1. Introduction
Tissue engineering combines engineering and life sciences to create biological substitutes to repair, support, or improve tissue function [
1]. This objective involves three basic elements: a dependable cell source, ideal chemical setting, and scaffold that works well with biological systems [
2]. Recent studies have focused on creating structures that imitate both the physical and biological characteristics of the extracellular matrix (ECM), which are crucial for tissue regeneration, particularly in chronic wound therapy [
3]. Wound dressings are essential in managing wounds, as they shield the area from outside elements and help speed up the healing process. Effective wound dressings should soak up extra liquids, prevent infections, maintain a moist healing setting, enable gas exchange, and be non-harmful, compatible, and able to break down [
4]. Furthermore, they must be simple to replace, avoid sticking to the wound, and support tissue regeneration and angiogenesis [
5].
The different types of wound dressings available include films, foams, sponges, hydrogels, and nanofiber membranes. Among these options, nanofiber membranes show great potential because of their small pore size, high porosity, and capacity to facilitate the transfer of gases and liquids [
6]. Their versatility for different biomedical uses is due to the fact that their physical and mechanical properties can be modified [
7].
Nanofibers are becoming increasingly acknowledged for their capabilities in wound dressings because of their distinctive characteristics, including a high surface-area-to-volume ratio, small pore size, and high porosity [
8]. These properties allow nanofibers to effectively protect against microbial infections, while promoting the exchange of gases and fluids at the wound site. These features closely mimic the extracellular matrix (ECM), offering an optimal environment for cell adhesion, proliferation, and tissue regeneration [
9]. Electrospinning is a widely utilized technique for producing nanofibers, allowing for the customization of wound dressings by incorporating various bioactive agents, such as antibacterial compounds, growth factors, and drugs, thereby accelerating and enhancing the healing process. Additionally, the structural flexibility of nanofibers facilitates the controlled and sustained release of therapeutic agents, which is particularly beneficial for the treatment of chronic wounds [
10].
The integration of Collagen into needleless electrospun wound dressings presents a promising strategy for healing chronic wounds [
11]. Collagen is the most abundant protein in the body and plays a critical role in wound healing by facilitating cellular adhesion, proliferation, and differentiation [
12]. The primary types of fibrillar Collagen in the skin are types I, III, and V, whereas types XII, XIV, XVI, and VI are present in smaller amounts. Nonfibrillar types IV and XVIII are located in the basement membrane. Combining the natural regenerative properties of Collagen with the structural support of electrospun nanofibers creates an ideal environment for cell growth, particularly in chronic wound healing [
13]. During the healing process, fibroblasts produce Collagen, which is then gradually reorganized into complex structures that enhance the tensile strength of the newly formed skin. Initially, Collagen III is synthesized, followed by Collagen I, the dominant form in mature skin [
14]. Enzymes such as lysyl oxidase further support the maturation and alignment of Collagen fibers, resulting in stronger and more resilient tissue [
15]. This natural healing process emphasizes the potential of Collagen-based dressings to improve wound healing. In addition, the combination of Collagen-based dressings with stem cells, particularly mesenchymal stem cells (MSCs), may further enhance wound healing [
16]. MSCs have a great potential in regenerative medicine thanks to their ability to self-renew and differentiate into various cell types. MSCs, which can be obtained from human and animal tissues such as bone marrow, adipose tissue, and umbilical cord, express specific surface markers such as CD29, CD44, CD73, and CD90. At the same time, they have immunomodulatory properties and secrete cytokines and anti-inflammatory molecules, making MSCs a promising therapeutic tool in the treatment of chronic diseases and tissue repair [
17]. The combination of Collagen and MSCs is an important innovative approach to provide effective healing, especially in chronic wound treatments [
18].
1.1. Needless Electrospinning
Needleless electrospinning, often referred to as free-surface electrohydrodynamic jetting, is a self-organizing technique that generates nanofibers by destabilizing liquid polymer solutions through the application of electrostatic forces, a phenomenon known as electrohydrodynamic destabilization [
19]. This process leads to the stretching and thinning of the polymer droplets, resulting in the formation of jets. At the peak of the polymer solution, a cone structure known as the Taylor cone is formed, from which jets of charged particles are emitted. In contrast to needle electrospinning, which can produce only a single Taylor cone per needle, needleless electrospinning allows for the generation of multiple Taylor cones simultaneously (see
Figure 1) [
20].
This enhances the quantity of polymer jets produced, facilitating the large-scale manufacturing of nanofibers [
22]. When the spinneret is partially immersed in the polymer solution and undergoes rotational motion, a thin film of the solution is formed on its surface. This rotation generates initial perturbations in the solution layer, resulting in localized increases in polymer concentration, which subsequently serve as nucleation points for further disturbance growth. Consequently, conical protrusions develop on the surface of the solution. Upon the application of a high voltage, the free surface of the polymer solution experiences instabilities in the electric field distribution at irregular intervals, resulting in the accumulation of electric charges on the surface protrusions. These charged protrusions give rise to polymer jets that orient perpendicular to the electric field. As the jets are ejected, they encounter increasing drag forces from the surrounding gas, which decelerates their velocity and leads to the formation of Taylor cones [
23]. From these cones, polymer jets are expelled toward the collector. During this process, solvent evaporation occurs, resulting in the solidification of the polymer jets into nanofibers, which ultimately deposit onto the collector’s surface [
24].
1.2. Wound Healing Activities of Collagen
In the process of wound healing, fibroblasts, including both resident fibroblasts and those converted from myeloid cells, serve as the primary source of newly produced Collagen [
25]. In older adults, the skin shows a reduced density of Collagen, which becomes more cross-linked and fragmented over time. This, along with cellular aging, results in a remodeling of Collagen fibers that increases skin stiffness [
26]. Furthermore, aging skin has a higher proportion of Collagen type III. Advanced imaging methods, such as Fourier-transform infrared imaging, scanning electron microscopy, and histological staining, demonstrate that the Collagen structure in aged skin is characterized by fragmented, clustered, and coarse fiber bundles that are mainly oriented parallel to the skin surface [
27]. These age-related alterations, including a reduction in Collagen synthesis and an increase in non-enzymatic cross-linking, significantly influence the skin’s mechanical properties, rendering it more susceptible to complications during wound healing [
28].
Collagen is essential to the wound healing process by providing both structural support and promoting tissue regeneration. When a wound occurs, the body activates a sequence of phases to repair the damaged tissue, and Collagen plays a vital role in each stage [
29].
Figure 2 illustrates the four phases of wound healing, including hemostasis, inflammation, proliferation, and remodeling. Each phase is critical for the proper recovery of skin tissue, with specific cellular activities and structural changes that contribute to healing [
30].
During the hemostasis and inflammation phase, the body works to prevent further blood loss by clotting the wound. An inflammatory response is triggered, where immune cells such as neutrophils and macrophages are mobilized to the wound site. These immune cells clean the area by removing bacteria, debris, and damaged tissue. During this time, Collagen production begins, with Type III Collagen being generated as a temporary scaffold to support the early stages of healing [
31]. As the wound enters the proliferation phase, fibroblasts become more active and increase Collagen production significantly. This new Collagen forms a robust extracellular matrix (ECM), which helps support new tissue growth. The ECM provides a structural foundation for cell adhesion and migration, essential for tissue repair and regeneration. Over time, the initially synthesized Collagen Type III is progressively replaced by Collagen Type I, which exhibits greater strength and is more abundant in healthy cutaneous tissue. [
32]. During the final remodeling phase, the Collagen fibers realign and become more organized, contributing to the restoration of the wound’s tensile strength. Enzymes like
lysyl oxidase facilitate cross-linking between the Collagen fibers, further enhancing tissue strength and elasticity. This process can continue for months after the wound has closed, ultimately restoring up to 80–85% of the original skin strength in well-healed wounds. Throughout this entire process, Collagen’s role is critical in providing the necessary framework for tissue regeneration, promoting wound closure, and reducing the risk of scarring and infection [
15].
This study explores the integration of Type I Collagen scaffolds with mesenchymal stem cells (MSCs) as a novel therapeutic strategy for managing chronic wounds. Collagen serves as a substrate that closely mimics the extracellular matrix (ECM), promoting MSC adhesion, growth, and differentiation. This approach is particularly beneficial for diabetic wounds, which often exhibit delayed healing and a higher risk of infection. By combining the regenerative abilities of MSCs with the biocompatibility of Collagen, the treatment is expected to accelerate wound healing, reduce inflammation, and enhance overall recovery.
2. Materials and Methods
2.1. Materials
Cell cultures utilized hASCs (human adipose tissue-derived stem cells) from passages 2 to 4, obtained from ScienCell Research Laboratories (Carlsbad, CA, USA). Cells were plated in T75 culture-treated flasks at approximately 1 million cells per flask, and the culture medium was changed every 3–4 days during culture. The collagen peptide powder used for the experiment was sourced from Peptan, derived from bovine, porcine, and fish origins by Darling Ingredients (Shanghai, China) with CAS number 92113-31-0. Pullulan, with a purity of 99%, was purchased with product number P4516 from Sigma-Aldrich (Prague, Czech Republic).
2.2. Methods
Distilled water was utilized to dissolve both Collagen and Pullulan. The concentration of Collagen in the solution was 50 mg/mL, while Pullulan was dissolved at a concentration of 20 mg/mL. The solutions were placed on a magnetic stirrer with heating capability at 40 °C for 20–30 min to ensure complete dissolution. Pullulan and Collagen solutions were prepared at a 1:1 volume ratio. The resulting mixture was heated to 40 °C to facilitate complete dissolution and subsequently cooled to 37 °C to maintain cell viability. A cell pellet containing 1 × 106 mesenchymal stem cells is added to the Pullulan/Collagen solution, resulting in a polymeric mixture that contains the cells and is ready for further processing. The Pullulan/Collagen cell mixture is loaded into the reservoir of a needleless electrospinning device. The collector plate, which is a Petri dish, is positioned approximately 7.5 cm away from the spinning rotating wire electrode, with an applied voltage of 10 kV. The electrospinning process is conducted for no longer than 15 min.
Control cells utilizing the same Pullulan/Collagen combination were cultured, and, subsequently, the same type of mesenchymal stem cells (MSCs) was sprayed into an empty Petri dish at an identical rate as the experimental setup, but without any voltage applied during the process. This approach enabled the researchers to establish a baseline and determine whether the act of spraying itself (without electrospinning) affected cell viability or morphology. The electrospinning process was never conducted for a duration exceeding 15 min.
Figure 3 illustrates the three main phases involved in the preparation and processing of Pullulan/Collagen scaffolds integrated with cells for wound healing applications. The LDH assay was performed on the cell lysates of both control and electrospun groups.
2.3. Morphological Analysis
2.3.1. Spinnability of Pullulan/Collagen (Fiber Morphology)
Pullulan/Collagen spinnability examined without P2–P4 of adipose tissue-derived stem cells (hASCs) using a scanning electron microscope (SEM). The Phenom scanning electron microscope (SEM), manufactured in Prague, Czech Republic, utilizes a focused electron beam to examine the surface of a specimen, thereby producing a detailed, high-resolution image. For this analysis, a secondary electron (SE) detector was utilized, which is optimal for capturing fine surface details and providing high-resolution images of the nanofibers’ surface morphology. The samples were secured to the SEM sample stage using conductive tape and analyzed under an accelerating voltage of 10 kV following sputter-coating with gold. Fiber diameters were determined by measuring 100 randomly selected points per SEM image, with statistical analysis conducted to ensure accuracy. The results are reported with confidence intervals to reflect variability, with each image encompassing 100 fibers. The analysis of these measurements was conducted utilizing version 1.8.0 of ImageJ software (version 1.8.0), which was developed by the National Institute of Mental Health in Bethesda, MD, USA.
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Characterisation
The composition of the scaffolds was analyzed using an attenuated total reflectance (ATR) attachment and a Thermo Scientific Nicolet iS 50 FTIR (Thermo Fisher, Waltham, MA, USA). After obtaining the data, it was processed and plotted using Python (version 3.9.7, Python Software Foundation, Wilmington, DE, USA).
2.4. Cellular Viability and Cytotoxicity Assays
2.4.1. Cellular Viability Analysis
The evaluation of cell viability was conducted utilizing a live/dead assay kit, which was subsequently analyzed through fluorescence microscopy Nikon Eclipse Ti2 (Prague, Czech Republic). Approximately six hours after the electrospinning process, the culture medium was meticulously removed from each well. The cells were then treated with a solution comprising calcein (2 µM) and ethidium (4 µM) in phosphate-buffered saline (PBS) for a duration of 10 min at 37 °C. Following this incubation period, the samples were rinsed with PBS to eliminate any residual staining agents, allowing for the visualization of the cells to determine their viability under fluorescence microscopy. This investigation assesses the viability of cells within the Pullulan/Collagen scaffold. Cellular viability is essential for wound healing processes, as viable cells facilitate tissue regeneration. The scaffold’s capacity to support cell survival indicates its potential efficacy in promoting wound healing.
2.4.2. Cytotoxicity Test
After 48 h post-electrospinning, the culture medium was removed, and the cells were subjected to a washing procedure utilizing PBS. A Lactate Dehydrogenase (LDH) cytotoxicity assay (Cytotox96 kit, Promega, Madison, WI, USA) was performed on the adherent cells according to the manufacturer’s protocol. This assay evaluates cell viability by measuring LDH activity in the cell lysate, indicating cellular membrane integrity and potential cytotoxic effects. For the LDH cytotoxicity assay, Triton X-100 was utilized as the positive control, representing 100% cell death, while untreated cells (cell culture without chemical exposure) served as the negative control, representing 0% cell death. The Triton X-100 disrupts the cell membrane, ensuring complete cell lysis, while the untreated cells maintain intact membranes, providing a baseline for cytotoxicity comparison [
33]. Cytotoxicity testing assesses the potential of scaffold materials to compromise cellular membrane integrity. Elevated cytotoxicity may result in cellular death and tissue deterioration. Consequently, materials exhibiting low cytotoxicity are more conducive to wound healing processes, as preservation of cellular membrane integrity facilitates the maintenance of cellular function.
2.5. Gene Expression by Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5.1. Gene Expression
One week post-electrospinning, RNA extraction was performed utilizing the RNeasy Plus Mini Kit (QIAGEN, Germantown, MD, USA), in accordance with the manufacturer’s protocol. Subsequently, RT-PCR was conducted following the methodology delineated in the One-Step RT-PCR kit (QIAGEN, Germantown, MD, USA). To assess gene expression, the transcription of pluripotency markers SOX2 and OCT4 was analyzed.
2.5.2. Immunocytochemistry
On the second day, fluorescence microscopy was employed to assess cellular morphology. The cells were fixed with 4% paraformaldehyde (PFA) in PBS (pH 7.4) for 15 min at room temperature. Following three PBS rinses, the samples were permeabilized using 0.1% (v/v) Triton X-100 for 10 min, followed by three additional PBS washes. Subsequently, the cells were stained with Phalloidin 488 to visualize f-actin and DAPI to highlight the nuclei. The staining agents were obtained from Life Technologies (Carlsbad, CA, USA). Cell visualization was performed using a Nikon Eclipse Ti2 fluorescence microscope (Prague, Czech Republic).
2.6. Cell Incorporation
The incorporation of cells within the scaffold, the cells were pre-labeled with a green CMFDA cell tracker dye (Invitrogen, Germantown, MD, USA) before the electrospinning process. After electrospinning, the cells were labeled with DAPI for nuclear staining. The samples were imaged using CytoViva’s enhanced darkfield transmitted light condenser (NA 1.2–1.4) paired with the Dual Mode Fluorescence (DMF) module for better visualization. These imaging components were mounted on an Olympus BX51 upright microscope, using a 100X oil UPL Fluorite objective (NA 0.60–1.30), which was optimized for darkfield imaging. The illumination source was a Prior Lumen 200 with a metal halide lamp and adjustable light attenuation for precise control. Optical images were captured using a DAGE-MTI XLMCT cooled CCD camera, equipped with a 7.4 µm pixel size, ensuring high-resolution image acquisition.
2.7. Apoptosis and Necrosis Evaluation
The MSC and HSF nuclei (2 × 105 cells/well) were dyed with PI after 24 h of incubation and then investigated by flow cytometers. Apoptotic cells were studied in an Annexin V and PI double dye (Sigma-Aldrich, Burlington, MA, USA) protocol and quantified in a flow cytometer. In fact, early apoptosis cells tend to expose phosphatidylserine (PS) on the extracellular surface of plasma membranes that can be specifically targeted by Annexin V. Moreover, PI can permeabilize chromosomes in late apoptosis cells. Dead cells (V+/PI− and V−/PI+) and apoptosis/necrosis (V+/PI and V/PI+) cells were detected by BD Biosciences’ BD FACScalibur flowcytometer and analyzed by FlowJo 7.6 software (Bennett Dickinson, Canton, OH, USA). This test evaluates the processes of programmed cell death (apoptosis) or sudden cell death (necrosis) of cells. During wound healing, cells need to continue their functions without dying and survive in the wound area. Low rates of apoptosis and necrosis indicate that the scaffold provides a protective environment for the cells.
2.8. Evaluation of Monocyte and Platelet Responses
Utilizing the Percoll protocol (Sigma-Aldrich, USA), human monocytes were isolated from the whole blood of healthy volunteers [
34]. The monocytes (1 × 105 cells/well) were seeded in a 24-well plate that had been pre-coated with each nanocomposite and cultured in RPMI medium supplemented with 10% FBS and 1% (
v/
v) antibiotics (comprising 10,000 U/mL penicillin G and 10 mg/mL streptomycin) for 96 h at 37 °C. Subsequently, the cells were harvested utilizing a 0.05% trypsin solution. The cell morphology of the monocytes and macrophages was observed through a microscope. The monocyte conversion yield was calculated according to the following formula: monocyte conversion yield (%) = ((Macrophages)/(Monocytes + Macrophages)) × 100%. To further investigate the inflammatory response, immunofluorescence staining was performed utilizing a primary anti-CD68 antibody (GeneTex Inc., Irvine, CA, USA) to examine CD68, which functions as a marker for macrophages. To investigate platelet adherence and activation by the different nanomaterials, 2 × 106 cells/well of platelets were seeded for 24 h of incubation. Subsequently, the cells were fixed with a 2.5% glutaraldehyde solution for 8 h. The platelets were subsequently washed with PBS and dehydrated through a series of alcohol solutions, ranging from 30% to 100%, with each step conducted at room temperature (RT) for 10 min. Following dehydration, each sample was desiccated and sputter-coated with gold to enhance conductivity. The platelet morphology, influenced by the different materials, was examined using a SEM Phenom (Prague, Czech Republic), which provided high-resolution surface images. Macrophages are immune cells that regulate inflammation and contribute to tissue repair. However, excessive macrophage activity may result in chronic inflammation and impaired wound healing. A low number of CD68-positive cells suggests that the skeleton facilitates wound healing by mitigating inflammation. Platelet activation is associated with blood coagulation. Excessive platelet activation can lead to undesirable thrombus formation in the wound and hinder the healing process. The low platelet activation observed in the Pullulan/Collagen framework exerts a beneficial effect on wound healing by minimizing blood coagulation.
2.9. Statistics
All statistical analyses were conducted utilizing Python, employing libraries such as NumPy and SciPy for data processing and evaluation. Quantitative data are presented as mean ± SD from three independent experiments, with statistical analysis conducted using ANOVA to determine the significance of differences (* p < 0.05).
3. Results
3.1. Material Characterization
The scanning electron microscopy (SEM) image presented in
Figure 4 provides a detailed view of the nanofibrous architecture, revealing a highly organized and interconnected network.
Figure 4 presents a comparative analysis of COL and PUL fibers, focusing on their structural and dimensional characteristics through SEM micrographs and fiber diameter distributions. The SEM images reveal that COL fibers exhibit a more uniform and tightly interconnected network, while PUL fibers display a looser and less compact structure with visibly thicker fibers. This suggests a significant difference in the fiber fabrication or material composition between the two types.
The diameter distribution analysis shows that COL fibers have an average diameter of 167.03 ± 40.04 nm, with a relatively narrow distribution, indicating a higher degree of uniformity in fiber formation. In contrast, PUL fibers have a larger average diameter of 274 ± 20 nm, with a broader distribution, reflecting greater variability in fiber thickness. The more consistent diameter of COL fibers aligns with their denser network structure, while the variability in PUL fibers corresponds to their more open, irregular arrangement. These differences in fiber diameter and morphology are likely to influence their respective mechanical and functional properties.
Figure 5 shows that the Col/Pul composite fibers exhibit a smooth and uniform morphology, with no apparent bead formation, in contrast to the pure Pullulan fibers shown earlier. The blending of Collagen and Pullulan appears to have resulted in well-formed, continuous fibers, indicating that the combination of these polymers in the specified ratio has improved the fiber structure by stabilizing the electrospinning process. The absence of bead defects suggests that the viscosity and solution properties are optimal for this fiber composition. The fiber diameter distribution on the right further quantifies the characteristics of the Col/Pul fibers. The average fiber diameter is measured at 280 ± 102 nm, indicating a relatively fine fiber formation. The distribution graph shows that most fibers fall within the 200–300 nm range, with a few thicker fibers reaching up to 500 nm or slightly higher. The relatively broad distribution suggests some variability in fiber size, but the majority of the fibers are consistently thin, which is typical for electrospun nanofibers.
The combination of Pullulan and Collagen at this ratio produces nanofibers with a smooth, uniform morphology and an average diameter suitable for applications such as tissue engineering or biomaterials, where controlled fiber diameter and morphology are crucial.
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
The FTIR spectrum for pure Pul (red line) shows characteristic absorption bands at several key regions.
Figure 6 shows the FTIR spectra of Pul, Col, and their combination (Pul + Col), highlighting the characteristic absorption peaks of each material. The shifts in the absorption bands after the combination of Pul and Col indicate molecular interactions between the two components, as evidenced by changes in the C–O–C, C–O, and CH
2/CH
3 stretching regions. These shifts suggest the formation of a composite material with modified functional group interactions compared to their individual spectra. A peak at 929 cm
−1 represents the C–O–C bond, characteristic of ether groups, while the band at 1029 cm
−1 corresponds to the C–O stretching vibrations. Additionally, a peak at 2931 cm
−1 is attributed to the CH
2 and CH
3 groups, typical of alkyl chains in the Pul structure. These peaks reflect the functional groups present in Pul, highlighting its typical structural components.
For pure Col, the spectrum displays prominent absorption bands. A peak at 1540 cm−1 corresponds to the amide II vibration, and another at 1655 cm−1 indicates the amide I vibration. Additionally, the broad peak around 3200 cm−1 is due to the O–H stretching vibrations, commonly associated with hydroxyl groups or hydrogen-bonded N–H groups in proteins like Collagen. These bands confirm the presence of peptide bonds and hydroxyl groups in the pure Collagen sample. When Pul is combined with Col shifts in key absorption bands are observed. The C–O–C vibration from Pul shifts from 929 cm−1 to 910 cm−1, indicating an interaction between Pul and Col, possibly due to hydrogen bonding or other intermolecular forces. Similarly, the C–O stretching vibration shifts from 1029 cm−1 to 1033 cm−1, while the CH2/CH3 group shifts from 2931 cm−1 to 2935 cm−1, suggesting slight structural alterations upon combination. Furthermore, the amide II band of Col shifts from 1540 cm−1 to 1550 cm−1 in the combined sample, further indicating interaction between the two materials at the molecular level.
In the Pul/Col sample, the amide peaks exhibited significant attenuation or absence. This phenomenon can be attributed to the robust molecular interactions between Pullulan and Collagen, presumably through hydrogen bonding. Pullulan, a polysaccharide, possesses numerous hydroxyl groups capable of forming hydrogen bonds with the amide groups in Collagen. These interactions may alter the electron distribution surrounding the peptide bonds, effectively reducing or shifting the vibrational modes responsible for the amide I and II peaks. Consequently, the FTIR spectrum of the composite may demonstrate attenuated or displaced amide peaks compared to pure Collagen. The suppression or absence of these peaks suggests that the Pullulan/Collagen scaffold forms a more integrated composite structure, wherein the individual identities of the biopolymers are partially obscured by their interactions.
These observed shifts confirm the interaction between Pul and Col, likely due to physical or chemical bonding, and suggest that the materials have formed a composite structure with modified functional groups compared to their pure forms.
3.3. Cellular Viability
Control cells were administered at a uniform rate onto the Petri dish, devoid of any voltage application. In this study, the Pullulan/Collagen polymeric solution was dissolved in distilled water, which resulted in a significant improvement in cell viability. The group achieved 99% viability compared to the control, while the Pullulan/cell scaffold alone had a 91% viability rate. The use of distilled water as a solvent for the Pullulan/Collagen solution appears to be a critical factor in promoting cell viability while maintaining the stemness of the mesenchymal stem cells, making it a promising approach for tissue engineering applications. Importantly, the differences between groups were not statistically significant (p > 0.05), suggesting that the observed variations in cell viability are within the margin of experimental error.
3.4. Cytotoxicity
Cell cytotoxicity was evaluated using lactate dehydrogenase (LDH) release. LDH is an enzyme that is released when the cell membrane is compromised, making it a reliable marker for cell death and cytotoxicity.
Cytotoxicity was calculated using the following formula [
33]:
Absorbance of Negative Control represents 0% cytotoxicity (indicating cells are viable).
Absorbance of Positive Control represents 100% cytotoxicity (indicating complete cell death).
Absorbance of Sample is the absorbance measured for the tested biomaterial.
The LDH levels depicted in
Figure 7 indicate that cells cultured on the Pullulan surface exhibited moderate cytotoxicity. This observation is evident in the control group, where LDH release was marginally lower than the 100% dead cell benchmark, suggesting a significant level of cell death. For cells cultured on the Collagen surface, LDH release was comparable to that of Pullulan, albeit slightly elevated. The minimal difference between the control and maximum LDH levels suggests that Collagen alone induced a mild cytotoxic effect.
Conversely, a substantial reduction in cytotoxicity was observed when cells were cultured on the Pullulan/Collagen combination. In this group, LDH release was significantly lower compared to cells cultured on either Pullulan or Collagen alone. This observation indicates that the combination of these two biomaterials created a more favorable environment for cell survival. The decreased LDH release suggests that fewer cells experienced membrane damage, reflecting enhanced cell viability in the Pullulan/Collagen group.
These findings suggest that the Pullulan/Collagen combination exerts a protective effect on cells, as evidenced by the reduced LDH levels relative to the individual biomaterials. This decrease in cytotoxicity indicates that the combined scaffold provides a more supportive environment for cell growth and survival. Consequently, the Pullulan/Collagen scaffold demonstrates significant potential as a more biocompatible option for tissue engineering and other biomedical applications where minimizing cell death is critical.
Additionally, further investigation into the protective role of the Pullulan/Collagen scaffold was conducted by electrospinning cells using only culture media, as illustrated in
Figure 8. In this instance, cell viability decreased markedly to 40% compared to cells directly sprayed onto a Petri dish with the same media. This result underscores the beneficial effect of dissolving the Pullulan/Collagen solution in distilled water, which appears to enhance cell viability by improving the biocompatibility of the scaffold.
The rationale for encapsulating cells within the Pullulan/Collagen scaffold pertains to providing a three-dimensional environment that mimics the natural extracellular matrix (ECM), thereby promoting enhanced cell adhesion, proliferation, and differentiation, which are crucial for chronic wound healing. Encapsulation augments the interactions between the cells and the scaffold, thus improving the structural integrity and functional outcomes in tissue regeneration.
In
Figure 7, LDH levels indicate the cytotoxicity and stress induced by the scaffold, demonstrating that without proper encapsulation, cells may experience elevated stress and reduced viability. Conversely,
Figure 8 illustrates that encapsulating cells within the scaffold significantly enhances cell viability, mitigating cytotoxic effects and creating a more favorable environment for wound healing.
3.5. Gene Expression by RT-PCR (SOX2 and OCT4 Markers)
The expression of SOX2 and OCT4 genes is analyzed using Reverse Transcription Polymerase Chain Reaction (RT-PCR) to confirm the pluripotency of stem cells. This method measures the levels of mRNA from these genes, which are critical markers of self-renewal and the ability of stem cells to differentiate into various cell types. The retention of SOX2 and OCT4 expression is essential to ensure that cells maintain their pluripotent properties, even after processes like electrospinning.
Seven days after electrospinning, staining with Oil Red O, toluidine blue, and Alizarin Red S was performed to assess adipogenic, chondrogenic, and osteogenic differentiation. All cells tested negative for these stains, indicating no differentiation into adipocytes, chondrocytes, or osteoblasts. Moreover, PCR analysis revealed no significant changes in the expression of stem cell markers SOX2 and OCT4, confirming that the stemness of the cells was preserved both before and after electrospinning (see
Figure 9).
SOX2 expression, the fold change for cells cultured on both Collagen and Pullulan/Collagen scaffolds is close to 1.0. This indicates that the expression of SOX2 remains relatively stable across these conditions when normalized to the control group. However, there is a slight increase in SOX2 expression in the Pullulan/Collagen group compared to the Collagen-only group, with a fold change slightly above 1.0. This suggests a potential upregulation of SOX2 when Pullulan is incorporated into the scaffold. Despite this, the error bars show considerable overlap, implying that the difference in SOX2 expression between the two groups is likely not statistically significant.
OCT4 expression follows a similar trend, with both scaffold types showing a fold change around 1.0 when compared to the control. The Pullulan/Collagen scaffold group again shows a slightly higher expression of OCT4 compared to the Collagen-only scaffold. However, like SOX2, the differences between the two groups are small, and the overlap in error bars indicates that the observed changes are probably not statistically significant. Both Collagen and Pullulan/Collagen scaffolds seem to maintain the pluripotency of stem cells effectively, with the Pullulan/Collagen scaffold showing a potential, though small, enhancement in SOX2 and OCT4 expression. Nonetheless, the differences are not large enough to suggest a clear advantage of the Pullulan/Collagen scaffold over the Collagen-only scaffold without further investigation.
The rationale for utilizing the Pullulan/Collagen composite scaffold, as opposed to Collagen alone (
Figure 9), is predicated on the synergistic benefits of combining these two biopolymers. While Collagen is well-established for its role in promoting cell adhesion, proliferation, and differentiation, Pullulan contributes additional mechanical strength and flexibility, thereby enhancing the overall stability of the scaffold. This combination enables the scaffold to more closely approximate the extracellular matrix (ECM), thus creating a more conducive environment for cell growth and tissue regeneration, which is particularly significant for chronic wound healing.
Figure 9 demonstrates the superior performance of the Pullulan/Collagen scaffold in maintaining cell viability and stemness compared to Collagen alone, further substantiating the utilization of this composite material in tissue engineering applications. In this study, “biocompatibility” refers to cell viability, which assesses the scaffold’s capacity to support the survival and growth of mesenchymal stem cells (MSCs); cytotoxicity, which measures the absence of deleterious effects on the cells as determined by LDH assays; and immune response, which evaluates the scaffold’s ability to minimize inflammation and platelet activation, as observed through macrophage activity and thrombogenicity assays. This distinction ensures clarity in describing the scaffold’s performance across various biological aspects.
3.6. Immunocytochemistry
Immunocytochemistry test is used to assess the morphology and structural integrity of the cells. In this case, the test compares the actin filament organization between the control group (
Figure 10a) and cells cultured on a Pullulan/Collagen scaffold (
Figure 10b). The presence of the scaffold introduces variations in cytoskeletal alignment, reflecting how cells adapt to the 3D environment, compared to the more uniform arrangement in the control (2D surface). Despite the changes in alignment, cell viability and structural integrity (cytoskeleton and nuclei) remain intact, indicating the scaffold supports healthy cell attachment and growth.
In this experiment, Phalloidin 488 was used to label the actin filaments in green, while DAPI stained the nuclei blue. The comparison between cells in the control group and those cultured on a Pullulan/Collagen scaffold reveals distinct differences in cell morphology and cytoskeletal organization. In the control group, shown in
Figure 10a, the actin filaments exhibit a dense and uniform alignment, indicative of healthy cells strongly attached to the culture surface. The nuclei, stained with DAPI, are evenly distributed, further supporting that the cells are proliferating normally and forming a typical two-dimensional monolayer. Since there is no scaffold present in the control, the cells spread out uniformly on the flat surface, leading to a well-organized cytoskeletal structure.
In contrast, the cells cultured on the Pullulan/Collagen scaffold, depicted in
Figure 10b, display a more varied cytoskeletal arrangement. Although the actin cytoskeleton remains intact, the alignment and distribution of the filaments are less uniform compared to the control. The three-dimensional nature of the scaffold affects the morphology of the cells, leading to more complex structural adaptations as the cells interact with the scaffold. Despite this, the nuclei are well preserved, and their distribution appears normal, suggesting that the scaffold does not compromise cell viability.
This study highlights the influence of the Pullulan/Collagen scaffold on the structural organization of ADSCs. While the scaffold alters the traditional flat monolayer arrangement seen in the control, it provides a supportive environment for cell attachment and growth. The cells adapt to the three-dimensional scaffold without losing cytoskeletal integrity, making it a promising material for tissue engineering applications, where a three-dimensional environment more closely mimics natural tissue conditions and is essential for effective cell growth and differentiation.
3.7. CytoViva Microscopy for Cell Incorporation
The test was performed to assess the integration and spatial distribution of adipose-derived stem cells (ADSCs) within a highly organized Pullulan/Collagen scaffold produced by electrospinning. CytoViva imaging was employed to visualize how effectively cells become embedded within the scaffold, and whether the scaffold’s architecture provides a suitable environment for cell attachment and growth. The scaffold’s ability to support cell viability and maintain cell function is critical for its potential applications in tissue engineering, where maintaining a three-dimensional cellular organization is essential for mimicking natural tissue environments.
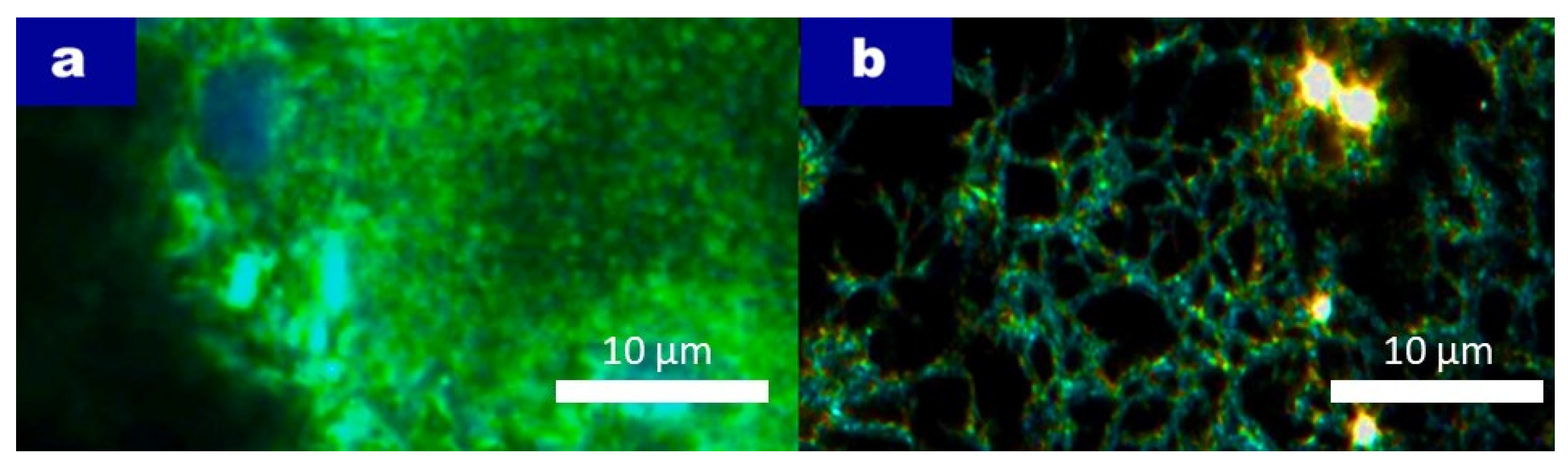
Figure 11 shows the CytoViva imaging, and the scaffold displayed a highly organized structure, which allowed for the successful embedding of cells within its architecture. As shown in
Figure 11a, cells pre-stained with CellTracker Green CMFDA prior to electrospinning were observed as evenly distributed throughout the scaffold. The green fluorescence indicates the presence of viable cells within the scaffold matrix, while the blue DAPI staining reveals the location of cell nuclei, confirming the integration of individual cells into the scaffold network. The pores in the scaffold appear to have diameters of approximately 10 µm, which is a suitable size for allowing cell penetration while maintaining scaffold stability.
In
Figure 11b, where no pre-staining was performed, the CytoViva image highlights the scaffold’s three-dimensional structure. The scaffold’s ability to support cells by providing sufficient surface area and space for attachment is clearly visible. The scaffold’s structure allows cells to spread and embed within the pores, which is essential for tissue regeneration applications where a robust cell–scaffold interaction is required.
These results confirm that the Pullulan/Collagen scaffold provides a conducive environment for cell integration and suggests its potential use in tissue engineering, as it supports not only cell attachment but also penetration into a three-dimensional space essential for the development of functional tissue structures.
3.8. Apoptosis and Necrosis Evaluation by Annexin V/PI Staining
In this study, the effects of various nanocomposites, especially Pullulan, Collagen, and Pullulan/Collagen electrospun scaffolds, on mesenchymal stem cells (MSCs) were investigated. Cell apoptosis and necrosis were assessed using Annexin V/PI double staining assay and then analyzed by flow cytometry. An apoptosis study was performed to investigate the biocompatibility of Pullulan/Collagen scaffolds and early apoptosis (Annexin V-positive); late apoptosis/necrosis (PI-positive) and cell survival rates were determined. The results were used to evaluate the effects of electrospun scaffolds on cell viability and the biocompatibility of the scaffold (see
Figure 12). Annexin V binds to phosphatidylserine on the cell membrane, indicating early apoptosis, while propidium iodide (PI) stains necrotic or late apoptotic cells in which membrane integrity has been compromised.
Figure 12 MSC serves as a control group (adipose tissue-derived stem cells (hASCs) were grown in standard culture medium in Pullulan/Collagen scaffolds without voltage application), where no scaffold was introduced. Only 0.81% of the cells were Annexin V-positive, and no PI-positive cells were detected. These results suggest that in the absence of any scaffold, the stem cells maintain high viability, with minimal apoptotic or necrotic effects.
Pul displays the results of MSCs cultured with Pullulan scaffolds. Early apoptosis was observed in 0.38% of cells, while necrosis or late apoptosis was significantly higher, with 2.35% of cells being PI-positive. These results suggest that Pullulan scaffolds might induce a higher degree of cell stress compared to other scaffolds, particularly leading to necrotic cell death.
The Pul-Col represents MSCs cultured with the Pullulan/Collagen composite scaffold. In this case, early apoptosis (Annexin V-positive cells) slightly increased to 0.39%, while late apoptosis/necrosis (PI-positive cells) decreased to 1.29%. This suggests that the Pullulan/Collagen scaffold may provide slightly better biocompatibility compared to pure Collagen, with a lower proportion of necrotic cells.
Col and MSCs were cultured with Collagen-based scaffolds. The results showed a low percentage of cells undergoing apoptosis, with 0.19% of the cells being Annexin V-positive, indicating early apoptosis. Additionally, 1.91% of the cells were PI-positive, suggesting a relatively higher level of necrosis or late apoptosis. These results demonstrate that the Collagen scaffold may induce a small degree of cellular stress, but overall, cell viability remains reasonably high.
By analyzing both early apoptotic (Annexin V-positive) and late apoptotic/necrotic (PI-positive) populations, we can better elucidate the scaffold’s capacity to maintain cell viability and minimize cell death, which is crucial for chronic wound healing applications.
The low percentage (~1–2%) of Annexin V- and PI-positive cells underscores the minimal cell death under scaffold conditions, indicating high cell viability and scaffold biocompatibility. The Annexin V/PI staining assay reveals that the Pullulan/Collagen scaffold demonstrates the highest biocompatibility with MSCs, as evidenced by the lower levels of late apoptosis and necrosis compared to the individual components. Collagen alone induces moderate necrosis, while Pullulan appears to have the most pronounced effect on necrosis. These findings suggest that combining Pullulan and Collagen into a composite scaffold may mitigate the adverse effects observed with each material individually, thereby enhancing cell survival and promoting scaffold biocompatibility.
3.9. Monocyte and Platelet Activities
The evaluation of platelet activation and monocyte activity is essential to determine the biocompatibility of nanocomposites, particularly for medical applications. Excessive platelet activation can lead to thrombosis, while heightened monocyte activity may trigger inflammation, both of which pose risks for implants or wound healing materials. These tests ensure that the material does not cause unwanted blood clotting or provoke harmful immune responses, making them crucial for assessing the safety and effectiveness of the nanocomposites in clinical settings.
In the present study, the effects of Pullulan (Pul), Collagen (Col), and Pul-Col nanocomposites on macrophage and platelet activation were investigated. In this analysis, which was performed to evaluate the biocompatibility of various material combinations, we particularly focused on CD68 expression and platelet activation.
To examine macrophage activation, CD68 protein was demonstrated by immunofluorescent staining (
Figure 13a). The number of CD68-positive cells showed a significant difference between the Pul-Col group and pure Pullulan and pure Collagen groups. CD68 expression increased by approximately 1.25-fold in both the pure Pullulan and pure Collagen groups, whereas the Pullulan/Collagen–MSC group exhibited a significant reduction, with the CD68 expression at approximately 0.75-fold, indicating reduced macrophage activation.
Platelet morphology was evaluated using scanning electron microscopy (SEM) (
Figure 13b). In these images, the degree of platelet spreading on the surface was used as an indicator of platelet activation. In the pure Pullulan and pure Collagen groups, platelets were observed to spread over a larger surface, indicating that these materials lead to higher platelet activation. In the Pul-Col group, platelet spreading was significantly reduced, indicating a lower level of platelet activation. This suggests that Pullulan/Collagen composites present a lower thrombogenic risk, making them more suitable for biomedical applications where minimizing blood clot formation is critical.
The quantitative data analyze macrophage activation and platelet behavior in more detail (
Figure 13c). In terms of CD68 expression, the macrophage activation increased by approximately 1.25-fold in the pure Collagen and pure Pullulan groups. In contrast, the Pul-Col-MSC group remained below this value and remained at approximately 0.75-fold. In the platelet adhesion analysis, it was observed that platelets adhered more to the materials in the pure Pullulan and pure Collagen groups. While platelet adhesion was 1.00 times in the pure Pullulan group, this value was around 1.25 times in the pure Collagen group. On the other hand, in the Pul-Col-MSC group, platelet adhesion rate was determined as 0.75 times. When platelet activation data were analyzed, this activation rate was approximately 1.25-fold in the pure Collagen and pure Pullulan groups, while this rate remained at the level of 0.50-fold in the Pul-Col-MSC group.
These findings generally show that Pul-Col nanocomposites reduce both inflammatory response and platelet activation, increase biocompatibility, and are a potential candidate for clinical applications. In particular, it is concluded that Pullulan/Collagen nanocomposites provide lower macrophage activation and platelet adhesion when used as biomaterials, and therefore may be preferred in biomedical applications such as wound healing.
4. Discussion
The results of this study highlight the potential of Pullulan/Collagen nanofiber scaffolds as a promising biomaterial for chronic wound healing applications. A key innovation in this scaffold design was the use of distilled water as a solvent for Collagen, which offers distinct biological advantages over traditional solvents like acetic acid. The ability of water to maintain Collagen’s biocompatibility by preserving higher cell viability is particularly significant. Acetic acid, though effective for Collagen dissolution, introduces complications due to its acidic nature, which can compromise cellular environments. The necessity of additional neutralization steps in acetic acid systems may contribute to cytotoxicity and reduced scaffold performance. In contrast, distilled water eliminates the need for such steps, thus ensuring a biocompatible environment that supports cellular function and the retention of MSC stemness.
The structural properties of the Pullulan/Collagen scaffold, as revealed through scanning electron microscopy (SEM) and Fourier-transform infrared spectroscopy (FTIR) analysis, further underscore its suitability for tissue engineering. The smooth, bead-free fiber morphology of the composite scaffold, coupled with the observed molecular interactions between Collagen and Pullulan, indicates a stable and robust material that can support cellular integration and proliferation. The fiber diameter data, with Collagen at 167.03 ± 40.04 nm and Pullulan at 274 ± 20 nm, suggests that the combination of these two biopolymers results in a balanced fiber structure that retains favorable mechanical properties while optimizing biological performance. The composite fibers, with an average diameter of 280 ± 102 nm, provide an ideal scaffold environment conducive to cell attachment and proliferation.
Biological testing, including cell viability, apoptosis, necrosis, and cytotoxicity assays, confirmed the superior performance of the Pullulan/Collagen scaffold. The significantly higher survival rate of 99% in the Pullulan/Collagen scaffold compared to 91% for Pullulan alone demonstrates the critical role of Collagen in enhancing cell proliferation and maintaining a supportive environment for MSCs. Furthermore, the reduced necrosis rate and lower levels of lactate dehydrogenase (LDH) cytotoxicity in the Pullulan/Collagen scaffold emphasize its biocompatibility and safety for clinical applications. These findings align with previous studies that suggest Collagen, when used in a neutral solvent system, can improve scaffold–cell interactions and reduce cytotoxic stress on cells.
Immunogenicity and thrombogenicity assessments provide further evidence of the scaffold’s suitability for regenerative medicine. The reduced macrophage activation, as indicated by lower CD68 expression levels, points to a favorable immune response, which is crucial for chronic wound healing applications where prolonged inflammation can impede tissue regeneration. Additionally, the lower platelet activation and adhesion observed with the Pullulan/Collagen scaffold suggest a reduced risk of thrombosis, making the scaffold a safer option for clinical use in wounds prone to excessive clotting.
These findings collectively suggest that the Pullulan/Collagen scaffold, developed using a water-based solvent system, not only improves the mechanical and structural properties of the material but also enhances its biological performance. The ability to minimize cytotoxicity, preserve cell viability, and modulate immune responses presents a strong case for the scaffold’s potential in clinical applications for chronic wound care and tissue engineering. Future studies should investigate the long-term performance of this scaffold in vivo, including its degradation profile, tissue integration, and the functional outcomes of wound healing in clinical models. The successful translation of this material into clinical use could provide significant advancements in regenerative medicine, offering patients a safer and more effective option for wound care and tissue repair.