Revolutionizing rheumatoid arthritis treatment with emerging cutaneous drug delivery systems: Overcoming the challenges and paving the way forward. Nanoscale 2025, 65–87. [Google Scholar] [CrossRef]
- Dhudum, P.; Dandekar, P.; Kadam, S. Revolutionizing drug discovery: A comprehensive review of AI applications. Drug Discov. Today 2024, 29, 103456. [Google Scholar] [CrossRef]
- Ali, K.A.; Rasool, M.F.; Zahid, M. Influence of artificial intelligence in modern pharmaceutical formulation. Artif. Intell. Med. 2024, 148, 102345. [Google Scholar]
| Thematic Grouping | Thematic Group Description | |
|---|---|---|
| 1 | Material and Microneedle Design | ML and simulation tools enable predictive material design (e.g., hydrogels, nanocomposites) for specific mechanical, electrical, and drug-release properties; accelerates bio-device innovation. |
| 2 | Microneedles for Diagnostics and Therapy | Integration of AI with microneedle devices for real-time biomarker diagnostics, adaptive drug release, and closed-loop feedback systems; enables personalized, minimally invasive therapy via biomarker sensing and intelligent control. |
| 3 | Microneedles for Drug Delivery | Focus on hydrogel-forming and polymer-based microneedles that are biocompatible, dissolvable, and suitable for sustained drug delivery; advances in drug dosing enhance comfort and efficacy. |
| 4 | Microneedles for Drug Development | AI tools (ANN, CNN, etc.) applied to optimize drug formulations, delivery systems, and bioavailability; revolutionizes pharma R&D with predictive analytics and rapid prototyping. |
| 5 | Microneedles Sensors for Health and Agriculture | AI-powered biosensors embedded in wearable tech monitor plant/human hydration, stress etc., offering real-time, adaptive health insights, especially for agriculture, sports and chronic care. |
| (A) Thematic Group 5: Microneedle Sensors for Health and Agriculture | |||||||||
| # | Author (Year) | AI/ML Both | Hydrogel Microneedle HMN | AI/ML Techniques & Algorithms | Key AI/ML Role/Purpose Application | AI/ML Integration Innovation in HMN | AI-Enhanced HMN Features | AI-Enhanced Targeted Design Features | |
| 1 | Kharb et al. [37] | Both | MN | RNN, ML algorithms DL, CNN, edge AI, RL, clustering | Predictive analytics, noise reduction, personalized health insights, Bio-signal processing, | AI enhances data analysis, predictive modeling, and real-time feedback for wearables | Improved disease prediction, real-time monitoring, user compliance, dynamic calibration | Sensor calibration, multimodal data fusion, adaptive feedback | |
| 2 | Ermis et al. [38] | Both | HMN | ML (predictive modeling, calibration algorithms) SVM, NN, DL | Real-time biomarker analysis, personalized insights, biosignal feedback | ML optimizes microneedle sampling efficiency, Real-time biosignal analysis | Accuracy, real-time response | Dynamic calibration for skin variability, Signal robustness, hydration mapping | |
| 3 | A. Sen et al. [39] | Both | HMN | Predictive analytics, trend detection, ML alerts, (Lacks in-depth AI/ML details) | Glucose prediction, treatment personalization | Indirect (via wearable analytics) | Remote tracking, early alerts | Glucose sensing, insulin release | |
| 4 | Kachouei et al. [40] | Both | MN | AI, ML, DL, Pattern Recognition Predictive analytics, CRISPR-Cas12a | Pathogen detection, crop optimization Decision support, real-time alerts, prediction | IoT-AI fusion, smartphone-based real-time sensing, wireless data transmission | Real-time decisions & detection, predictive alerts, smart integration, scalable systems | Customizable sensing, VOC detection, hormone fluctuation, nitrate detection, | |
| 5 | Fucheng Zhang et al. [41] | Both | MN | ANN, deep learning, edge computing Data fusion algorithms, IoT, ML-enabled optimization | Predictive analytics, data fusion, stability compensation, precision agriculture, real-time plant monitoring | No AI focus; ML integrated into multimodal, wearable, implantable plant sensors | Stability, multimodal data fusion; microclimate sensing, disease/stress prediction | Environmental adaptability, accuracy Plant hormone detection, stress prediction | |
| 6 | Ausri et al. [42] | Both | HMN | SVM, Random Forest | Ketone detection modeling | AI-enhanced biosensor for metabolic monitoring | AI-tuned sensor thresholds for diabetic patients | Ketone monitoring & early DKA prediction | |
| (B) Thematic Group 5: Microneedle Sensors for Health and Agriculture | |||||||||
| Author (Year) | Materials Used | Dataset/ Data Type/ Size | Targeted Application | Target Drug/Biosensing Parameter | Targeted Parameter/Sickness | Results | Limitations | Future Work | |
| 1 | Kharb et al. [37] | Graphene, biodegradable polymers, flexible bioelectronics, Flexible sensors, nanocomposites | Empirical (patient datasets), Large-scale IoT | Biosensing, drug delivery, Biosensing + diagnostics | Lactate, VOCs Glucose, cortisol, sweat ions | Metabolic disorders, chronic diseases, Diabetes, depression, neurostress | High early detection accuracy, less invasive; personalized, real-time predictive diagnostics | data privacy, environmental variability, power, clinical reliability | AI-IoT closed-loop systems, AI twins, federated learning, edge-powered wearables |
| 2 | Ermis et al. [38] | PDMS, graphene, PVA, chitosan, silk fibroin | Empirical (sweat, blood, tears) | Biosensing (glucose, cortisol, electrolytes) | Glucose, cortisol, Na+, K+ Electrolytes, | Diabetes, stress, dehydration | 95% correlation with blood tests, High accuracy, low latency | Power constraints, data security, Motion artifacts, privacy | Biodegradable materials, AI-driven closed-loop systems, blockchain, personalization, hormone tracking |
| 3 | A. Sen et al. [39] | Chitosan, PLGA, graphene, PANI, CNT, ZnO, metal oxides | In vitro/in vivo studies Secondary data discussed | Drug delivery & biosensing | Glucose, Insulin, metformin, GLP-1, sulfonylureas | Diabetes mellitus | Reduced injections, Better glycemic control, wearable convenience | Scalability, biocompatibility, Scale-up issues, toxicity concerns, regulatory compliance | Smart polymers, nanocomposites, digital health, AI-health integration, digital medicine |
| 4 | Kachouei et al. [40] | Graphene, CNTs, polymers, Au NPs PDA, MN, nanofibers | Empirical (field data), streaming data, low-volume | Food safety, plant health, disease detection, sustainability, stress monitoring | Toxins, Pathogens, VOCs, Hormones, Nitrate | Heavy metals, pathogens, VOCs, Foodborne illness, plant stress | Early disease detection, reduced pesticide use, Highly accurate, responsive | High cost, energy inefficiency Scalability, cost, infra-structure | Solar sensors, blockchain, 5G, green tech, AI-automated models |
| 5 | Fucheng Zhang et al. [41] | Graphene, MXene, LIG, PI, AgNO3 PVA, conductive hydrogel, CNTs, sensors | Empirical/field data (large-scale) Empirical + Real-field data | Crop monitoring, soil analysis Crop growth, drought, stress sensing | NH4+, H2O2, VOCs, ethylene Salicylic acid, ethylene, nitrate, VOCs | Crop diseases, nutrient deficiency, drought, plant stress | Ethylene LOD: 0.084 ppm; VOC accuracy: 97% High stretchability, energy harvesting, accuracy | Cost, durability, environmental impact Sensor longevity, calibration stability | Self-powered sensors, biodegradable materials Integration of AI-powered feedback loops, data mining, closed-loop optimization |
| 6 | Ausri et al. [42] | DA-HA hydrogel MNs, PEDOT:PSS, NAD+, HBD | Porcine & rat ketone levels + ML model | Continuous ketone sensing | No drug (biosensor only) | Type 1 Diabetes (DKA) | MAD: 0.184 mM, R2 = 0.95, MARD: 7.68% | 2 hr tests only, animal models | Longer trials, CGM integration, multiplex sensing |
| Development Stage | AI/ML Application | Description | Benefits |
|---|---|---|---|
| 1. Material Discovery & Optimization | Data-Driven Material Selection | AI models analyze datasets of hydrogel compositions to predict optimal formulations. | Faster material discovery, improved biocompatibility. |
| Predictive Modeling | ML predicts hydrogel behavior under various conditions (hydration, pH, temperature). | Reduces trial-and-error experimentation. | |
| High-Throughput Screening | Deep learning and reinforcement learning identify promising hydrogel candidates. | Accelerates discovery of novel materials. | |
| Multi-Objective Optimization | Genetic algorithms balance mechanical strength, biodegradability, and drug release. | Tailored microneedles for specific applications. | |
| 2. Structural & Mechanical Design | Generative Design | AI-driven topology optimization for novel microneedle geometries. | Enhances penetration efficiency and drug delivery. |
| FEA | ML predicts microneedle stress distribution and mechanical failure points. | Improves durability and reliability. | |
| Bio-Inspired Design | AI mimics biological structures for better adhesion and penetration. | Increased effectiveness and patient comfort. | |
| Swelling Dynamics Prediction | AI models hydrogel swelling behavior for optimal drug diffusion. | Ensures controlled fluid absorption and drug release. | |
| 3. Manufacturing Process Optimization | Process Control & Automation | AI optimizes 3D printing, micro-molding, and photopolymerization. | Reduces defects, improves fabrication efficiency. |
| Defect Detection | ML-based image recognition detects microscopic defects in microneedles. | Enhances quality control, reduces waste. | |
| 3D Printing Optimization | AI refines printing techniques and layer-by-layer deposition. | Ensures structural integrity and printability. | |
| Self-Correcting Fabrication Systems | Reinforcement learning enables real-time process adjustments. | Reduces variability, increases yield. | |
| 4. Biomedical Applications & Performance Enhancement | Drug Delivery Optimization | AI predicts drug release kinetics based on hydrogel properties. | Enables sustained and controlled drug release. |
| Personalized Medicine | ML analyzes patient data to tailor microneedle formulations. | Enhances treatment efficacy, reduces side effects. | |
| Biosensing Integration | AI optimizes sensor placement and biomarker detection algorithms. | Improves real-time health monitoring accuracy. | |
| AI-Assisted Pharmacokinetics Modeling | Deep learning models simulate drug absorption and metabolism. | Speeds up drug development and regulatory approval. | |
| 5. Advanced AI Integration | AI-Powered Smart Microneedles | Embedded AI sensors adjust drug delivery in real-time. | Enables adaptive and responsive therapy. |
| Blockchain for Data Security | Secure storage and sharing of microneedle treatment data. | Ensures regulatory compliance and patient privacy. | |
| Hybrid AI-Physics Models | AI-driven simulations combined with physics-based modeling. | Enhances predictive accuracy for microneedle performance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urifa, J.; Shah, K.W. Early Insights into AI and Machine Learning Applications in Hydrogel Microneedles: A Short Review. Micro 2025, 5, 48. https://doi.org/10.3390/micro5040048
Urifa J, Shah KW. Early Insights into AI and Machine Learning Applications in Hydrogel Microneedles: A Short Review. Micro. 2025; 5(4):48. https://doi.org/10.3390/micro5040048
Chicago/Turabian StyleUrifa, Jannah, and Kwok Wei Shah. 2025. "Early Insights into AI and Machine Learning Applications in Hydrogel Microneedles: A Short Review" Micro 5, no. 4: 48. https://doi.org/10.3390/micro5040048
APA StyleUrifa, J., & Shah, K. W. (2025). Early Insights into AI and Machine Learning Applications in Hydrogel Microneedles: A Short Review. Micro, 5(4), 48. https://doi.org/10.3390/micro5040048







