Abstract
The dental environment is being polluted with metals from dental materials in many ways, mainly due to aerosol-generating procedures; this could affect the long-term well-being of dentists, dental students, and dental personnel. The current dental pollution incorporates metallic nanoparticles, which are highly reactive and quickly become airborne, especially those particles that become unbound in the bulk composition. In addition, liquid mercury or mercury vapors may be released from dental amalgam, causing concerns in the dental community. In our study, we reviewed the behavior of metallic elements present in dental materials, their routes of exposure, and their potentially toxic effects on the dental team. This review found that skin and lung disorders are the most harmful effects of metallic exposure for dentists, dental students, and dental personnel. Therefore, chronic exposure to low concentrations of metals in the dental environment, especially in nanosized forms, should be further investigated to improve the environmental matrix, material choice, and safety protocols.
1. Introduction
The use of restorative materials to replace missing dental structures has been the base of dental care since its beginnings [1]. Despite the recent development of new biomaterials, such as composites and ceramics, the old metallic restorative materials remain used in current dental practices. One reason is that metallic elements confer excellent mechanical properties and durability for fillings, crowns, bridges, and dental implants [2,3,4]. These metallic restorations are presented mainly as alloys containing several metals, including mercury, silver, tin, copper, gold, nickel, zinc, aluminum, chromium, cobalt, iron, manganese, titanium, palladium, platinum, iridium, ruthenium, beryllium, gallium, indium, molybdenum, beryllium, lithium, strontium, barium, bismuth, and zirconium, as well as rare metals, such as rhodium and osmium [3,5,6,7,8,9,10], and promising metals such as niobium [11]. The examples of metallic components in dental materials are summarized in Table 1.

Table 1.
Metallic elements in dental materials.
With advances in nanotechnology, the risk of toxicity and metal absorption from dental materials has increased. For example, nanometals ranging from 5 to 260 nm were incorporated into dental materials to improve physicochemical properties and antibacterial purposes, but the reactivity of the metal particulates also increased [4,12,13]. Another technological advance was the introduction of computer-assisted design and computer-assisted manufacturing (CAD/CAM) in dental offices. This technology allows ceramic restorations to be milled chairside in a same-day visit. Even though there is no available data to prove the toxicity, it is possible to assume that the presence of airborne nano and microparticles originating from the CAD/CAM milling could also aggravate the air quality of the dental environment.
Metal ions from restorations, cements, dental prostheses, implants, and dental appliances may leach into the patient’s saliva due to material degradation in the oral cavity [9,14,15,16,17]. However, the exposure to metals is even higher for dentists, dental students, dental technicians, and dental assistants than for patients [18]. Dental professionals and students are constantly exposed to aerosols, vapors, and particulates containing metals during dental procedures such as dental materials manipulation, removal, polishing, finishing, trituration, condensation, scaling, and in the adjustments of dental fillings and cement [19,20,21], prostheses [22], and dental appliances [23,24,25].
The quality of the dentistry environment is affected when nanoscale metal particles or metal vapors (e.g., mercury) surround the air and surfaces of dental spaces and laboratories [26,27]. Furthermore, the toxicological reliability of metals in dental materials is unclear, especially for their potentially harmful effects on dental professionals in long-term exposure. Therefore, based on the current literature, this paper reviewed the metallic elements in dental materials and the toxicological potential for dentists, dental students, and dental personnel.
2. Toxicological Potential of Metallic Elements Present in Dental Materials
2.1. Routes for the Spread of Metals in Dentistry
How metallic ions are spread in the dental field can be divided into the following: aerosol, droplets, splatter, and vapor. Slow or high-speed dental drills and ultrasonic scaling dental equipment generate metal spreading in the dental environment [28]. Dental drills are used with diamond or carbide burs for dental amalgam removal, adjustments of restorations, prostheses, dental implants, orthodontic appliances, and diamond disks or points for polishing and finishing restorations and scaling, using an ultrasonic scaler with a tip coupled for ultrasonic scaling. These dental treatment procedures release nanometals and micrometals in a suspension of air–water aerosols containing particles ≤ 5 µm in diameter, droplets in the 5–100 µm range, and splatter with sizes ≥ 100 µm into the dental environment [29,30,31,32,33].
Among the metals listed in Table 1, mercury is a unique liquid metal able to volatilize at room temperature. This particular characteristic attracts attention to the risk of exposure to materials containing mercury. In dentistry, dental amalgam restoration comprises approximately 50% wt. of elemental mercury [34,35]. When liquid mercury (Hg) is mixed with a metal alloy, the Hg reacts with intermetallic Ag3Sn (γ-phase), forming Ag2Hg3 (γ1-phase), Sn8Hg (γ2-phase), and the unreacted alloy Ag3Sn (γ-phase). The γ-phase and γ1-phase are mechanically strong, but the γ2-phase is soft and unstable, which leads to metal leaching. [36]. Therefore, dental amalgam degradation may be attributed to the γ2-phase. Mercury vapor may be released during mixing, placement, condensation, polishing, finishing, and dental amalgam removal.
According to Warnick D. et al., 2019 [37], amalgam particulates still release significant amounts of mercury vapor after removing the amalgam filling. The literature also shows remaining mercury vaporization in dental instruments, opened capsules, work surfaces and tools, and amalgam residues after procedures with dental amalgam. Bulk mercury may spill from defective amalgamators and mercury dispensers or leak from amalgam capsules [21,26,38].
2.2. Routes of Exposure to Metals in the Dental Environment
The three primary routes of occupational and nonoccupational exposure to metals in dentistry include (1) inhalation, (2) ingestion, and (3) dermal/ocular exposure. Figure 1 illustrates the possible routes of exposure, pathways to organs, and primary target organs of the human body for metallic elements in the dental environment.
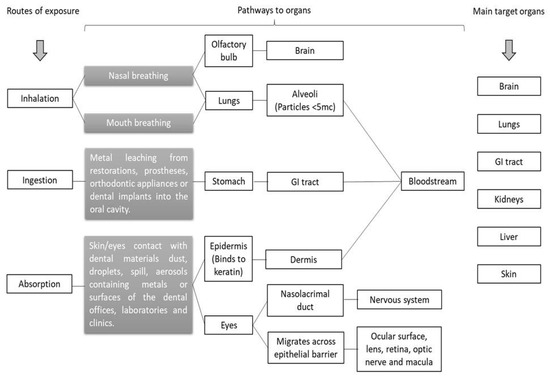
Figure 1.
Routes of exposure, pathways to organs, and primary target organs of the human body for metallic elements in the dental materials and dental environment.
For dentists, dental students, and dental personnel, inhalation and dermal/ocular absorption are the main routes of exposure to metallic elements in the dental environment. Ingestion is a route of exposure to metallic elements that often occurs in dental patients. The ingestion of metal ions happens due to dental material degradation in the oral cavity [9,14,15,16,17].
After inhalation and the dermal/ocular absorption pathway, metal ions reach several organs. Some metal elements (e.g., Cu, Cr, Fe, Mn, Mg, and Zn) are essential for the physiological functions of the human body, while others (e.g., Li, Cr, Sr, Ag, Ba, and Hg) are nonessential [39]. The metallic elements are nonbiodegradable. When the metal ions are in excess, they are widely distributed in different organs, and bioaccumulation in the human system is a concern.
Metals have a long half-life due to the chemical properties of the elements, especially nonessential elements. In general, metals in excess may be trapped in vital organs by covalent chemical bonds with organic groups, which generate intracellular granules in an insoluble form to be excreted through the organism’s feces or for long-term storage that may cause toxic effects [40]. For example, mercury binds to sulphydryl protein groups in the central nervous system (CNS).
2.3. Potential Metal Toxicity in the Dental Environment
A dental environment can include a dental clinic, preclinical teaching laboratories, dental laboratories, and hospital dental departments. The metal exposure in these environments is affected by the type of dental procedure and properties of the environment matrix (air quality and room temperature). However, the properties of the metallic ions (pH, redox potential, and the possible formation of complex metallic compounds) are essential factors in determining the potential toxicity [41]. In addition, other important factors can affect the exposure–response relationship, such as the route and time of exposure, biological factors, skin permeability, and the diet of exposed individuals [42,43,44]. Therefore, dentists, dental students, and dental personnel should evaluate the already reported toxic effects of metal exposure in the dental environment (Table 2) and consider the potential risks of the metallic elements to which they are exposed (Table 3).

Table 2.
Summary of adverse health effects on dentists, dental students, and dental personnel associated with metal exposure.

Table 3.
The potentially toxic effects of metallic elements on humans.
Notice the potential of metal spreading increases in dental procedures involving high-speed handpiece or ultrasound scaler usage. According to J. Matys et al., 2020 [106], these instruments generate intensive dental aerosol during dental procedures. In dental clinics and preclinical laboratories, where a group of dentists or students perform dental practices in the same room, the risk of metal exposure should be evaluated as the metal spread will be more intense in this environment. Thus, a specific room for dental procedures involving dental amalgam is suggested to prevent mercury vapor spread.
Metallic ion mobility, related to dental material composition, is particularly concerning from a potential toxicological point of view. Unbound nanoparticles in the bulk composition are more reactive and can become quickly airborne [107]. Furthermore, airborne metal particles are highly insoluble at a physiological pH, which can increase the risk of lung toxicity for metal dust from dental materials in dentists, dental students, and dental personnel [108].
The independent experiments of Checchi L et al., 2005 [108], using bicarbonate dust in variable dimensions (~1–300 µm), showed that a certified personal respirator could be more effective than high-quality surgical masks in dental settings. Currently, the release of nanomaterials into the dental market has increased. For this reason, a study in the U.S.A. that monitored indoor particulates in dental offices in California found that 67% of the particulates had an average particle size of <100 nm in size and there were 37 % of PM0.3–PM10 [109].
More research about barrier efficiency against ultrafine metal particulates is necessary to protect dentists, dental students, and dental personnel in dental clinics and preclinical laboratories. Recently, Liu et al., 2019 [27] found that almost all of the particles produced by tooth drilling and grinding were ≤1 μm. The central vacuum systems and protective surgical masks have limited efficacy against these suspended particulate matters.
Thus, dental procedures should not be underestimated in terms of the risk of metal spreading into the environment and adverse effects on human health. For better conduct of metal spread prevention, dental clinics, and preclinical laboratories should define a guideline detailing a risk procedure for metal spread, a specific treatment area (enclosed or nonenclosed operatory), the necessity of essential equipment (use or nonuse air filtration and evacuation), and the type of personal protective equipment (PPE) for the operator, patient, and others present in the same room, as suggested in Table 4.

Table 4.
Guideline of risk of procedure for metal spread.
3. General Recommendations
Several measures have been proposed to reduce and eliminate occupational and nonoccupational hazards in the form of exposure to metallic ions from dental materials in dentists to dental students and dental personnel [37,110,111,112,113]. The main measures could be listed as the following:
3.1. Air Quality Precautions
- Use adequately designed ventilation systems, including local exhaust ventilation.
- Use air filtration equipment for dental procedures involving dental amalgam and make sure the filters are appropriate.
- Perform air monitoring periodically to ensure that the occupational (dental workers) and nonoccupational (dental students) exposure limit is not exceeded.
3.2. Good Hygiene Practices
- Clean hands vigorously to create friction in an appropriate sink. Use soap for your hands and a nailbrush to clean your fingernails. Afterward, rinse well to remove all of the soap and dry your hands thoroughly using a paper towel.
- Hand hygiene should be performed immediately before starting a clinical session, before putting on gloves, and following the removal of gloves. Hand hygiene should also be performed at anytime hands are contaminated with visible metal dust or liquid mercury.
- Wearing jewelry is not recommended as it can chemically bond with other metals released during dental procedures.
- Observe periodically if the working team or dental students follow good hygiene practices for quality control.
3.3. Personal Protective Equipment (PPE)
- Dentists, dental students, dental personnel, and patients should wear protective clothing (ideally an impermeable full-body covering for procedures with dental amalgam).
- Use a dental dam sealed correctly in the patient’s mouth with a saliva ejector to remove contaminated saliva or liquid debris from the oral cavity.
- The protective mask or respirator should be selected according to the type of metal involved in the dental material or procedure, and as guided by the local, national, or international regulatory agency of occupational and nonoccupational health and safety recommendations. Each agency has its guidelines recommending different types of masks or respirators. The barrier choice should be based on the air monitoring results of the dental clinic or laboratory, respecting the threshold limit value (TLV), permissible exposure limit (PEL), recommended exposure limit (REL), minimal risk level (MRL), or reference concentration (RfC) of the agency. The most common agencies used in North America are the National Institute for Occupational Safety and Health (NIOSH), the Occupational Safety and Health Administration (OSHA), the American Conference of Governmental Industrial Hygienists (ACGIH), the United States Environmental Protection Agency, and the California Environmental Protection Agency. The mask or respirator should cover the mouth and nose completely.
- Regarding protective eyewear, prescription safety glasses, safety glasses over corrective lenses, or loupes with side shields should be used during all preclinical and clinical procedures.
- Bouffant, impermeable dedicated footwear (covering the entire foot) and a face shield are also recommended.
- The patient’s skin and clothing should be protected, providing a whole-body, impermeable barrier, and a complete head/face/neck barrier under/around the dam should also be used.
- Use high-volume suction and continually add water spray to the site where the amalgam is removed.
3.4. Health and Educational Surveillance
- Perform medical monitoring of metals levels in the working team or dental students.
- Periodically observe if the working team or dental students are following the local quality control guidelines.
- Review the local guidelines periodically or when updated.
3.5. Substitution with Less Harmful Products
- It is reasonable to choose metal-free materials when appropriate. The use of alternative materials to dental amalgams has been encouraged in Europe to reduce environmental and human exposure to mercury [113]. Composite resins and metal-free glass ionomer cement are excellent alternative materials for dental restorations.
- Metal-free crowns (porcelain-based ceramic, quartz, glass, or resin, through zirconium and lithium disilicate) are suggested instead of metallic crowns (made of gold, platinum, copper, nickel, or chromium) when indicated.
- The cobalt–chromium (Co–Cr) alloy is indicated for fabricating metallic frameworks of removable partial dentures instead of Ni–Cr alloy to reduce allergic reactions. The additional use of allergenic metals should be reduced if possible.
3.6. Cleaning Dental Instruments and Surfaces
- For dental procedures involving dental amalgam, use mercury decontaminant to clean instruments, countertops, and surfaces after the clinical session.
- For dental procedures involving other metal types, clean instruments with water, soap, and a brush.
- Clean spills of mercury using commercial mercury spill clean-up kits. Afterward, check mercury vapor levels in the dental operatory.
- Clean handpieces according to the manufacturer’s instructions. For handpieces contaminated with mercury, it is recommended to wipe the handpieces with mercury decontaminant before starting the manufacturer’s instructions.
The literature outlines occupational adverse effects of metal exposure for dental workers, including dentists and dental personnel. However, there is a lack of discussion regarding dental student exposures to hazardous metals. Dental students perform daily dental procedures in preclinical laboratories and dental clinics. Extra hours of their regular practices are common in preclinical laboratories. However, the limits of exposure to metallic elements for this population have not been established since they are not considered dental workers.
This review was carried out with a critical eye to expand the safety and health of the dental community, including dental students. Therefore, for dentists, dental students, and dental personnel, we highlight the importance of following the procedural risk guidelines to reduce the spread of metals and also general recommendations for clinical sections.
4. Conclusions
Metallic pollution from dental materials in dental environments has become more prevalent and dangerous with the advance of nanotechnology. Nanometals increase the risk of toxicity and absorption. This review found that skin and lung disorders are the most harmful effects associated with exposure to metallic elements by dentists, dental students, and dental personnel. Therefore, guidelines for reducing risk in dental procedures are encouraged to reduce the daily metal intake through inhalation and dermal/ocular absorption in dentistry.
Author Contributions
Conceptualization, A.C.B.C.J.F. and R.F.; methodology, A.C.B.C.J.F.; formal analysis, A.C.B.C.J.F. and R.F.; investigation, A.C.B.C.J.F.; resources, R.F.; writing—original draft preparation, A.C.B.C.J.F.; writing—review and editing A.C.B.C.J.F. and R.F.; project administration, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sakaguchi, R.; Ferracane, J.; Powers, J. Craig’s Restorative Dental Materials, 14th ed.; Mosby: Maryland Heights, MO, USA, 2019; p. 352. [Google Scholar]
- Anusavice, K. Phillips’ Science of Dental Materials, 12th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2013. [Google Scholar]
- Sacher, E.; Franc, R. Dental Biomaterials. In World Scientific Series: From Biomaterials towards Medical Devices; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2019; Volume 2. [Google Scholar]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Givan, D.A. Precious metals in dentistry. Dent. Clin. N. Am. 2007, 51, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Panpisut, P.; Liaqat, S.; Zacharaki, E.; Xia, W.; Petridis, H.; Young, A.M. Dental Composites with Calcium/Strontium Phosphates and Polylysine. PLoS ONE 2016, 11, e0164653. [Google Scholar] [CrossRef]
- Zhou, M.; Drummond, J.L.; Hanley, L. Barium and strontium leaching from aged glass particle/resin matrix dental composites. Dent. Mater. 2005, 21, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Borg, J.; Damidot, D.; Salvadori, E.; Pilecki, P.; Zaslansky, P.; Darvell, B.W. Colour and chemical stability of bismuth oxide in dental materials with solutions used in routine clinical practice. PLoS ONE 2020, 15, e0240634. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Grech, J.; Antunes, E. Zirconia in dental prosthetics: A literature review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Balbinot, G.S.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Niobium silicate particles as bioactive fillers for composite resins. Dent. Mater 2020, 36, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Fidalgo, T.K.S.; da Costa, L.P.; Maia, L.C.; Balan, L.; Anselme, K.; Ploux, L.; Thiré, R.M.S.M. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J. Dent. 2018, 69, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Santos, H.E.S.; Garbossa, M.; Santos, C. Mechanical Properties of Zirconia Y-TZP Core Veneered for Dentistry Applications. J. Ceram. Sci. Technol. 2017, 8, 525–530. [Google Scholar]
- Berdouses, E.; Vaidyanathan, T.K.; Dastane, A.; Weisel, C.; Houpt, M.; Shey, Z. Mercury Release from Dental Amalgams: An in vitro Study Under Controlled Chewing and Brushing in an Artificial Mouth. J. Dent. Res. 1995, 74, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Mocnik, P.; Kosec, T.; Kovac, J.; Bizjak, M. The effect of pH, fluoride and tribocorrosion on the surface properties of dental archwires. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 682–689. [Google Scholar] [CrossRef]
- Bajsman, A.; Vukovic, A.; Zukic, S.; Jakupovic, S. Dental Amalgam: Do We Have Enough Proves “pro” et “contra”. Mater. Socio-Med. 2010, 22, 222. [Google Scholar]
- Siddharth, R.; Gautam, R.; Chand, P.; Agrawal, K.K.; Singh, R.D.; Singh, B.P. Quantitative analysis of leaching of different metals in human saliva from dental casting alloys: An in vivo study. J. Indian Prosthodont. Soc. 2015, 15, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Ozcan, M.; Maleki Dizaj, S.; Sharifi, S.; Al-Haj Husain, N.; Eftekhari, A.; Ahmadian, E. A review on potential toxicity of dental material and screening their biocompatibility. Toxicol. Mech. Methods 2019, 29, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.G.; Summitt, J.B.; Chung, A.K.; Osborne, J.W. Amalgam at the new millennium. J. Am. Dent. Assoc. 1998, 129, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Kralj, P.; Veber, M.; Sinagra, E. Characterization and analyses of acid-extractable and leached trace elements in dental cements. Int. Endod. J. 2012, 45, 737–743. [Google Scholar] [CrossRef]
- Warwick, R.; O’Connor, A.; Lamey, B. Mercury vapour exposure during dental student training in amalgam removal. J. Occup. Med. Toxicol. 2013, 8, 27. [Google Scholar] [CrossRef]
- Keinan, D.; Mass, E.; Zilberman, U. Absorption of nickel, chromium, and iron by the root surface of primary molars covered with stainless steel crowns. Int. J. Dent. 2010, 2010, 326124. [Google Scholar] [CrossRef]
- Wendl, B.; Wiltsche, H.; Lankmayr, E.; Winsauer, H.; Walter, A.; Muchitsch, A.; Jakse, N.; Wendl, M.; Wendl, T. Metal release profiles of orthodontic bands, brackets, and wires: An in vitro study. J. Orofac. Orthop. 2017, 78, 494–503. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Chojnacka, K.; Wozniak, B.; Downarowicz, P. Release of metal ions from orthodontic appliances: An in vitro study. Biol. Trace Elem. Res. 2012, 146, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Gopikrishnan, G.; Melath, A.; Ajith, V.V.; Mathews, N.B. A Comparative Study of Bio Degradation of Various Orthodontic Arch Wires: An In Vitro Study. J. Int. Oral Health 2015, 7, 12–17. [Google Scholar]
- Liu, M.H.; Chen, C.T.; Chuang, L.C.; Lin, W.M.; Wan, G.H. Removal efficiency of central vacuum system and protective masks to suspended particles from dental treatment. PLoS ONE 2019, 14, e0225644. [Google Scholar] [CrossRef] [PubMed]
- Innes, N.; Johnson, I.G.; Al-Yaseen, W.; Harris, R.; Jones, R.; Kc, S.; McGregor, S.; Robertson, M.; Wade, W.G.; Gallagher, J.E. A systematic review of droplet and aerosol generation in dentistry. J. Dent. 2021, 105, 103556. [Google Scholar] [CrossRef]
- Han, P.; Li, H.; Walsh, L.J.; Ivanovski, S. Splatters and Aerosols Contamination in Dental Aerosol Generating Procedures. Appl. Sci. 2021, 11, 1914. [Google Scholar] [CrossRef]
- Shiu, E.Y.C.; Leung, N.H.L.; Cowling, B.J. Controversy around airborne versus droplet transmission of respiratory viruses: Implication for infection prevention. Curr. Opin. Infect. Dis. 2019, 32, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Grantham, M.; Pantelic, J.; Bueno de Mesquita, P.J.; Albert, B.; Liu, F.; Ehrman, S.; Milton, D.K.; EMIT Consortium. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 2018, 115, 1081–1086. [Google Scholar] [CrossRef]
- Leggat, P.A.; Kedjarune, U. Bacterial aerosols in the dental clinic: A review. Int. Dent. J. 2001, 51, 39–44. [Google Scholar] [CrossRef]
- James, R.; Mani, A.K. Dental Aerosols: A Silent Hazard in Dentistry. Int. J. Sci. Res. 2016, 5, 1761–1763. [Google Scholar]
- Estrich, C.G.; Lipman, R.D.; Araujo, M.W.B. Dental amalgam restorations in nationally representative sample of US population aged >/=15 years: NHANES 2011–2016. J. Public Health Dent. 2021, 81, 327–330. [Google Scholar] [CrossRef]
- Adabo, G.L. Mercury Toxicity. In Dental Biomaterials; World Scientific: Singapore, 2019; pp. 125–145. [Google Scholar]
- Davies, R.A.; Ardalan, S.; Mu, W.-H.; Tian, K.; Farsaikiya, F.; Darvell, B.W.; Chass, G.A. Geometric, electronic and elastic properties of dental silver amalgam γ-(Ag3Sn), γ1-(Ag2Hg3), γ2-(Sn8Hg) phases, comparison of experiment and theory. Intermetallics 2010, 18, 756–760. [Google Scholar] [CrossRef]
- Warwick, D.; Young, M.; Palmer, J.; Ermel, R.W. Mercury vapor volatilization from particulate generated from dental amalgam removal with a high-speed dental drill-a significant source of exposure. J. Occup. Med. Toxicol. 2019, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, N.; Bettiol, S.S.; Isham, A.; Hoang, H.; Crocombe, L.A. A Review of Mercury Exposure and Health of Dental Personnel. Saf. Health Work 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- van Gestel, C.A.M. Environmental Toxicology; Vrije Universiteit Amsterdam: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- Li, L.; Li, D. Inter-Individual Variability and Non-linear Dose-Response Relationship in Assessing Human Health Impact From Chemicals in LCA: Addressing Uncertainties in Exposure and Toxicological Susceptibility. Front. Sustain. 2021, 2, 648138. [Google Scholar] [CrossRef]
- Hostynek, J.J. Factors determining percutaneous metal absorption. Food Chem. Toxicol. 2003, 41, 327–345. [Google Scholar] [CrossRef]
- Bjørklund, G.; Hilt, B.; Dadar, M.; Lindh, U.; Aaseth, J. Neurotoxic effects of mercury exposure in dental personnel. Basic Clin. Pharmacol. Toxicol. 2019, 124, 568–574. [Google Scholar] [CrossRef]
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M.; Aaseth, J. Mercury exposure and its effects on fertility and pregnancy outcome. Basic Clin. Pharmacol. Toxicol. 2019, 125, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Bengtsson, U.; Chirumbolo, S.; Kern, J.K. Concerns about environmental mercury toxicity: Do we forget something else? Environ. Res. 2017, 152, 514–516. [Google Scholar] [CrossRef]
- Anglen, J.; Gruninger, S.E.; Chou, H.; Weuve, J.; Turyk, M.E.; Freels, S.; Stayner, L.T. Occupational mercury exposure in association with prevalence of multiple sclerosis and tremor among US dentists. J. Am. Dent. Assoc. 2015, 146, 659–668.e1. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.M.; Wang, Y.; Gillespie, B.; Werner, R.; Franzblau, A.; Basu, N. Methylmercury and elemental mercury differentially associate with blood pressure among dental professionals. Int. J. Hyg. Environ. Health 2013, 216, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Björkman, L.; Brokstad, K.A.; Moen, K.; Jonsson, R. Minor changes in serum levels of cytokines after removal of amalgam restorations. Toxicol. Lett. 2012, 211, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lindbohm, M.; Ylöstalo, P.; Sallmén, M.; Henriks-Eckerman, M.; Nurminen, T.; Forss, H.; Taskinen, H. Occupational exposure in dentistry and miscarriage. Occup. Environ. Med. 2007, 64, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bittner, A.C.; Echeverria, D.; Woods, J.S.; Aposhian, H.V.; Naleway, C.; Martin, M.D.; Mahurin, R.K.; Heyer, N.J.; Cianciola, M. Behavioral effects of low-level exposure to Hg0 among dental professionals: A cross-study evaluation of psychomotor effects. Neurotoxicol. Teratol. 1998, 20, 429–439. [Google Scholar] [CrossRef]
- Echeverria, D.; Heyer, N.J.; Martin, M.D.; Naleway, C.A.; Woods, J.S.; Bittner, A.C. Behavioral effects of low-level exposure to Hg ∘ among dentists. Neurotoxicol. Teratol. 1995, 17, 161–168. [Google Scholar] [CrossRef]
- Ngim, C.H.; Foo, S.C.; Boey, K.W. Chronic neurobehavioural effects of elemental. Br. J. Ind. Med. 1992, 49, 782–790. [Google Scholar]
- Bjørklund, G. Mercury in the dental office. Risk evaluation of the occupational environment in dental care. Tidsskr Nor Laegeforen 1991, 111, 948–951. [Google Scholar]
- Bauer, J.G. Action of mercury in dental exposures to mercury. Oper. Dent. 1985, 10, 104–113. [Google Scholar]
- Shapiro, I.M.; Cornblath, D.R.; Sumner, A.J.; Uzzell, B.; Spitz, L.K.; Ship, I.I.; Bloch, P. Neurophysiological and neuropsychological function in mercury-exposed dentists. Lancet 1982, 319, 1147–1150. [Google Scholar] [CrossRef]
- Warshaw, E.M.; Ruggiero, J.L.; Atwater, A.R.; DeKoven, J.G.; Zug, K.A.; Reeder, M.J.; Silverberg, J.I.; Taylor, J.S.; Pratt, M.D.; Maibach, H.I.; et al. Occupational Contact Dermatitis in Dental Personnel: A Retrospective Analysis of the North American Contact Dermatitis Group Data, 2001 to 2018. Dermatitis 2022, 33, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Heratizadeh, A.; Werfel, T.; Schubert, S.; Geier, J.; Ludwig, A.; Bircher, A.; Köhler, A. Contact sensitization in dental technicians with occupational contact dermatitis. Data of the Information Network of Departments of Dermatology (IVDK) 2001–2015. Contact Dermat. 2018, 78, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Wrangsjö, K.; Swartling, C.; Meding, B. Occupational dermatitis in dental personnel: Contact dermatitis with special reference to (meth)acrylates in 174 patients: Occupational dermatitis in dental personnel. Contact Dermat. 2001, 45, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wallenhammar, L.M.; Örtengren, U.; Andreasson, H.; Barregård, L.; Björkner, B.; Karlsson, S.; Wrangsjö, K.; Meding, B. Contact allergy and hand eczema in Swedish dentists. Contact Dermat. 2000, 43, 192–199. [Google Scholar] [CrossRef]
- Hill, J.G.; Grimwood, R.E.; Hermesch, C.B.; Marks, J.G. Prevalence of occupationally related hand dermatitis in dental workers. J. Am. Dent. Assoc. 1998, 129, 212–217. [Google Scholar] [CrossRef]
- Rustemeyer, T.; Frosch, P.J. Occupational skin diseases in dental laboratory technicians: (I). Clinical picture and causative factors. Contact Dermat. 1996, 34, 125–133. [Google Scholar] [CrossRef]
- Okamoto, M.; Tominaga, M.; Shimizu, S.; Yano, C.; Masuda, K.; Nakamura, M.; Zaizen, Y.; Nouno, T.; Sakamoto, S.; Yokoyama, M.; et al. Dental Technicians’ Pneumoconiosis. Intern. Med. 2017, 56, 3323–3326. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Luo, X.; Zhang, K.; Cao, N.; Liu, K.; Li, X.; Zhu, Y. Cytotoxic effects of dental prosthesis grinding dust on RAW264.7 cells. Sci. Rep. 2020, 10, 14364. [Google Scholar] [CrossRef]
- Seldén, A.I.; Persson, B.; Bornberger-Dankvardt, S.I.; Winström, L.E.; Bodin, L.S. Exposure to cobalt chromium dust and lung disorders in dental technicians. Thorax 1995, 50, 769–772. [Google Scholar] [CrossRef]
- Martin, S.; Wendy, G. Human Health Effects of Heavy Metals. Environ. Sci. Technol. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Kaye, G.W.C.; Laby, T.H. Tables of Physical and Chemical Constants, 15th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1986. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Hoboken, NJ, USA, 2011. [Google Scholar]
- ATSDR. Toxicological Profile for Mercury; ATSDR: Atlanta, GA, USA, 1999.
- Li, R.; Wu, H.; Ding, J.; Fu, W.; Gan, L.; Li, Y. Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Sci. Rep. 2017, 7, 46545. [Google Scholar] [CrossRef] [PubMed]
- WHO. Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications, European Series; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2000; p. 91. [Google Scholar]
- ATSDR. ATSDR Toxicological Profile for Nickel; ATSDR: Atlanta, GA, USA, 2017.
- Denkhaus, E.; Salnikow, K. Nickel essentiality, toxicity, and carcinogenicity. Crit. Rev. Oncol./Hematol. 2002, 42, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Low nickel diet in dermatology. Indian J. Dermatol. 2013, 58, 240. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Silver; ATSDR: Atlanta, GA, USA, 1990.
- ATSDR. Toxicological Profile for Copper; ATSDR: Atlanta, GA, USA, 2004.
- Tsafrir, J. Copper Toxicity: A Common Cause of Psychiatric Symptoms. Available online: https://www.psychologytoday.com/intl/blog/holistic-psychiatry/201709/copper-toxicity-common-cause-psychiatric-symptoms (accessed on 19 July 2022).
- Barnhart, J. Occurrences, Uses, and Properties of Chromium. Regul. Toxicol. Pharmacol. 1997, 26, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Chromium; ATSDR: Atlanta, GA, USA, 2012.
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Straeten, C.V.D.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Lauwerys, R.; Lison, D. Health risks associated with cobalt exposure—An overview. Sci. Total Environ. 1994, 150, 1–6. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile of Cobalt; ATSDR: Atlanta, GA, USA, 2004.
- ATSDR. Toxicological Profile for Zinc; ATSDR: Atlanta, GA, USA, 2005.
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Dobson, A.W.; Erikson, K.M.; Aschner, M. Manganese Neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1012, 115–128. [Google Scholar] [CrossRef]
- Marcus, J.B. Chapter 7—Vitamin and Mineral Basics: The ABCs of Healthy Foods and Beverages, Including Phytonutrients and Functional Foods: Healthy Vitamin and Mineral Choices, Roles and Applications in Nutrition, Food Science and the Culinary Arts. In Culinary Nutrition; Marcus, J.B., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 279–331. [Google Scholar]
- Iregren, A. Psychological test performance in foundry workers exposed to low levels of manganese. Neurotoxicol. Teratol. 1990, 12, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Roels, H.A.; Ghyselen, P.; Buchet, J.P.; Ceulemans, E.; Lauwerys, R.R. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br. J. Ind. Med. 1992, 49, 25–34. [Google Scholar] [CrossRef]
- Mergler, D.; Huel, G.; Bowler, R.; Iregren, A.; Bélanger, S.; Baldwin, M.; Tardif, R.; Smargiassi, A.; Martin, L. Nervous system dysfunction among workers with long-term exposure to manganese. Environ. Res. 1994, 64, 151–180. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Manganese; ATSDR: Atlanta, GA, USA, 2012.
- Melber, C.; Keller, D.; Mangelsdorf, I.; International Programme on Chemical Safety. Palladium; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Kielhorn, J.; Melber, C.; Keller, D.; Mangelsdorf, I. Palladium—A review of exposure and effects to human health. Int. J. Hyg. Environ. Health 2002, 205, 417–432. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Aluminium; ATSDR: Atlanta, GA, USA, 2008.
- Shaw, C.A.; Tomljenovic, L. Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol. Res. 2013, 56, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Momcilović, B. A case report of acute human molybdenum toxicity from a dietary molybdenum supplement—A new member of the “Lucor metallicum” family. Arh. Hig. Rada Toksikol. 1999, 50, 289–297. [Google Scholar]
- ATSDR. ATSDR Toxicological Profile for Molybdenum; ATSDR: Atlanta, GA, USA, 2017.
- Nakano, M.; Omae, K.; Tanaka, A.; Hirata, M.; Michikawa, T.; Kikuchi, Y.; Yoshioka, N.; Nishiwaki, Y.; Chonan, T. Causal Relationship between Indium Compound Inhalation and Effects on the Lungs. J. Occup. Health 2009, 51, 513–521. [Google Scholar] [CrossRef]
- Hoet, P.; De Graef, E.; Swennen, B.; Seminck, T.; Yakoub, Y.; Deumer, G.; Haufroid, V.; Liso, D. Occupational exposure to indium: What does biomonitoring tell us? Toxicol. Lett. 2012, 213, 122–128. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium allergy: Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef]
- Decker, A.; Daly, D.; Scher, R.K. Role of Titanium in the Development of Yellow Nail Syndrome. Ski. Appendage Disord. 2015, 1, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Ataya, A.; Kline, K.P.; Cope, J.; Alnuaimat, H. Titanium exposure and yellow nail syndrome. Respir. Med. Case Rep. 2015, 16, 146–147. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Urciuoli, B.; Starace, M.; Tosti, A.; Balestri, R. Yellow nail syndrome: Clinical experience in a series of 21 patients: 21 patients with the yellow nail syndrome. J. Dtsch. Dermatol. Ges. 2014, 12, 131–137. [Google Scholar] [CrossRef]
- Matys, J.; Grzech-Lesniak, K. Dental Aerosol as a Hazard Risk for Dental Workers. Materials 2020, 13, 5109. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, W.M. Solubilization of Metal Particles and Lung Toxicity. SM J. Environ. Toxicol. 2015, 1, 1002. [Google Scholar]
- Wu, X.; Apte, M.G.; Bennett, D.H. Indoor Particle Levels in Small- and Medium-Sized Commercial Buildings in California. Environ. Sci. Technol. 2012, 46, 12355–12363. [Google Scholar] [CrossRef]
- Colson, D.G. A Safe Protocol for Amalgam Removal. J. Environ. Public Health 2012, 2012, 517391–517394. [Google Scholar] [CrossRef]
- Occupational Exposure to Elemental Mercury in Dentistry. 2012. Available online: https://wedocs.unep.org/20.500.11822/31736 (accessed on 2 February 2023).
- World Dental Federation. Mercury Hygiene Guidance. 2007. Available online: https://preprod.fdiworlddental.org/mercury-hygiene-guidance (accessed on 3 February 2023).
- Canadian Centre for Occupational and Safety. OSH Answers Fact Sheets, Mercury. 2023. Available online: https://www.ccohs.ca/oshanswers/chemicals/chem_profiles/mercury.html (accessed on 7 February 2023).
- Communication from the Commission to the European Parliament and the Council on the Review of the Community Strategy Concerning Mercury. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52010DC0723 (accessed on 2 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).