Nanometals and Metal Ion Pollution from Dental Materials in Dental Environment
Abstract
1. Introduction
2. Toxicological Potential of Metallic Elements Present in Dental Materials
2.1. Routes for the Spread of Metals in Dentistry
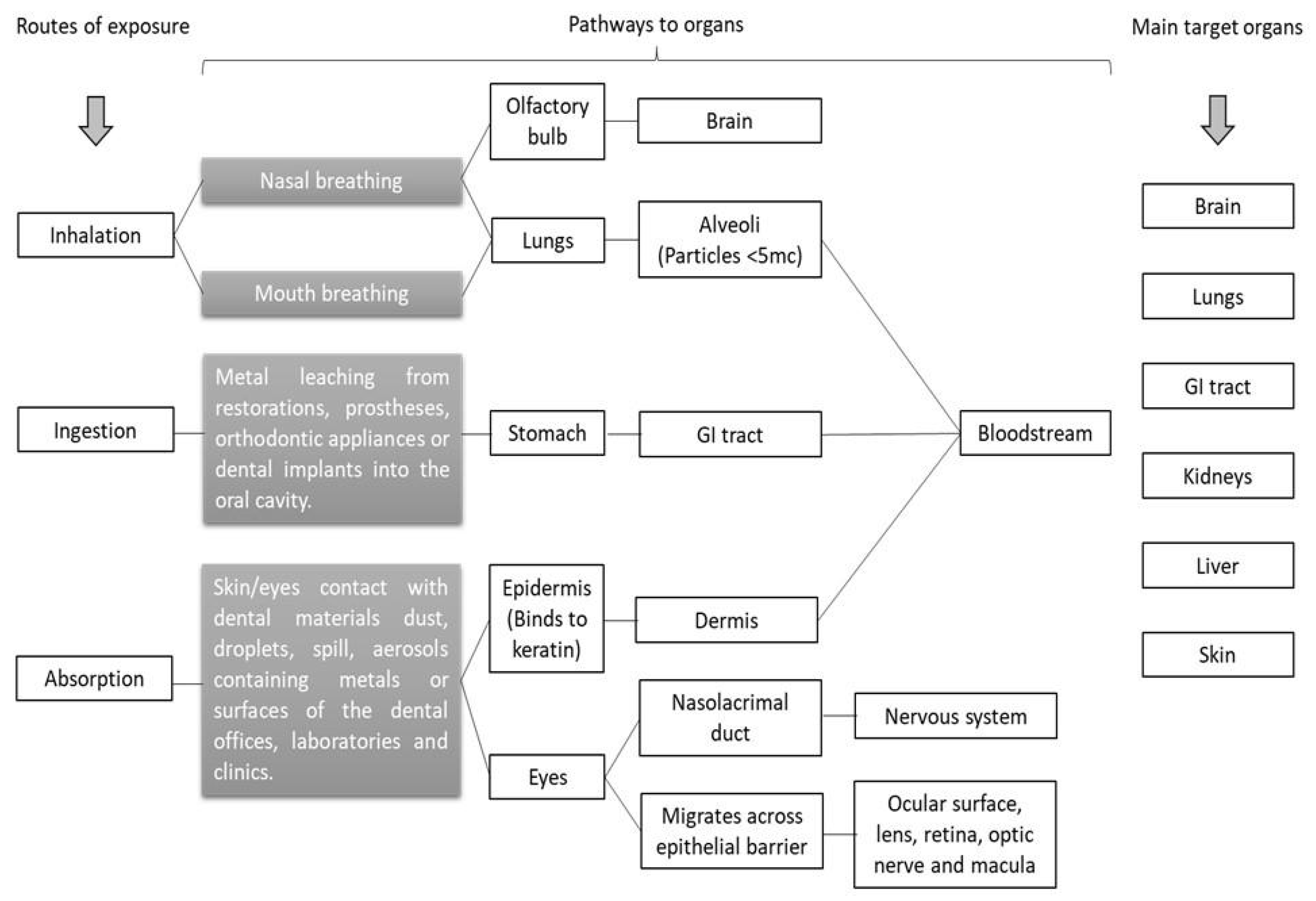
2.2. Routes of Exposure to Metals in the Dental Environment
2.3. Potential Metal Toxicity in the Dental Environment
3. General Recommendations
3.1. Air Quality Precautions
- Use adequately designed ventilation systems, including local exhaust ventilation.
- Use air filtration equipment for dental procedures involving dental amalgam and make sure the filters are appropriate.
- Perform air monitoring periodically to ensure that the occupational (dental workers) and nonoccupational (dental students) exposure limit is not exceeded.
3.2. Good Hygiene Practices
- Clean hands vigorously to create friction in an appropriate sink. Use soap for your hands and a nailbrush to clean your fingernails. Afterward, rinse well to remove all of the soap and dry your hands thoroughly using a paper towel.
- Hand hygiene should be performed immediately before starting a clinical session, before putting on gloves, and following the removal of gloves. Hand hygiene should also be performed at anytime hands are contaminated with visible metal dust or liquid mercury.
- Wearing jewelry is not recommended as it can chemically bond with other metals released during dental procedures.
- Observe periodically if the working team or dental students follow good hygiene practices for quality control.
3.3. Personal Protective Equipment (PPE)
- Dentists, dental students, dental personnel, and patients should wear protective clothing (ideally an impermeable full-body covering for procedures with dental amalgam).
- Use a dental dam sealed correctly in the patient’s mouth with a saliva ejector to remove contaminated saliva or liquid debris from the oral cavity.
- The protective mask or respirator should be selected according to the type of metal involved in the dental material or procedure, and as guided by the local, national, or international regulatory agency of occupational and nonoccupational health and safety recommendations. Each agency has its guidelines recommending different types of masks or respirators. The barrier choice should be based on the air monitoring results of the dental clinic or laboratory, respecting the threshold limit value (TLV), permissible exposure limit (PEL), recommended exposure limit (REL), minimal risk level (MRL), or reference concentration (RfC) of the agency. The most common agencies used in North America are the National Institute for Occupational Safety and Health (NIOSH), the Occupational Safety and Health Administration (OSHA), the American Conference of Governmental Industrial Hygienists (ACGIH), the United States Environmental Protection Agency, and the California Environmental Protection Agency. The mask or respirator should cover the mouth and nose completely.
- Regarding protective eyewear, prescription safety glasses, safety glasses over corrective lenses, or loupes with side shields should be used during all preclinical and clinical procedures.
- Bouffant, impermeable dedicated footwear (covering the entire foot) and a face shield are also recommended.
- The patient’s skin and clothing should be protected, providing a whole-body, impermeable barrier, and a complete head/face/neck barrier under/around the dam should also be used.
- Use high-volume suction and continually add water spray to the site where the amalgam is removed.
3.4. Health and Educational Surveillance
- Perform medical monitoring of metals levels in the working team or dental students.
- Periodically observe if the working team or dental students are following the local quality control guidelines.
- Review the local guidelines periodically or when updated.
3.5. Substitution with Less Harmful Products
- It is reasonable to choose metal-free materials when appropriate. The use of alternative materials to dental amalgams has been encouraged in Europe to reduce environmental and human exposure to mercury [113]. Composite resins and metal-free glass ionomer cement are excellent alternative materials for dental restorations.
- Metal-free crowns (porcelain-based ceramic, quartz, glass, or resin, through zirconium and lithium disilicate) are suggested instead of metallic crowns (made of gold, platinum, copper, nickel, or chromium) when indicated.
- The cobalt–chromium (Co–Cr) alloy is indicated for fabricating metallic frameworks of removable partial dentures instead of Ni–Cr alloy to reduce allergic reactions. The additional use of allergenic metals should be reduced if possible.
3.6. Cleaning Dental Instruments and Surfaces
- For dental procedures involving dental amalgam, use mercury decontaminant to clean instruments, countertops, and surfaces after the clinical session.
- For dental procedures involving other metal types, clean instruments with water, soap, and a brush.
- Clean spills of mercury using commercial mercury spill clean-up kits. Afterward, check mercury vapor levels in the dental operatory.
- Clean handpieces according to the manufacturer’s instructions. For handpieces contaminated with mercury, it is recommended to wipe the handpieces with mercury decontaminant before starting the manufacturer’s instructions.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Sakaguchi, R.; Ferracane, J.; Powers, J. Craig’s Restorative Dental Materials, 14th ed.; Mosby: Maryland Heights, MO, USA, 2019; p. 352. [Google Scholar]
- Anusavice, K. Phillips’ Science of Dental Materials, 12th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2013. [Google Scholar]
- Sacher, E.; Franc, R. Dental Biomaterials. In World Scientific Series: From Biomaterials towards Medical Devices; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2019; Volume 2. [Google Scholar]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Givan, D.A. Precious metals in dentistry. Dent. Clin. N. Am. 2007, 51, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Panpisut, P.; Liaqat, S.; Zacharaki, E.; Xia, W.; Petridis, H.; Young, A.M. Dental Composites with Calcium/Strontium Phosphates and Polylysine. PLoS ONE 2016, 11, e0164653. [Google Scholar] [CrossRef]
- Zhou, M.; Drummond, J.L.; Hanley, L. Barium and strontium leaching from aged glass particle/resin matrix dental composites. Dent. Mater. 2005, 21, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Borg, J.; Damidot, D.; Salvadori, E.; Pilecki, P.; Zaslansky, P.; Darvell, B.W. Colour and chemical stability of bismuth oxide in dental materials with solutions used in routine clinical practice. PLoS ONE 2020, 15, e0240634. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Grech, J.; Antunes, E. Zirconia in dental prosthetics: A literature review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Balbinot, G.S.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Niobium silicate particles as bioactive fillers for composite resins. Dent. Mater 2020, 36, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Fidalgo, T.K.S.; da Costa, L.P.; Maia, L.C.; Balan, L.; Anselme, K.; Ploux, L.; Thiré, R.M.S.M. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J. Dent. 2018, 69, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Santos, H.E.S.; Garbossa, M.; Santos, C. Mechanical Properties of Zirconia Y-TZP Core Veneered for Dentistry Applications. J. Ceram. Sci. Technol. 2017, 8, 525–530. [Google Scholar]
- Berdouses, E.; Vaidyanathan, T.K.; Dastane, A.; Weisel, C.; Houpt, M.; Shey, Z. Mercury Release from Dental Amalgams: An in vitro Study Under Controlled Chewing and Brushing in an Artificial Mouth. J. Dent. Res. 1995, 74, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Mocnik, P.; Kosec, T.; Kovac, J.; Bizjak, M. The effect of pH, fluoride and tribocorrosion on the surface properties of dental archwires. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 682–689. [Google Scholar] [CrossRef]
- Bajsman, A.; Vukovic, A.; Zukic, S.; Jakupovic, S. Dental Amalgam: Do We Have Enough Proves “pro” et “contra”. Mater. Socio-Med. 2010, 22, 222. [Google Scholar]
- Siddharth, R.; Gautam, R.; Chand, P.; Agrawal, K.K.; Singh, R.D.; Singh, B.P. Quantitative analysis of leaching of different metals in human saliva from dental casting alloys: An in vivo study. J. Indian Prosthodont. Soc. 2015, 15, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Ozcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.G.; Summitt, J.B.; Chung, A.K.; Osborne, J.W. Amalgam at the new millennium. J. Am. Dent. Assoc. 1998, 129, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Kralj, P.; Veber, M.; Sinagra, E. Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int. Endod. J. 2012, 45, 737–743. [Google Scholar] [CrossRef]
- Warwick, R.; O’Connor, A.; Lamey, B. Mercury vapour exposure during dental student training in amalgam removal. J. Occup. Med. Toxicol. 2013, 8, 27. [Google Scholar] [CrossRef]
- Keinan, D.; Mass, E.; Zilberman, U. Absorption of nickel, chromium, and iron by the root surface of primary molars covered with stainless steel crowns. Int. J. Dent. 2010, 2010, 326124. [Google Scholar] [CrossRef]
- Wendl, B.; Wiltsche, H.; Lankmayr, E.; Winsauer, H.; Walter, A.; Muchitsch, A.; Jakse, N.; Wendl, M.; Wendl, T. Metal release profiles of orthodontic bands, brackets, and wires: An in vitro study. J. Orofac. Orthop. 2017, 78, 494–503. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K.; Wozniak, B.; Downarowicz, P. Release of metal ions from orthodontic appliances: An in vitro study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishnan, G.; Melath, A.; Ajith, V.V.; Mathews, N.B. A Comparative Study of Bio Degradation of Various Orthodontic Arch Wires: An In Vitro Study. J. Int. Oral Health 2015, 7, 12–17. [Google Scholar]
- Liu, M.H.; Chen, C.T.; Chuang, L.C.; Lin, W.M.; Wan, G.H. Removal efficiency of central vacuum system and protective masks to suspended particles from dental treatment. PLoS ONE 2019, 14, e0225644. [Google Scholar] [CrossRef] [PubMed]
- Innes, N.; Johnson, I.G.; Al-Yaseen, W.; Harris, R.; Jones, R.; Kc, S.; McGregor, S.; Robertson, M.; Wade, W.G.; Gallagher, J.E. A systematic review of droplet and aerosol generation in dentistry. J. Dent. 2021, 105, 103556. [Google Scholar] [CrossRef]
- Han, P.; Li, H.; Walsh, L.J.; Ivanovski, S. Splatters and Aerosols Contamination in Dental Aerosol Generating Procedures. Appl. Sci. 2021, 11, 1914. [Google Scholar] [CrossRef]
- Shiu, E.Y.C.; Leung, N.H.L.; Cowling, B.J. Controversy around airborne versus droplet transmission of respiratory viruses: Implication for infection prevention. Curr. Opin. Infect. Dis. 2019, 32, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Grantham, M.; Pantelic, J.; Bueno de Mesquita, P.J.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K.; EMIT Consortium. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef]
- Leggat, P.A.; Kedjarune, U. Bacterial aerosols in the dental clinic: A review. Int. Dent. J. 2001, 51, 39–44. [Google Scholar] [CrossRef]
- James, R.; Mani, A.K. Dental Aerosols: A Silent Hazard in Dentistry. Int. J. Sci. Res. 2016, 5, 1761–1763. [Google Scholar]
- Estrich, C.G.; Lipman, R.D.; Araujo, M.W.B. Dental amalgam restorations in nationally representative sample of US population aged >/=15 years: NHANES 2011–2016. J. Public Health Dent. 2021, 81, 327–330. [Google Scholar] [CrossRef]
- Adabo, G.L. Mercury Toxicity. In Dental Biomaterials; World Scientific: Singapore, 2019; pp. 125–145. [Google Scholar]
- Davies, R.A.; Ardalan, S.; Mu, W.-H.; Tian, K.; Farsaikiya, F.; Darvell, B.W.; Chass, G.A. Geometric, electronic and elastic properties of dental silver amalgam γ-(Ag3Sn), γ1-(Ag2Hg3), γ2-(Sn8Hg) phases, comparison of experiment and theory. Intermetallics 2010, 18, 756–760. [Google Scholar] [CrossRef]
- Warwick, D.; Young, M.; Palmer, J.; Ermel, R.W. Mercury vapor volatilization from particulate generated from dental amalgam removal with a high-speed dental drill-a significant source of exposure. J. Occup. Med. Toxicol. 2019, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, N.; Bettiol, S.S.; Isham, A.; Hoang, H.; Crocombe, L.A. A Review of Mercury Exposure and Health of Dental Personnel. Saf. Health Work 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- van Gestel, C.A.M. Environmental Toxicology; Vrije Universiteit Amsterdam: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Li, L.; Li, D. Inter-Individual Variability and Non-linear Dose-Response Relationship in Assessing Human Health Impact From Chemicals in LCA: Addressing Uncertainties in Exposure and Toxicological Susceptibility. Front. Sustain. 2021, 2, 648138. [Google Scholar] [CrossRef]
- Hostynek, J.J. Factors determining percutaneous metal absorption. Food Chem. Toxicol. 2003, 41, 327–345. [Google Scholar] [CrossRef]
- Bjørklund, G.; Hilt, B.; Dadar, M.; Lindh, U.; Aaseth, J. Neurotoxic effects of mercury exposure in dental personnel. Basic Clin. Pharmacol. Toxicol. 2019, 124, 568–574. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M.; Aaseth, J. Mercury exposure and its effects on fertility and pregnancy outcome. Basic Clin. Pharmacol. Toxicol. 2019, 125, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Bengtsson, U.; Chirumbolo, S.; Kern, J.K. Concerns about environmental mercury toxicity: Do we forget something else? Environ. Res. 2017, 152, 514–516. [Google Scholar] [CrossRef]
- Anglen, J.; Gruninger, S.E.; Chou, H.; Weuve, J.; Turyk, M.E.; Freels, S.; Stayner, L.T. Occupational mercury exposure in association with prevalence of multiple sclerosis and tremor among US dentists. J. Am. Dent. Assoc. 2015, 146, 659–668.e1. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.M.; Wang, Y.; Gillespie, B.; Werner, R.; Franzblau, A.; Basu, N. Methylmercury and elemental mercury differentially associate with blood pressure among dental professionals. Int. J. Hyg. Environ. Health 2013, 216, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Björkman, L.; Brokstad, K.A.; Moen, K.; Jonsson, R. Minor changes in serum levels of cytokines after removal of amalgam restorations. Toxicol. Lett. 2012, 211, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lindbohm, M.; Ylöstalo, P.; Sallmén, M.; Henriks-Eckerman, M.; Nurminen, T.; Forss, H.; Taskinen, H. Occupational exposure in dentistry and miscarriage. Occup. Environ. Med. 2007, 64, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bittner, A.C.; Echeverria, D.; Woods, J.S.; Aposhian, H.V.; Naleway, C.; Martin, M.D.; Mahurin, R.K.; Heyer, N.J.; Cianciola, M. Behavioral effects of low-level exposure to Hg0 among dental professionals: A cross-study evaluation of psychomotor effects. Neurotoxicol. Teratol. 1998, 20, 429–439. [Google Scholar] [CrossRef]
- Echeverria, D.; Heyer, N.J.; Martin, M.D.; Naleway, C.A.; Woods, J.S.; Bittner, A.C. Behavioral effects of low-level exposure to Hg ∘ among dentists. Neurotoxicol. Teratol. 1995, 17, 161–168. [Google Scholar] [CrossRef]
- Ngim, C.H.; Foo, S.C.; Boey, K.W. Chronic neurobehavioural effects of elemental. Br. J. Ind. Med. 1992, 49, 782–790. [Google Scholar]
- Bjørklund, G. Mercury in the dental office. Risk evaluation of the occupational environment in dental care. Tidsskr Nor Laegeforen 1991, 111, 948–951. [Google Scholar]
- Bauer, J.G. Action of mercury in dental exposures to mercury. Oper. Dent. 1985, 10, 104–113. [Google Scholar]
- Shapiro, I.M.; Cornblath, D.R.; Sumner, A.J.; Uzzell, B.; Spitz, L.K.; Ship, I.I.; Bloch, P. Neurophysiological and neuropsychological function in mercury-exposed dentists. Lancet 1982, 319, 1147–1150. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Ruggiero, J.L.; Atwater, A.R.; DeKoven, J.G.; Zug, K.A.; Reeder, M.J.; Silverberg, J.I.; Taylor, J.S.; Pratt, M.D.; Maibach, H.I.; et al. Occupational Contact Dermatitis in Dental Personnel: A Retrospective Analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermatitis 2022, 33, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Heratizadeh, A.; Werfel, T.; Schubert, S.; Geier, J.; Ludwig, A.; Bircher, A.; Köhler, A. Contact sensitization in dental technicians with occupational contact dermatitis. Data of the Information Network of Departments of Dermatology (IVDK) 2001–2015. Contact Dermat. 2018, 78, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Wrangsjö, K.; Swartling, C.; Meding, B. Occupational dermatitis in dental personnel: Contact dermatitis with special reference to (meth)acrylates in 174 patients: Occupational dermatitis in dental personnel. Contact Dermat. 2001, 45, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wallenhammar, L.M.; Örtengren, U.; Andreasson, H.; Barregård, L.; Björkner, B.; Karlsson, S.; Wrangsjö, K.; Meding, B. Contact allergy and hand eczema in Swedish dentists. Contact Dermat. 2000, 43, 192–199. [Google Scholar] [CrossRef]
- Hill, J.G.; Grimwood, R.E.; Hermesch, C.B.; Marks, J.G. Prevalence of occupationally related hand dermatitis in dental workers. J. Am. Dent. Assoc. 1998, 129, 212–217. [Google Scholar] [CrossRef]
- Rustemeyer, T.; Frosch, P.J. Occupational skin diseases in dental laboratory technicians: (I). Clinical picture and causative factors. Contact Dermat. 1996, 34, 125–133. [Google Scholar] [CrossRef]
- Okamoto, M.; Tominaga, M.; Shimizu, S.; Yano, C.; Masuda, K.; Nakamura, M.; Zaizen, Y.; Nouno, T.; Sakamoto, S.; Yokoyama, M.; et al. Dental Technicians’ Pneumoconiosis. Intern. Med. 2017, 56, 3323–3326. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Luo, X.; Zhang, K.; Cao, N.; Liu, K.; Li, X.; Zhu, Y. Cytotoxic effects of dental prosthesis grinding dust on RAW264.7 cells. Sci. Rep. 2020, 10, 14364. [Google Scholar] [CrossRef]
- Seldén, A.I.; Persson, B.; Bornberger-Dankvardt, S.I.; Winström, L.E.; Bodin, L.S. Exposure to cobalt chromium dust and lung disorders in dental technicians. Thorax 1995, 50, 769–772. [Google Scholar] [CrossRef]
- Martin, S.; Wendy, G. Human Health Effects of Heavy Metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Kaye, G.W.C.; Laby, T.H. Tables of Physical and Chemical Constants, 15th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1986. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Hoboken, NJ, USA, 2011. [Google Scholar]
- ATSDR. Toxicological Profile for Mercury; ATSDR: Atlanta, GA, USA, 1999.
- Li, R.; Wu, H.; Ding, J.; Fu, W.; Gan, L.; Li, Y. Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Sci. Rep. 2017, 7, 46545. [Google Scholar] [CrossRef] [PubMed]
- WHO. Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications, European Series; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2000; p. 91. [Google Scholar]
- ATSDR. ATSDR Toxicological Profile for Nickel; ATSDR: Atlanta, GA, USA, 2017.
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol./Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Low nickel diet in dermatology. Indian J. Dermatol. 2013, 58, 240. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Silver; ATSDR: Atlanta, GA, USA, 1990.
- ATSDR. Toxicological Profile for Copper; ATSDR: Atlanta, GA, USA, 2004.
- Tsafrir, J. Copper Toxicity: A Common Cause of Psychiatric Symptoms. Available online: https://www.psychologytoday.com/intl/blog/holistic-psychiatry/201709/copper-toxicity-common-cause-psychiatric-symptoms (accessed on 19 July 2022).
- Barnhart, J. Occurrences, Uses, and Properties of Chromium. Regul. Toxicol. Pharmacol. 1997, 26, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Chromium; ATSDR: Atlanta, GA, USA, 2012.
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Straeten, C.V.D.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Lauwerys, R.; Lison, D. Health risks associated with cobalt exposure—An overview. Sci. Total Environ. 1994, 150, 1–6. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile of Cobalt; ATSDR: Atlanta, GA, USA, 2004.
- ATSDR. Toxicological Profile for Zinc; ATSDR: Atlanta, GA, USA, 2005.
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Dobson, A.W.; Erikson, K.M.; Aschner, M. Manganese Neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1012, 115–128. [Google Scholar] [CrossRef]
- Marcus, J.B. Chapter 7—Vitamin and Mineral Basics: The ABCs of Healthy Foods and Beverages, Including Phytonutrients and Functional Foods: Healthy Vitamin and Mineral Choices, Roles and Applications in Nutrition, Food Science and the Culinary Arts. In Culinary Nutrition; Marcus, J.B., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 279–331. [Google Scholar]
- Iregren, A. Psychological test performance in foundry workers exposed to low levels of manganese. Neurotoxicol. Teratol. 1990, 12, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Roels, H.A.; Ghyselen, P.; Buchet, J.P.; Ceulemans, E.; Lauwerys, R.R. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br. J. Ind. Med. 1992, 49, 25–34. [Google Scholar] [CrossRef]
- Mergler, D.; Huel, G.; Bowler, R.; Iregren, A.; Bélanger, S.; Baldwin, M.; Tardif, R.; Smargiassi, A.; Martin, L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994, 64, 151–180. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Manganese; ATSDR: Atlanta, GA, USA, 2012.
- Melber, C.; Keller, D.; Mangelsdorf, I.; International Programme on Chemical Safety. Palladium; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Kielhorn, J.; Melber, C.; Keller, D.; Mangelsdorf, I. Palladium—A review of exposure and effects to human health. Int. J. Hyg. Environ. Health 2002, 205, 417–432. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Aluminium; ATSDR: Atlanta, GA, USA, 2008.
- Shaw, C.A.; Tomljenovic, L. Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol. Res. 2013, 56, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Momcilović, B. A case report of acute human molybdenum toxicity from a dietary molybdenum supplement—A new member of the “Lucor metallicum” family. Arh. Hig. Rada Toksikol. 1999, 50, 289–297. [Google Scholar]
- ATSDR. ATSDR Toxicological Profile for Molybdenum; ATSDR: Atlanta, GA, USA, 2017.
- Nakano, M.; Omae, K.; Tanaka, A.; Hirata, M.; Michikawa, T.; Kikuchi, Y.; Yoshioka, N.; Nishiwaki, Y.; Chonan, T. Causal Relationship between Indium Compound Inhalation and Effects on the Lungs. J. Occup. Health 2009, 51, 513–521. [Google Scholar] [CrossRef]
- Hoet, P.; De Graef, E.; Swennen, B.; Seminck, T.; Yakoub, Y.; Deumer, G.; Haufroid, V.; Liso, D. Occupational exposure to indium: What does biomonitoring tell us? Toxicol. Lett. 2012, 213, 122–128. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium allergy: Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef]
- Decker, A.; Daly, D.; Scher, R.K. Role of Titanium in the Development of Yellow Nail Syndrome. Ski. Appendage Disord. 2015, 1, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Ataya, A.; Kline, K.P.; Cope, J.; Alnuaimat, H. Titanium exposure and yellow nail syndrome. Respir. Med. Case Rep. 2015, 16, 146–147. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Urciuoli, B.; Starace, M.; Tosti, A.; Balestri, R. Yellow nail syndrome: Clinical experience in a series of 21 patients: 21 patients with the yellow nail syndrome. J. Dtsch. Dermatol. Ges. 2014, 12, 131–137. [Google Scholar] [CrossRef]
- Matys, J.; Grzech-Lesniak, K. Dental Aerosol as a Hazard Risk for Dental Workers. Materials 2020, 13, 5109. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, W.M. Solubilization of Metal Particles and Lung Toxicity. SM J. Environ. Toxicol. 2015, 1, 1002. [Google Scholar]
- Wu, X.; Apte, M.G.; Bennett, D.H. Indoor Particle Levels in Small- and Medium-Sized Commercial Buildings in California. Environ. Sci. Technol. 2012, 46, 12355–12363. [Google Scholar] [CrossRef]
- Colson, D.G. A Safe Protocol for Amalgam Removal. J. Environ. Public Health 2012, 2012, 517391–517394. [Google Scholar] [CrossRef]
- Occupational Exposure to Elemental Mercury in Dentistry. 2012. Available online: https://wedocs.unep.org/20.500.11822/31736 (accessed on 2 February 2023).
- World Dental Federation. Mercury Hygiene Guidance. 2007. Available online: https://preprod.fdiworlddental.org/mercury-hygiene-guidance (accessed on 3 February 2023).
- Canadian Centre for Occupational and Safety. OSH Answers Fact Sheets, Mercury. 2023. Available online: https://www.ccohs.ca/oshanswers/chemicals/chem_profiles/mercury.html (accessed on 7 February 2023).
- Communication from the Commission to the European Parliament and the Council on the Review of the Community Strategy Concerning Mercury. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52010DC0723 (accessed on 2 February 2023).
| Element | Atomic Mass | Density (G/Cm3) | Metal Type | Commonly Present in | ||
|---|---|---|---|---|---|---|
| Prostheses | Restorative Materials + Cements | Implants | ||||
| Calcium (Ca) | 40.08 | 1.54 | Alkaline Earth Metal | X | X | |
| Strontium (Sr) | 87.62 | 2.64 | Alkaline Earth Metal | X | ||
| Barium (Ba) | 137.33 | 3.62 | Alkaline Earth Metal | X | ||
| Magnesium (Mg) | 24.30 | 1.73 | Alkaline earth Metal | X | ||
| Gold (Au) | 196.97 | 19.32 | Transition Metal | X | X | |
| Palladium (Pd) | 106.42 | 12.02 | Transition Metal | X | X | |
| Platinum (Pt) | 195.08 | 21.45 | Transition Metal | X | ||
| Iridium (Ir) | 192.22 | 22.65 | Transition Metal | X | ||
| Ruthenium (Ru) | 101.07 | 12.48 | Transition Metal | X | ||
| Rhodium (Rh) | 102.91 | 12.41 | Transition Metal | X | ||
| Copper (Cu) | 63.55 | 8.92 | Transition Metal | X | X | |
| Titanium (Ti) | 47.87 | 4.51 | Transition Metal | X | X | X |
| Beryllium (Be) | 9.01 | 1.85 | Transition Metal | X | X | |
| Chromium (Cr) | 51.99 | 7.15 | Transition Metal | X | X | X |
| Iron (Fe) | 55.84 | 7.87 | Transition Metal | X | X | |
| Manganese (Mn) | 54.93 | 7.30 | Transition Metal | X | ||
| Molybdenum (Mo) | 95.95 | 10.20 | Transition Metal | X | ||
| Nickel (Ni) | 58.69 | 8.91 | Transition Metals | X | X | |
| Zinc (Zn) | 65.4 | 7.13 | Transition Metal | X | ||
| Mercury (Hg) | 200.59 | 13.53 | Transition Metal | X | ||
| Osmium (Os) | 190.2 | 22.57 | Transition Metal | X | ||
| Vanadium (V) | 50.94 | 6.0 | Transition Metal | X | ||
| Zirconium (Zr) | 91.22 | 6.52 | Transition Metal | X | X | |
| Niobium (Nb) | 92.90 | 8.57 | Transition Metal | X | ||
| Tantalum (Ta) | 180.94 | 16.4 | Transition metal | X | ||
| Silver (Ag) | 107.87 | 10.49 | Post-transition Metal | X | X | X |
| Aluminum (Al) | 26.98 | 2.70 | Post-transition Metal | X | X | X |
| Gallium (Ga) | 69.72 | 5.91 | Post-transition Metal | X | ||
| Indium (In) | 114.81 | 7.31 | Post-transition Metal | X | ||
| Bismuth (Bi) | 208.98 | 9.80 | Post-transition Metal | X | ||
| Tin (Sn) | 118.71 | 7.28 | Post-transition Metal | X | X | |
| Indium (In) | 114.82 | 7.31 | Post-transition Metal | X | X | |
| Element | Adverse Health Effects Associated with Metal Exposure in the Dental Environment | References |
|---|---|---|
| Hg | Risk of neurological and sensory symptoms (memory loss, fatigue, attention deficits, neurobehavioral problems, reduced cognitive flexibility, reduced psychomotor speed, and sleep problems) | G. Bjørklund et al., 2019 [45] |
| Risk of fertility issues, congenital deficits or abnormalities, and spontaneous abortion | G. Bjørklund et al., 2019 [46] | |
| Pathogenetic role in neurological disorders, particularly in pregnant women | G. Bjørklund et al., 2017 [47] | |
| Increased risk of tremor | J. Anglen et al., 2015 [48] | |
| Risk of reduction in systolic blood pressure | Goodrich et al., 2013 [49] | |
| Risk of reduction in Th1-type proinflammatory markers in serum | L. Björkman et al., 2012 [50] | |
| Risk of miscarriage | Lindbohm et al., 2007 [51] | |
| A deficit in psychomotor performance (hand steadiness) | A.C. Bittner et al., 1998 [52] | |
| Behavioral deficits, tension, fatigue, and confusion | D. Echeverria et al., 1995 [53] | |
| Risk of problems in visual memory, verbal memory, visuomotor coordination speed, visuomotor coordination and concentration, optical scanning, and motor speed | C.H. Ngim et al., 1992 [54] | |
| Risk of reproductive issues, renal function changes, allergies, immunotoxicological effects, and glioblastoma (brain cancer) | G. Bjørklund et al., 1991 [55] | |
| Risk of headaches, fatigue, malaise, weakness, irritability, depression, loss of memory, the feeling of hopelessness, tremor, decreased reflexes, loss of fine motor control, visual disturbances, lens and retina pigmentation, digestive disturbances, diarrhea, poor appetite, nausea, stomatitis, metallic taste, sore mouth, an increase in nasal secretion and saliva, burning tongue, red palms, and eczema | J.G. Bauer et al., 1985 [56] | |
| Risk of polyneuropathies, mild visuographic dysfunction, and symptom-distress | Shapiro et al., 1982 [57] | |
| Ni | Allergy | E.M. Warshaw et al., 2022 [58] |
| Allergy | T. Werfel et al., 2018 [59] | |
| Contact allergy | K. Wrangsjö et al., 2001 [60] | |
| Hand eczema and allergy | L.M. Wallenhammar et al., 2000 [61] | |
| Hand dermatitis | J.G. Hill et al., 1998 [62] | |
| Allergy, contact dermatitis, and hand eczema | T. Rustemeyer et al., 1996 [63] | |
| Risk of pneumoconiosis | M. Okamoto et al., 2017 [64] | |
| Ag | Risk of pneumoconiosis | M. Okamoto et al., 2017 [64] |
| Cu | Contact dermatitis | K. Wrangsjö et al., 2001 [60] |
| Cr | Toxic to RAW264.7 cells (monocyte/macrophage cell line) | W. Wang et al., 2020 [65] |
| Risk of pneumoconiosis | M. Okamoto et al., 2017 [64] | |
| Risk of lung disorders | A.I. Seldén et al., 1995 [66] | |
| Co | Allergy | L.M. Wallenhammar et al., 2000 [61] |
| Contact allergy | K. Wrangsjö et al., 2001 [60] | |
| Allergy, contact dermatitis, and hand eczema | T. Rustemeyer et al., 1996 [63] | |
| Toxic to RAW264.7 cells (monocyte/macrophage cell line) | W. Wang et al., 2020 [65] | |
| Risk of pneumoconiosis | M. Okamoto et al., 2017 [64] | |
| Risk of lung disorders | A.I. Seldén et al., 1995 [66] | |
| Pd | Contact dermatitis | T. Werfel et al., 2018 [59] |
| Allergy, contact dermatitis, and hand eczema | T. Rustemeyer et al., 1996 [63] | |
| Sn | Contact Dermatitis | T. Werfel et al., 2018 [59] |
| Al | Toxic to RAW264.7 cells (monocyte/macrophage cell line) | W. Wang et al., 2020 [65] |
| Risk of pneumoconiosis | M. Okamoto et al., 2017 [64] | |
| Mo | Toxic to RAW264.7 cells (monocyte/macrophage cell line) | W. Wang et al., 2020 [65] |
| Risk of lung disorders | A.I. Seldén et al., 1995 [66] | |
| In | Risk of pneumoconiosis | M. Okamoto et al., 2017 [64] |
| Ti | Risk of pneumoconiosis | M. Okamoto et al., 2017 [64] |
| Element | Potential Toxic Effects of Metal Elements on Humans | References |
|---|---|---|
| Hg | Risk of lung and eye irritation, diarrhea, rashes, and vomiting Risk of DNA damage Risk of reproductive problems (congenital disabilities miscarriages, and sperm damage in men) Risk of neurological disorders, learning disabilities, speech defects, memory loss, tremors and muscle incoordination, deafness, vision complications, and personality changes, Risk of paralysis, insanity, coma, and death Risk of congenital disabilities through a toxic effect on an embryo or fetus | [40,67,68,69,70,71] |
| Ni | Allergic reactions Risk of respiratory problems (asthma, lung embolisms, and respiratory failure) Risk of heart disorders Ni inhalation may cause: Risk of cancer | [40,68,69,72,73,74,75] |
| Ag | Contact with silver liquid may cause: Risk of allergic dermatitis, skin irritation, and argyria Risk of corneal injury Silver inhalation may cause: Risk of dizziness, headaches, drowsiness, confusion, and staggering Risk of respiratory issues Risk of unconsciousness, coma, and death Silver ingestion may cause: Risk of nausea and vomiting, diarrhea, and stomach discomfort Risk of narcosis, brain damage, and cardiac abnormalities | [40,67,68,69,76] |
| Cu | Risk of flu-like symptoms Risk of diarrhea, vomiting, eye irritation, dizziness, and oral mucosa irritation Risk of acute gastroenteritis Oral intake will cause hepatic and, Risk of hepatocellular degeneration, kidney disease, insomnia, anxiety, agitation, and necrosis Risk of Wilson’s disease (symptoms: lack of appetite, fatigue, jaundice, Kayser–Fleisher rings, speech impairment, difficulty in swallowing, uncontrolled poisoning, brain damage, demyelination, and hepatic cirrhosis) Risk of death | [40,68,69,77,78] |
| Cr | Cr ingestion may cause: Risk of nausea and vomiting, fever, diarrhea, gastrointestinal ulceration, vertigo, toxic nephritis, liver damage, and coma Cr (VI) inhalation or repeated skin contact may cause: Risk of allergic contact dermatitis and eczema, gingivitis, irritation of mucous membranes, bronchitis, and liver and kidney disease, Risk of respiratory issues (sinusitis, pneumonia, and lung cancer) Risk of chrome holes in the forearms, hands, fingers, and nose Risk of cancer Risk of death | [40,67,72,79,80,81] |
| Co | Risk of skin and respiratory issues allergic dermatitis Co-inhalation may cause: Risk of congestion, wheezing, asthma, respiratory irritation, lung function reduction, edema, pneumonia, fibrosis, and lung hemorrhage Risk of nausea, vomiting, diarrhea, renal congestion, and cardiac and liver disorders | [40,72,82,83,84] |
| Zn | Risk of nausea and vomiting, fatigue, anemia, neutropenia, stomach cramps, epigastric pain, copper deficiency, decrease in high-density lipoprotein (HDL) cholesterol, pancreatic complications, and impaired immune function | [40,68,69,85,86] |
| Mn | Risk of weakness, lethargy, decreased blood pressure, dullness, tremors, akathisia, dystonia, anxiety, motor disorders, and lack of facial expression Risk of neurological disorders and behavioral changes Risk of manganism Risk of mimicry of Parkinson’s disease Mn inhalation may cause: Risk of reproductive problems (sperm damage and loss of sex drive) and pneumonia | [40,68,69,72,87,88,89,90,91,92] |
| Pd | Risk of allergy or contact dermatitis Risk of eyes and oral mucosa irritation (stomatitis or mucositis) and oral lichen planus | [93,94] |
| Al | Risk of liver and kidney dysfunction Risk of lung damage and pulmonary fibrosis Risk of leukocytosis, osteomalacia, hypoparathyroidism, and colitis Risk of central nervous system damage Risk of amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinsonism dementia complex (ALS–PDC), and listlessness | [40,72,95,96] |
| Mo | Risk of headache, fatigue, weakness, appetite reduction, hypochromic microcytic anemia, and anorexia Risk of listlessness, chest pain, myalgia, and arthralgia, Risk of testicular atrophy Risk of copper deficiency | [40,68,69,97,98] |
| In | Risk of interstitial pneumonia and pulmonary and systemic diseases | [99,100] |
| Ti | Risk of intermittent coughing and respiratory diseases (bronchial asthma, chronic sinusitis, chronic bronchitis, chronic obstructive lung disease, chronic rhinitis, nasal septum deviation, nasal polyposis, recurrent pneumonia, recurrent pleural effusion, and acute pulmonary edema) Risk of yellow nail syndrome Risk of inflammation reactions and hypersensitivity Risk of systemic disease, cardiac failure, and death | [101,102,103,104,105] |
| Sn | Risk of interstitial pneumonia | [99] |
| Low-Risk Procedure | Moderate Risk Procedure | High-Risk Procedure |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.C.B.C.J.; França, R. Nanometals and Metal Ion Pollution from Dental Materials in Dental Environment. Micro 2023, 3, 471-483. https://doi.org/10.3390/micro3020031
Fernandes ACBCJ, França R. Nanometals and Metal Ion Pollution from Dental Materials in Dental Environment. Micro. 2023; 3(2):471-483. https://doi.org/10.3390/micro3020031
Chicago/Turabian StyleFernandes, Ana Carla B. C. J., and Rodrigo França. 2023. "Nanometals and Metal Ion Pollution from Dental Materials in Dental Environment" Micro 3, no. 2: 471-483. https://doi.org/10.3390/micro3020031
APA StyleFernandes, A. C. B. C. J., & França, R. (2023). Nanometals and Metal Ion Pollution from Dental Materials in Dental Environment. Micro, 3(2), 471-483. https://doi.org/10.3390/micro3020031







