F2 Laser-Induced Micro-Reticulated Structural Changes of Amorphous Carbon Thin Films
Abstract
1. Introduction
2. Experimental Procedure
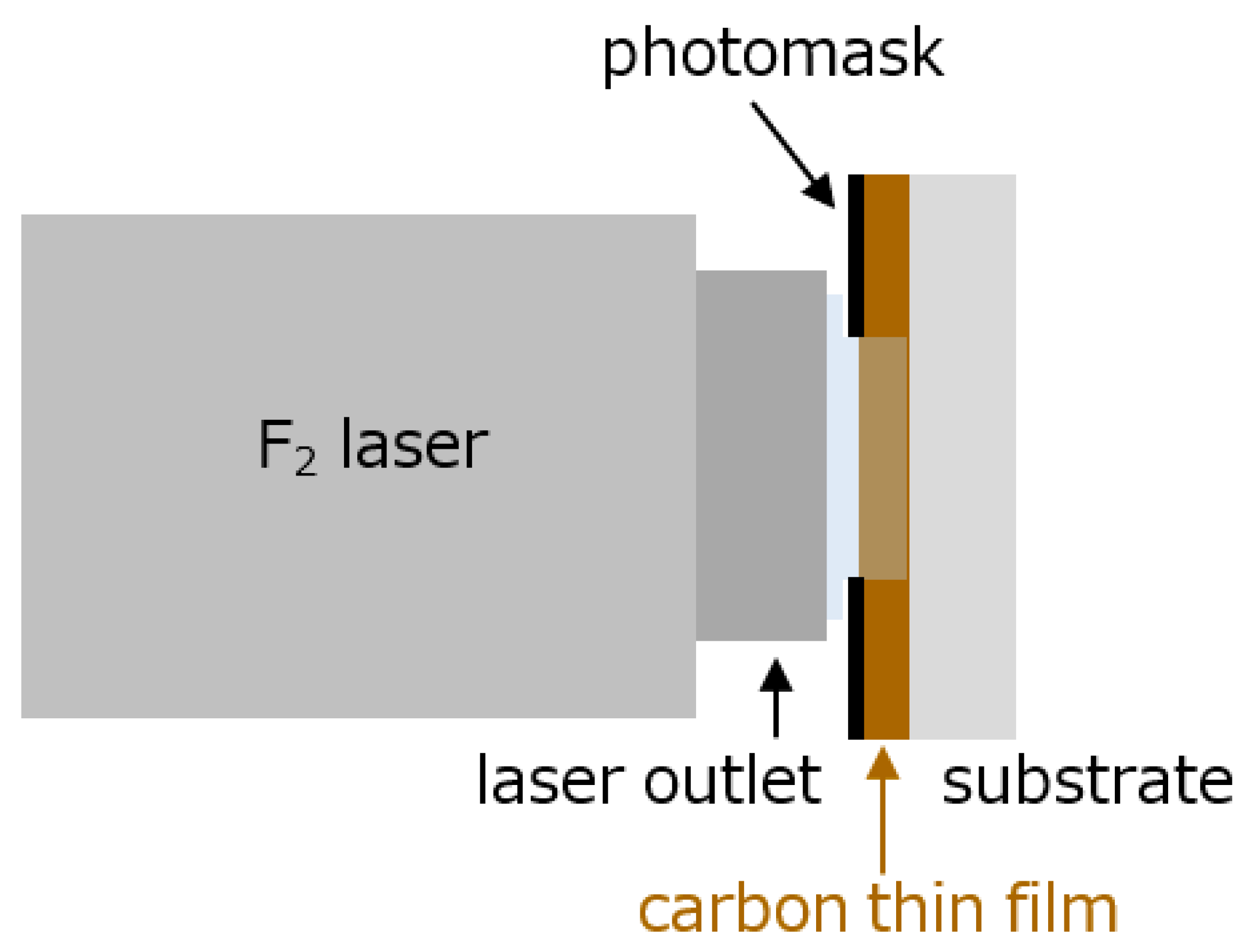
2.1. Samples Obtaining
2.2. Characterization
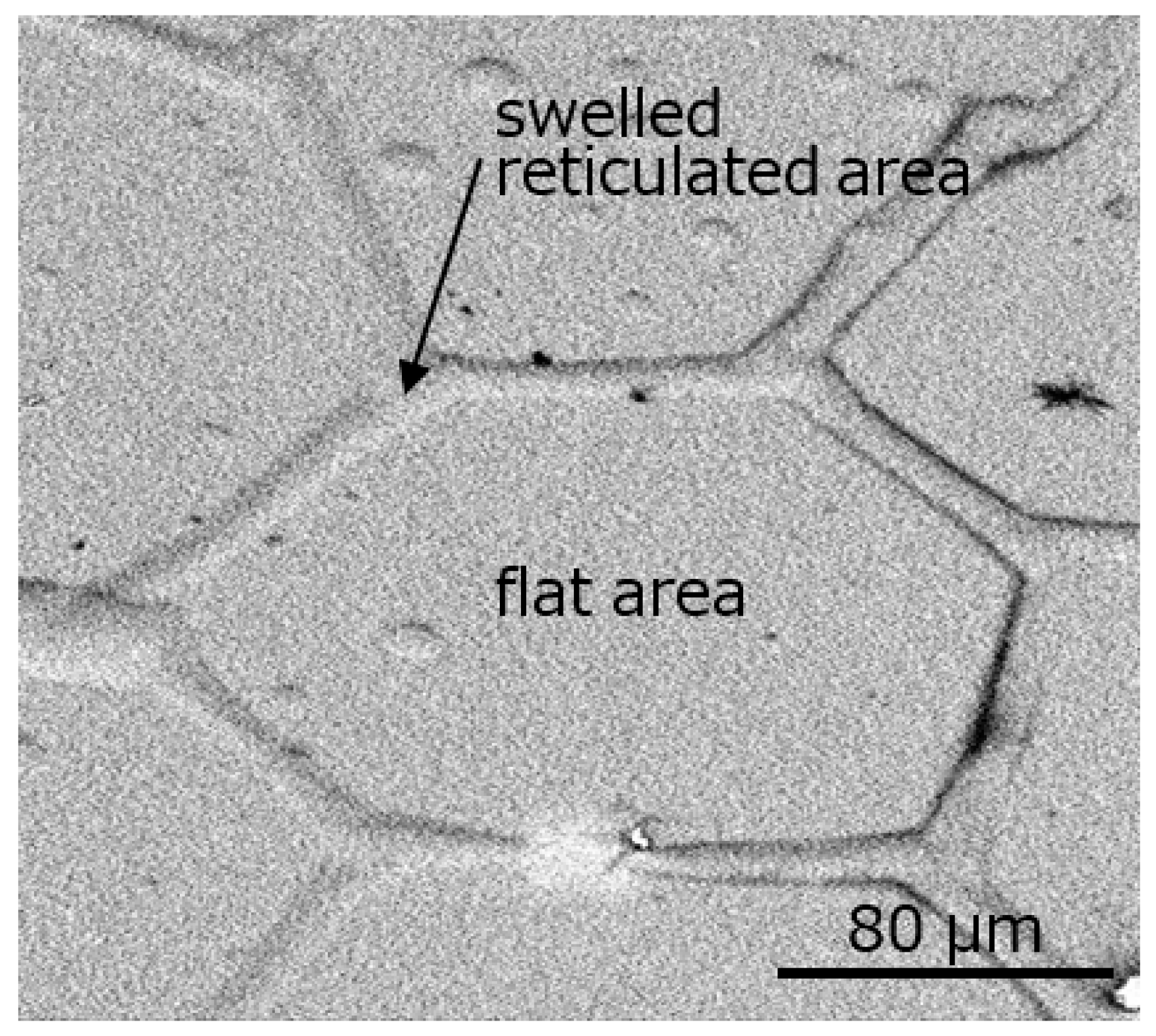
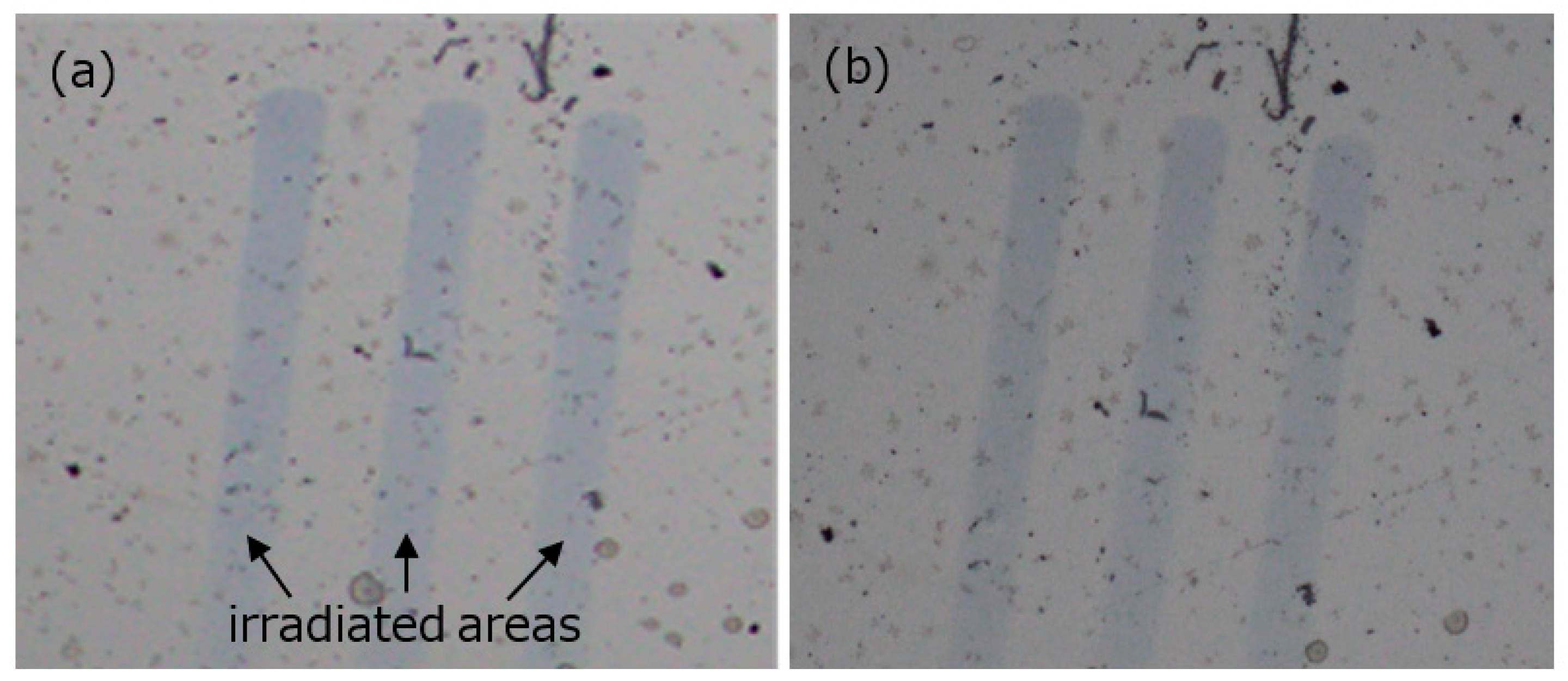
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, H.; Liu, P.; Park, Y.; Choi, G.; Choi, P.-P.; Sohn, H. Pulsed laser-assisted additive manufacturing of Ti-6Al-4V for in-situ grain refinement. Sci. Rep. 2022, 12, 22247. [Google Scholar] [CrossRef]
- Chakraborty, S.; Park, H.-Y.; Ahn, S.I. Copper laser patterning on a flexible substrate using a cost-effective 3D printer. Sci. Rep. 2022, 12, 21149. [Google Scholar] [CrossRef]
- Ródenas, A.; Gu, M.; Corrielli, G.; Paiè, P.; John, S.; Kar, A.K.; Osellame, R. Three-dimensional femtosecond laser nanolithography of crystals. Nat. Photonics 2019, 13, 105–109. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Chen, P.; Zhou, C.; Ma, J.; Wei, D.; Wang, H.; Niu, B.; Fang, X.; Wu, D.; et al. Femtosecond laser writing of lithium niobate ferroelectric nanodomains. Nature 2022, 609, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Suttmann, O.; Obata, K.; Nakajima, Y.; Hohnholz, A.; Koch, J.; Terakawa, M.; Overmeyer, L. UV laser photo-polymerization of elastic 2D/3D structures using photo-curable PDMS (Polydimethylsiloxane). J. Laser Micro. Nanoeng. 2017, 12, 153–158. [Google Scholar] [CrossRef]
- Zazo, R.; Solis, J.; Sanchez-Gil, J.A.; Ariza, R.; Serna, R.; Siegel, J. Deep UV laser induced periodic surface structures on silicon formed by self-organization of nanoparticles. Appl. Surf. Sci. 2020, 520, 146307. [Google Scholar] [CrossRef]
- Meinertz, J.; Fricke-Begemann, T.; Ihlemann, J. Micron and sub-micron gratings on glass by UV laser ablation. Physica. Procedia. 2013, 41, 708–712. [Google Scholar] [CrossRef]
- Iwasaki, K.; Yoshida, T.; Okoshi, M. Near-superhydrophobic silicone microcapsule arrays encapsulating ionic liquid electrolytes for micro-power storage assuming use in seawater. Sci. Rep. 2022, 12, 18264. [Google Scholar] [CrossRef]
- Takao, H.; Okoshi, M.; Inoue, N. Fabrication of SiO2-humps on silicone rubber using F2 laser. Jpn. J. Appl. Phys. 2002, 41, L1088–L1089. [Google Scholar] [CrossRef]
- Okoshi, M.; Kimura, T.; Takao, H.; Inoue, N.; Yamashita, T. Photochemical modification of silicone films using F2 laser for selective chemical etching. Jpn. J. Appl. Phys. 2004, 43, 3438–3442. [Google Scholar] [CrossRef]
- Okoshi, M.; Li, J.; Herman, P.R. 157-nm F2-laser writing of silica optical waveguides in silicone rubber. Opt. Lett. 2005, 30, 2730–2732. [Google Scholar] [CrossRef]
- Nojiri, H.; Okoshi, M. Surface texturing effect on crack suppression of SiO2 film formed by F2 laser-induced photochemical surface modification of silicone on polycarbonate under heat resistance test. Jpn. J. Appl. Phys. 2017, 56, 085502. [Google Scholar] [CrossRef]
- Okoshi, M.; Awaihara, Y.; Yamashita, T.; Inoue, N. F2 laser induced surface modification of iron thin films to obtain corrosion resistance. Jpn. J. Appl. Phys. 2014, 53, 022702. [Google Scholar] [CrossRef]
- Okoshi, M.; Awaihara, Y.; Yamashita, T.; Inoue, N. Fabrication of hydrophobic and corrosion resistant iron thin film by interference exposure using 157 nm F2 laser. Mater. Lett. 2015, 139, 300–302. [Google Scholar] [CrossRef]
- Okoshi, M.; Iwai, K.; Nojiri, H.; Inoue, N. F2 laser induced modification of aluminum thin films into transparent aluminum oxide. Jpn. J. Appl. Phys. 2012, 51, 122701. [Google Scholar] [CrossRef]
- Losero, E.; Jagannath, S.; Pezzoli, M.; Goblot, V.; Babashah, H.; Lashuel, H.A.; Galland, C.; Quack, N. Neuronal growth on high-aspect-ratio diamond nanopillar arrays for biosensing applications. Sci. Rep. 2023, 13, 5909. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, A. Fullerenes make connections. Nat. Synth. 2023, 2, 78. [Google Scholar] [CrossRef]
- Tian, H.; Ma, Y.; Li, Z.; Cheng, M.; Ning, S.; Chen, J.; Zhou, W.; Liu, L.; Wang, E.; Pei, J.; et al. Disorder-tuned conductivity in amorphous monolayer carbon. Nature 2023, 615, 56–61. [Google Scholar] [CrossRef]
- Ban, M.; Chen, J. Fabrication of plane-type axon guidance substrates by applying diamond-like carbon thin film deposition. Sci. Rep. 2023, 13, 8489. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Wang, C.; Lu, Y.; Hao, J. High temperature tribology behavior of silicon and nitrogen doped hydrogenated diamond-like carbon (DLC) coatings. Tribol. Int. 2022, 175, 107845. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kim, J.-I.; Lee, W.-Y.; Kim, J. Friction properties of thick tetrahedral amorphous carbon coating with different surface defects under dry contact conditions. Appl. Surf. Sci. 2023, 550, 149332. [Google Scholar] [CrossRef]
- Lai, C.Q.; Lim, G.Y.; Tai, K.J.; Lim, K.J.D.; Yu, L.; Kanaujia, P.K.; Seetoh, P.I. Exceptional energy absorption characteristics and compressive resilience of functional carbon foams scalably and sustainably derived from additively manufactured kraft paper. Addit. Manuf. 2022, 58, 102992. [Google Scholar] [CrossRef]
- Yoshikawa, M. Raman spectra of diamondlike amorphous carbon films. Mater. Sci. Forum. 1990, 52–53, 365–386. [Google Scholar]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Balabanyan, V.Y.; Tsiskarashvili, A.V. Antibacterial properties of modified a-C and ta-C coatings: The effects of the sp2/sp3 ratio, oxidation, nitridation, and silver incorporation. Appl. Phys. A 2022, 128, 929. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Mallet-Ladeira, P.; Puech, P.; Toulouse, C.; Cazayous, M.; Ratel-Ramond, N.; Weisbecker, P.; Vignoles, G.L.; Monthioux, M. A Raman study to obtain crystallite size of carbon materials: A better alternative to the Tuinstra–Koenig law. Carbon 2014, 80, 629–639. [Google Scholar] [CrossRef]
- Zkria, A.; Haque, A.; Egiza, M.; Abubakr, E.; Murasawa, K.; Yoshitake, T.; Narayan, J. Laser-induced structure transition of diamond-like carbon coated on cemented carbide and formation of reduced graphene oxide. MRS Commun. 2019, 9, 910–915. [Google Scholar] [CrossRef]
- Kudryashov, S.I.; Karabutov, A.A.; Kudryashova, M.A.; Beketov, V.I.; Zorov, N.B. Laser-induced phase transitions of carbon. Mendeleev. Commun. 1998, 8, 29–30. [Google Scholar] [CrossRef]
- Nojiri, H.; Okoshi, M. Crack suppression of silica glass formed by zoned F2 laser-induced photochemical surface modification of hard silicone thin film coating on polycarbonate. Jpn. J. Appl. Phys. 2016, 55, 122701. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoshi, M. F2 Laser-Induced Micro-Reticulated Structural Changes of Amorphous Carbon Thin Films. Micro 2023, 3, 602-609. https://doi.org/10.3390/micro3020041
Okoshi M. F2 Laser-Induced Micro-Reticulated Structural Changes of Amorphous Carbon Thin Films. Micro. 2023; 3(2):602-609. https://doi.org/10.3390/micro3020041
Chicago/Turabian StyleOkoshi, Masayuki. 2023. "F2 Laser-Induced Micro-Reticulated Structural Changes of Amorphous Carbon Thin Films" Micro 3, no. 2: 602-609. https://doi.org/10.3390/micro3020041
APA StyleOkoshi, M. (2023). F2 Laser-Induced Micro-Reticulated Structural Changes of Amorphous Carbon Thin Films. Micro, 3(2), 602-609. https://doi.org/10.3390/micro3020041






