A Review of Materials for the Removal of Micro- and Nanoplastics from Different Environments
Abstract
1. Introduction
2. Materials Used for Removal of Micro- and Nanoplastics
2.1. Conventional and Novel Techniques for MP/NP Removal
2.2. Biological Method/Bioinspired Based Materials
2.3. Activated Carbon, Biochar, and Polymeric Adsorbents
2.4. Functionalized Magnetic Nanoparticles
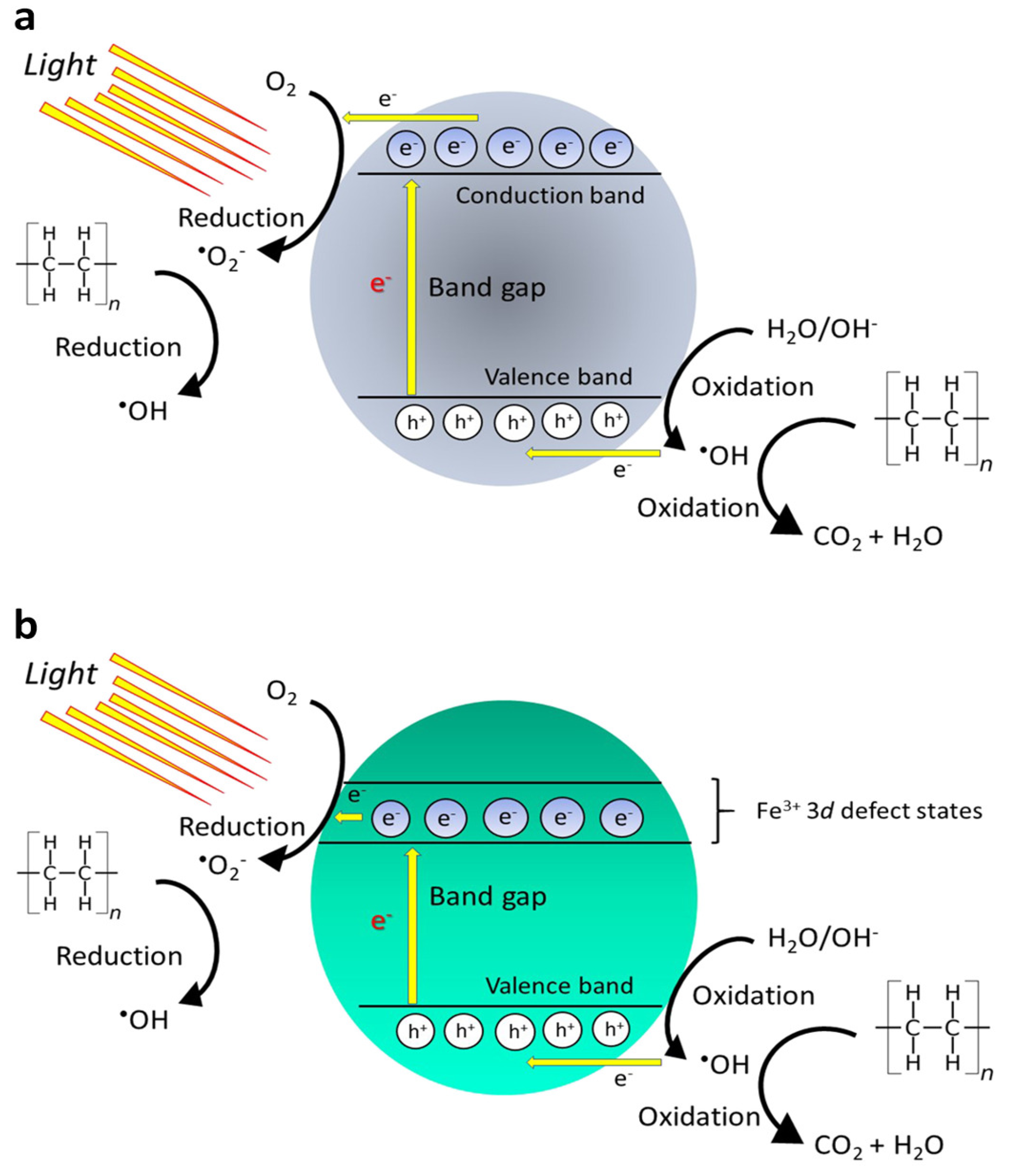
2.5. Photocatalytic and Electrochemical Materials
2.6. Filtration Materials
2.7. Electrocoagulation
2.8. Applications in Different Environmental Matrices
3. Emerging Technologies and Novel Materials
3.1. Novel Laser-Based Technology
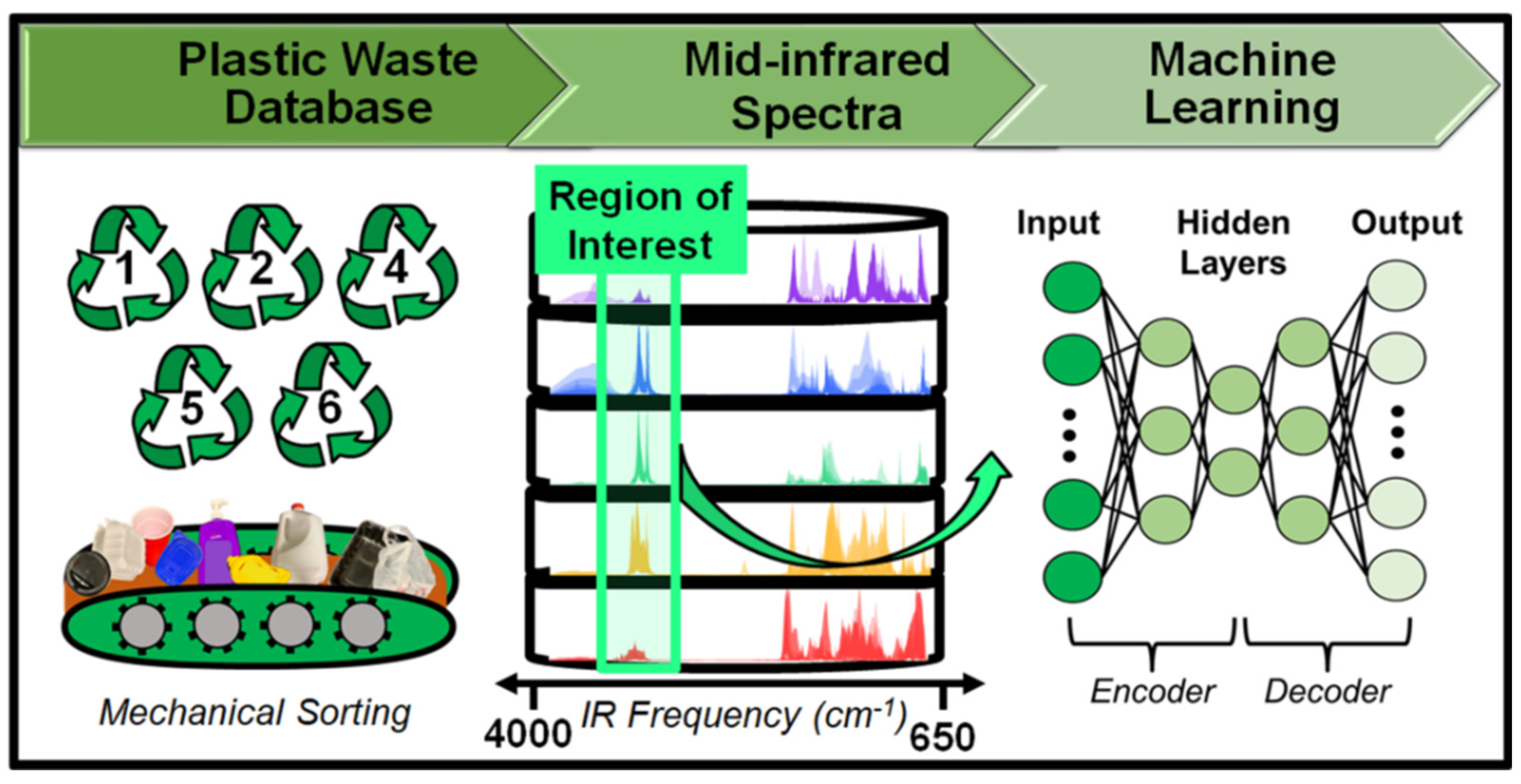
3.2. AI-Driven Solution for the Removal of Nano and Microplastic
3.3. Promising and Scalable Materials for MP/NP Removal
4. Conclusions
- Combining multiple removal mechanisms (e.g., adsorption + magnetic capture) for better efficacy.
- Developing decentralized or small-scale systems for areas without centralized wastewater treatment.
- Conducting pilot-scale studies and life-cycle assessments to evaluate feasibility and environmental impact.
- Supporting international efforts to establish standardized detection methods and regulatory guidelines
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedrero, D.; Carlos, E.; Francisca, F.; Roberto, R.; Sonia, A. Efficient removal of nanoplastics from water using mesoporous metal organic frameworks. Sep. Purif. Technol. 2024, 333, 125816. [Google Scholar] [CrossRef]
- Xu, Y.; Ou, Q.; Wang, X.; Hou, F.; Li, P.; van der Hoek, J.P.; Liu, G. Assessing the mass concentration of microplastics and nanoplastics in wastewater treatment plants by pyrolysis gas chromatography–mass spectrometry. Environ. Sci. Technol. 2023, 57, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Enyoh, C.E.; Fadare, O.O.; Paredes, M.; Wang, Q.; Verla, A.W.; Shafea, L.; Chowdhury, T. An overview of physical, chemical and biological methods for removal of microplastics. In Microplastics Pollution in Aquatic Media: Occurrence, Detection, and Removal; Springer: Berlin/Heidelberg, Germany, 2022; pp. 273–289. [Google Scholar]
- Oleksiuk, K.; Krupa-Kotara, K.; Wypych-Ślusarska, A.; Głogowska-Ligus, J.; Spychała, A.; Słowiński, J. Microplastic in food and water: Current knowledge and awareness of consumers. Nutrients 2022, 14, 4857. [Google Scholar] [CrossRef] [PubMed]
- Enyoh, C.E.; Devi, A.; Kadono, H.; Wang, Q.; Rabin, M.H. The plastic within: Microplastics invading human organs and bodily fluids systems. Environments 2023, 10, 194. [Google Scholar] [CrossRef]
- Cverenkárová, K.; Valachovičová, M.; Mackuľak, T.; Žemlička, L.; Bírošová, L. Microplastics in the food chain. Life 2021, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, Q.; Chen, Z. Carbon-based adsorbents for micro/nano-plastics removal: Current advances and perspectives. Water Emerg. Contam. Nanoplast. 2024, 3, 11. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Al-Muqbel, D.; Al-Othman, A.; Halalsheh, N.; Tawalbeh, M. Insights into the removal of microplastics from water using biochar in the era of COVID-19: A mini review. Case Stud. Chem. Environ. Eng. 2021, 4, 100151. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, C.; Huang, Q.-X.; Chi, Y.; Yan, J.-H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, Y.; Zhou, G.; Zhang, Y.; Yue, J.; Wang, X.; Yang, Z.; Chen, J.; Wang, Q.; Zhang, J. Mechanisms of polystyrene nanoplastics adsorption onto activated carbon modified by ZnCl2. Sci. Total Environ. 2023, 876, 162763. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Cao, Y.; Sathish, C.; Guan, X.; Wang, S.; Palanisami, T.; Vinu, A.; Yi, J. Advances in magnetic materials for microplastic separation and degradation. J. Hazard. Mater. 2024, 461, 132537. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Li, H.; Offiong, N.-A.O.; Bi, Y.; Zhou, R.; Ren, H. A systematic review of electrocoagulation technology applied for microplastics removal in aquatic environment. Chem. Eng. J. 2023, 456, 141078. [Google Scholar] [CrossRef]
- Chellasamy, G.; Kiriyanthan, R.M.; Maharajan, T.; Radha, A.; Yun, K. Remediation of microplastics using bionanomaterials: A review. Environ. Res. 2022, 208, 112724. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Huo, P.; Wang, H.; Weiqiang, Z.; Wan, Y. A review on plasmonic-based heterojunction photocatalysts for degradation of organic pollutants in wastewater. J. Mater. Sci. 2023, 58, 6474–6515. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Choi, W.; Lim, G. Electrokinetic-assisted filtration for fast and highly efficient removal of microplastics from water. Chem. Eng. J. 2023, 452, 139152. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, J. Application of artificial intelligence in the analysis of microplastics. In Analysis of Microplastics and Nanoplastics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 225–246. [Google Scholar]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Sundbæk, K.B.; Koch, I.D.W.; Villaro, C.G.; Rasmussen, N.S.; Holdt, S.L.; Hartmann, N.B. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J. Appl. Phycol. 2018, 30, 2923–2927. [Google Scholar] [CrossRef]
- Yuan, F.; Yue, L.; Zhao, H.; Wu, H. Study on the adsorption of polystyrene microplastics by three-dimensional reduced graphene oxide. Water Sci. Technol. 2020, 81, 2163–2175. [Google Scholar] [CrossRef] [PubMed]
- Almomani, F.; Bhosale, R.; Khraisheh, M.; Almomani, T. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP). Appl. Surf. Sci. 2020, 506, 144924. [Google Scholar] [CrossRef]
- Ramirez Arenas, L.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ. 2021, 791, 148175. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Xiang, M.; Wang, W.; Su, Z.; Liu, H.; Mao, Y.; Chen, Y.; Zhang, P. Engineering 3D graphene-like carbon-assembled layered double oxide for efficient microplastic removal in a wide pH range. J. Hazard. Mater. 2022, 433, 128672. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, T.; Mustafa, B.; Mackenzie, K.; Ali, W.; Sabir, R.I.; Anum, W.; Gaurav, G.K.; Riaz, U.; Liu, X.; Peng, L. Recent developments in microplastic contaminated water treatment: Progress and prospects of carbon-based two-dimensional materials for membranes separation. Chemosphere 2023, 316, 137704. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khandelwal, N.; Ganie, Z.A.; Tiwari, E.; Darbha, G.K. Eco-friendly magnetic biochar: An effective trap for nanoplastics of varying surface functionality and size in the aqueous environment. Chem. Eng. J. 2021, 418, 129405. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Yu, Y.-X. Adsorptive removal of Cr3+, Cu2+, and Ni2+ ions by magnetic Fe3O4@ alkali-treated coal fly ash. Desalination Water Treat. 2018, 123, 277–287. [Google Scholar] [CrossRef]
- Urso, M.; Ussia, M.; Pumera, M. Breaking polymer chains with self-propelled light-controlled navigable hematite microrobots. Adv. Funct. Mater. 2021, 31, 2101510. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic Extraction of Microplastics from Environmental Samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of microplastics from water by magnetic nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef]
- Aragón, D.; García-Merino, B.; Barquín, C.; Bringas, E.; Rivero, M.J.; Ortiz, I. Advanced green capture of microplastics from different water matrices by surface-modified magnetic nanoparticles. Sep. Purif. Technol. 2025, 354, 128813. [Google Scholar] [CrossRef]
- Bhore, R.K.; Kamble, S.B. Nano adsorptive extraction of diverse microplastics from the potable and seawater using organo-polyoxometalate magnetic nanotricomposites. J. Environ. Chem. Eng. 2022, 10, 108720. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A.; Abdulrasheed, A.A. A review on recent progression of photocatalytic desulphurization study over decorated photocatalysts. J. Ind. Eng. Chem. 2019, 74, 172–186. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Ching Ng, A.M. Strategies for improving the efficiency of semiconductor metal oxide photocatalysis. Mater. Horiz. 2014, 1, 400–410. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Hernández-Ramírez, A.; Medina-Ramírez, I. Photocatalytic Semiconductors; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwak, S.-Y.; Suzuki, T. Photocatalytic degradation of flexible PVC/TiO2 nanohybrid as an eco-friendly alternative to the current waste landfill and dioxin-emitting incineration of post-use PVC. Polymer 2006, 47, 3005–3016. [Google Scholar] [CrossRef]
- An, Y.; Hou, J.; Liu, Z.; Peng, B. Enhanced solid-phase photocatalytic degradation of polyethylene by TiO2–MWCNTs nanocomposites. Mater. Chem. Phys. 2014, 148, 387–394. [Google Scholar] [CrossRef]
- Saifuddin, M.; Ghaffari, Y.; Park, S.Y.; Kim, C.G. Rapid surface degradation of co-axially arranged polypropylene globules by nanoporous carbonized TiO2 assisted with UV-C. Environ. Res. 2022, 212, 113422. [Google Scholar] [CrossRef]
- Tian, L.; Chen, Q.; Jiang, W.; Wang, L.; Xie, H.; Kalogerakis, N.; Ma, Y.; Ji, R. A carbon-14 radiotracer-based study on the phototransformation of polystyrene nanoplastics in water versus in air. Environ. Sci. Nano 2019, 6, 2907–2917. [Google Scholar] [CrossRef]
- Nabi, I.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. Iscience 2020, 23, 101326. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Busquets, R.; Choi, I.-C.; Lee, S.-H.; Kim, J.-K.; Campos, L.C. Photocatalytic degradation of polyamide 66; evaluating the feasibility of photocatalysis as a microfibre-targeting technology. Water 2020, 12, 3551. [Google Scholar] [CrossRef]
- Saquib, M.; Muneer, M. TiO2-mediated photocatalytic degradation of a triphenylmethane dye (gentian violet), in aqueous suspensions. Dye. Pigment. 2003, 56, 37–49. [Google Scholar] [CrossRef]
- Ebrahimbabaie, P.; Yousefi, K.; Pichtel, J. Photocatalytic and biological technologies for elimination of microplastics in water: Current status. Sci. Total Environ. 2022, 806, 150603. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-M.; Sin, J.-C.; Zeng, H.; Lin, H.; Li, H.; Chai, Y.-Y.; Choong, M.-K.; Mohamed, A.R. Green synthesis of Fe-ZnO nanoparticles with improved sunlight photocatalytic performance for polyethylene film deterioration and bacterial inactivation. Mater. Sci. Semicond. Process. 2021, 123, 105574. [Google Scholar] [CrossRef]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane processes for microplastic removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef] [PubMed]
- Golgoli, M.; Khiadani, M.; Shafieian, A.; Sen, T.K.; Hartanto, Y.; Johns, M.; Zargar, M. Microplastics fouling and interaction with polymeric membranes: A review. Chemosphere 2021, 283, 131185. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, L.; Chen, B.; Zhu, X. Low-pressure driven electrospun membrane with tuned surface charge for efficient removal of polystyrene nanoplastics from water. J. Membr. Sci. 2020, 614, 118470. [Google Scholar] [CrossRef]
- LaRue, R.J.; Patterson, B.; O’Brien, S.; Latulippe, D.R. Evaluation of membrane fouling by microplastic particles in tertiary wastewater treatment processes. ACS EST Water 2022, 2, 955–966. [Google Scholar] [CrossRef]
- Aslan, T.; Arslan, S.; Eyvaz, M.; Güçlü, S.; Yüksel, E.; Koyuncu, I. A novel nanofiber microfiltration membrane: Fabrication and characterization of tubular electrospun nanofiber (TuEN) membrane. J. Membr. Sci. 2016, 520, 616–629. [Google Scholar] [CrossRef]
- Wang, Z.; Crandall, C.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Microfiltration performance of electrospun nanofiber membranes with varied fiber diameters and different membrane porosities and thicknesses. Polymer 2017, 114, 64–72. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, B.K.; Pramanik, S.K.; Monira, S. Understanding the fragmentation of microplastics into nano-plastics and removal of nano/microplastics from wastewater using membrane, air flotation and nano-ferrofluid processes. Chemosphere 2021, 282, 131053. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, A.; Campbell, A.J.; Adeyemi, O.G. The potential role of membrane technology in the removal of microplastics from wastewater. J. Appl. Membr. Sci. Technol. 2021, 25, 31–53. [Google Scholar] [CrossRef]
- Mustapha, S.; Tijani, J.; Elabor, R.; Salau, R.; Egbosiuba, T.; Amigun, A.; Shuaib, D.; Sumaila, A.; Fiola, T.; Abubakar, Y. Technological approaches for removal of microplastics and nanoplastics in the environment. J. Environ. Chem. Eng. 2024, 12, 112084. [Google Scholar] [CrossRef]
- Akarsu, C.; Deniz, F. Electrocoagulation/electroflotation process for removal of organics and microplastics in laundry wastewater. CLEAN–Soil Air Water 2021, 49, 2000146. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, S. Enhancing microplastics removal from wastewater using electro-coagulation and granule-activated carbon with thermal regeneration. Processes 2021, 9, 617. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Almatrafi, E.; Hu, T.; Zhou, C.; Song, B.; Zeng, Z.; Zeng, G. Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem. Eng. J. 2022, 428, 131161. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.; Niu, Y.; Xu, D.; Wang, J.; Han, J.; Wang, H. Removal of microplastics and attached heavy metals from secondary effluent of wastewater treatment plant using interpenetrating bipolar plate electrocoagulation. Sep. Purif. Technol. 2022, 290, 120905. [Google Scholar] [CrossRef]
- Tiwari, E.; Singh, N.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Application of Zn/Al layered double hydroxides for the removal of nano-scale plastic debris from aqueous systems. J. Hazard. Mater. 2020, 397, 122769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Chen, Y.; Miao, C.; Wang, Y.-R.; Gao, G.-K.; Yang, R.-X.; Zhu, H.-J.; Wang, J.-H.; Li, S.-L.; Lan, Y.-Q. Metal–organic framework-based foams for efficient microplastics removal. J. Mater. Chem. A 2020, 8, 14644–14652. [Google Scholar] [CrossRef]
- Yen, P.-L.; Hsu, C.-H.; Huang, M.-L.; Liao, V.H.-C. Removal of nano-sized polystyrene plastic from aqueous solutions using untreated coffee grounds. Chemosphere 2022, 286, 131863. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Martin, C.; Corona, E.; Mahadik, G.A.; Duarte, C.M. Adhesion to coral surface as a potential sink for marine microplastics. Environ. Pollut. 2019, 255, 113281. [Google Scholar] [CrossRef] [PubMed]
- Kuoppamäki, K.; Pflugmacher Lima, S.; Scopetani, C.; Setälä, H. The Ability of Selected Filter Materials in Removing Nutrients, Metals, and Microplastics from Stormwater in Biofilter Structures; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 465–475. [Google Scholar]
- Chazovachii, P.T.; Rieland, J.M.; Sheffey, V.V.; Jugovic, T.M.; Zimmerman, P.M.; Eniola-Adefeso, O.; Love, B.J.; McNeil, A.J. Using adhesives to capture microplastics from water. ACS EST Eng. 2021, 1, 1698–1704. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Usman, J.; Othman, M.H.D.; Ismail, A.F.; Goh, P.S.; Gangasalam, A.; Adam, M.R. Low-cost silica based ceramic supported thin film composite hollow fiber membrane from guinea corn husk ash for efficient removal of microplastic from aqueous solution. J. Hazard. Mater. 2022, 424, 127298. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suta, T.; Usuelli, M.; Handschin, S.; Canelli, G.; Bagnani, M.; Mezzenga, R. Sustainable removal of microplastics and natural organic matter from water by coagulation–flocculation with protein amyloid fibrils. Environ. Sci. Technol. 2021, 55, 8848–8858. [Google Scholar] [CrossRef]
- Wang, P.; Huang, Z.; Chen, S.; Jing, M.; Ge, Z.; Chen, J.; Yang, S.; Chen, J.; Fang, Y. Sustainable removal of nano/microplastics in water by solar energy. Chem. Eng. J. 2022, 428, 131196. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Llorca-Isern, N. A robust and anticorrosion non-fluorinated superhydrophobic aluminium surface for microplastic removal. Sci. Total Environ. 2021, 760, 144090. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Zhang, J.; Bhingarde, A.; Matotek, T.; Barrett, J.; Hardesty, B.D.; Holl, M.M.B.; Khoo, B.L. A portable purification system for the rapid removal of microplastics from environmental samples. Chem. Eng. J. 2022, 428, 132614. [Google Scholar] [CrossRef]
- Cunha, C.; Faria, M.; Nogueira, N.; Ferreira, A.; Cordeiro, N. Marine vs freshwater microalgae exopolymers as biosolutions to microplastics pollution. Environ. Pollut. 2019, 249, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Leung, M.M.-L.; Fang, J.K.-H.; Chua, S.L. Engineering a microbial ‘trap and release’mechanism for microplastics removal. Chem. Eng. J. 2021, 404, 127079. [Google Scholar] [CrossRef]
- Zhou, H.; Mayorga-Martinez, C.C.; Pumera, M. Microplastic removal and degradation by mussel-inspired adhesive magnetic/enzymatic microrobots. Small Methods 2021, 5, 2100230. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Peldszus, S.; Van Dyke, M.I.; Huck, P.M. Removal of polystyrene microplastic spheres by alum-based coagulation-flocculation-sedimentation (CFS) treatment of surface waters. Chem. Eng. J. 2021, 422, 130023. [Google Scholar] [CrossRef]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Kleinberg, K.A. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem. Eng. J. 2020, 386, 123807. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Ding, Y.; Hu, C.; Liu, H.; Qu, J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 2019, 78, 267–275. [Google Scholar] [CrossRef]
- Prokopova, M.; Novotna, K.; Pivokonska, L.; Cermakova, L.; Cajthaml, T.; Pivokonsky, M. Coagulation of polyvinyl chloride microplastics by ferric and aluminium sulphate: Optimisation of reaction conditions and removal mechanisms. J. Environ. Chem. Eng. 2021, 9, 106465. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Na, S.-H.; Kim, M.-J.; Kim, J.-T.; Jeong, S.; Lee, S.; Chung, J.; Kim, E.-J. Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res. 2021, 202, 117417. [Google Scholar] [CrossRef]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Franco Castillo, I.; Mitchell, S.G.; Güttel, R.; Streb, C. Water purification and microplastics removal using magnetic polyoxometalate-supported ionic liquid phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef] [PubMed]
- Radford, F.; Zapata-Restrepo, L.M.; Horton, A.A.; Hudson, M.D.; Shaw, P.J.; Williams, I.D. Developing a systematic method for extraction of microplastics in soils. Anal. Methods 2021, 13, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leiniö, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive oil-based method for the extraction, quantification and identification of microplastics in soil and compost samples. Sci. Total Environ. 2020, 733, 139338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.M.B.; Knutsen, H.; Mahat, S.; Wade, E.J.; Arp, H.P.H. Facilitating microplastic quantification through the introduction of a cellulose dissolution step prior to oxidation: Proof-of-concept and demonstration using diverse samples from the Inner Oslofjord, Norway. Mar. Environ. Res. 2020, 161, 105080. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Murphy, F.; Ewins, C. Validation of density separation for the rapid recovery of microplastics from sediment. Anal. Methods 2017, 9, 1491–1498. [Google Scholar] [CrossRef]
- Duan, J.; Han, J.; Zhou, H.; Lau, Y.L.; An, W.; Wei, P.; Cheung, S.G.; Yang, Y.; Tam, N.F.-y. Development of a digestion method for determining microplastic pollution in vegetal-rich clayey mangrove sediments. Sci. Total Environ. 2020, 707, 136030. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, R.; Tsuchiya, M.; Lindsay, D.J.; Kitahashi, T.; Fujikura, K.; Fukushima, T. A new small device made of glass for separating microplastics from marine and freshwater sediments. PeerJ 2019, 7, e7915. [Google Scholar] [CrossRef] [PubMed]
- Farshidianfar, A.; Nabavi, S.F.; Farshidianfar, M.H. The Laser Manufacturing Process: Fundamentals of Process and Applications; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Cui, M.; Xiong, S.; Yang, N.; Wang, Y.; Wang, Z.; Luo, M.; Yao, C.; Deguchi, Y. Applications of laser-induced breakdown spectroscopy in industrial measurement and monitoring: Multi-technology combination. Appl. Spectrosc. Rev. 2024, 60, 243–291. [Google Scholar] [CrossRef]
- Sommer, C.; Schneider, L.; Nguyen, J.; Prume, J.; Lautze, K.; Koch, M. Identifying microplastic litter with Laser Induced Breakdown Spectroscopy: A first approach. Mar. Pollut. Bull. 2021, 171, 112789. [Google Scholar] [CrossRef]
- Michel, A.P.; Morrison, A.E.; Preston, V.L.; Marx, C.T.; Colson, B.C.; White, H.K. Rapid identification of marine plastic debris via spectroscopic techniques and machine learning classifiers. Environ. Sci. Technol. 2020, 54, 10630–10637. [Google Scholar] [CrossRef] [PubMed]
- Brunnbauer, L.; Jirku, M.; Quarles, C.D., Jr.; Limbeck, A. Capabilities of simultaneous 193 nm-LIBS/LA-ICP-MS imaging for microplastics characterization. Talanta 2024, 269, 125500. [Google Scholar] [CrossRef] [PubMed]
- Pořízka, P.; Brunnbauer, L.; Porkert, M.; Rozman, U.; Marolt, G.; Holub, D.; Kizovský, M.; Benešová, M.; Samek, O.; Limbeck, A. Laser-based techniques: Novel tools for the identification and characterization of aged microplastics with developed biofilm. Chemosphere 2023, 313, 137373. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, H.; Bashir, S. Laser-Induced Breakdown Spectroscopy for the Identification of Microplastics Collected from Arabian Sea of Pakistan. Water Air Soil Pollut. 2024, 235, 70. [Google Scholar] [CrossRef]
- Sunil, M.; Pallikkavaliyaveetil, N.; Gopinath, A.; Chidangil, S.; Kumar, S.; Lukose, J. Machine learning assisted Raman spectroscopy: A viable approach for the detection of microplastics. J. Water Process Eng. 2024, 60, 105150. [Google Scholar] [CrossRef]
- López-Rosales, A.; Ferreiro, B.; Andrade, J.; Fernández-Amado, M.; González-Pleiter, M.; López-Mahía, P.; Rosal, R.; Muniategui-Lorenzo, S. A reliable method to determine airborne microplastics using quantum cascade laser infrared spectrometry. Sci. Total Environ. 2024, 913, 169678. [Google Scholar] [CrossRef] [PubMed]
- Parobková, V.; Holub, D.; Kizovský, M.; Kalčíková, G.; Rozman, U.; Urík, M.; Novotný, K.; Samek, O.; Zikmund, T.; Pořízka, P. Raman microspectroscopy and laser-induced breakdown spectroscopy for the analysis of polyethylene microplastics in human soft tissues. Heliyon 2024, 10, e37844. [Google Scholar] [CrossRef]
- Meng, X.; Chen, S.; Li, D.; Song, Y.; Sun, L. Identification of marine microplastics based on laser-induced fluorescence and principal component analysis. J. Hazard. Mater. 2024, 465, 133352. [Google Scholar] [CrossRef] [PubMed]
- Román-Zas, C.; Ferreiro, B.; Terán-Baamonde, J.; Busto, M.E.D.C.; Andrade, J.M.; Muniategui, S. Measurement of tyre-based microplastics using traditional and quantum cascade laser-based infrared spectrometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 327, 125321. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, B.; He, M.; Zhou, Y.; Lei, L.; Han, J.; Zhou, B.; Hu, L.; Hu, B. Nanoplastics and nano-ZnO facilitate Cd accumulation in zebrafish larvae via a distinct pathway: Revelation by LA-ICP-MS imaging. Chin. Chem. Lett. 2025, 36, 109908. [Google Scholar] [CrossRef]
- Sima, J.; Song, J.; Du, X.; Lou, F.; Zhu, Y.; Lei, J.; Huang, Q. Complete degradation of polystyrene microplastics through non-thermal plasma-assisted catalytic oxidation. J. Hazard. Mater. 2024, 480, 136313. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xiong, Y.; Jia, H.; Han, L.; Yin, K. Superb microplastics separation performance of graphene oxide tuned by laser bombardment. J. Hazard. Mater. 2024, 461, 132599. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-Y.; Sugita, N.; Shin, B.-S. Fe3O4/Laser-Induced graphene as an adsorbent for microplastics emitted from household wastewater. Int. J. Precis. Eng. Manuf.-Green Technol. 2023, 10, 807–818. [Google Scholar] [CrossRef]
- Pizzichetti, A.R.P.; Pablos, C.; Álvarez-Fernández, C.; Reynolds, K.; Stanley, S.; Marugán, J. Kinetic and mechanistic analysis of membrane fouling in microplastics removal from water by dead-end microfiltration. J. Environ. Chem. Eng. 2023, 11, 109338. [Google Scholar] [CrossRef]
- Dong, H.; Huang, X.; Wu, Z.; Li, P.; Silvain, J.-F.; Hussain, K.A.; Cui, B.; Li, Y.; Lu, Y. Generation of nano-to-microplastics from polypropylene surfaces via femtosecond laser ablation in liquids with different viscosities. Appl. Surf. Sci. 2024, 670, 160661. [Google Scholar] [CrossRef]
- Jin, H.; Kong, F.; Li, X.; Shen, J. Artificial intelligence in microplastic detection and pollution control. Environ. Res. 2024, 262, 119812. [Google Scholar] [CrossRef] [PubMed]
- Lubongo, C.; Bin Daej, M.A.A.; Alexandridis, P. Recent Developments in Technology for Sorting Plastic for Recycling: The Emergence of Artificial Intelligence and the Rise of the Robots. Recycling 2024, 9, 59. [Google Scholar] [CrossRef]
- Cairone, S.; Hasan, S.W.; Choo, K.-H.; Li, C.-W.; Zarra, T.; Belgiorno, V.; Naddeo, V. Integrating artificial intelligence modeling and membrane technologies for advanced wastewater treatment: Research progress and future perspectives. Sci. Total Environ. 2024, 944, 173999. [Google Scholar] [CrossRef]
- Wen, S.; Yuan, Y.; Chen, J. A vision detection scheme based on deep learning in a waste plastics sorting system. Appl. Sci. 2023, 13, 4634. [Google Scholar] [CrossRef]
- Kroell, N.; Chen, X.; Greiff, K.; Feil, A. Optical sensors and machine learning algorithms in sensor-based material flow characterization for mechanical recycling processes: A systematic literature review. Waste Manag. 2022, 149, 259–290. [Google Scholar] [CrossRef]
- Dhulekar, P.; Gandhe, S.; Mahajan, U.P. Development of bottle recycling machine using machine learning algorithm. In Proceedings of the 2018 International Conference on Advances in Communication and Computing Technology (ICACCT), Sangamner, India, 11 November 2018; pp. 515–519. [Google Scholar]
- Sidharth, R.; Rohit, P.; Vishagan, S.; Karthika, R.; Ganesan, M. Deep learning based smart garbage classifier for effective waste management. In Proceedings of the 2020 5th International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 10 July 2020; pp. 1086–1089. [Google Scholar]
- Fang, C.; Luo, Y.; Naidu, R. Microplastics and nanoplastics analysis: Options, imaging, advancements and challenges. TrAC Trends Anal. Chem. 2023, 166, 117158. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.-P.; Chen, P.; Li, J.-Y.; Liu, D.; Chu, X.-L. Combining spectroscopy and machine learning for rapid identification of plastic waste: Recent developments and future prospects. J. Clean. Prod. 2023, 431, 139771. [Google Scholar] [CrossRef]
- Sarker, M.A.B.; Imtiaz, M.H.; Holsen, T.M.; Baki, A.B. Real-time detection of microplastics using an ai camera. Sensors 2024, 24, 4394. [Google Scholar] [CrossRef] [PubMed]
- Bogue, R. The role of robots in environmental monitoring. Ind. Robot. Int. J. Robot. Res. Appl. 2023, 50, 369–375. [Google Scholar] [CrossRef]
- Ayesh, M.; Qidwai, U. Detection and Collection of Waste Using a Partially Submerged Aquatic Robot. In Proceedings of the SAI Intelligent Systems Conference, Amsterdam, The Netherlands, 1–2 September 2022; pp. 133–149. [Google Scholar]
- Martinez-Hernandez, U.; West, G.; Assaf, T. Low-Cost Recognition of Plastic Waste Using Deep Learning and a Multi-Spectral Near-Infrared Sensor. Sensors 2024, 24, 2821. [Google Scholar] [CrossRef] [PubMed]
- Neo, E.R.K.; Low, J.S.C.; Goodship, V.; Debattista, K. Deep learning for chemometric analysis of plastic spectral data from infrared and Raman databases. Resour. Conserv. Recycl. 2023, 188, 106718. [Google Scholar] [CrossRef]
- Babajamaaty, G. Numerical Simulation of Microplastics Transport in a Part of Fraser River and Detection of Accumulation Zones Based on Clustering Methods. Master’s Thesis, Université d’Ottawa/University of Ottawa, Ottawa, ON, Canada, 16 May 2023. [Google Scholar]
- Pojunas, D. Bayesian Learning of Spatiotemporal Source Distribution for Beached Microplastic in the Gulf of Mexico. Master’s Thesis, University of Arkansas, Fayetteville, AR, USA, December 2023. [Google Scholar]
- Lin, J.-y.; Liu, H.-t.; Zhang, J. Recent advances in the application of machine learning methods to improve identification of the microplastics in environment. Chemosphere 2022, 307, 136092. [Google Scholar] [PubMed]
- Bułkowska, K.; Zielińska, M.; Bułkowski, M. Blockchain-Based Management of Recyclable Plastic Waste. Energies 2024, 17, 2937. [Google Scholar] [CrossRef]
- Bernat, K. Post-consumer plastic waste management: From collection and sortation to mechanical recycling. Energies 2023, 16, 3504. [Google Scholar] [CrossRef]
- Das, K.P.; Chauhan, P.; Staudinger, U.; Satapathy, B.K. Exploring sustainable adsorbents to mitigate micro-/nano-plastic contamination: Perspectives on electrospun fibrous constructs, biochar, and aerogels. Environ. Sci. Adv. 2024, 3, 1217–1243. [Google Scholar] [CrossRef]
- Yakoubi, S. Sustainable Revolution: AI-Driven Enhancements for Composite Polymer Processing and Optimization in Intelligent Food Packaging. Food Bioprocess Technol. 2024, 18, 82–107. [Google Scholar] [CrossRef]
- Ganie, Z.A.; Khandelwal, N.; Tiwari, E.; Singh, N.; Darbha, G.K. Biochar-facilitated remediation of nanoplastic contaminated water: Effect of pyrolysis temperature induced surface modifications. J. Hazard. Mater. 2021, 417, 126096. [Google Scholar] [CrossRef] [PubMed]
- Mondal, U.S.; Karmakar, A.; Paul, A.; Paul, S. Emerging Applications of Magnetic Nanomaterials in the Remediation of Microplastics from the Aquatic Environment. In Remediation of Plastic and Microplastic Waste; CRC Press: Boca Raton, FL, USA, 2024; pp. 261–274. [Google Scholar]
- Sacco, N.A.; Zoppas, F.M.; Devard, A.; González Muñoz, M.d.P.; García, G.; Marchesini, F.A. Recent advances in microplastics removal from water with special attention given to photocatalytic degradation: Review of scientific research. Microplastics 2023, 2, 278–303. [Google Scholar] [CrossRef]
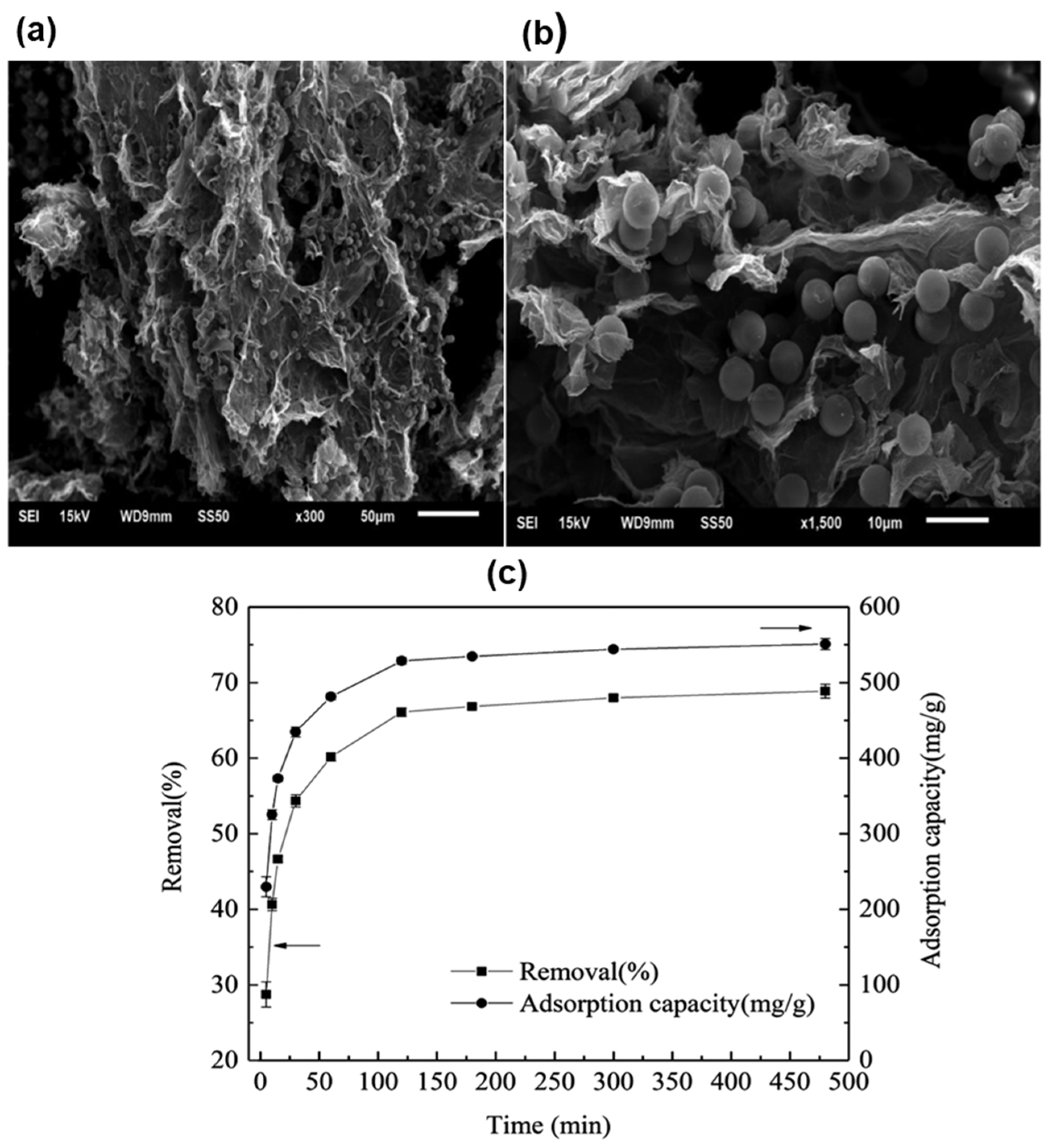
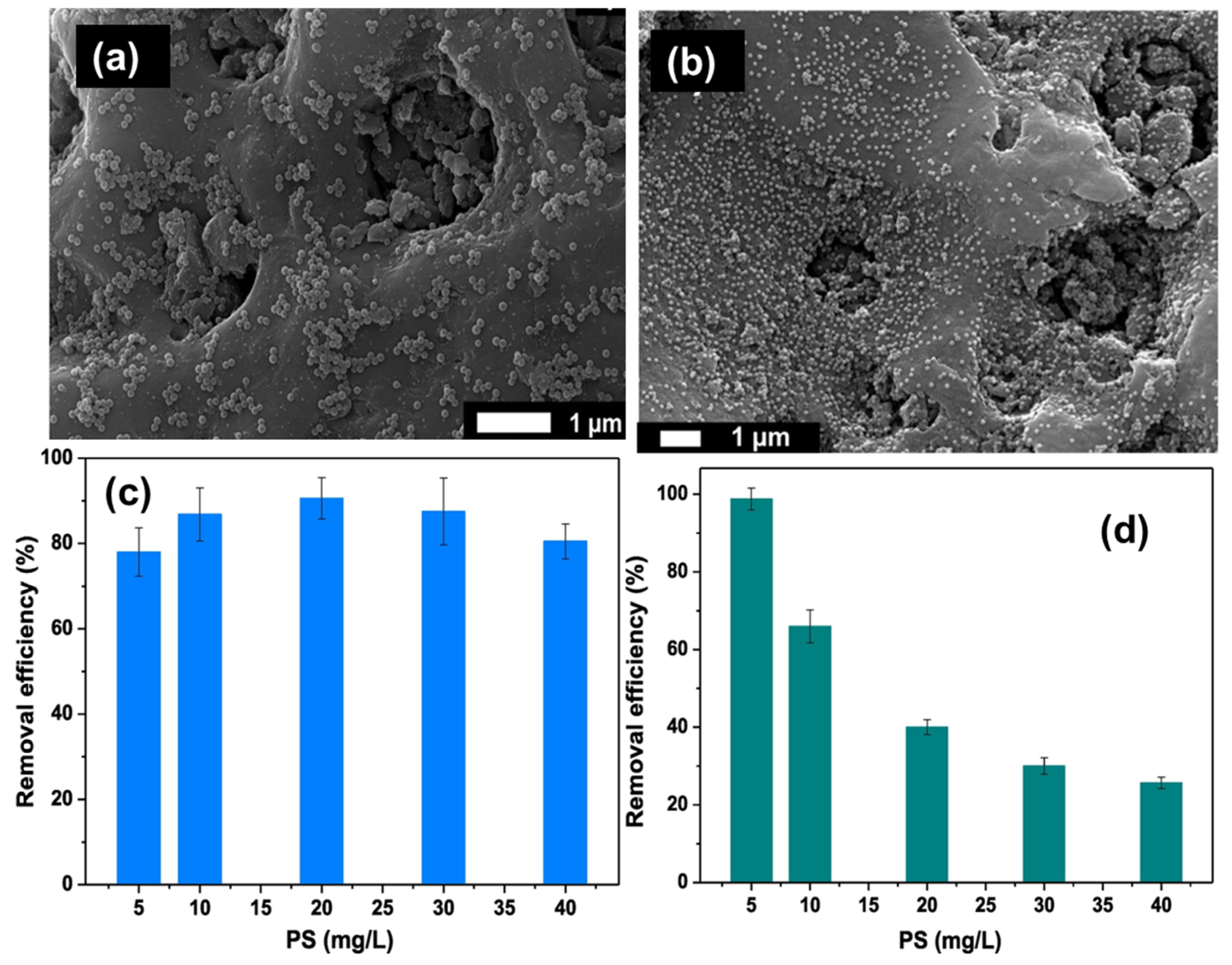


| Removal Process | Removal Techniques | Materials | Types of MP/NPs | MP/NPs Size | % MP/NPs Removed | Reference |
|---|---|---|---|---|---|---|
| Natural water | ||||||
| Adsorption, Photocatalytic and Electrochemical Materials | Biochar and Modified Magnetic Biochar (MBC) | MBCs | PS | 1 µm | 94.81 | [11] |
| Mg-MBCs | PS | 1 µm | 98.75 | |||
| Zn-MBCs) | PS | 1 µm | 99.46 | |||
| Sponge | Chitin and graphene oxide (ChGO) based sponge | PS | 1 µm | 92.2 | [13] | |
| 3 dimensional graphene | Three-dimensional reduced graphene oxide (3D RGO) | Monodisperse PS | 5 µm | 56.08 | [23] | |
| Oat protein sponges | Oat protein isolates | PS | 1 µm | 81.2 | [11] | |
| Zirconium-based MOF foam with Zn-Al LDH | Zn-Al LDH | PS | 55 nm | 100 | [63] | |
| Zirconium metal organic frame work based foam | UiO-66-OH@MF-3 | PVDF | ~260 nm | 95.5 ± 1.2 | [64] | |
| PMMA | ~325 nm | |||||
| PS | ~183 nm | |||||
| Granular activated carbon (GAC) | Granular coconut shell based Activated Carbon | PS latex NPs | 90 ± 7 nm | 90 | [25] | |
| Coffee grounds | Coffee grounds biomass | PS (fluo-NP) | 100 nm | 74 | [65] | |
| Hydrophobic Fe nanoparticles | Modified Fe nanoparticles | MPs | 1–8 nm | 74–105 | [31] | |
| 200 µm–1 nm | 59–100 | |||||
| <20 µm | ~90 | |||||
| Magnetic carbon nanotubes | M-CNTs | PE, PET, and PA | 48 µm | 100 | [66] | |
| Filtration | Electrocoagulation | Reactor, electrodes | PE | 300–355 µm | 90–100 | [67] |
| Biofilter structures | Crushed light-expanded clay aggregates with and without biochar | PE | 100 µm | 100 | [68] | |
| Pressure-sensitive adhesive | Zirconium silicate beads coated with poly (2- ethylhexyl acrylate) | PS | 10 µm | 99 | [69] | |
| Silica-based ceramic hollow fiber microporous membrane | Guinea cornhusk ash (GCHA) | PVC, PVP, PAN, PMMA | - | 88–97.2 | [70] | |
| Natural Bioflocculant | Lysozyme amyloid fibrils | PS | 500 nm | 93.4–98 | [71] | |
| Solar energy | Spherical K5 glass balls | PS | 60 nm | 74 | [72] | |
| Marine water | ||||||
| Non-fluorinated | Combining anodization and liquid phase of lauric acid | PP | 262 ± 4 µm | >99 | [73] | |
| MP concentrator (MPC) | Patterned PDMS bonded with oxygen plasma | PS | 1–20 µm | ≥90 | [74] | |
| Microbes | Extracellular polymeric substances (EPS) | PMMA, PS | 106–250 µm | N/A | [75] | |
| Bacterial biofilm | EPS | MPs | 106–300 µm | N/A | [76] | |
| PDA@Fe3O4 (MagRobots) | Coating Fe3O4 nanoparticles | MPs solution | - | N/A | [77] | |
| Drinking water | ||||||
| Filtration | Alum-based coagulation-flocculation-sedimentation (CFS) | CFS | PS | 3, 6, 25, 45 45 and 90 µm | ~100 | [78] |
| Alum coagulation | CFS | PE, Rayon, Polyester | 1–5 µm | N/A | [79] | |
| Coagulation | Coagulant: AlCl3.6H2O | PE | <0.5–5 mm | 36.89 ± 1.06 | [80] | |
| Coagulation | Ferric and aluminium Sulphate coagulats | Pristine PVC | <50 µm | ~80 | [81] | |
| Coagulation | Fe-based coagulants | PE | 0.5–5 nm | 87.66–90.91 | [82] | |
| Sand filtration | Coagulation | PS | 10, 20, 45 and 90 µm | 77.4–95.3 | [83] | |
| Magnetic Separation | Magnetic polyoxometalate ionic-liquids | PS | N/A | 90 | [84] | |
| Soil | ||||||
| Filtration | Density Separation | NaCl, ZnCl2, DI water, NaI | PE, PP, PET, PAN | 0.02–0.25 mm | N/A | [85] |
| Filtration | Filter papers | PET, PP.LDPE, PVC, HDPE, PS | 0.25–1 mm | 51–99 | [86] | |
| Oil-based extraction | Coaster and olive oil | PS, PE, PVC, PET, polyurethane and poly-carbonate | 5 μm to 300 μm | 90–97% | [87] | |
| Flotation method | DI water | PE, PP | >100 μm | 90 | [88] | |
| Sediments | ||||||
| Filtration and/or sieving | Oxidizing digestion | H2O2 | PS, fibers | size > 1 mm | 65.8–98 | [88] |
| Alkaline digestion | A mixture of urea: thiourea: NaOH | PET, nylon | >45 μm | 100 | [89] | |
| Density separation | NaCl, NaBr, NaI, ZnBr2 | PS, Nylon, PVP, HDPE, PET, Mixed MPs | <1 mm | 91–99 | [90] | |
| Heat assisted density separation | Sodium dihydrogen phosphate solution | PS, PE, PVC, PP, PET, Polyamide | 0.1 to 1 mm | 93 | [67] | |
| Digestion method | H2O2 | PP, PS, PE, PET and PA | >1 mm | 100 | [91] | |
| JAMSTEC MPs- Sediment separator | Small device | PE, PP, PVC, PET, PS | <1000 µm | 92–98 | [92] | |
| Sieving Method | Sodium polytungstate (SPT) | 62 MPs | 5 mm–250 μm | 97 | [67] | |
| Removal Method | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Filtration (Membrane, Nanofiber, Ultrafiltration) | High efficiency in removing MPs/NPs, well-established technology | Prone to fouling, high maintenance costs | [49,50,55] |
| Adsorption (Activated Carbon, Biochar, Graphene-based materials) | Cost-effective, high adsorption capacity | Requires regeneration, limited selectivity for different MPs/NPs | [7,8,11] |
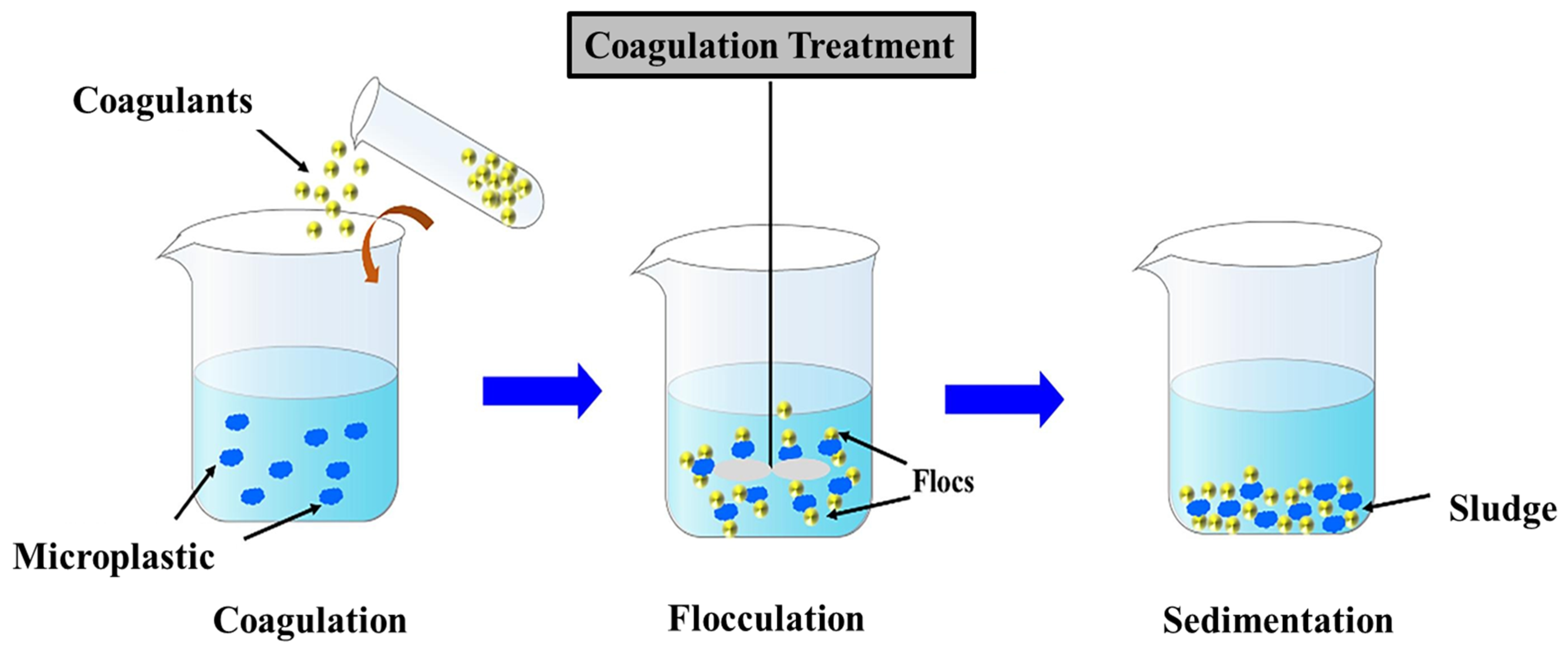
| Coagulation & Flocculation | Simple and scalable for wastewater treatment | Ineffective for small-sized NPs, generates sludge | [58,59,60] |
| Electrocoagulation | High removal efficiency, minimal chemical use | Energy-intensive, requires electrode maintenance | [15,61,62] |
| Photocatalysis (TiO2, ZnO, Fe-based catalysts) | Can degrade MPs into harmless byproducts | Requires light source, slow reaction time | [35,36,44] |
| Magnetic Nanoparticles | Rapid and selective removal, reusable | High synthesis cost, requires optimization | [31,32,33] |
| AI-driven Optimization | Enhances process efficiency and automation | Requires advanced infrastructure, high initial cost | [19,111,112] |
| Laser-based Removal | Potentially efficient for microplastic degradation | Still in research phase, high energy demand | [13,106,108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enyoh, C.E.; Devi, A.; Maduka, T.O.; Tyagi, L.; Rana, S.; Akuwudike, I.S.; Wang, Q. A Review of Materials for the Removal of Micro- and Nanoplastics from Different Environments. Micro 2025, 5, 17. https://doi.org/10.3390/micro5020017
Enyoh CE, Devi A, Maduka TO, Tyagi L, Rana S, Akuwudike IS, Wang Q. A Review of Materials for the Removal of Micro- and Nanoplastics from Different Environments. Micro. 2025; 5(2):17. https://doi.org/10.3390/micro5020017
Chicago/Turabian StyleEnyoh, Christian Ebere, Arti Devi, Tochukwu Oluwatosin Maduka, Lavista Tyagi, Sohel Rana, Ifunanya Scholastica Akuwudike, and Qingyue Wang. 2025. "A Review of Materials for the Removal of Micro- and Nanoplastics from Different Environments" Micro 5, no. 2: 17. https://doi.org/10.3390/micro5020017
APA StyleEnyoh, C. E., Devi, A., Maduka, T. O., Tyagi, L., Rana, S., Akuwudike, I. S., & Wang, Q. (2025). A Review of Materials for the Removal of Micro- and Nanoplastics from Different Environments. Micro, 5(2), 17. https://doi.org/10.3390/micro5020017