Chemistry and Physics of Wet Foam Stability for Porous Ceramics: A Review
Abstract
1. Introduction
2. Colloidal Suspensions
2.1. Destabilization of Colloidal Suspension
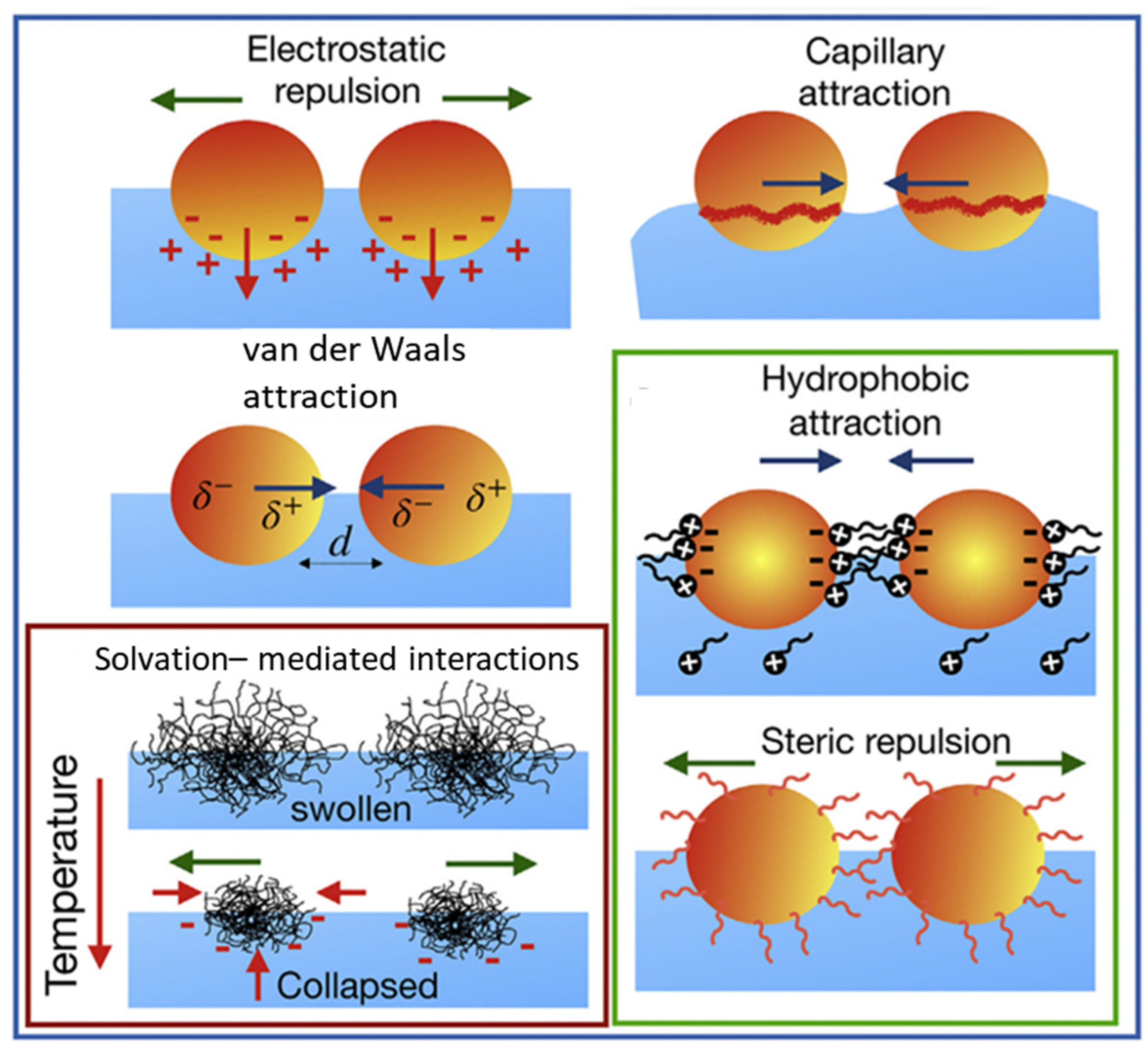
2.2. Inter-Particle Behavior in Colloidal Suspension
2.3. Hydrophobization of Colloidal Particles
3. The Chemistry and Physics of Wet Foam
3.1. Chemistry of Wet Foam from Colloidal Suspension
3.2. Hydrophobization of Particles through Surface Modification
4. Physics of Wet Foam from Colloidal Suspension
4.1. Physical Phenomena of the Bubble
4.2. Stabilizing Mechanisms of Bubbles
4.3. Wet Foam Stability
5. Microstructure
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scheffler, M.; Colombo, P. (Eds.) Cellular Ceramics: Structure, Manufacturing, Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gauckler, L.J.; Waeber, M.M.; Conti, C.; Jacob-Duliere, M. Ceramic foam for molten metal filtration. JOM 1985, 37, 47–50. [Google Scholar] [CrossRef]
- Wong, J.C.H.; Tervoort, E.; Busato, S.; Gonzenbach, U.T.; Studart, A.R.; Ermanni, P.; Gauckler, L.J. Designing macroporous polymers from particle-stabilized foams. J. Mater. Chem. 2010, 20, 5628–5640. [Google Scholar] [CrossRef]
- Pugh, R.J. Handbook of Applied Surface and Colloid Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Gauckler, L.J.; Graule, T.; Baader, F. Ceramic forming using enzyme catalyzed reactions. Mater. Chem. Phys. 1999, 61, 78–102. [Google Scholar] [CrossRef]
- Lewis, J.A. Colloidal processing of ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Young, A.C.; Omatete, O.O.; Janney, M.A.; Menchhofer, P.A. Gelcasting of alumina. J. Am. Ceram. Soc. 1991, 74, 612–618. [Google Scholar] [CrossRef]
- Green, D.J.; Colombo, P. Cellular ceramics: Intriguing structures, novel properties, and innovative applications. MRS Bull. 2003, 28, 296–300. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Krauss Juillerat, F.; Borcard, F.; Staedler, D.; Scaletta, C.; Applegate, L.A.; Comas, H.; Gauckler, L.J.; Gerber-Lemaire, S.; Juillerat-Jeanneret, L.; Gonzenbach, U.T. Functionalization of microstructured open-porous bioceramic scaffolds with human fetal bone cells. Bioconjugate Chem. 2012, 23, 2278–2290. [Google Scholar] [CrossRef]
- Kearsley, E.P.; Wainwright, P.J. The effect of high fly ash content on the compressive strength of foamed concrete. Cem. Concr. Res. 2001, 31, 105–112. [Google Scholar] [CrossRef]
- Amran, Y.M.; Farzadnia, N.; Ali, A.A. Properties and applications of foamed concrete; a review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- Amin, M.; Subri, M.; Subardi, A. Fabrication of Porous Ceramic Composite Clay-Zeolite Membranes via Replica Template Technique. In Proceedings of the 6th Mechanical Engineering, Science and Technology International Conference (MEST 2022), Surakarta, Indonesia, 20–22 December 2022; pp. 179–188. [Google Scholar]
- Schelm, K.; Fey, T.; Dammler, K.; Betke, U.; Scheffler, M. Hierarchical-porous ceramic foams by a combination of replica and freeze technique. Adv. Eng. Mater. 2019, 21, 1801362. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Zhang, M.; Xiu, Z.; Li, J.G.; Li, J.; Xie, M.; Chen, J.; Sun, X. High-strength macro-porous alumina ceramics with regularly arranged pores produced by gel-casting and sacrificial template methods. J. Mater. Sci. 2019, 54, 10119–10129. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Chen, Z.; Liu, Y.; Guo, J.; Zhang, W.; Rao, P.; Li, G. Recent progress in the pore size control of silicon carbide ceramic membranes. Ceram. Int. 2022, 48, 8960–8971. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Steinlin, D.; Tervoort, E.; Gauckler, L.J. Processing of particle-stabilized wet foams into porous ceramics. J. Am. Ceram. Soc. 2007, 90, 3407–3414. [Google Scholar] [CrossRef]
- Kim, I.J.; Park, J.G.; Han, Y.H.; Kim, S.Y.; Shackelford, J.F. Wet foam stability from colloidal suspension to porous ceramics: A review. J. Korean Ceram. Soc. 2019, 56, 211–232. [Google Scholar] [CrossRef]
- Pokhrel, A.; Seo, D.N.; Lee, S.T.; Kim, I.J. Processing of porous ceramics by direct foaming: A review. J. Korean Ceram. Soc. 2013, 50, 93–102. [Google Scholar] [CrossRef]
- Akartuna, I.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Macroporous ceramics from particle-stabilized emulsions. Adv. Mater. 2008, 20, 4714–4718. [Google Scholar] [CrossRef]
- Basnet, B.; Sarkar, N.; Park, J.G.; Mazumder, S.; Kim, I.J. Al2O3–TiO2/ZrO2–SiO2 based porous ceramics from particle-stabilized wet foam. J. Adv. Ceram. 2017, 6, 129–138. [Google Scholar] [CrossRef]
- Jang, W.Y.; Park, J.G.; Basnet, B.; Woo, K.T.; Han, I.S.; Kim, I.J. Highly porous SiC ceramics from particle-stabilized suspension. J. Aust. Ceram. Soc. 2017, 53, 657–665. [Google Scholar] [CrossRef]
- Bhaskar, S.; Park, J.G.; Kim, I.J.; Kang, B.H.; Lim, T.Y. ZrTiO4 porous ceramics fabricated from particle-stabilized wet foam by direct foaming. J. Korean Phys. Soc. 2016, 68, 77–82. [Google Scholar] [CrossRef]
- Bhaskar, S.; Park, J.G.; Lee, K.S.; Kim, S.Y.; Kim, I.J. Thermal and mechanical behavior of ZrTiO4-TiO2 porous ceramics by direct foaming. Ceram. Int. 2016, 42, 14395–14402. [Google Scholar] [CrossRef]
- Salvini, V.R.; Vivaldini, D.O.; Spinelli, D.; Pandolfelli, V.C. Green and Reliable Macro-Porous Ceramic Processing. Adv. Mater. Sci. Environ. Energy Technol. III 2014, 250, 87–98. [Google Scholar]
- Bhaskar, S.; Seo, D.N.; Park, J.G.; Cho, G.H.; Kang, B.H.; Lim, T.Y.; Kim, I.J. Al2O3-TiO2 porous ceramics from particle-stabilized wet foam by direct foaming. J. Ceram. Process. Res. 2015, 16, 643–647. [Google Scholar]
- Sarkar, N.; Park, J.G.; Mazumder, S.; Aneziris, C.G.; Kim, I.J. Processing of particle stabilized Al2TiO5–ZrTiO4 foam to porous ceramics. J. Eur. Ceram. Soc. 2015, 35, 3969–3976. [Google Scholar] [CrossRef]
- Pokhrel, A.; Zhao, W.; Kim, I.J. Wet Foam Stabilized by Amphiphiles to Tailor the Microstructure of Porous Ceramics. Key Eng. Mater. 2012, 512, 288–292. [Google Scholar] [CrossRef]
- Juillerat, F.K.; Gonzenbach, U.T.; Studart, A.R.; Gauckler, L.J. Self-setting particle-stabilized foams with hierarchical pore structures. Mater. Lett. 2010, 64, 1468–1470. [Google Scholar] [CrossRef]
- Sarkar, N.; Park, J.G.; Mazumder, S.; Pokhrel, A.; Aneziris, C.G.; Kim, I.J. Effect of amphiphile chain length on wet foam stability of porous ceramics. Ceram. Int. 2015, 41, 4021–4027. [Google Scholar] [CrossRef]
- Jang, W.Y.; Park, J.G.; Han, I.S.; Lim, H.M.; Lim, T.Y.; Kim, I.J. Effect of surfactant on wet foam stability to SiC porous ceramics. J. Ceram. Process. Res. 2017, 18, 887–893. [Google Scholar]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing routes to macroporous ceramics: A review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Stabilization of foams with inorganic colloidal particles. Langmuir 2006, 22, 10983–10988. [Google Scholar] [CrossRef]
- Basnet, B.; Lim, H.M.; Lee, K.S.; Kim, I.J. Effects of Carbon Fiber on Mechanical Behaviour of Al2O3 Porous Ceramics. J. Korean Ceram. Soc. 2019, 56, 513–520. [Google Scholar] [CrossRef]
- Seeber, B.S.M.; Gonzenbach, U.T.; Gauckler, L.J. Mechanical properties of highly porous alumina foams. J. Mater. Res. 2013, 28, 2281–2287. [Google Scholar] [CrossRef]
- Sarkar, N.; Park, J.G.; Mazumder, S.; Pokhrel, A.; Aneziris, C.G.; Kim, I.J. Al2TiO5–mullite porous ceramics from particle stabilized wet foam. Ceram. Int. 2015, 41, 6306–6311. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Drenckhan, W.; Hutzler, S. Structure and energy of liquid foams. Adv. Colloid Interface Sci. 2015, 224, 1–16. [Google Scholar] [CrossRef]
- Megias-Alguacil, D.; Tervoort, E.; Cattin, C.; Gauckler, L.J. Contact angle and adsorption behavior of carboxylic acids on α-Al2O3 surfaces. J. Colloid Interface Sci. 2011, 353, 512–518. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Akartuna, I.; Tervoort, E.; Gauckler, L.J. Materials from foams and emulsions stabilized by colloidal particles. J. Mater. Chem. 2007, 17, 3283–3289. [Google Scholar] [CrossRef]
- Wong, J.C.; Tervoort, E.; Busato, S.; Gonzenbach, U.T.; Studart, A.R.; Ermanni, P.; Gauckler, L.J. Macroporous polymers from particle-stabilized foams. J. Mater. Chem. 2009, 19, 5129–5133. [Google Scholar] [CrossRef]
- Hill, C.; Eastoe, J. Foams: From nature to industry. Adv. Colloid Interface Sci. 2017, 247, 496–513. [Google Scholar] [CrossRef]
- Murray, B.S.; Ettelaie, R. Foam stability: Proteins and nanoparticles. Curr. Opin. Colloid Interface Sci. 2004, 9, 314–320. [Google Scholar] [CrossRef]
- Murray, B.S. Stabilization of bubbles and foams. Curr. Opin. Colloid Interface Sci. 2007, 12, 232–241. [Google Scholar] [CrossRef]
- Bhaskar, S.; Park, J.G.; Lee, M.J.; Lim, T.Y.; Han, I.S.; Kim, I.J. ZrO2–TiO2 porous ceramics from particle stabilized wet foam by colloidal processing. J. Ceram. Soc. Jpn. 2016, 124, 106–110. [Google Scholar] [CrossRef]
- Byun, Y.M.; Lee, G.W.; Lee, K.S.; Park, J.G.; Kim, I.J. Mechanical properties of carbon fiber-reinforced Al2O3 porous ceramics. J. Korean Ceram. Soc. 2021, 58, 269–275. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Abd Hamid, S.B. Stable monodisperse nanomagnetic colloidal suspensions: An overview. Colloids Surf. B Biointerfaces 2015, 133, 388–411. [Google Scholar] [CrossRef]
- Yu, W.; Kanj, M.Y. Review of foam stability in porous media: The effect of coarsening. J. Pet. Sci. Eng. 2022, 208, 109698. [Google Scholar] [CrossRef]
- Guzmán, E.; Martínez-Pedrero, F.; Calero, C.; Maestro, A.; Ortega, F.; Rubio, R.G. A broad perspective to particle-laden fluid interfaces systems: From chemically homogeneous particles to active colloids. Adv. Colloid Interface Sci. 2022, 302, 102620. [Google Scholar] [CrossRef]
- Maestro, A. Tailoring the interfacial assembly of colloidal particles by engineering the mechanical properties of the interface. Curr. Opin. Colloid Interface Sci. 2019, 39, 232–250. [Google Scholar] [CrossRef]
- Pokhrel, A.; Park, J.G.; Zhao, W.; Kim, I.J. Functional porous ceramics using amphiphilic molecule. J. Ceram. Process. Res. 2012, 13, 420–424. [Google Scholar]
- Morris, G.; Pursell, M.R.; Neethling, S.J.; Cilliers, J.J. The effect of particle hydrophobicity, separation distance and packing patterns on the stability of a thin film. J. Colloid Interface Sci. 2008, 327, 138–144. [Google Scholar] [CrossRef]
- Maestro, A.; Guzmán, E.; Santini, E.; Ravera, F.; Liggieri, L.; Ortega, F.; Rubio, R.G. Wettability of silica nanoparticle–surfactant nanocomposite interfacial layers. Soft Matter. 2012, 8, 837–843. [Google Scholar] [CrossRef]
- Fey, J. A Qualitative Analysis of Bioactive Compounds (Saponins) in Mauby Tree Bark (Colubrina arborescens) Using Gas Chromatography/Mass Spectrometry (GC/MS); California State University, Long Beach: Northridge, CA, USA, 2019. [Google Scholar]
- Ahmad, R.; Ha, J.H.; Song, I.H. Processing Methods for the Preparation of Porous Ceramics. J. Powder Mater. 2014, 21, 389–398. [Google Scholar] [CrossRef]
- Studart, A.R.; Libanori, R.; Moreno, A.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Unifying Model for the Electrokinetic and Phase Behavior of Aqueous Suspensions Containing Short and Long Amphiphiles. Langmuir 2011, 27, 11835–11844. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P. Conventional and novel processing methods for cellular ceramics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 109–124. [Google Scholar] [CrossRef]
- Schramm, L.L.; Marangoni, D.G. Surfactants: Fundamentals and Applications in the Petroleum. Industry 2000, 3–24. [Google Scholar]
- Gonzenbach, U.T.; Studart, A.R.; Tervoort, E.; Gauckler, L.J. Ultrastable particle-stabilized foams. Angew. Chem. Int. Ed. 2006, 45, 3526–3530. [Google Scholar] [CrossRef]
- Salimi, A.; Makhmalzadeh, B.S.; Esfahani, G. Polymeric Micelle as a New Carrier in Oral Drug Delivery Systems. Asian J. Pharm. 2017, 11, 704–711. [Google Scholar]
- Birdi, K.S. Surface and Colloid Chemistry: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Saint-Jalmes, A. Physical chemistry in foam drainage and coarsening. Soft Matter 2006, 2, 836–849. [Google Scholar] [CrossRef]
- Stocco, A.; Rio, E.; Binks, B.P.; Langevin, D. Aqueous foams stabilized solely by particles. Soft Matter 2011, 7, 1260–1267. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, M.; Xu, J.; Wang, Q.; Fan, Z. Stabilization of water-in-octane nano-emulsion. Part I: Stabilized by mixed surfactant systems. Fuel 2010, 89, 2838–2843. [Google Scholar] [CrossRef]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics; John Wiley & Sons: Hoboken, NJ, USA, 1976; Volume 17. [Google Scholar]
- Denkov, N.; Tcholakova, S.; Politova-Brinkova, N. Physicochemical control of foam properties. Curr. Opin. Colloid Interface Sci. 2020, 50, 101376. [Google Scholar] [CrossRef]
- Wilson, A.J. (Ed.) Foams: Physics, Chemistry, and Structure; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Pokhrel, A.; Park, J.G.; Nam, J.S.; Cheong, D.S.; Kim, I.J. Stabilization of wet foams for porous ceramics using amphiphilic particles. J. Korean Ceram. Soc. 2011, 48, 463–466. [Google Scholar] [CrossRef]
- Gonzenbach, U.T. Particle-Stabilized Foams. Doctoral Dissertation, ETH Zurich, Zurich, Switzerland, 2006. [Google Scholar]
- Miller, C.A. Antifoaming in aqueous foams. Curr. Opin. Colloid Interface Sci. 2008, 13, 177–182. [Google Scholar] [CrossRef]
- Karakashev, S.I.; Grozdanova, M.V. Foams and antifoams. Adv. Colloid Interface Sci. 2012, 176, 1–17. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants-similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Ribeiro, A.; Lopes, J.C.B.; Dias, M.M.; Barreiro, M.F. Pickering emulsions based in inorganic solid particles: From product development to food applications. Molecules 2023, 28, 2504. [Google Scholar] [CrossRef]
- Horozov, T.S. Foams and foam films stabilised by solid particles. Curr. Opin. Colloid Interface Sci. 2008, 13, 134–140. [Google Scholar] [CrossRef]
- Tang, F.Q.; Xiao, Z.; Tang, J.A.; Jiang, L. The Effect of SiO2 Particles upon Stabilization of Foam. J. Colloid Interface Sci. 1989, 131, 498–502. [Google Scholar] [CrossRef]
- Fujii, S.; Iddon, P.D.; Ryan, A.J.; Armes, S.P. Aqueous Particulate Foams Stabilized Solely with Polymer Latex Particles. Langmuir 2006, 22, 7512–7520. [Google Scholar] [CrossRef]
- Alargova, R.G.; Warhadpande, D.S.; Paunov, V.N.; Velev, O.D. Foam superstabilization by polymer microrods. Langmuir 2004, 20, 10371–10374. [Google Scholar] [CrossRef] [PubMed]
- Guillermic, R.M.; Salonen, A.; Emile, J.; Saint-Jalmes, A. Surfactant foams doped with laponite: Unusual behaviour induced by aging and confinement. Soft Matter 2009, 5, 4975–4982. [Google Scholar] [CrossRef]
- Guevara, J.S.; Mejia, A.F.; Shuai, M.; Chang, Y.W.; Mannan, M.S.; Cheng, Z. Stabilization of Pickering foams by high-aspect-ratio nano-sheets. Soft Matter 2013, 9, 1327–1336. [Google Scholar] [CrossRef]
- Vignati, E.; Piazza, R.; Lockhart, T.P. Pickering emulsions: Interfacial tension, colloidal layer morphology, and trapped-particle motion. Langmuir 2003, 19, 6650–6656. [Google Scholar] [CrossRef]
- Wasan, D.; Nikolov, A. Thin liquid films containing micelles or nanoparticles. Curr. Opin. Colloid Interface Sci. 2008, 13, 128–133. [Google Scholar] [CrossRef]
- Sethumadhavan, G.; Nikolov, A.; Wasan, D. Stability of films with nanoparticles. J. Colloid Interface Sci. 2004, 272, 167–171. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Von Klitzing, R. Disjoining pressure in thin liquid foam and emulsion films—New concepts and perspectives. J. Phys. Condens. Matter 2003, 15, R1197. [Google Scholar] [CrossRef]
- Carn, F.; Colin, A.; Pitois, O.; Vignes-Adler, M.; Backov, R. Foam Drainage in the Presence of Nanoparticle−Surfactant Mixtures. Langmuir 2009, 25, 7847–7856. [Google Scholar] [CrossRef]
- Dickinson, E.; Ettelaie, R.; Kostakis, T.; Murray, B.S. Factors controlling the formation and stability of air bubbles stabilized by partially hydrophobic silica nanoparticles. Langmuir 2004, 20, 8517–8525. [Google Scholar] [CrossRef]
- Kostakis, T.; Ettelaie, R.; Murray, B.S. Effect of high salt concentrations on the stabilization of bubbles by silica particles. Langmuir 2006, 22, 1273–1280. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Drenckhan, W.; Salonen, A.; Rodrigues, J.A.; Iniguez-Palomares, R.; Rio, E.; Langevin, D. On the long-term stability of foams stabilised by mixtures of nano-particles and oppositely charged short chain surfactants. Soft Matter 2012, 8, 11085–11097. [Google Scholar] [CrossRef]
- Lesov, I.; Tcholakova, S.; Denkov, N. Drying of particle-loaded foams for production of porous materials: Mechanism and theoretical modeling. RSC Adv. 2014, 4, 811–823. [Google Scholar] [CrossRef]
- Juillerat, F.K.; Gonzenbach, U.T.; Gauckler, L.J. Tailoring the hierarchical pore structures in self-setting particle-stabilized foams made from calcium aluminate cement. Mater. Lett. 2012, 70, 152–154. [Google Scholar] [CrossRef]
- Koczo, K.; Lobo, L.A.; Wasan, D.T. Effect of oil on foam stability: Aqueous foams stabilized by emulsions. J. Colloid Interface Sci. 1992, 150, 492–506. [Google Scholar] [CrossRef]
- Cohen-Addad, S.; Krzan, M.; Höhler, R.; Herzhaft, B. Rigidity percolation in particle-laden foams. Phys. Rev. Lett. 2007, 99, 168001. [Google Scholar] [CrossRef]
- Rouyer, F.; Louvet, N.; Fritz, C.; Pitois, O. Transport of coarse particles in liquid foams: Coupling of confinement and buoyancy effects. Soft Matter 2011, 7, 4812–4820. [Google Scholar] [CrossRef]
- Louvet, N.; Höhler, R.; Pitois, O. Capture of particles in soft porous media. Phys. Rev. E 2010, 82, 041405. [Google Scholar] [CrossRef]
- Guignot, S.; Faure, S.; Vignes-Adler, M.; Pitois, O. Liquid and particles retention in foamed suspensions. Chem. Eng. Sci. 2010, 65, 2579–2585. [Google Scholar] [CrossRef]
- Goyon, J.; Bertrand, F.; Pitois, O.; Ovarlez, G. Shear induced drainage in foamy yield-stress fluids. Phys. Rev. Lett. 2010, 104, 128301. [Google Scholar] [CrossRef]
- Sarkar, N.; Park, J.G.; Seo, D.N.; Mazumder, S.; Pokhrel, A.; Aneziris, C.G.; Kim, I.J. Influence of amphiphile on foam stability of Al2O3-SiO2 colloidal suspension to porous ceramics. J. Ceram. Process. Res. 2015, 16, 392–396. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- de Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Garimella, A.; Ashri, A.; Kumar, A.; Kumar, A.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Evaluation of microstructure and mechanical properties of magnesium alloy based porous bone substitutes. Mater. Today Proc. 2022, 56, 2708–2713. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E.C. Bone healing materials in the treatment of recalcitrant nonunions and bone defects. Int. J. Mol. Sci. 2022, 23, 3352. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.S.R.; Elsayed, H.; Tadier, S.; Montembault, A.; Maire, E.; David, L.; Delair, T.; Colombo, P.; Gremillard, L. Fabrication and characterization of hardystonite-chitosan biocomposite scaffolds. Ceram. Int. 2019, 45, 8804–8814. [Google Scholar] [CrossRef]
- Kocyło, E.; Franchin, G.; Colombo, P.; Chmielarz, A.; Potoczek, M. Hydroxyapatite-coated ZrO2 scaffolds with a fluorapatite intermediate layer produced by direct ink writing. J. Eur. Ceram. Soc. 2021, 41, 920–928. [Google Scholar] [CrossRef]
- Rohanová, D.; Horkavcová, D.; Helebrant, A.; Boccaccini, A.R. Assessment of in vitro testing approaches for bioactive inorganic materials. J. Non-Cryst. Solids 2016, 432, 53–59. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.; Myszka, B.; Boccaccini, A.R.; Dysthe, D.K. Setting behavior and bioactivity assessment of calcium carbonate cements. J. Am. Ceram. Soc. 2019, 102, 6980–6990. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Bernard, S.; Xue, W.; Bose, S. Calcium Phosphate-Based Resorbable Ceramics: Influence of MgO, ZnO, and SiO2 Dopants. J. Am. Ceram. Soc. 2006, 89, 2675–2688. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]










| Predominant State of Surface Hydroxyl Groups | Surfactant | Particles/IEP | Solvent/pH | Solid Content (vol.%) |
|---|---|---|---|---|
 |  Butyric acid | Al2O3/9.4 | Water/pH 4.75 | 35 |
 |  Propyl gallate | Al2O3/9.4 | Water/pH 9.9 | 35 |
 |  Hexylamine | SiO2/1.5 | Water/pH 10.6 | 35 |
| Sample | Surfactant | θ (°) | γ (mN/m) | (J) | (mPa) | Wet Foam Stability (%) |
|---|---|---|---|---|---|---|
| Al2O3 | Propyl gallate | 86.94 | 22.67 | 1.0 × 10−12 | 0.56 | 50−65 |
| Al2O3–SiO2 | Propyl gallate | 63.90–86.91 | 23.77–70.32 | 1.3 × 10−12–6.9 × 10−13 | 0.52–1.31 | >89 |
| Propionic acid | 56.46–74.58 | 28.44–34.85 | 3.6 × 10−13–8.1 × 10−13 | 0.63–0.86 | >89 | |
| Butyric acid | 56.29–68.80 | 20.94–27.45 | 2.0 × 10−13–5.3 × 10−13 | 0.60–0.76 | >85 | |
| Valeric acid | 61.63–70.13 | 20.30–22.27 | 3.2 × 10−13–4.4 × 10−13 | 1.37–1.64 | >90 | |
| Al2O3–TiO2 | Propyl gallate | 47.45–69.03 | 7.76–136.06 | 1.5 × 10−13–1.6 × 10−12 | 0.11–2.80 | >94 |
| Al2O3–SiO2–TiO2 | Propyl gallate Hexylamine | 45.99–55.23 | 23.56–56.13 | 2.2 × 10−13–2.7 × 10−12 | 1.30–2.23 | >92 |
| Al2O3–TiO2–ZrO2 | Propyl gallate Hexylamine | 45.75–74.08 | 38.50–56.02 | 2.6 × 10−13–1.0 × 10−12 | 1.37–2.23 | >87 |
| Al2O3–TiO2–ZrO2–SiO2 | Propyl gallate | 45.85–74.94 | 27.50–56.15 | × –7.6 × 108 | 1.45–3.46 | >85 |
| ZrO2–TiO2 | Propyl gallate | 54.92–65.82 | 68.4–79.49 | 9.3 × 10−12–1.7 × 10−11 | 1.06–2.05 | >84 |
| SiO2 | Hexylamine | 38.96–70.88 | 13.23–46.94 | 2.3 × 10−14–.6 × 10−13 | 0.30–0.99 | >83 |
| SiC | Hexylamine | 31.52–57.33 | 10.28–33.17 | 4.2 × 10−16–1.4 × 10−14 | 0.25–0.59 | >88 |
| SiC–SiO2 | Hexylamine | 49.01–70.98 | 48.19–81.90 | 8.1 × 10−13–2.8 × 10−12 | 1.06–1.87 | >82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatema, K.N.; Biswas, M.R.U.D.; Park, J.G.; Kim, I.J. Chemistry and Physics of Wet Foam Stability for Porous Ceramics: A Review. Micro 2024, 4, 552-571. https://doi.org/10.3390/micro4040034
Fatema KN, Biswas MRUD, Park JG, Kim IJ. Chemistry and Physics of Wet Foam Stability for Porous Ceramics: A Review. Micro. 2024; 4(4):552-571. https://doi.org/10.3390/micro4040034
Chicago/Turabian StyleFatema, Kamrun Nahar, Md Rokon Ud Dowla Biswas, Jung Gyu Park, and Ik Jin Kim. 2024. "Chemistry and Physics of Wet Foam Stability for Porous Ceramics: A Review" Micro 4, no. 4: 552-571. https://doi.org/10.3390/micro4040034
APA StyleFatema, K. N., Biswas, M. R. U. D., Park, J. G., & Kim, I. J. (2024). Chemistry and Physics of Wet Foam Stability for Porous Ceramics: A Review. Micro, 4(4), 552-571. https://doi.org/10.3390/micro4040034





