Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
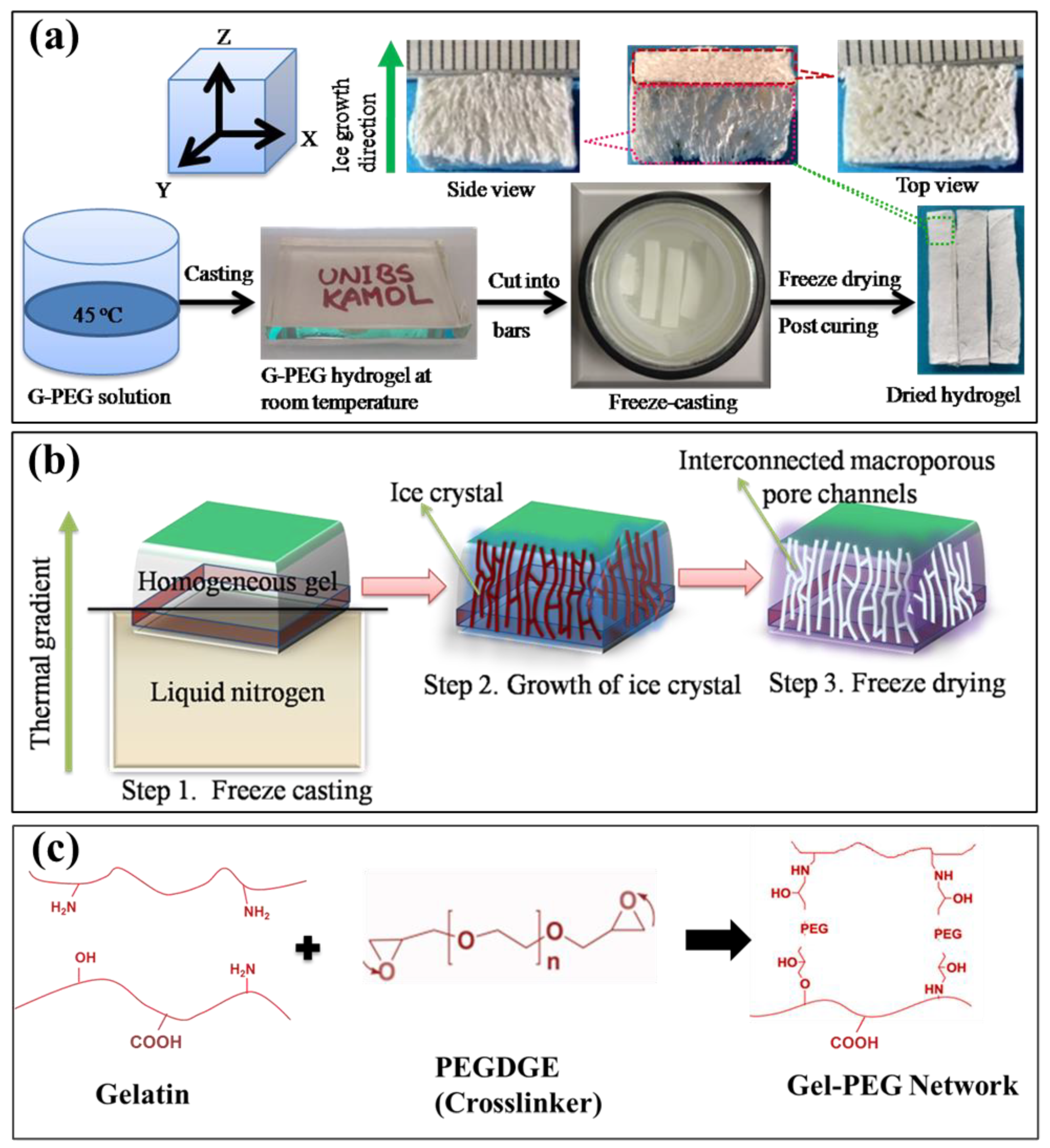
2.2.1. Formation of Gel-PEG Hybrid Hydrogel
2.2.2. Physical Properties Measurements
Gel Fraction of Gel-PEG Hybrid Hydrogel
Measurement of Apparent Density and Porosity of the Gel-PEG Hybrid Hydrogel
Swelling Ratio (%) of Gel-PEG Hybrid Hydrogel
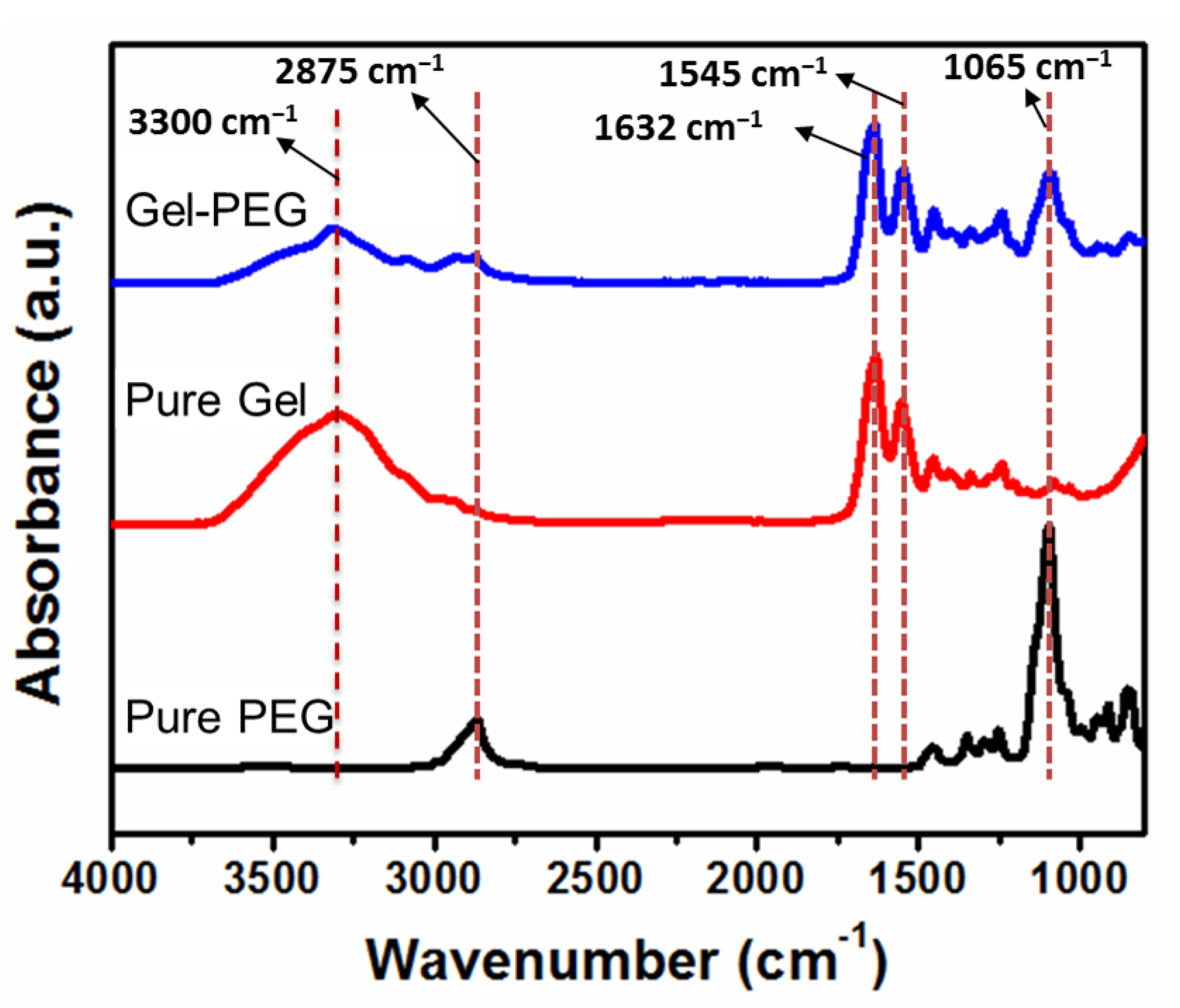
2.2.3. Structural Characterization
2.2.4. Morphological Analysis (Stereomicroscope and SEM)
2.2.5. Mechanical Properties of the Gel-PEG Hybrid Hydrogel
2.2.6. Hydrolytic Degradation of Gel-PEG Hybrid Hydrogel
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Formation of Anisotropic Gel-PEG Hybrid Hydrogel
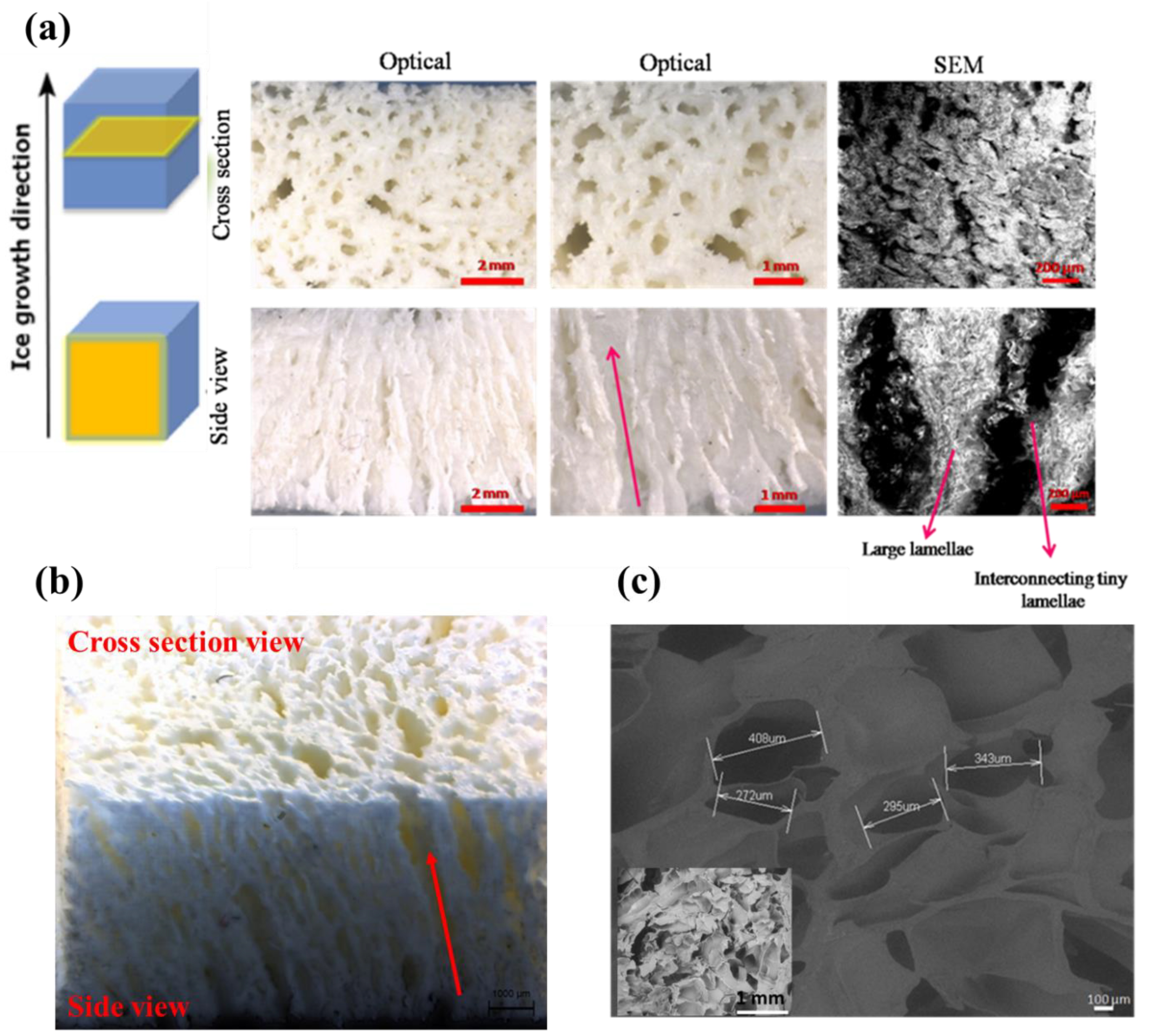
3.2. Macro and Micro-Structures of Anisotropic Gel-PEG Hybrid Hydrogel
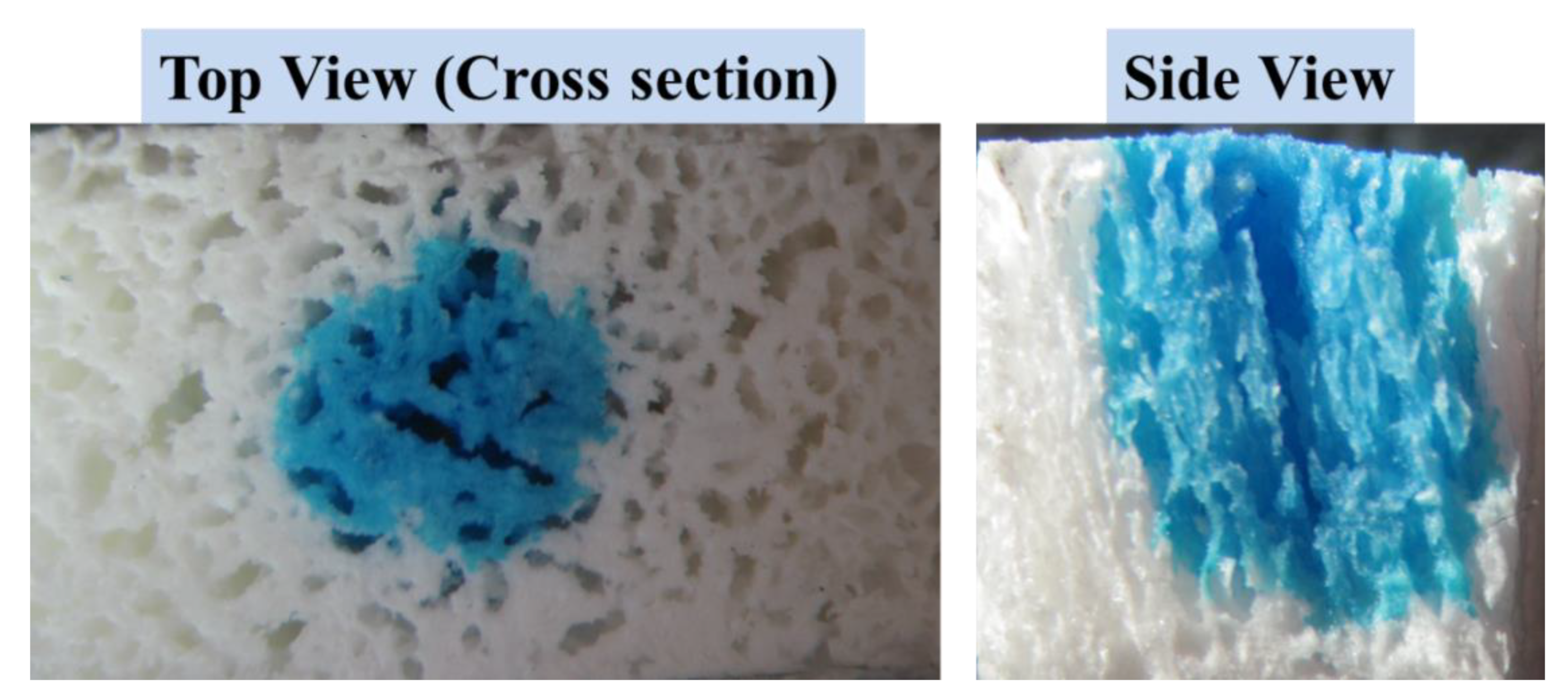
3.2.1. Skin Formation during Freeze-Drying
3.2.2. Microscopic Analysis of Gel-PEG Hybrid Hydrogel without Skin
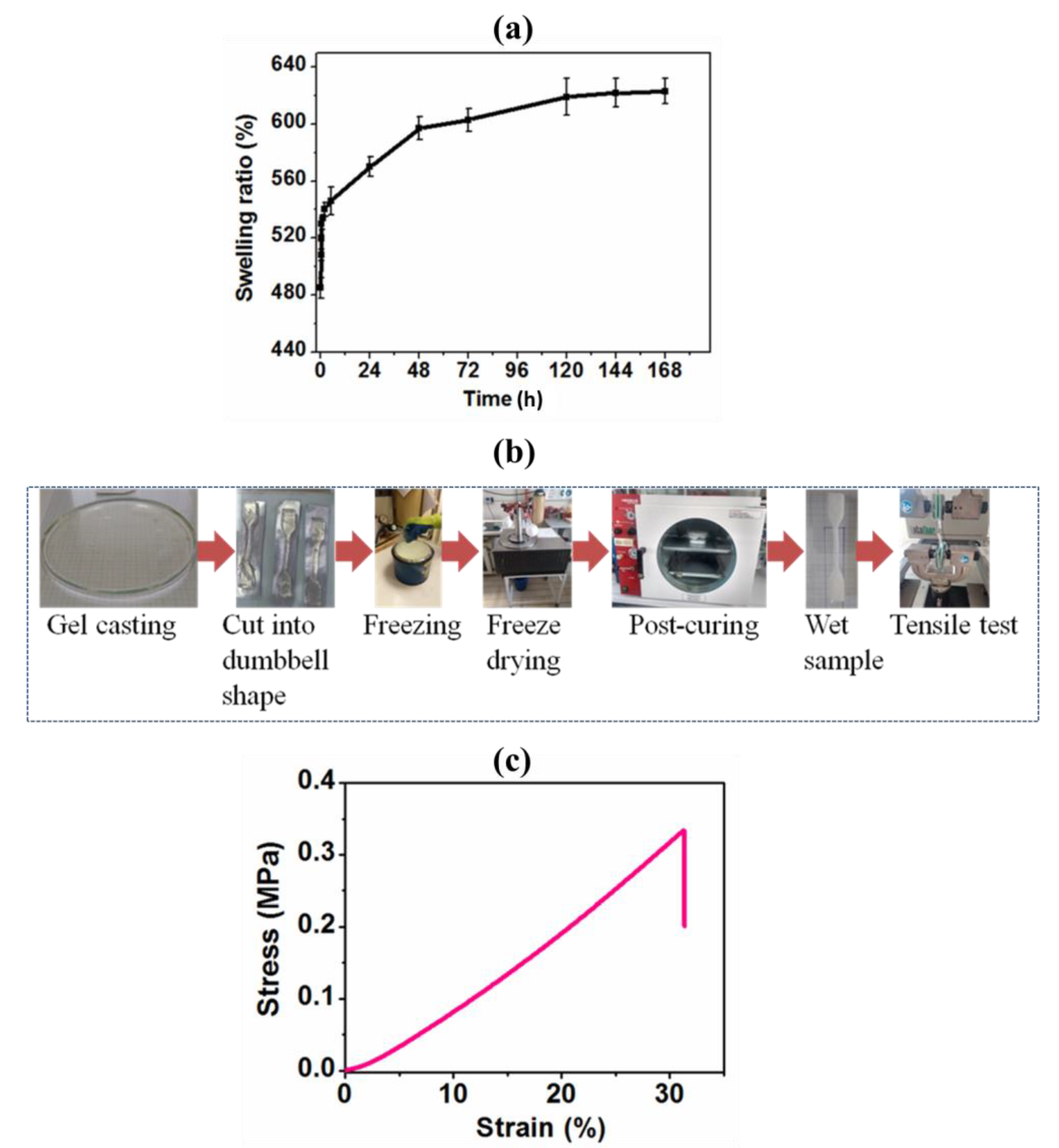
3.3. Physical Properties and Swelling Ratio (%) of Gel-PEG Hybrid Hydrogel
3.4. Mechanical Properties of Gel-PEG Hybrid Hydrogel
3.4.1. Tensile Mechanical Properties
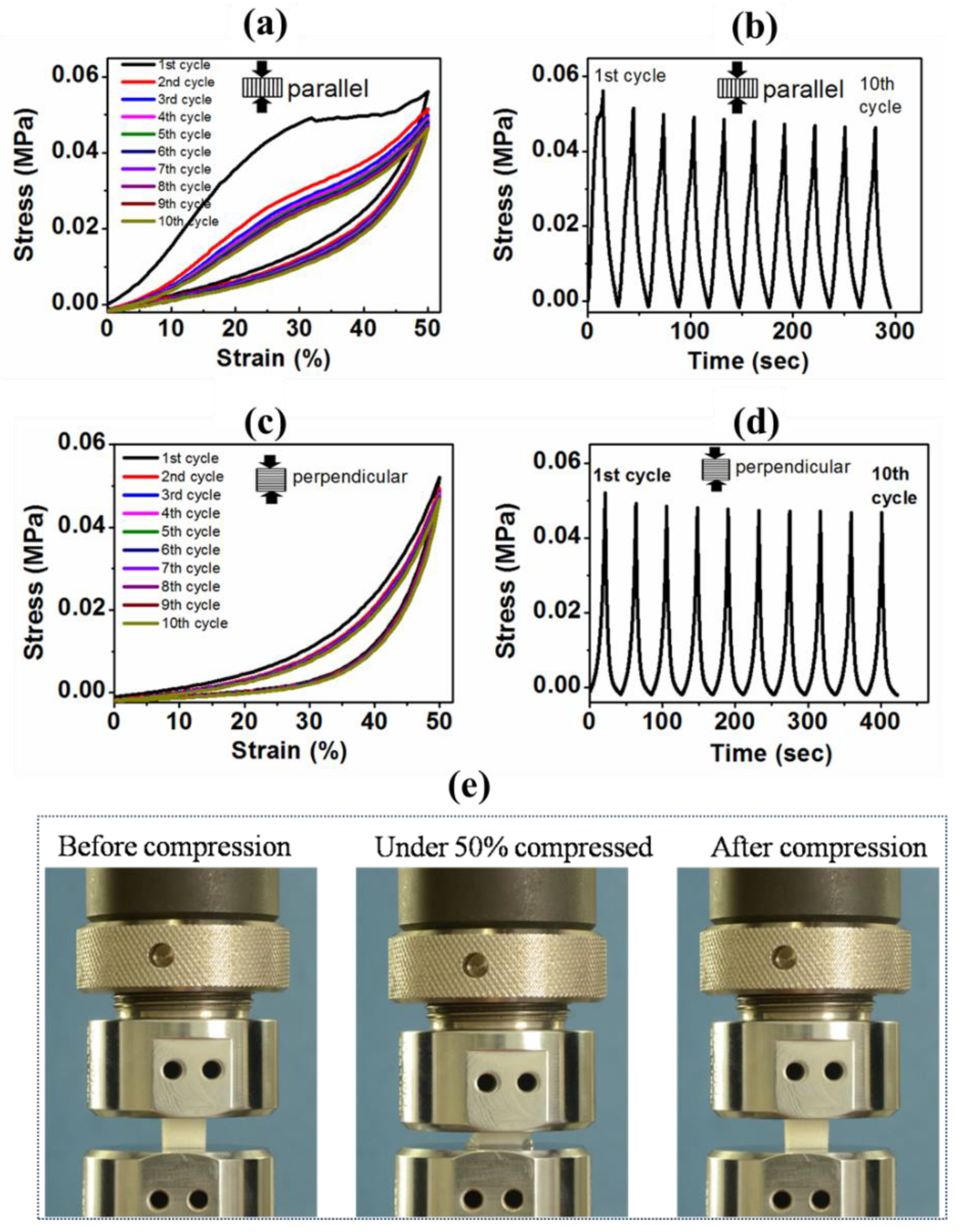
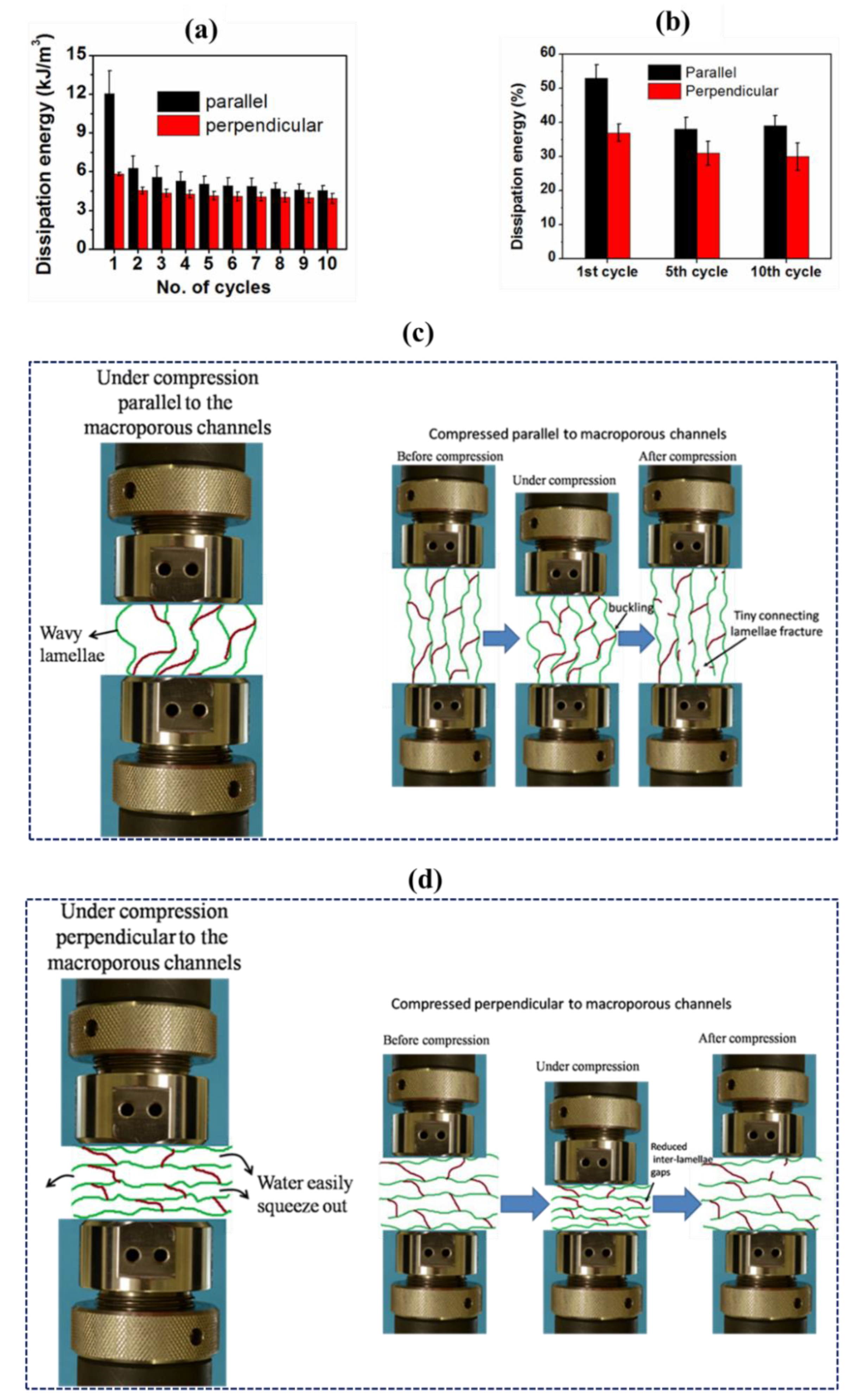
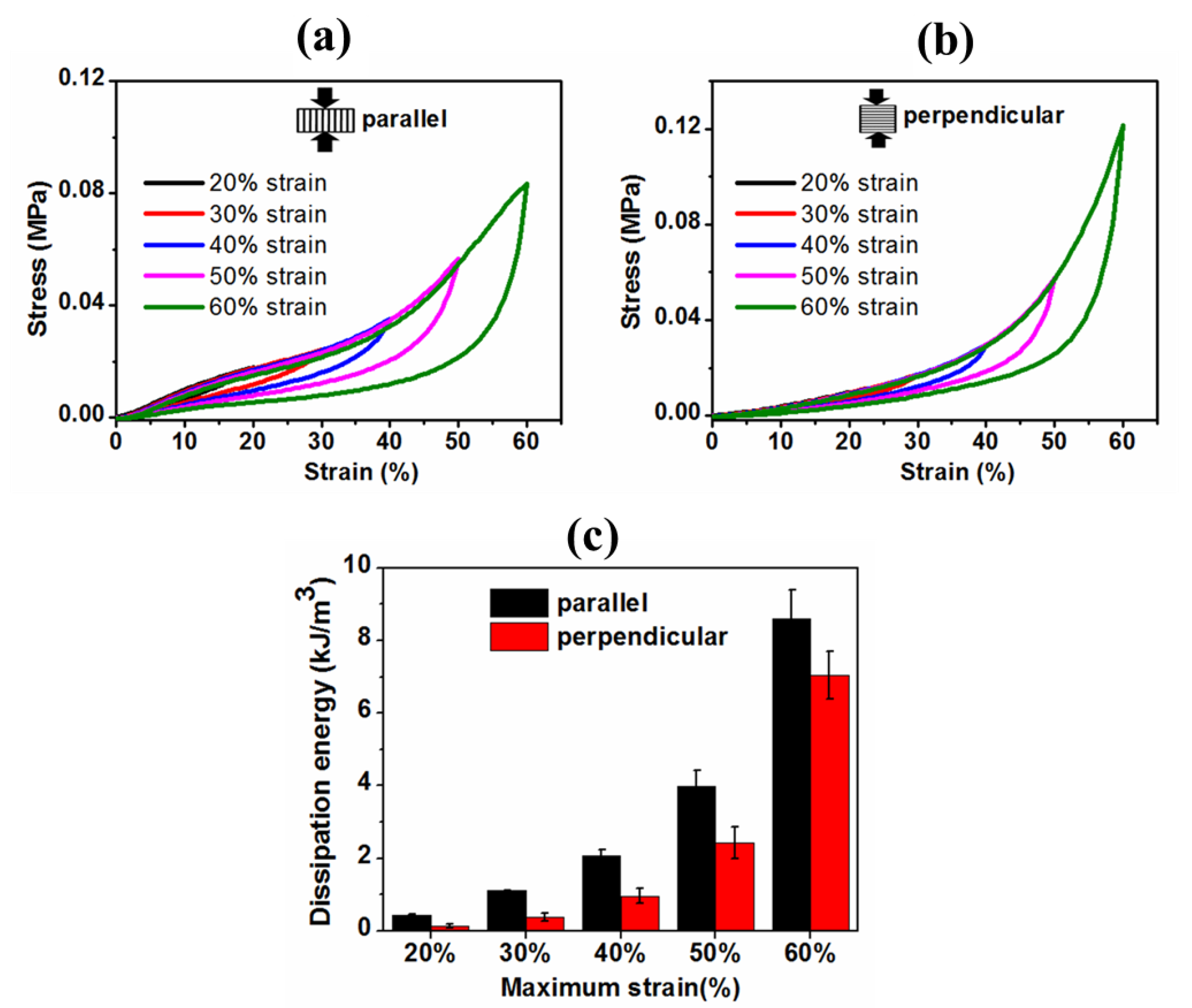
3.4.2. Cyclic Compression and Compressive Mechanical Properties
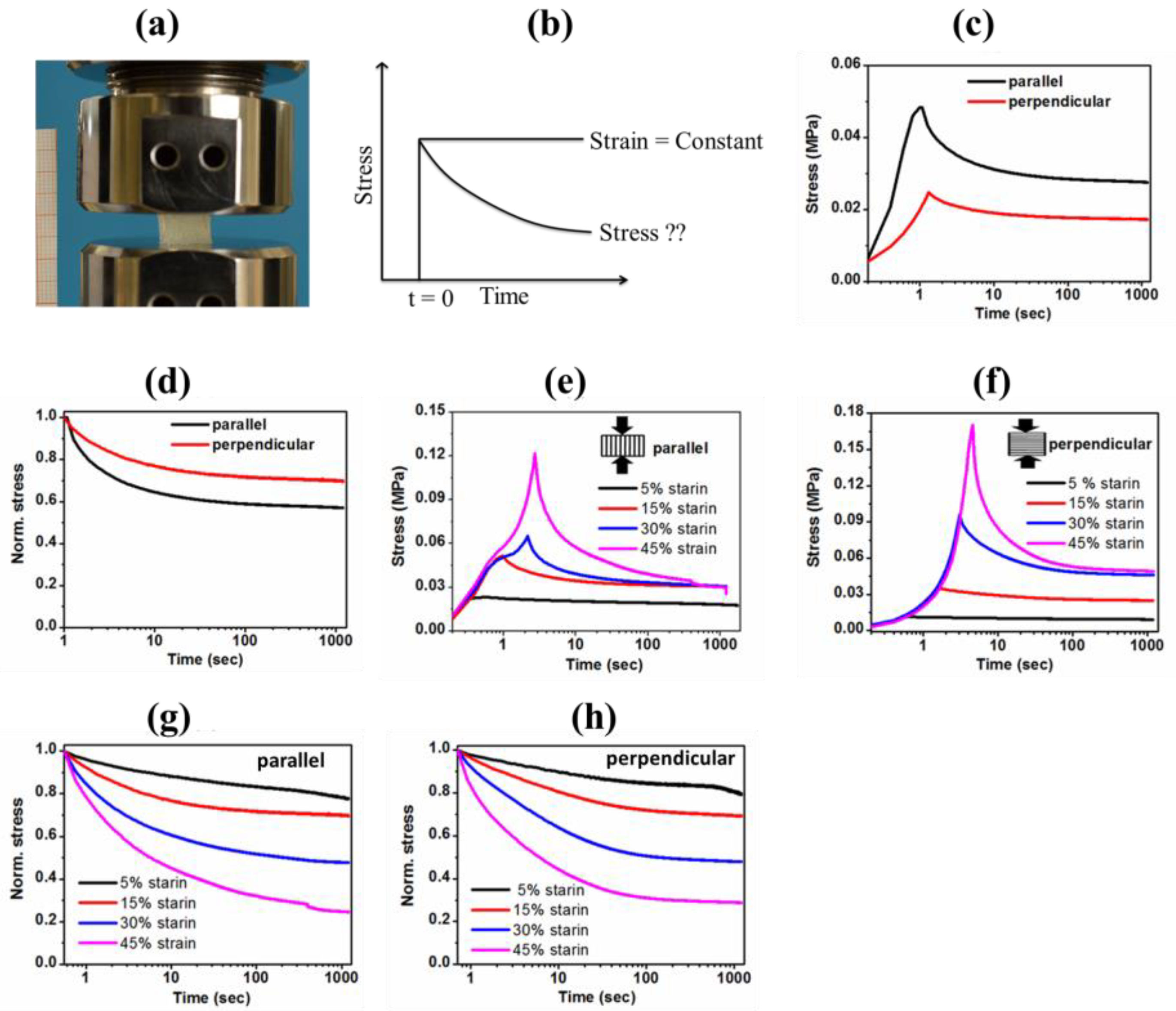
3.4.3. Stress Relaxation Behavior of the Anisotropic Gel-PEG Hybrid Hydrogel
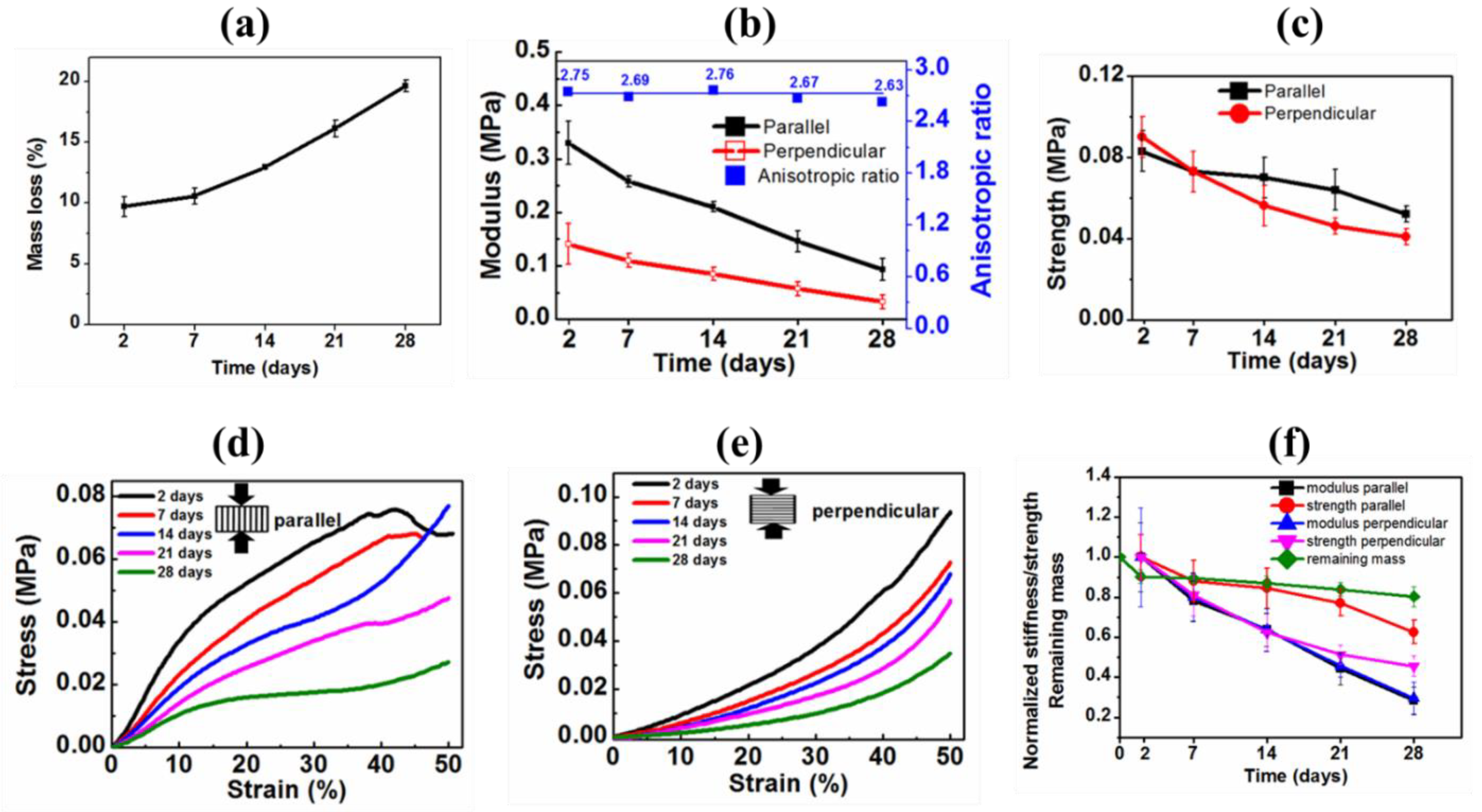
3.4.4. Hydrolytic Mass Loss and Associated Mechanical Properties of Gel-PEG Hybrid Hydrogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadtler, K.; Singh, A.; Wolf, M.T.; Wang, X.; Pardoll, D.M.; Elisseeff, J.H. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater. 2016, 1, 16040. [Google Scholar] [CrossRef]
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.; et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Transplantation. Available online: https://www.who.int/health-topics/transplantation#tab=tab_2 (accessed on 15 January 2023).
- Dey, K.; Agnelli, S.; Re, F.; Russo, D.; Lisignoli, G.; Manferdini, C.; Bernardi, S.; Gabusi, E.; Sartore, L. Rational Design and Development of Anisotropic and Mechanically Strong Gelatin—Based Stress Relaxing Hydrogels for Osteogenic/Chondrogenic Differentiation. Macromol. Biosci. 2019, 19, 1900099. [Google Scholar] [CrossRef]
- Dey, K.; Roca, E.; Ramorino, G.; Sartore, L. Progress in the mechanical modulation of cell functions in tissue engineering. Biomater. Sci. 2020, 8, 7033–7081. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R.; Borenstein, J.; Vacanti, J.P. Microscale technologies for tissue engineering and biology. Proc. Natl. Acad. Sci. USA 2006, 103, 2480–2487. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Serzanti, M.; Ginestra, P.; Scarì, G.; Dell’Era, P.; Sartore, L. Preparation and properties of high performance gelatin-based hydrogels with chitosan or hydroxyethyl cellulose for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 183–192. [Google Scholar] [CrossRef]
- Elkhoury, K.; Russell, C.S.; Sanchez-Gonzalez, L.; Mostafavi, A.; Williams, T.J.; Kahn, C.; Peppas, N.A.; Arab-Tehrany, E.; Tamayol, A. Soft-Nanoparticle functionalization of natural hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2019, 8, 1900506. [Google Scholar] [CrossRef]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef]
- Cai, M.H.; Chen, X.Y.; Fu, L.Q.; Du, W.L.; Yang, X.; Mou, X.Z.; Hu, P.Y. Design and development of hybrid hydrogels for biomedical applications: Recent trends in anticancer drug delivery and tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xing, X.; Li, S.; Wu, X.; Jia, Q.; Tu, H.; Bian, H.; Lu, A.; Zhang, L.; Yang, H.; et al. Anisotropic Hybrid Hydrogels Constructed via the Noncovalent Assembly for Biomimetic Tissue Scaffold. Adv. Funct. Mater. 2022, 32, 2112685. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Z.; Xu, D.; Zhao, Y. Electroconductive and Anisotropic Structural Color Hydrogels for Visual Heart-on-a-Chip Construction. Adv. Sci. 2022, 9, 2105777. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.; De France, K.J.; Kopera, B.; Machado, V.R.; Rosenfeldt, S.; Reyes, L.; Chan, K.J.; Forster, S.; Cranston, E.D.; Hoare, T.; et al. Composite hydrogels with tunable anisotropic morphologies and mechanical properties. Chem. Mater. 2016, 28, 3406–3415. [Google Scholar] [CrossRef]
- Choi, S.; Choi, Y.; Kim, J. Anisotropic hybrid hydrogels with superior mechanical properties reminiscent of tendons or ligaments. Adv. Funct. Mater. 2019, 29, 1904342. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Engelmayr, G.C.; Cheng, M.; Bettinger, C.J.; Borenstein, J.T.; Langer, R.; Freed, L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008, 7, 1003–1010. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of anisotropic hydrogels and their applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Marelli, B.; Ghezzi, C.E.; James-Bhasin, M.; Nazhat, S.N. Fabrication of injectable, cellular, anisotropic collagen tissue equivalents with modular fibrillar densities. Biomaterials 2015, 37, 183–193. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, J.; Qi, G.; He, C.; Wang, H. Anisotropic hydrogels fabricated with directional freezing and radiation-induced polymerization and crosslinking method. Mater. Lett. 2012, 89, 104–107. [Google Scholar] [CrossRef]
- Li, T.; Hou, J.; Wang, L.; Zeng, G.; Wang, Z.; Yu, L.; Yang, Q.; Yin, J.; Long, M.; Chen, L.; et al. Bioprinted anisotropic scaffolds with fast stress relaxation bioink for engineering 3D skeletal muscle and repairing volumetric muscle loss. Acta Biomater. 2023, 156, 21. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Khuu, N.; Kheiri, S.; Kumacheva, E. Structurally anisotropic hydrogels for tissue engineering. Trends Chem. 2021, 3, 1002. [Google Scholar] [CrossRef]
- Chen, Z.; Khuu, N.; Xu, F.; Kheiri, S.; Yakavets, I.; Rakhshani, F.; Morozova, S.; Kumacheva, E. Printing Structurally Anisotropic Biocompatible Fibrillar Hydrogel for Guided Cell Alignment. Gels 2022, 8, 685. [Google Scholar] [CrossRef]
- Wang, M.; Yang, C.; Deng, H.; Du, Y.; Xiao, L.; Shi, X. Electrically induced anisotropic assembly of chitosan with different molecular weights. Carbohydr. Polym. 2023, 304, 120494. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Z.Z.; Lv, W.; Liu, B.; Wei, J.; Lv, X.; Luo, Y.; Nishihara, H.; Yang, Q.H. A directional strain sensor based on anisotropic microhoneycomb cellulose nanofiber-carbon nanotube hybrid aerogels prepared by unidirectional freeze drying. Small 2019, 15, 1805363. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Jeon, I. Biomimetic anisotropic hydrogels: Advanced fabrication strategies, extraordinary functionalities, and broad applications. Prog. Mater. Sci. 2022, 124, 100870. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Chen, K.; Wu, X.; Zong, T.; Feng, C.; Zhang, D. Anisotropic hydrogels with enhanced mechanical and tribological performance by magnetically oriented nanohybrids. Chem. Eng. J. 2022, 430, 133036. [Google Scholar] [CrossRef]
- Xing, J.; Liu, N.; Xu, N.; Chen, W.; Xing, D. Engineering complex anisotropic scaffolds beyond simply uniaxial alignment for tissue engineering. Adv. Funct. Mater. 2022, 32, 2110676. [Google Scholar] [CrossRef]
- Wang, W.; Deng, X.; Luo, C. Anisotropic hydrogels with high-sensitivity and self-adhesion for wearable sensors. J. Mater. Chem. C 2023, 11, 196–203. [Google Scholar] [CrossRef]
- Huang, J.; Wu, D.; Xiong, X. Preparation of a composite hydrogel of polyvinyl alcohol/chitosan fiber with anisotropic properties for sustained drug release. J. Appl. Polym. Sci. 2022, 139, e53199. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Wang, Z.; Hou, J.; Liu, S.; Yang, Q.; Yu, L.; Guo, W.; Wang, Y.; Guo, B.; et al. Injectable remote magnetic nanofiber/hydrogel multiscale scaffold for functional anisotropic skeletal muscle regeneration. Biomaterials 2022, 285, 121537. [Google Scholar] [CrossRef] [PubMed]
- Tognato, R.; Bonfrate, V.; Giancane, G.; Serra, T. Fabrication of anisotropic collagen-based substrates for potential use in tissue engineering. Smart Mater. Struct. 2022, 31, 074001. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Borsani, E.; Sartore, L. Degradation-Dependent Stress Relaxing Semi-Interpenetrating Networks of Hydroxyethyl Cellulose in Gelatin-PEG Hydrogel with Good Mechanical Stability and Reversibility. Gels 2021, 7, 277. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Sartore, L. Effects of gamma sterilization on the physicomechanical and thermal properties of gelatin-based novel hydrogels. Polym. Eng. Sci. 2019, 59, 2533–2540. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Sartore, L. Dynamic freedom: Substrate stress relaxation stimulates cell responses. Biomater. Sci. 2019, 7, 836–842. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Wei, M.; Luo, Y.; Zhu, X.; Liu, Y. Compressive property and energy absorption characteristic of open-cell ZA22 foams. Mater. Des. 2009, 30, 87–90. [Google Scholar] [CrossRef]
- Suhr, J.; Victor, P.; Ci, L.; Sreekala, S.; Zhang, X.; Nalamasu, O.; Ajayan, P.M. Fatigue resistance of aligned carbon nanotube arrays under cyclic compression. Nat. Nanotechnol. 2007, 2, 417–421. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.P.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Bernardi, S.; Re, F.; Bosio, K.; Dey, K.; Almici, C.; Malagola, M.; Guizzi, P.; Sartore, L.; Russo, D. Chitosan-Hydrogel polymeric scaffold acts as an independent primary inducer of osteogenic differentiation in human mesenchymal stromal cells. Materials 2020, 13, 3546. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [CrossRef]
- Manferdini, C.; Gabusi, E.; Sartore, L.; Dey, K.; Agnelli, S.; Almici, C.; Bianchetti, A.; Zini, N.; Russo, D.; Re, F.; et al. Chitosan-based scaffold counteracts hypertrophic and fibrotic markers in chondrogenic differentiated mesenchymal stromal cells. J. Tissue Eng. Regen. Med. 2019, 13, 1896–1911. [Google Scholar] [CrossRef]
- Ji, C.; Khademhosseini, A.; Dehghani, F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials 2011, 32, 9719. [Google Scholar] [CrossRef]
- Sharma, A.; Bhat, S.; Nayak, V.; Kumar, A. Efficacy of supermacroporous poly (ethylene glycol)–gelatin cryogel matrix for soft tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 298. [Google Scholar] [CrossRef]
- Gu, L.; Li, T.; Song, X.; Yang, X.; Li, S.; Chen, L.; Liu, P.; Gong, X.; Chen, C.; Sun, L. Preparation and characterization of methacrylated gelatin/bacterial cellulose composite hydrogels for cartilage tissue engineering. Regen. Biomater. 2020, 7, 195. [Google Scholar] [CrossRef]
- Todros, S.; Spadoni, S.; Barbon, S.; Stocco, E.; Confalonieri, M.; Porzionato, A.; Pavan, P.G. Compressive Mechanical Behavior of Partially Oxidized Polyvinyl Alcohol Hydrogels for Cartilage Tissue Repair. Bioengineering 2022, 9, 789. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Yang, W.; Song, Y. Compressive mechanical properties and microstructure of PVA–HA hydrogels for cartilage repair. RSC Adv. 2016, 6, 20166. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Lin, G.; Tang, B.; Li, X.; Zhang, J.; Ding, X.; Zhou, G. Advances in mechanical properties of hydrogels for cartilage tissue defect repair. Macromol. Biosci. 2023, 2200539. [Google Scholar] [CrossRef]
- Taheri, S.; Ghazali, H.S.; Ghazali, Z.S.; Bhattacharyya, A.; Noh, I. Progress in biomechanical stimuli on the cell-encapsulated hydrogels for cartilage tissue regeneration. Biomater. Res. 2023, 27, 22. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, W.; Shao, Z.; Wang, Z.; Chang, B.; Ding, X.; Yang, Y. Biodegradable glass fiber reinforced PVA hydrogel for cartilage repair: Mechanical properties, ions release behavior and cell recruitment. J. Mat. Res. Technol. 2023, 23, 154. [Google Scholar] [CrossRef]
- Demott, C.J.; Jones, M.R.; Chesney, C.D.; Yeisley, D.J.; Culibrk, R.A.; Hahn, M.S.; Grunlan, M.A. Ultra-High modulus hydrogels mimicking cartilage of the human body. Macromol. Biosci. 2022, 22, 2200283. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Wang, Y.; Shi, Y.; Wang, F.; Lin, G. Research progress on mechanical properties and wear resistance of cartilage repair hydrogel. Mater. Des. 2022, 216, 110575. [Google Scholar] [CrossRef]
- Benitez-Duif, P.A.; Breisch, M.; Kurka, D.; Edel, K.; Gökcay, S.; Stangier, D.; Tillmann, W.; Hijazi, M.; Tiller, J.C. Ultrastrong poly (2-oxazoline)/poly (acrylic acid) double-network hydrogels with cartilage-like mechanical properties. Adv. Funct. Mater. 2022, 32, 2204837. [Google Scholar] [CrossRef]
- Romischke, J.; Scherkus, A.; Saemann, M.; Krueger, S.; Bader, R.; Kragl, U.; Meyer, J. Swelling and mechanical characterization of polyelectrolyte hydrogels as potential synthetic cartilage substitute materials. Gels 2022, 8, 296. [Google Scholar] [CrossRef]
- Hao, M.; Wang, Y.; Li, L.; Liu, Y.; Bai, Y.; Zhou, W.; Lu, Q.; Sun, F.; Li, L.; Feng, S.; et al. Tough engineering hydrogels based on swelling–freeze–thaw method for artificial cartilage. ACS Appl. Mater. Interfaces 2022, 14, 25093. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, J.S.; Kim, W.K.; Lee, W.; Kim, N.; Song, C.U.; Jung, J.J.; Song, J.E.; Khang, G. Evaluation of hyaluronic acid/agarose hydrogel for cartilage tissue engineering biomaterial. Macromol. Res. 2020, 28, 979. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Youn, J.; Lee, Y.; Kim, W.; Choe, S.; Song, J.; Reis, R.L.; Khang, G. Development and evaluation of gellan gum/silk fibroin/chondroitin sulfate ternary injectable hydrogel for cartilage tissue engineering. Biomolecules 2021, 11, 1184. [Google Scholar] [CrossRef]
- Phatchayawat, P.P.; Khamkeaw, A.; Yodmuang, S.; Phisalaphong, M. 3D bacterial cellulose-chitosan-alginate-gelatin hydrogel scaffold for cartilage tissue engineering. Biochem. Eng. J. 2022, 184, 108476. [Google Scholar] [CrossRef]


| Composition (wt%) | Physical Properties | ||||
|---|---|---|---|---|---|
| Sample | Gelatin (Gel) | PEG | Apparent Density (g/cc) | Porosity (%) | Gel Fraction (%) |
| Gel-PEG | 81 | 19 | 0.162 ± 0.01 | 75.12 ± 2.12 | 90.30 ± 2.0 |
| Tensile Properties | |
|---|---|
| Tensile elastic modulus (MPa) | 0.863 ± 0.13 |
| Tensile strength (MPa) | 0.380 ± 0.11 |
| Elongation at break (%) | 27.00 ± 4.98 |
| Properties | Parallel | Perpendicular |
|---|---|---|
| Compressive elastic modulus (MPa) | 0.33 ± 0.04 | 0.12 ± 0.03 |
| Compressive stress (MPa) at 50% strain | 0.08 ± 0.02 | 0.09 ± 0.01 |
| Compression energy (kJ/m3) | 13.73 ± 4.2 | 14.49 ± 0.5 |
| Relaxation energy (kJ/m3) | 8.71 ± 3.6 | 9.89 ± 1.2 |
| Dissipation energy (kJ/m3) | 5.03 ± 0.63 | 4.60 ± 1.2 |
| Percentage of energy dissipation (%) | 38.21 ± 7.0 | 31.76 ± 8.0 |
| Compressed Parallel to the Macroporous Channels | Compressed Perpendicular to the Macroporous Channels | ||||
|---|---|---|---|---|---|
| Time (Days) | Modulus (MPa) | Stress (MPa) at 50% Strain | Modulus (MPa) | Stress (MPa) at 50% Strain | Anisotropic Ratio E‖/E⊥ |
| 2 | 0.330 ± 0.04 | 0.083 ± 0.01 | 0.120 ± 0.03 | 0.09 ± 0.01 | 2.75 |
| 7 | 0.258 ± 0.01 | 0.073 ± 0.01 | 0.096 ± 0.01 | 0.073 ± 0.01 | 2.69 |
| 14 | 0.211 ± 0.01 | 0.070 ± 0.01 | 0.0763 ± 0.01 | 0.0562 ± 0.01 | 2.76 |
| 21 | 0.146 ± 0.02 | 0.064 ± 0.001 | 0.0548 ± 0.01 | 0.0462 ± 0.002 | 2.67 |
| 28 | 0.093 ± 0.02 | 0.052 ± 0.004 | 0.0355 ± 0.01 | 0.0410 ± 0.004 | 2.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dey, K.; Agnelli, S.; Sartore, L. Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application. Micro 2023, 3, 434-457. https://doi.org/10.3390/micro3020029
Dey K, Agnelli S, Sartore L. Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application. Micro. 2023; 3(2):434-457. https://doi.org/10.3390/micro3020029
Chicago/Turabian StyleDey, Kamol, Silvia Agnelli, and Luciana Sartore. 2023. "Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application" Micro 3, no. 2: 434-457. https://doi.org/10.3390/micro3020029
APA StyleDey, K., Agnelli, S., & Sartore, L. (2023). Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application. Micro, 3(2), 434-457. https://doi.org/10.3390/micro3020029









