Unveiling the Intricacies of Microbial Pigments as Sustainable Alternatives to Synthetic Colorants: Recent Trends and Advancements
Abstract
1. Introduction
2. Microbial Pigments: Production and Market
3. Habitats of Pigmented Microorganisms
4. Analytics of Bio-Pigments
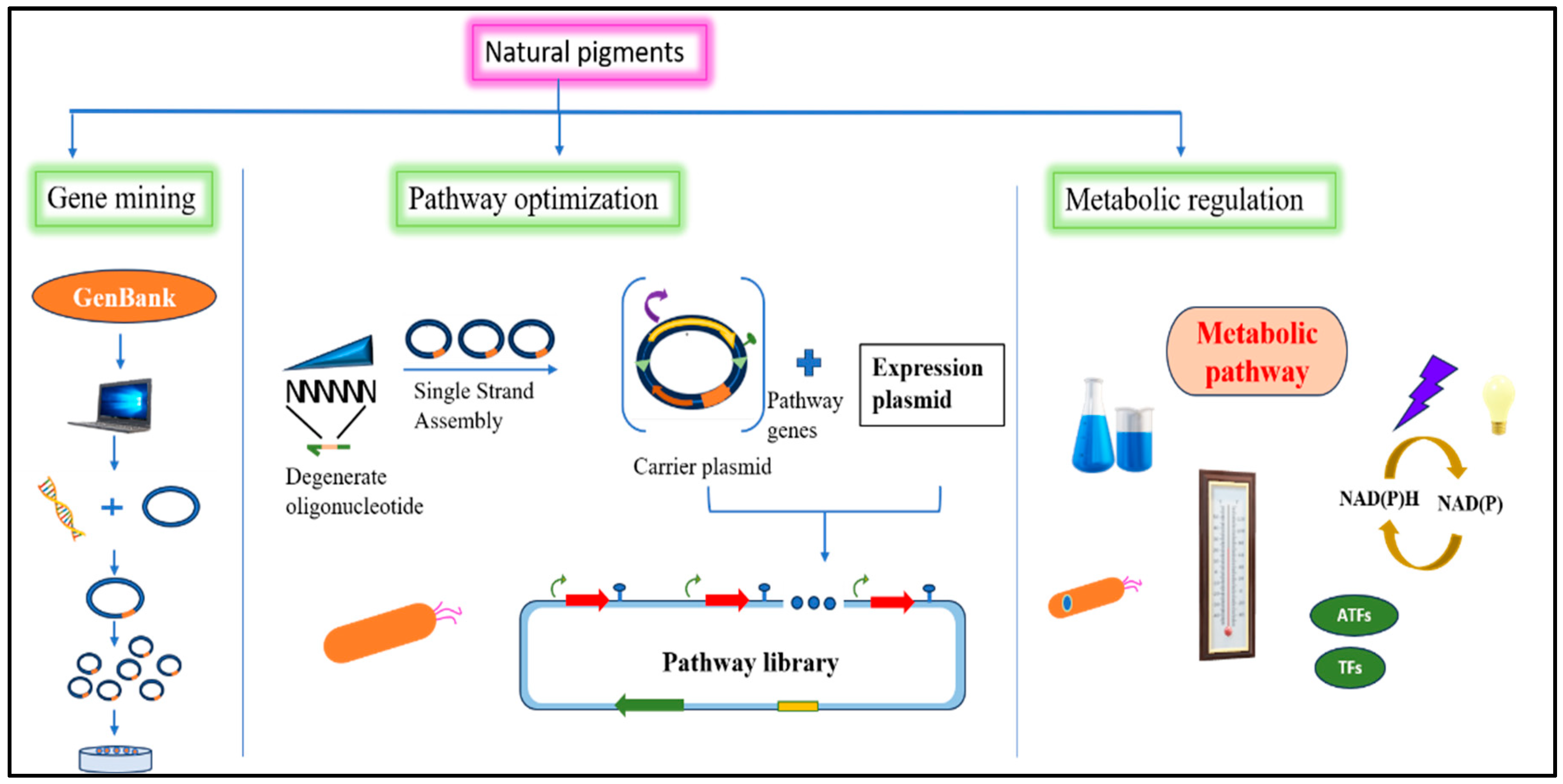
5. Genetically Modified Organisms in the Pigment Industry
5.1. Metabolic Engineering
5.2. Gene Cloning
6. Significance of Pigmented Bacteria
6.1. Cosmetic Industries
6.2. Food and Pharmaceutical Industries
6.3. Therapeutic Significance
6.3.1. Antibacterial Properties
6.3.2. Antifungal Properties
6.3.3. Anticancer Properties
6.3.4. Antiparasitic Properties
6.3.5. Immunosuppressing Properties
7. Bio-Colorants as Bio-Indicators
8. Limitations of Pigmented Microbes
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vila, E.; Hornero-Méndez, D.; Azziz, G.; Lareo, C.; Saravia, V. Carotenoids from heterotrophic bacteria isolated from fildes peninsula, king george island, antarctica. Biotechnol. Rep. 2019, 21, e00306. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Shukla, A.K.; Kumari, A.; Dhewa, T.; Kumar, A. Nutritional evaluation of probiotics enriched rabadi beverage (PERB) and molecular mapping of digestive enzyme with dietary fibre for exploring the therapeutic potential. Food Humanit. 2023, 2, 100221. [Google Scholar] [CrossRef]
- Rather, L.J.; Mir, S.S.; Ganie, S.A.; Li, Q. Research progress, challenges, and perspectives in microbial pigment production for industrial applications—A review. Dye. Pigment. 2023, 210, 110989. [Google Scholar] [CrossRef]
- US Foods. Drug Administration Center for Food Safety and Applied Nutrition. Agency Response Lett. GRAS Not. No GRN 2002, 105. [Google Scholar] [CrossRef]
- Fabre, C.; Santerre, A.; Loret, M.; Baberian, R.; Pareilleux, A.; Goma, G. Production and food applications of the red pigments of Monascus ruber. J. Food Sci. 1993, 58, 1099–1102. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Napathorn, S.C.; Kim, B.S. Valorization of organic wastes using bioreactors for polyhydroxyalkanoate production: Recent advancement, sustainable approaches, challenges, and future perspectives. Int. J. Biol. Macromol. 2023, 247, 125743. [Google Scholar] [CrossRef]
- Singh, A.; Kushwaha, A.; Goswami, S.; Tripathi, A.; Bhasney, S.M.; Goswami, L.; Hussain, C.M. Roadmap from microalgae to biorefinery: A circular bioeconomy approach. In Emerging Trends to Approaching Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–360. [Google Scholar]
- Kushwaha, A.; Yadav, A.N.; Singh, B.; Dwivedi, V.; Kumar, S.; Goswami, L.; Hussain, C.M. Life cycle assessment and techno-economic analysis of algae-derived biodiesel: Current challenges and future prospects. In Waste-to-Energy Approaches Towards Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–372. [Google Scholar]
- Wehrs, M.; Tanjore, D.; Eng, T.; Lievense, J.; Pray, T.R.; Mukhopadhyay, A. Engineering robust production microbes for large-scale cultivation. Trends Microbiol. 2019, 27, 524–537. [Google Scholar] [CrossRef]
- Goswami, L.; Kayalvizhi, R.; Dikshit, P.K.; Sherpa, K.C.; Roy, S.; Kushwaha, A.; Rajak, R.C. A critical review on prospects of bio-refinery products from second and third generation biomasses. Chem. Eng. J. 2022, 448, 137677. [Google Scholar] [CrossRef]
- Uddin, F. Environmental hazard in textile dyeing wastewater from local textile industry. Cellulose 2021, 28, 10715–10739. [Google Scholar] [CrossRef]
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2019, 46, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Ramesh, C.; Mohanraju, R.; Murthy, K.; Karthick, P. Molecular characterization of marine pigmented bacteria showing antibacterial activity. Indian J. Mar. Sci. 2017, 46, 2081–2087. [Google Scholar]
- Zhang, X.; Yao, T.; Tian, L.; Xu, S.; An, L. Phylogenetic and physiological diversity of bacteria isolated from Puruogangri ice core. Microb. Ecol. 2008, 55, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Khanafari, A.; Khavarinejad, D.; Mashinchian, A. Solar salt lake as natural environmental source for extraction halophilic pigments. Iran. J. Microbiol. 2010, 2, 103. [Google Scholar]
- Hermansson, M.; Jones, G.; Kjelleberg, S. Frequency of antibiotic and heavy metal resistance, pigmentation, and plasmids in bacteria of the marine air-water interface. Appl. Environ. Microbiol. 1987, 53, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Miteva, V.; Sheridan, P.; Brenchley, J. Phylogenetic and physiological diversity of microorganisms isolated from a deep Greenland glacier ice core. Appl. Environ. Microbiol. 2004, 70, 202–213. [Google Scholar] [CrossRef]
- Agogué, H.; Joux, F.; Obernosterer, I.; Lebaron, P. Resistance of marine bacterioneuston to solar radiation. Appl. Environ. Microbiol. 2005, 71, 5282–5289. [Google Scholar] [CrossRef]
- Yurkov, V.V.; Krieger, S.; Stackebrandt, E.; Beatty, J.T. Citromicrobium bathyomarinum, a novel aerobic bacterium isolated from deep-sea hydrothermal vent plume waters that contains photosynthetic pigment-protein complexes. J. Bacteriol. 1999, 181, 4517–4525. [Google Scholar] [CrossRef]
- Méndez-Zavala, A.; Contreras-Esquivel, J.; Lara-Victoriano, F.; Rodríguez-Herrera, R.; Aguilar, C. Fungal production of the red pigment using a xerophilic strain Penicillium purpurogenum GH-2. Rev. Mex. Ing. QuÍMica 2007, 6, 267–273. [Google Scholar]
- Likens, G.E. Plankton of Inland Waters; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Graham, R.D.; Rosser, J.M. Carotenoids in staple foods: Their potential to improve human nutrition. Food Nutr. Bull. 2000, 21, 404–409. [Google Scholar] [CrossRef]
- Thawornwiriyanun, P.; Tanasupawat, S.; Dechsakulwatana, C.; Techkarnjanaruk, S.; Suntornsuk, W. Identification of newly zeaxanthin-producing bacteria isolated from sponges in the Gulf of Thailand and their zeaxanthin production. Appl. Biochem. Biotechnol. 2012, 167, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Nugraheni, S.A.; Khoeri, M.M.; Kusmita, L.; Widyastuti, Y.; Radjasa, O.K. Characterization of carotenoid pigments from bacterial symbionts of seagrass Thalassia hemprichii. J. Coast. Dev. 2010, 14, 51–60. [Google Scholar]
- Azman, A.-S.; Mawang, C.-I.; Abubakar, S. Bacterial pigments: The bioactivities and as an alternative for therapeutic applications. Nat. Prod. Commun. 2018, 13, 1934578X1801301240. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial pigments: Sustainable compounds with market potential for pharma and food industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Bennett, J.; Bentley, R. Seeing Red: The Story of Prodigiosin; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Soliev, A.B.; Enomoto, K. Antitumor Pigments from Marine Bacteria. In Marine Biomaterials: Characterization, Isolation and Applications; Kim, S., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 149–171. [Google Scholar]
- Okazaki, T.; Kitahara, T.; Okami, Y. Studies on marine microorganisms. IV a new antibiotic SS-228 Y produced by chainia isolated from shallow sea mud. J. Antibiot. 1975, 28, 176–184. [Google Scholar] [CrossRef]
- Franks, A.; Haywood, P.; Holmström, C.; Egan, S.; Kjelleberg, S.; Kumar, N. Isolation and structure elucidation of a novel yellow pigment from the marine bacterium Pseudoalteromonas tunicata. Molecules 2005, 10, 1286–1291. [Google Scholar] [CrossRef]
- Pinkerton, D.M.; Banwell, M.G.; Garson, M.J.; Kumar, N.; de Moraes, M.O.; Cavalcanti, B.C. Antimicrobial and Cytotoxic Activities of Synthetically Derived Tambjamines C and E–J, BE-18591, and a Related Alkaloid from the Marine Bacterium Pseudoalteromonas tunicata. Chem. Biodivers. 2010, 7, 1311–1324. [Google Scholar] [CrossRef]
- Blackman, A.J.; Li, C. New tambjamine alkaloids from the marine bryozoan Bugula dentata. Aust. J. Chem. 1994, 47, 1625–1629. [Google Scholar] [CrossRef]
- Carté, B.; Faulkner, D.J. Defensive metabolites from three nembrothid nudibranchs. J. Org. Chem. 1983, 48, 2314–2318. [Google Scholar] [CrossRef]
- Carté, B.; Faulkner, D.J. Role of secondary metabolites in feeding associations between a predatory nudibranch, two grazing nudibranchs, and a bryozoan. J. Chem. Ecol. 1986, 12, 795–804. [Google Scholar] [CrossRef]
- Tarangini, K.; Mishra, S. Production, characterization and analysis of melanin from isolated marine Pseudomonas sp. using vegetable waste. Res. J. Eng. Sci. 2013, 2278, 9472. [Google Scholar]
- Bezirhan Arikan, E.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of bio-based pigments from food processing industry by-products (apple, pomegranate, black carrot, red beet pulps) using Aspergillus carbonarius. J. Fungi 2020, 6, 240. [Google Scholar] [CrossRef] [PubMed]
- Naik, C.; Srisevita, J.; Shushma, K.; Noorin, F.; Shilpa, A.; Muttanna, C. Peanut oil cake: A novel substrate for enhanced cell growth and prodigiosin production from Serratia marcescens CF-53. J. Res. Biol. 2012, 2, 549–557. [Google Scholar]
- Aruldass, C.A.; Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Brown sugar as a low-cost medium for the production of prodigiosin by locally isolated Serratia marcescens UTM1. Int. Biodeterior. Biodegrad. 2014, 95, 19–24. [Google Scholar] [CrossRef]
- Luti, K.J.; Yonis, R.W.; Mahmoud, S.T. An application of solid state fermentation and elicitation with some microbial cells for the enhancement of prodigiosin production by Serratia marcescens. Al-Nahrain J. Sci. 2018, 21, 98–105. [Google Scholar] [CrossRef]
- Sumathi, C.; MohanaPriya, D.; Swarnalatha, S.; Dinesh, M.; Sekaran, G. Production of prodigiosin using tannery fleshing and evaluating its pharmacological effects. Sci. World J. 2014, 2014, 290327. [Google Scholar] [CrossRef]
- Giri, A.V.; Anandkumar, N.; Muthukumaran, G.; Pennathur, G. A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol. 2004, 4, 11. [Google Scholar] [CrossRef]
- El-Bondkly, A.M.; El-Gendy, M.M.; Bassyouni, R.H. Overproduction and biological activity of prodigiosin-like pigments from recombinant fusant of endophytic marine Streptomyces species. Antonie Leeuwenhoek 2012, 102, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Aruldass, C.A.; Venil, C.K.; Ahmad, W.A. Violet pigment production from liquid pineapple waste by Chromobacterium violaceum UTM5 and evaluation of its bioactivity. Rsc Adv. 2015, 5, 51524–51536. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Yusof, N.Z.; Nordin, N.; Zakaria, Z.A.; Rezali, M.F. Production and characterization of violacein by locally isolated Chromobacterium violaceum grown in agricultural wastes. Appl. Biochem. Biotechnol. 2012, 167, 1220–1234. [Google Scholar] [CrossRef]
- Cassarini, M.; Crônier, D.; Besaury, L.; Rémond, C. Protein-rich agro-industrial co-products are key substrates for growth of Chromobacterium vaccinii and its violacein bioproduction. Waste Biomass Valorization 2022, 13, 4459–4468. [Google Scholar] [CrossRef]
- Joshi, V.K.; Attri, D.; Rana, N.S. (Eds.) Optimization of apple pomace based medium and fermentation conditions for pigment production by Sarcina sp. Indian J. Nat. Prod. Resour. 2011, 2, 421–427. [Google Scholar]
- Valduga, E.; Ribeiro, A.H.R.; Cence, K.; Colet, R.; Tiggemann, L.; Zeni, J. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain using agroindustrial substrates. Biocatal. Agric. Biotechnol. 2014, 3, 207–213. [Google Scholar] [CrossRef]
- Taskin, M.; Sisman, T.; Erdal, S.; Kurbanoglu, E.B. Use of waste chicken feathers as peptone for production of carotenoids in submerged culture of Rhodotorula glutinis MT-5. Eur. Food Res. Technol. 2011, 233, 657–665. [Google Scholar] [CrossRef]
- Nasrabadi, M.R.N.; Razavi, S.H. Optimization of β-carotene production by a mutant of the lactose-positive yeast Rhodotorula acheniorum from whey ultrafiltrate. Food Sci. Biotechnol. 2011, 20, 445–454. [Google Scholar] [CrossRef]
- Bonadio, M.d.P.; Freita, L.A.d.; Mutton, M.J.R. Carotenoid production in sugarcane juice and synthetic media supplemented with nutrients by Rhodotorula rubra l02. Braz. J. Microbiol. 2018, 49, 872–878. [Google Scholar] [CrossRef]
- Moreira, M.D.; Melo, M.M.; Coimbra, J.M.; Dos Reis, K.C.; Schwan, R.F.; Silva, C.F. Solid coffee waste as alternative to produce carotenoids with antioxidant and antimicrobial activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef]
- Kang, C.D.; An, J.Y.; Park, T.H.; Sim, S.J. Astaxanthin biosynthesis from simultaneous N and P uptake by the green alga Haematococcus pluvialis in primary-treated wastewater. Biochem. Eng. J. 2006, 31, 234–238. [Google Scholar] [CrossRef]
- Ramírez, J.; Obledo, N.; Arellano, M.; Herrera, E. Astaxanthin production by Phaffia rhodozyma in a fedbatch culture using a low cost medium feeding. e-Gnosis 2006, 4, 5. [Google Scholar]
- Haard, N. Astaxanthin formation by the yeast Phaffia rhodozyma on molasses. Biotechnol. Lett. 1988, 10, 609–614. [Google Scholar] [CrossRef]
- Meyer, P.; Du Preez, J. Astaxanthin production by a Phaffia rhodozyma mutant on grape juice. World J. Microbiol. Biotechnol. 1994, 10, 178–183. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, Y.H.; Han, J.K.; Park, J.H.; Lee, K.H.; Choi, H. Microorganism for Producing Riboflavin and Method for Producing Riboflavin Using the Same. U.S. Patent 7,166,456, 23 January 2007. [Google Scholar]
- El-Fouly, M.; Sharaf, A.; Shahin, A.; El-Bialy, H.A.; Omara, A. Biosynthesis of pyocyanin pigment by Pseudomonas aeruginosa. J. Radiat. Res. Appl. Sci. 2015, 8, 36–48. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Sudheer, S.; Gupta, V.K.; Bhat, R. Engineered microbes for pigment production using waste biomass. Curr. Genom. 2020, 21, 80–95. [Google Scholar] [CrossRef]
- Henke, N.A.; Wiebe, D.; Pérez-García, F.; Peters-Wendisch, P.; Wendisch, V.F. Coproduction of cell-bound and secreted value-added compounds: Simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum. Bioresour. Technol. 2018, 247, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Chi, S.; Li, Y.; Tan, S.; Qiang, S.; Chen, Z. Metabolic redesign of Rhodobacter sphaeroides for lycopene production. J. Agric. Food Chem. 2018, 66, 5879–5885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Q.; Sun, T.; Zhu, X.; Xu, H.; Tang, J. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab. Eng. 2013, 17, 42–50. [Google Scholar] [CrossRef]
- Kushwaha, A.; Goswami, L.; Singhvi, M.; Kim, B.S. Biodegradation of poly (ethylene terephthalate): Mechanistic insights, advances, and future innovative strategies. Chem. Eng. J. 2023, 457, 141230. [Google Scholar] [CrossRef]
- Singh, S.; Goswami, L.; Hussain, C.M. (Eds.) Waste-to-Energy Approaches towards Zero Waste: Interdisciplinary Methods of Controlling Waste; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Bharagava, R.N.; Goswami, L.; Kushwaha, A.; Hussain, C.M. (Eds.) Bio-Based Materials and Waste for Energy Generation and Resource Management: Volume 5 of Advanced Zero Waste Tools: Present and Emerging Waste Management Practices; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Yang, D.; Park, S.Y.; Lee, S.Y. Production of rainbow colorants by metabolically engineered Escherichia coli. Adv. Sci. 2021, 8, 2100743. [Google Scholar] [CrossRef] [PubMed]
- Getino, L.; Chamizo-Ampudia, A.; Martín, J.L.; Luengo, J.M.; Barreiro, C.; Olivera, E.R. Specific Gene Expression in Pseudomonas putida U Shows New Alternatives for Cadaverine and Putrescine Catabolism. Genes 2023, 14, 1897. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Y.; Zhang, C.; Yang, S.; Cui, J.Y.; Jiang, P.X.; Lou, K.; Xing, X.H. High crude violacein production from glucose by Escherichia coli engineered with interactive control of tryptophan pathway and violacein biosynthetic pathway. Microb. Cell Factories 2015, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Hsiao, H.C.; Hou, Y.C.; Hsieh, C.W.; Hsu, H.Y.; Chen, H.Y.; Lin, S.P. Improvement in violacein production by utilizing formic acid to induce quorum sensing in Chromobacterium violaceum. Antioxidants 2022, 11, 849. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Applications of prodigiosin extracted from marine red pigmented bacteria Zooshikella sp. and actinomycete Streptomyces sp. Microorganisms 2020, 8, 556. [Google Scholar] [CrossRef]
- Mühlmann, M.; Forsten, E.; Noack, S.; Büchs, J. Optimizing recombinant protein expression via automated induction profiling in microtiter plates at different temperatures. Microb. Cell Factories 2017, 16, 220. [Google Scholar] [CrossRef]
- Watstein, D.M.; McNerney, M.P.; Styczynski, M.P. Precise metabolic engineering of carotenoid biosynthesis in Escherichia coli towards a low-cost biosensor. Metab. Eng. 2015, 31, 171–180. [Google Scholar] [CrossRef]
- Choi, O.; Kang, B.; Lee, Y.; Lee, Y.; Kim, J. Pantoea ananatis carotenoid production confers toxoflavin tolerance and is regulated by Hfq-controlled quorum sensing. MicrobiologyOpen 2021, 10, e1143. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Nielsen, J. Development of fungal cell factories for the production of secondary metabolites: Linking genomics and metabolism. Synth. Syst. Biotechnol. 2017, 2, 5–12. [Google Scholar] [CrossRef]
- Pohl, C.; Kiel, J.A.; Driessen, A.J.; Bovenberg, R.A.; Nygard, Y. CRISPR/Cas9 based genome editing of Penicillium chrysogenum. ACS Synth. Biol. 2016, 5, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Almeida, C.; Vasconcelos, V.; Guedes, A.C. Cosmetic Potential of Pigments Extracts from the Marine Cyanobacterium Cyanobium sp. Mar. Drugs 2022, 20, 481. [Google Scholar] [CrossRef]
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial metabolites as a source of sunscreens and moisturizers: A comparison with current synthetic compounds. Eur. J. Phycol. 2017, 52, 43–56. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Videira, I.F.d.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Gillbro, J.; Olsson, M. The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef]
- Costin, G.-E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Takizawa, T.; Imai, T.; Onose, J.-I.; Ueda, M.; Tamura, T.; Mitsumori, K. Enhancement of hepatocarcinogenesis by kojic acid in rat two-stage models after initiation with N-bis (2-hydroxypropyl) nitrosamine or N-diethylnitrosamine. Toxicol. Sci. 2004, 81, 43–49. [Google Scholar] [CrossRef]
- García-Gavín, J.; González-Vilas, D.; Fernández-Redondo, V.; Toribio, J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermat. 2010, 62, 63–64. [Google Scholar] [CrossRef]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4752–4761. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Jung, E.Y.; Noh, D.O.; Suh, H.J. Physiological effects of formulation containing tannase-converted green tea extract on skin care: Physical stability, collagenase, elastase, and tyrosinase activities. Integr. Med. Res. 2014, 3, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lajis, A.F.B.; Ariff, A.B. Discovery of new depigmenting compounds and their efficacy to treat hyperpigmentation: Evidence from in vitro study. J. Cosmet. Dermatol. 2019, 18, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Keziah, S.M.; Hemalatha, M.; Devi, C.S. (Eds.) Pigment from Streptomyces bellus MSA1 isolated from marine sediments. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017. [Google Scholar]
- El-Naggar, N.E.-A.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef]
- Choksi, J.; Vora, J.; Shrivastava, N. Bioactive pigments from isolated bacteria and its antibacterial, antioxidant and sun protective application useful for cosmetic products. Indian J. Microbiol. 2020, 60, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Kiki, M.J. Biopigments of microbial origin and their application in the cosmetic industry. Cosmetics 2023, 10, 47. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Chandra, R.; Parra, R.; MNIqbal, H. Phycobiliproteins: A novel green tool from marine origin blue-green algae and red algae. Protein Pept. Lett. 2017, 24, 118–125. [Google Scholar] [CrossRef]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The realm of microbial pigments in the food color market. Front. Sustain. Food Syst. 2021, 5, 603892. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to synthetic pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Jurić, M.; Król-Kilińska, Ż.; Vlahoviček-Kahlina, K.; Vinceković, M.; Dragović-Uzelac, V. Sources, stability, encapsulation and application of natural pigments in foods. Food Rev. Int. 2022, 38, 1735–1790. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Optimization of culture conditions for flexirubin production by Chryseobacterium artocarpi CECT 8497 using response surface methodology. Acta Biochim. Pol. 2015, 62, 185–190. [Google Scholar] [CrossRef]
- Meruvu, H.; Dos Santos, J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021, 41, 1153–1177. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Jia, M.; Li, W.; Deng, J.; Ren, J.; Luo, F. Toward improvements for enhancement the productivity and color value of Monascus pigments: A critical review with recent updates. Crit. Rev. Food Sci. Nutr. 2022, 62, 7139–7153. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety evaluation of fungal pigments for food applications. J. Fungi 2021, 7, 692. [Google Scholar] [CrossRef]
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current perspective of yellowish-orange pigments from microorganisms—A review. J. Clean. Prod. 2018, 180, 168–182. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Pereira da Costa, D.; Campos Miranda-Filho, K. The use of carotenoid pigments as food additives for aquatic organisms and their functional roles. Rev. Aquac. 2020, 12, 1567–1578. [Google Scholar] [CrossRef]
- Dufossé, L. Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 113–132. [Google Scholar]
- Chatragadda, R.; Dufossé, L. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef]
- Nasseri, A.; Rasoul-Amini, S.; Morowvat, M.; Ghasemi, Y. Single cell protein: Production and process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Carlozzi, P.; Buccioni, A.; Minieri, S.; Pushparaj, B.; Piccardi, R.; Ena, A. Production of bio-fuels (hydrogen and lipids) through a photofermentation process. Bioresour. Technol. 2010, 101, 3115–3120. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, B.-K. Mass production of Rhodopseudomonas palustris as diet for aquaculture. Aquac. Eng. 2000, 23, 281–293. [Google Scholar] [CrossRef]
- Tian, Y.; Machado, P.A.; Fu, H.; Hahm, T.-S.; Wei, C.-I.; Lo, Y.M. Photosynthetic bioconversion of coenzyme Q10 using agrowaste generated from tobacco biorefinery for nonsmoking applications: A review. J. Food Drug Anal. 2012, 20, 81. [Google Scholar] [CrossRef]
- Wang, G.-S.; Grammel, H.; Abou-Aisha, K.; Sägesser, R.; Ghosh, R. High-level production of the industrial product lycopene by the photosynthetic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 2012, 78, 7205–7215. [Google Scholar] [CrossRef] [PubMed]
- Saejung, C.; Ampornpat, W. Production and nutritional performance of carotenoid-producing photosynthetic bacterium Rhodopseudomonas faecalis PA2 grown in domestic wastewater intended for animal feed production. Waste Biomass Valorization 2019, 10, 299–310. [Google Scholar] [CrossRef]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arct. Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Stafsnes, M.H.; Dybwad, M.; Brunsvik, A.; Bruheim, P. Large scale MALDI-TOF MS based taxa identification to identify novel pigment producers in a marine bacterial culture collection. Antonie Leeuwenhoek 2013, 103, 603–615. [Google Scholar] [CrossRef]
- Egan, S.; James, S.; Holmström, C.; Kjelleberg, S. Correlation between pigmentation and antifouling compounds produced by Pseudoalteromonas tunicata. Environ. Microbiol. 2002, 4, 433–442. [Google Scholar] [CrossRef]
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef]
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- Konzen, M.; De Marco, D.; Cordova, C.A.; Vieira, T.O.; Antonio, R.V.; Creczynski-Pasa, T.B. Antioxidant properties of violacein: Possible relation on its biological function. Bioorganic Med. Chem. 2006, 14, 8307–8313. [Google Scholar] [CrossRef] [PubMed]
- Matz, C.; Deines, P.; Boenigk, J.; Arndt, H.; Eberl, L.; Kjelleberg, S. Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol. 2004, 70, 1593–1599. [Google Scholar] [CrossRef]
- Soliev, A.B.; Hosokawa, K.; Enomoto, K. Bioactive pigments from marine bacteria: Applications and physiological roles. Evid.-Based Complement. Altern. Med. ECAM 2011, 2011, 670349. [Google Scholar] [CrossRef]
- Visca, P.; Imperi, F.; Lamont, I.L. Pyoverdine siderophores: From biogenesis to biosignificance. Trends Microbiol. 2007, 15, 22–30. [Google Scholar] [CrossRef]
- Borić, M.; Danevčič, T.; Stopar, D. Prodigiosin from Vibrio sp. DSM 14379, a new UV-protective pigment. Microb. Ecol. 2011, 62, 528–536. [Google Scholar] [CrossRef]
- Płonka, P.; Grabacka, M. Melanin synthesis in microorganisms: Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar] [CrossRef]
- Coyne, V.; Al-Harthi, L. Induction of melanin biosynthesis in Vibrio cholerae. Appl. Environ. Microbiol. 1992, 58, 2861–2865. [Google Scholar] [CrossRef]
- Nair, S.; Chandramohan, D.; Bharathi, P.L. Differential sensitivity of pigmented and non-pigmented marine bacteria to metals and antibiotics. Water Res. 1992, 26, 431–434. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-F.; Si, L.; Wang, Z.; Jingjie Zhu, Y.Z.; Shangguan, Y.; Lu, D. Genetic control of a transition from black to straw-white seed hull in rice domestication. Plant Physiol. 2011, 155, 1301–1311. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Van Leur, J. Genetic diversity in barley landraces from Syria and Jordan. Euphytica 1987, 36, 389–405. [Google Scholar] [CrossRef]
- Jana, B.; Mukherjee, S. Notes on the distribution of phytomelanin layer in higher plants—A short communication. J. Pharm. Biol. 2014, 4, 131–132. [Google Scholar]
- Pandey, A.; Dhakal, M. Phytomelanin in compositae. Curr. Sci. 2001, 80, 933–940. [Google Scholar]
- Panzella, L.; Eidenberger, T.; Napolitano, A.; d’Ischia, M. Black sesame pigment: DPPH assay-guided purification, antioxidant/antinitrosating properties, and identification of a degradative structural marker. J. Agric. Food Chem. 2012, 60, 8895–8901. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł. Antioxidant, antibacterial properties and the light barrier assessment of raw and purified melanins isolated from (watermelon) seeds. Herba Pol. 2018, 64, 25–36. [Google Scholar] [CrossRef]
- López, G.D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef]
- Montaner, B.; Pérez-Tomás, R. Prodigiosin-induced apoptosis in human colon cancer cells. Life Sci. 2001, 68, 2025–2036. [Google Scholar] [CrossRef]
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Stafsnes, M.H.; Josefsen, K.D.; Kildahl-Andersen, G.; Valla, S.; Ellingsen, T.E.; Bruheim, P. Isolation and characterization of marine pigmented bacteria from Norwegian coastal waters and screening for carotenoids with UVA-blue light absorbing properties. J. Microbiol. 2010, 48, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. A short history of the symposia on halophilic microorganisms: From Rehovot 1978 to Beijing 2010. In Halophiles and Hypersaline Environments: Current Research and Future Trends; Springer: Berlin/Heidelberg, Germany, 2011; pp. 373–382. [Google Scholar]
- Kapil, S.; Sharma, V. d-Amino acids in antimicrobial peptides: A potential approach to treat and combat antimicrobial resistance. Can. J. Microbiol. 2021, 67, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Heer, K.; Sharma, S. Microbial pigments as a natural color: A review. Int. J. Pharm. Sci. Res. 2017, 8, 1913–1922. [Google Scholar]
- Agematu, H.; Suzuki, K.; Tsuya, H. Massilia sp. BS-1, a novel violacein-producing bacterium isolated from soil. Biosci. Biotechnol. Biochem. 2011, 75, 2008–2010. [Google Scholar] [CrossRef] [PubMed]
- Selvameenal, L.; Radhakrishnan, M.; Balagurunathan, R. Antibiotic pigment from desert soil actinomycetes; biological activity, purification and chemical screening. Indian J. Pharm. Sci. 2009, 71, 499. [Google Scholar]
- Samrot, A.V.; Rio, A.J.; Kumar, S.S.; Samanvitha, S.K. Bioprospecting studies of pigmenting Pseudomonas aeruginosa SU-1, Microvirga aerilata SU14 and Bacillus megaterium SU15 isolated from garden soil. Biocatal. Agric. Biotechnol. 2017, 11, 330–337. [Google Scholar] [CrossRef]
- Agarwal, H.; Bajpai, S.; Mishra, A.; Kohli, I.; Varma, A.; Fouillaud, M. Bacterial Pigments and Their Multifaceted Roles in Contemporary Biotechnology and Pharmacological Applications. Microorganisms 2023, 11, 614. [Google Scholar] [CrossRef]
- El-Zawawy, N.A.; Kenawy, E.-R.; Ahmed, S.; El-Sapagh, S. Bioproduction and optimization of newly characterized melanin pigment from Streptomyces djakartensis NSS-3 with its anticancer, antimicrobial, and radioprotective properties. Microb. Cell Factories 2024, 23, 23. [Google Scholar] [CrossRef]
- Elkhateeb, W.; Daba, G. Fungal Pigments: Their Diversity, Chemistry, Food and Non-Food Applications. Appl. Microbiol. 2023, 3, 735–751. [Google Scholar] [CrossRef]
- Dixon, D.M.; McNeil, M.M.; Cohen, M.L.; Gellin, B.G.; La Montagne, J.R. Fungal infections: A growing threat. Public Health Rep. 1996, 111, 226. [Google Scholar] [PubMed]
- Andrighetti-Fröhner, C.; Antonio, R.; Creczynski-Pasa, T.; Barardi, C.; Simões, C. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. MemÓRias Do Inst. Oswaldo Cruz 2003, 98, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Moss, M. Bacterial pigments. Microbiologist 2002, 3, 10–12. [Google Scholar]
- Wagh, P.; Mane, R. Identification and characterization of extracellular red pigment producing Neisseria spp. isolated from soil sample. Int. J. Innov. Knowl. Concept. 2017, 5, 23–25. [Google Scholar]
- Shirata, A.; Tsukamoto, T.; Yasui, H.; Kato, H.; Hayasaka, S.; Kojima, A. Production of bluish-purple pigments by Janthinobacterium lividum isolated from the raw silk and dyeing with them. J. Sericultural Sci. Jpn. 1997, 66, 377–385. [Google Scholar]
- Lyakhovchenko, N.; Efimova, V.; Seleznev, A.; Ogneva, Z.; Solyanikova, I. (Eds.) Antifungal Activity of Gram-Negative Pigment-Forming Bacteria Against Aspergillus Unguis. In BIO Web of Conferences; EDP Sciences: Paris, France, 2023. [Google Scholar]
- Pan, M.-H.; Ho, C.-T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008, 37, 2558–2574. [Google Scholar] [CrossRef]
- Kawauchi, K.; Shibutani, K.; Yagisawa, H.; Kamata, H.; Nakatsuji, S.; Anzai, H. A Possible Immunosuppressant, Cycloprodigiosin Hydrochloride, Obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 1997, 237, 543–547. [Google Scholar] [CrossRef]
- Schneemann, I.; Wiese, J.; Kunz, A.L.; Imhoff, J.F. Genetic approach for the fast discovery of phenazine producing bacteria. Mar. Drugs 2011, 9, 772–789. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, Y.; Alfred, A.; Wei, P.; Yang, S. Anticancer effects of pyocyanin on HepG2 human hepatoma cells. Lett. Appl. Microbiol. 2014, 58, 541–548. [Google Scholar] [CrossRef]
- Vipin, C.; Ashwini, P.; Kavya, A.; Rekha, P. Overproduction of pyocyanin in Pseudomonas aeruginosa by supplementation of pathway precursor shikimic acid and evaluation of its activity. Res. J. Pharm. Technol. 2017, 10, 533–536. [Google Scholar] [CrossRef]
- Prashanthi, K.; Suryan, S.; Varalakshmi, K.N. In vitro anticancer property of yellow pigment from Streptomyces griseoaurantiacus JUACT 01. Braz. Arch. Biol. Technol. 2015, 58, 869–876. [Google Scholar] [CrossRef]
- Venil, C.K.; Khasim, A.R.; Aruldass, C.A.; Ahmad, W.A. Microencapsulation of flexirubin-type pigment by spray drying: Characterization and antioxidant activity. Int. Biodeterior. Biodegrad. 2016, 113, 350–356. [Google Scholar] [CrossRef]
- Maheswarappa, G.; Kavitha, D.; Vijayarani, K.; Kumanan, K. Prodigiosin as anticancer drug Produced from bacteria of termite gut. Indian J. Basic Appl. Med. Res. 2013, 1, 257–266. [Google Scholar]
- Lin, S.-R.; Weng, C.-F. PG-priming enhances doxorubicin influx to trigger necrotic and autophagic cell death in oral squamous cell carcinoma. J. Clin. Med. 2018, 7, 375. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Mitra, A.K.; Mawson, A.R. Neglected tropical diseases: Epidemiology and global burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef]
- Silva, T.R.; Duarte, A.W.; Passarini, M.R.; Ruiz, A.L.T.; Franco, C.H.; Moraes, C.B. Bacteria from Antarctic environments: Diversity and detection of antimicrobial, antiproliferative, and antiparasitic activities. Polar Biol. 2018, 41, 1505–1519. [Google Scholar] [CrossRef]
- Bilsland, E.; Tavella, T.A.; Krogh, R.; Stokes, J.E.; Roberts, A.; Ajioka, J. Antiplasmodial and trypanocidal activity of violacein and deoxyviolacein produced from synthetic operons. BMC Biotechnol. 2018, 18, 22. [Google Scholar] [CrossRef]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Chandrakant, N.; Laxmikant, S.; Satish, P. Nematicidal activity of microbial pigment from Serratia marcescens. Nat. Prod. Res. 2014, 28, 1399–1404. [Google Scholar] [CrossRef]
- Gutierrez-Dalmau, A.; Campistol, J.M. Immunosuppressive therapy and malignancy in organ transplant recipients: A systematic review. Drugs 2007, 67, 1167–1198. [Google Scholar] [CrossRef]
- Nakashima, T.; Kurachi, M.; Kato, Y.; Yamaguchi, K.; Oda, T. Characterization of bacterium isolated from the sediment at coastal area of Omura Bay in Japan and several biological activities of pigment produced by this isolate. Microbiol. Immunol. 2005, 49, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Chander, R.; Sainis, K. A novel prodigiosin-like immunosuppressant from an alkalophilic Micrococcus sp. Int. Immunopharmacol. 2003, 3, 159–167. [Google Scholar] [CrossRef] [PubMed]
- DeLange, R.J.; Glazer, A.N. Phycoerythrin fluorescence-based assay for peroxy radicals: A screen for biologically relevant protective agents. Anal. Biochem. 1989, 177, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-D.; Cheung, K. Phenotypic Expression of Vogesella Indigofera upon Exposure to Hexavalent Chromium, Cr6+; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Wong, L.; Teo, S. (Eds.) Naturally occurring carotenoids in cyanobacteria as a bioindicator for heavy metals detection. In Proceedings of the International Conference on Advances in Applied Science and Environmental Engineering–ASEE, Kuala Lumpur, Malaysia, 2–3 August 2014. [Google Scholar]
- Turcotte, F.; Mouget, J.-L.; Genard, B.; Lemarchand, K.; Deschênes, J.-S.; Tremblay, R. Prophylactic effect of Haslea ostrearia culture supernatant containing the pigment marennine to stabilize bivalve hatchery production. Aquat. Living Resour. 2016, 29, 401. [Google Scholar] [CrossRef]
- Jitmuang, A. Human Chromobacterium violaceum infection in Southeast Asia: Case reports and literature review. Southeast Asian J. Trop. Med. Public Health 2008, 39, 452. [Google Scholar]
- Oh, W.T.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W. Janthinobacterium lividum as an emerging pathogenic bacterium affecting rainbow trout (Oncorhynchus mykiss) fisheries in Korea. Pathogens 2019, 8, 146. [Google Scholar] [CrossRef]
- Petersen, L.M.; Tisa, L.S. Friend or foe? A review of the mechanisms that drive Serratia towards diverse lifestyles. Can. J. Microbiol. 2013, 59, 627–640. [Google Scholar] [CrossRef]
- Mahlen, S.D. Serratia infections: From military experiments to current practice. Clin. Microbiol. Rev. 2011, 24, 755–791. [Google Scholar] [CrossRef]
- Sharmin, S.; Kamal, S.M. Review on Chromobacterium violaceum, a rare but fatal bacteria needs special clinical attention. Anwer Khan Mod. Med. Coll. J. 2019, 10, 169–175. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Chen, J.; Liu, X.; Xiang, T.; Zhang, L. The red pigment prodigiosin is not an essential virulence factor in entomopathogenic Serratia marcescens. J. Invertebr. Pathol. 2016, 136, 92–94. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Revtovich, A.V.; Chen, Q.; Shah, K.N.; Cannon, C.L.; Kirienko, N.V. Pyoverdine-dependent virulence of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Front. Microbiol. 2019, 10, 2048. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Ju, X.-L.; Zhou, Y.-G. The variability of citrinin production in Monascus type cultures. Food Microbiol. 2005, 22, 145–148. [Google Scholar] [CrossRef]


| Pigment | Organism Involved | Substrate Used for Production | Production Rate (mg/L) | Reference |
|---|---|---|---|---|
| Melanin | Pseudomonas sp. | Vegetable waste | 2.79 | [40] |
| Aspergillus carbonarius | Apple, black carrot, pomegranate, red beet pulps | 61.84 | [41] | |
| Prodigiosin | Serratia marcescens | Peanut oil cake | 40,000 | [42] |
| Brown sugar | 8 | [43] | ||
| Wheat bran and sunflower oil | 240 | [44] | ||
| Tannery fleshing | 33,000 | [45] | ||
| Sesame seed | 17 | [46] | ||
| Streptomyces sp. | Dairy processing wastewater | 47,000 | [47] | |
| Violacein | Chromobacterium vilaceum | Liquid pineapple waste | 16.25 | [48] |
| Sugarcane waste | 820 | [49] | ||
| Rapeseed cake | 12.93 | [50] | ||
| Carotenoids | Sarcina sp. | Apple pomace | 12.87 | [51] |
| Sporidiobolus pararoseus | Corn steep liquor | 40 mg/L | [52] | |
| Rhodotorula glutinis | Chicken feathers | 92 | [53] | |
| Rhodotorula achenorium | Whey filtrate | 262 | [54] | |
| Rhodotorula rubra | Sugarcane juice | 30.39 mg/g | [55] | |
| Rhodotorula mucilaginosa | Coffee pulp | 16.36 | [56] | |
| Astaxanthin | Haematococcus pluvialis | Piggery wastewater | 83.9 | [57] |
| Phaffia rhodozyma | ||||
| Juice of date | 23.8 | [58] | ||
| molasses | 15.3 | [59] | ||
| Grape juice | 9.8 µg/mL | [60] | ||
| Riboflavin | Bacillus subtilis | Corn steep liquor | 26.8 | [61] |
| Pyocyanin | Pseudomonas aeruginosa | Grape seed | 4 µg/mL | [62] |
| Cotton seed meal | 4 µg/mL | [62] |
| Pigment Produced | Microbial Source | Therapeutic Application | Reference |
|---|---|---|---|
| Prodigiosin | Serratia marcescens | Human colon cancer cells | [141] |
| Violacein | C. violaceum | Colorectal cancer | [142] |
| Monascin | Monascus purpureus | Teratogenic effects on chicken embryos | [143] |
| Carotenoids | Haematococcus pluvialis | Food additives | [144] |
| Bromoalterochromide | Pseudoalteromonas maricaloris | Cytotoxic effect on sea urchins | [145] |
| Tambjamine | Atapozoa sp. | Ichthyodeterrent activities | [146] |
| Anthraquinones | Phoma multirostrata | Herbicidal activities | [147] |
| Astaxanthin | Phaffia rhodozyma | Anti-aging properties | [148] |
| Astaxanthin | Phaffia rhodozyma | Antiproliferative activity | [149] |
| Monascus | Red mold dioscorea | Anti-diabetic activity | [150] |
| Carotenoid | Streptomyces mediolani | Antioxidant activity | [151] |
| Astaxanthin | Haematococcus pluvialis | Anti-atherosclerotic activity | [152] |
| Anthraquinones | Phoma foveata | Inhibition of HIV | [147] |
| Prodigiosin | S. marcescens | Antitrypanosomal activity | [153] |
| Violacein | Chromobacterium violaceum | Anti-malarial activity | [154] |
| Flexirubin | Flavobacterium | Treatment of skin disease | [155] |
| Prodigiosin | S. marcescens | Antileishmanial activity | [156] |
| Violacein | Janthinobacterium lividum | Antiprotozoal activity | [125] |
| Violacein | Plasmodium falciparum | Antiparasitic activity | [154] |
| Prodigiosin | Hahella chejuensis | Anti-algicidal activity | [157] |
| Prodigiosin | S. marcescens | Antifouling activity | [158] |
| Flexirubin | Flavobacterium sp. | Anti-tuberculosis activity | [159] |
| Phycobilioproteins | Cyanobacterial sp. | Anti-alzhelmeric activity | [159] |
| Cycloprodigiosin hydrochloride | Pseudoalteromonas denitrificans | Immunosuppressive activity | [160] |
| Phenazine | Streptomyces sp. | Antiviral activities | [161] |
| Anthraquinone | Trichoderma harzianum | Antifungal activity | [161] |
| Phycocyanin | Spirulina | Phycofluoures for DNA probes | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anshi; Kapil, S.; Goswami, L.; Sharma, V. Unveiling the Intricacies of Microbial Pigments as Sustainable Alternatives to Synthetic Colorants: Recent Trends and Advancements. Micro 2024, 4, 621-640. https://doi.org/10.3390/micro4040038
Anshi, Kapil S, Goswami L, Sharma V. Unveiling the Intricacies of Microbial Pigments as Sustainable Alternatives to Synthetic Colorants: Recent Trends and Advancements. Micro. 2024; 4(4):621-640. https://doi.org/10.3390/micro4040038
Chicago/Turabian StyleAnshi, Shikha Kapil, Lalit Goswami, and Vipasha Sharma. 2024. "Unveiling the Intricacies of Microbial Pigments as Sustainable Alternatives to Synthetic Colorants: Recent Trends and Advancements" Micro 4, no. 4: 621-640. https://doi.org/10.3390/micro4040038
APA StyleAnshi, Kapil, S., Goswami, L., & Sharma, V. (2024). Unveiling the Intricacies of Microbial Pigments as Sustainable Alternatives to Synthetic Colorants: Recent Trends and Advancements. Micro, 4(4), 621-640. https://doi.org/10.3390/micro4040038






