1. Introduction
The tooth root cementum is one of the three hard tissues of the human tooth. This is a calcified tissue similar to bone; however, unlike the latter, it is devoid of blood vessels [
1,
2]. The cement covers the dentine throughout its entire length, from the cervical zone of the tooth to its root, in some cases directly in contact with the enamel [
3]. Cementum is the least durable of the hard tissues of the tooth, containing 45–50% inorganic and 50–55% organic substances (mainly collagen and glycoproteins) [
4].
The inner layer of the cementum has no cells (acellular cementum), but on top of it there is a cellular cementum in which there are cells, namely cementocytes and cementoblasts. The main function of this tissue is to form the supporting apparatus of the tooth—it provides attachment to the root and cervical zone of the peripheral sections of periodontal fibers [
5]. The latter, penetrating into the cementum, have the form of craters located in the center of dome-shaped structures elevated above the cementum surface. Cementum protects the root dentine from damaging effects [
6,
7] and caries [
8] and performs reparative functions in the formation of so-called resorption lacunae and in root fractures [
9,
10,
11]. It is cementum that makes it possible to maintain a constant total length of the tooth due to the constant deposition of tissue in the region of the root apex, thereby compensating for the abrasion of the crown as a result of enamel wear [
12,
13,
14].
Mechanical and micro-geometrical properties of tooth hard tissues determine the strength and normal functioning of the masticatory apparatus. Their values are important in compiling models and identifying the mechanisms of the chewing process and they can also be indicators of various pathologies. In modern mathematical modeling, as well as in treatment and prosthetics, the values of strength characteristics in local areas of the teeth should be taken into account when developing biocompatible materials, as well as implants based on them. In this regard, a number of scientists have performed works on the complex characterization of the properties of sound [
15,
16,
17] and pathologically altered tooth tissues [
18,
19,
20,
21], as well as biocompatible materials for substituting such tissues [
22,
23,
24]; however, most of these works include results only for enamel and dentine.
The hardness of cementum was first measured by Hodge and Mckay [
25] using the Brinell hardness test. Precision studies of the mechanical characteristics of cementum are associated with the pioneering work of Poolthong [
26], who assessed the hardness and Young’s modulus of two regions of the tooth: the middle third of the root and the apex region, using the nanoindentation method (Vickers indenter). Ho et al. [
27] studied the mechanical properties, chemical composition and microstructure of cementum at the junction with enamel. In their other work [
28], the authors investigated the mechanism of attachment of the periodontal ligament to the cementum of the tooth. In [
29] Ho et al. have conducted an extensive comparisons of the physical properties of rat and human cementum, root dentine and their interface, including the dentine–cementum junction. A detailed methodology, as well as comparison of several histological and immunohistochemical stains for imaging the cementum by light microscopy, was made by Foster [
30]. Jang et al. [
31], using the methods of mathematical statistics, found a correlation between the increase in Knoop hardness of cementum, obtained on a microhardness tester, and the age of patients, which indicated an ongoing process of mineralization of this tissue with age. Chutimanutskul et al. [
32] experimentally showed the closeness of the mechanical properties of the cementum of the left and right maxillary premolars. Malek et al. [
33] proposed a technique for mapping mechanical properties over the surface of the tooth root using two indenters, Berkovich and spherical one, and then investigated [
34] the effect of drying on the mechanical properties of cementum. Further studies by this group delved into the evaluation of the chemical composition of cementum [
35] and the mechanical properties of pathologically altered cementum [
36,
37]. A review of the mechanical properties of the individual cells of cementum was made by Radermacher et al. [
38]. Hinrichs et al. [
39] found some new aspects of the microstructure of acellular cementum using scanning electron microscopy. Arefnia et al. [
40] traced surface changes induced on enamel and cementum by different scaling and polishing techniques in vitro.
In the present work, the mechanical characteristics (reduced Young’s modulus, indentation hardness) obtained using the nanoindentation method with a Berkovich indenter installed, the average roughness and the maximum roughness height of three regions on the prepared section of the tooth root cementum were studied. For each region, images of the surface microrelief were obtained using an atomic force microscope (AFM). A study was made of the parameters of the topographic features of the cementum in the vicinity of the lacunae of cementocytes. Given that the functioning of the cementum is closely related to the dentine of the tooth root, a number of changes in the dentine near its border with the cementum, compared with the dentine of the crown part of the tooth, have been established. Particularly novel aspects of the research are: the simultaneous investigation of the mechanical properties of cementum alongside the parameters of its roughness; the study of the specific features of this tissue (such as cementocyte lacunas); comparison of the microgeometrical properties of dentine in the coronal part of the tooth with the vicinity of the junction with cementum; and AFM investigation of the anatomical parts of dentine–cementum junction with high resolution.
2. Materials and Methods
We used tooth №16 extracted from a patient (female) for orthodontic reasons as a sample. The local Independent Ethics Committee of Rostov State Medical University approved the study (protocol code 13/22, date of approval: 8 September 2022). The patient provided informed consent. After removal, the sample was kept in 1% NaClO solution for 10 min. The specimen was then placed in a sterile container filled with Hanks’ Balanced Salt Solution (HBSS) containing thymol to prevent demineralization of the tooth tissues. The ratio of thymol to HBSS was 1:1000. The container was stored in a pharmaceutical refrigerator at a temperature of 4 °C.
Before examining the properties of the tooth tissues, the sample preparation was carried out. First, the sample was placed in a hollow ABS plastic (acrylonitrile butadiene styrene) cylinder. Then the cylinder was filled with universal epoxy adhesive (Baza Klass, Staraya Stanitsa, Rostov Region, Russia); the ratio of hardener (polyethylene polyamine) to epoxy resin was 1:3. To prevent leakage of epoxy glue from under the cylinder, its base was smeared with a high-temperature sealant-gasket Red RTV Silicone Gasket Maker (Doctor Chemical Corporation, Longwan, Wenzhou, China).
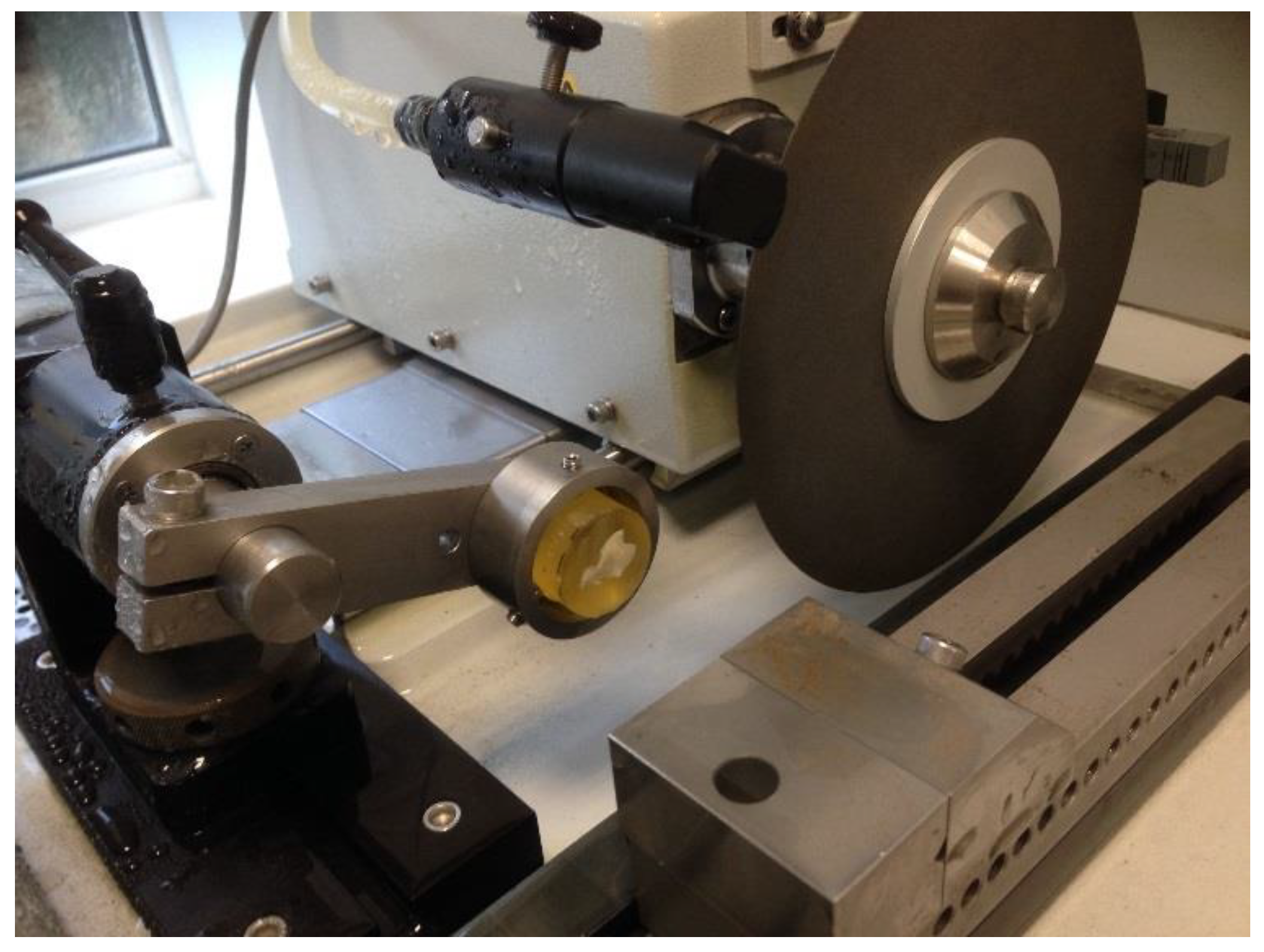
After the adhesive was cured for 24 h, the sample was placed in an Isomet 4000 linear precision cutting machine (Buehler, Lake Bluff, IL, USA). The sample was fixed with a holder for cylindrical samples with a diameter of 25.4 mm. Cutting was performed with a Buehler MetAbrase abrasive wheel with cutting fluid (10% solution of Buehler Cool 2 Cutting Fluid in running water) without foaming on the wheel. Before cutting the sample, the abrasive disk was straightened on a special bar. Cutting parameters were as follows: blade rotation speed 2500 rpm, 1 mm/min. Two cuts were made in such a way that the second cut formed a slice of the tooth (
Figure 1—the sample was moved relative to the disk using the micrometer built into the machine). The pulp chamber was cleared of soft tissue remnants.
Next, the sample was ground and polished on a MetaServ 250 grinding and polishing machine (Buehler, Shanghai, China) with a Vector LC 250 semi-automatic nozzle. Abrasive discs and paper were glued onto a Buehler MagnoPad magnetic disc installed in the machine. The loading on the sample at each stage of grinding and polishing was 10 N, the disk rotation speed was 200 rpm. The following stages of grinding and polishing were carried out:
- (1)
grinding using a Buehler CarbiMet abrasive disc based on SiC, grit P320 in the presence of distilled water for 1 min;
- (2)
similar to the previous step, but P400 grit, grinding time 2 min;
- (3)
grinding using Dexter abrasive paper (Shandong Boss Abrasives Manufacturing Co., Jinan, Shandong, China) based on SiC, grit P600, in the presence of distilled water for 1 min;
- (4)
similar to the previous step, but grit P1000, grinding time 30 s;
- (5)
polishing using an Buehler UltraPad lint-free dense cloth disc; a MetaDi oil-based single-crystal Buehler diamond suspension, particle diameter 6 µm, was applied to the disc in the presence of Buehler MetaDi Fluid lubricant. The polishing time was ~2 min;
- (6)
similar to the previous stage, but with particle diameter 1 µm;
- (7)
polishing using a disk made of a porous, chemically resistant synthetic fabric Buehler ChemoMet; a sol-gel suspension of Buehler MasterPrep based on Al particles with a diameter of 0.05 μm was applied to the disk in the presence of MetaDi Fluid lubricant. The polishing time was 2 min.
After sample preparation, the sample was placed in physiological saline, in which it was also stored between subsequent experiments in a pharmaceutical refrigerator at a temperature of 4–6 °C.
The indentation hardness H and the reduced modulus of elasticity E were measured on a 750 Ubi nanoindentation test device (Hysitron, Bruker, Eden Prairie, MN, USA). In all experiments, a Berkovich diamond indenter with a tip radius of 200 nm was used, along with a load P = 1000 µN. Load profile was as follows: 10 s loading/10 s unloading time. The area of the contact zone between the indenter tip and the sample was calibrated on a standard calibration sample of fused silica. All measurements were made in air.
Using AFM Dimension FastScan (Bruker, Eden Prairie, MN, USA), images of the relief of the surface of tooth tissues were obtained. When performing a scan, the device worked in the PeakForce Tapping QNM (Quantitative NanoMechanics) mode. In this mode, there is a constant control of the force with which the probe acts on the sample, while the depth of deformation of the sample was very small. The surface relief was studied using a diamond probe on a D300 silicon cantilever (TipsNano, Zelenograd, Moscow, Russia) with a tip radius of 45 nm and cantilever stiffness of 55.10 N/m, with image resolution 256 × 256 pixels. The speed of the probe movement along the surface was 1.99 µm/s. The rigidity of the console was specifically qualified using the microscope software options.
3. Results
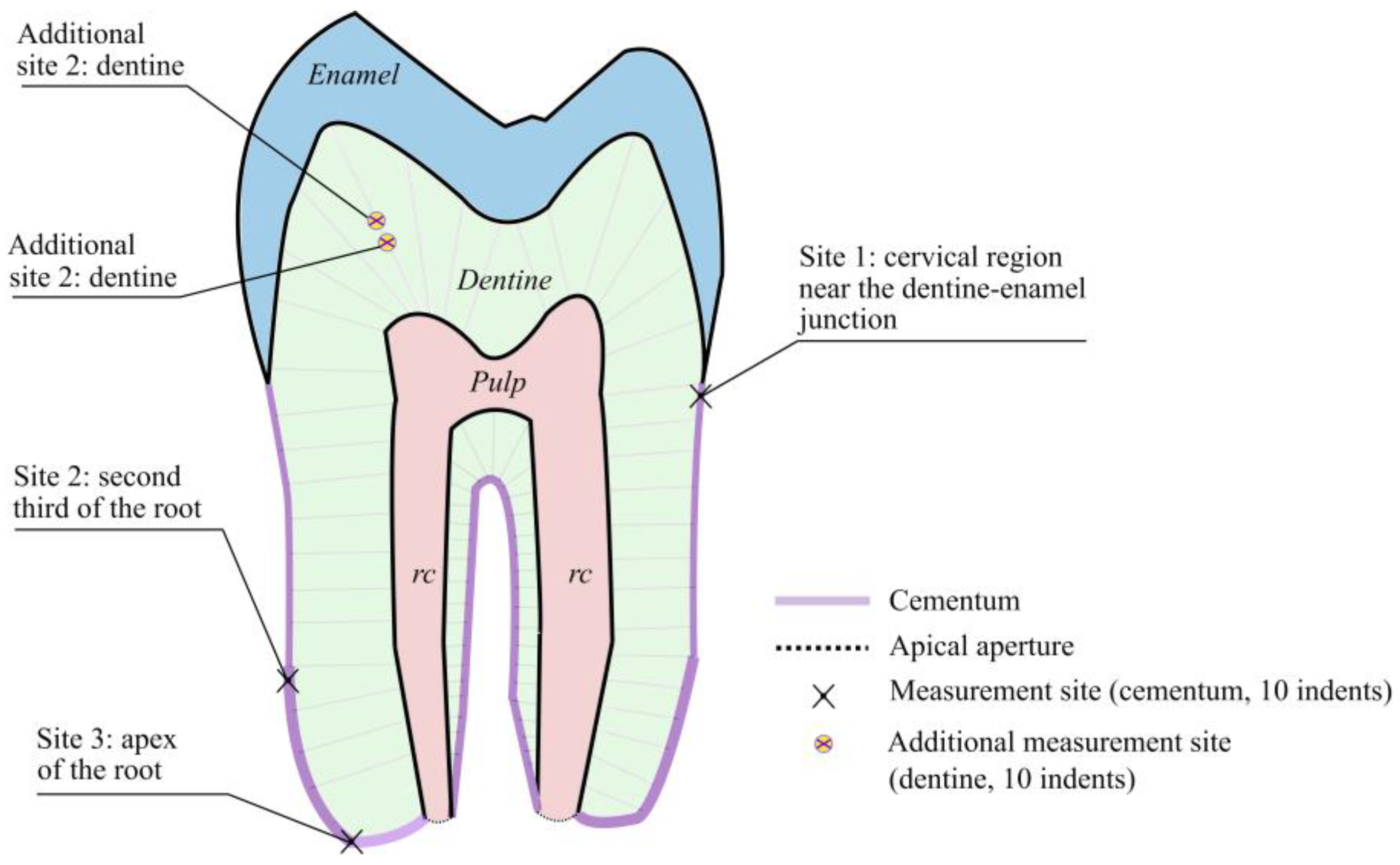
Three measurement sites were selected for the study of cementum on the following areas of the tooth root: the cervical region near the dentine–enamel junction, the second third of the tooth root and the apex of the tooth root (marked with an X in
Figure 2). On each section, 10 measurements were carried out with the same loading parameters on an area of 80 μm × 80 µm, then the results were averaged. The analysis of the experimental results was carried out according to the Oliver–Pharr method [
41]. In addition, for each section, the average roughness
Ra was obtained in a field of 80 μm × 80 μm in the mode of using the indenter as an AFM probe. The measurement results are listed in
Table 1.
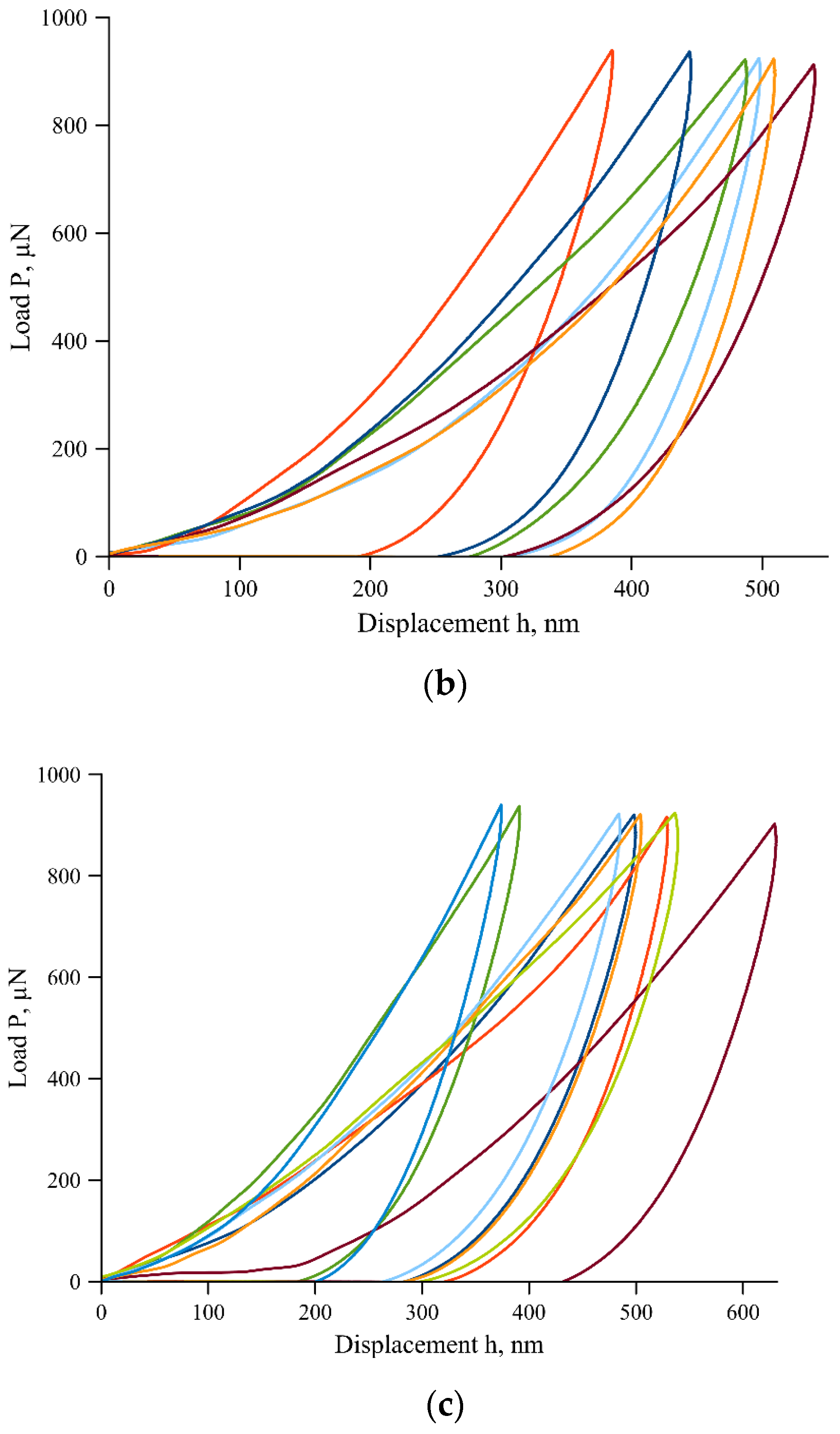
The indentation force-displacement curves for each of the sites are shown in
Figure 3. For the measurement site 2 (cementum in the second third of the tooth root), near the junction of dentine and cementum, images were obtained in the 750 Ubi device in the scanning mode with a diamond probe, as well as an image from an optical microscope (
Figure 4).
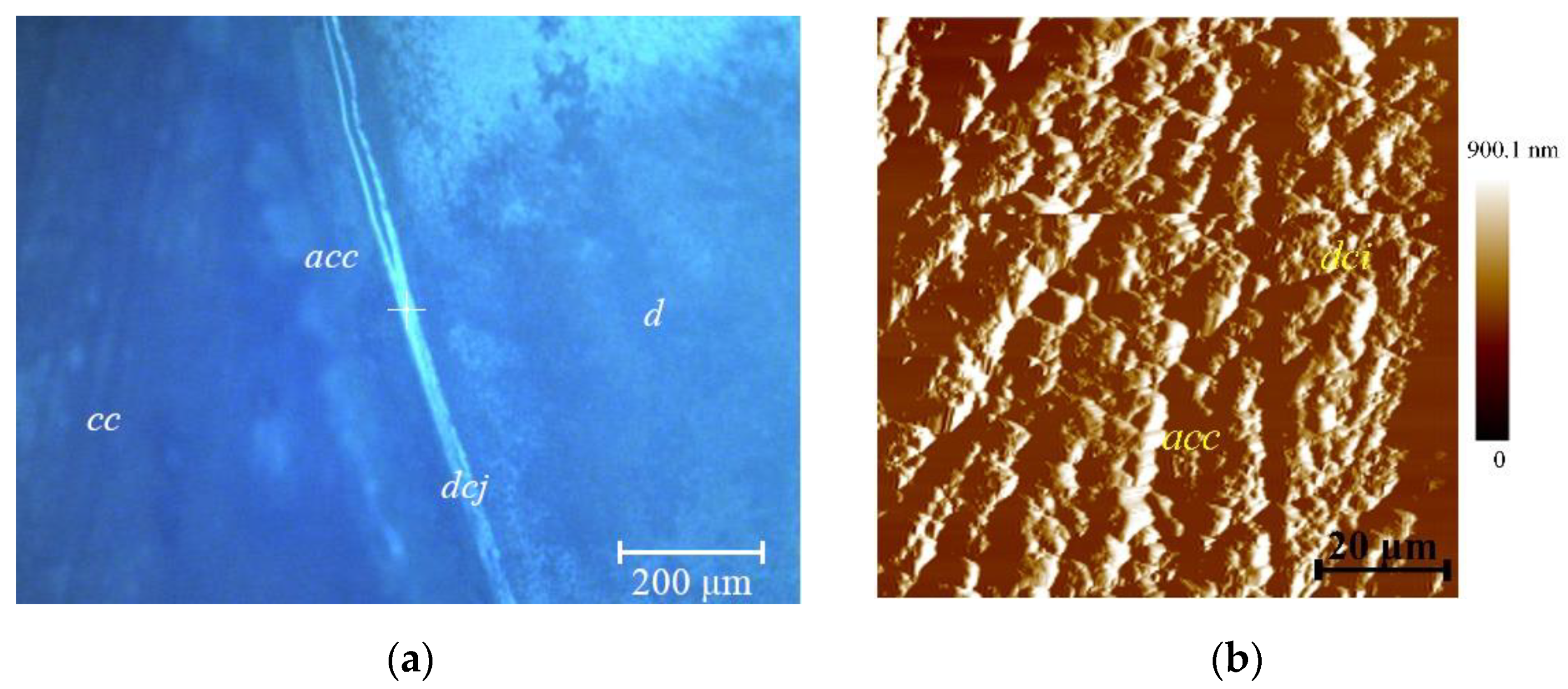
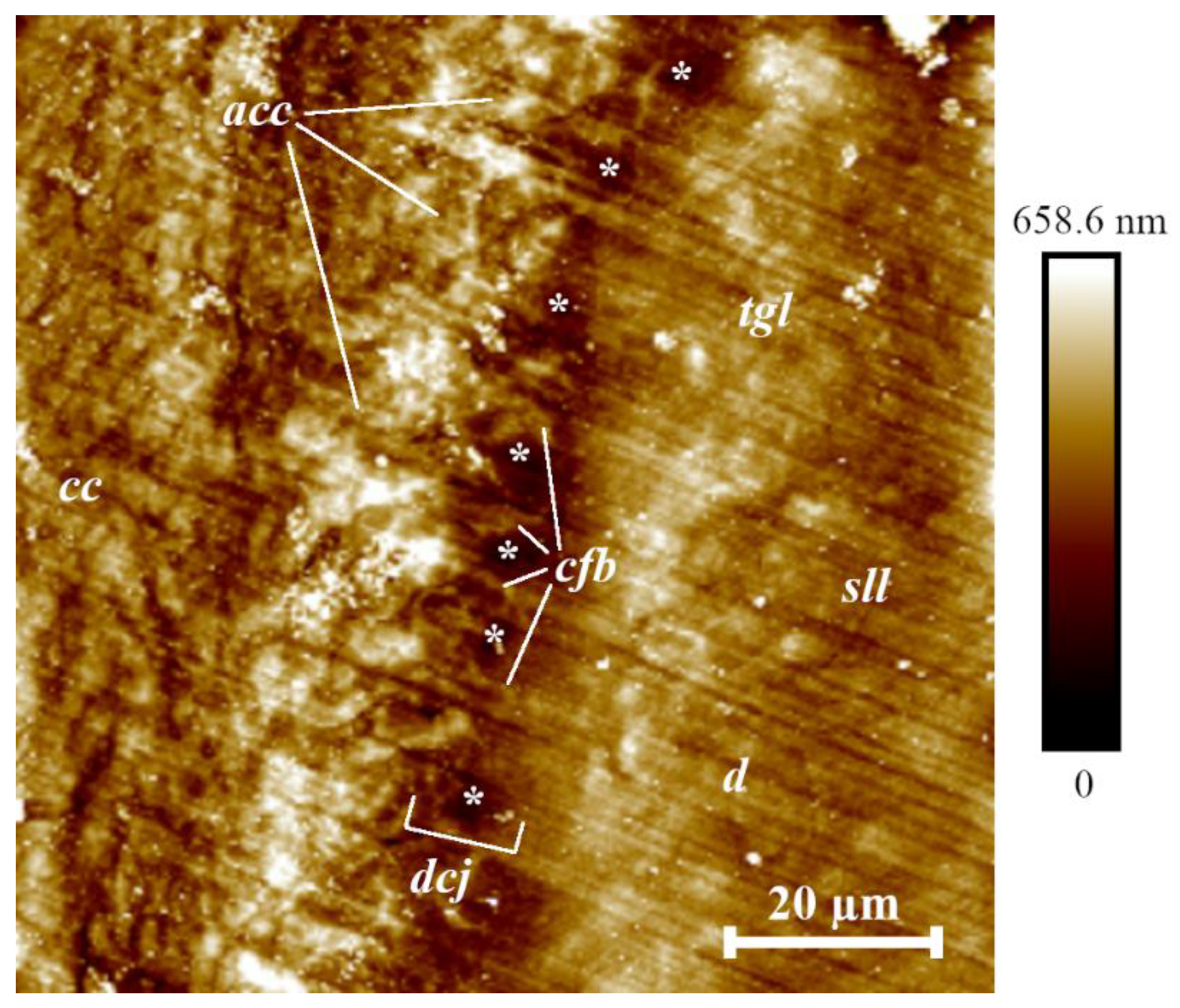
Figure 5 shows the topography of the cementum, the dentine–cementum junction, and dentine in the vicinity of measurement site 1 (cervical area near the dentine–enamel junction). Image processing was performed using NanoScope Analysis (Bruker, Eden Prairie, MN, USA) software. The main substance of the cementum is formed by the structural units of collagen fibers/collagen fibrils and gluing matrix. The matrix contains a carbohydrate-protein complex. Collagen fibers in this case have a different origin—one group is directly produced in the cementum, the other is woven from the periodontal ligament (Sharpey’s fibers) [
42,
43]. Dentine in the figure is located to the right of the border, cementum to the left. To analyze the results of the study, the average surface roughness
Ra for acellular cementum, cellular cementum, dentine–cementum junction and dentine near this interface was measured.
Due to the large number of irregularities on the surface of the sample, the measurement of roughness in one direction is not representative enough. Thus, the average roughness
Ra and the maximum roughness height
Rt were measured in two directions, along the dentine–cementum junction and perpendicular to it. Five profiles were built for each of the directions (each profile was an average of another 10 pixels around it). After that, the mean values (from 10 profiles) with standard deviations were calculated and the results are listed in
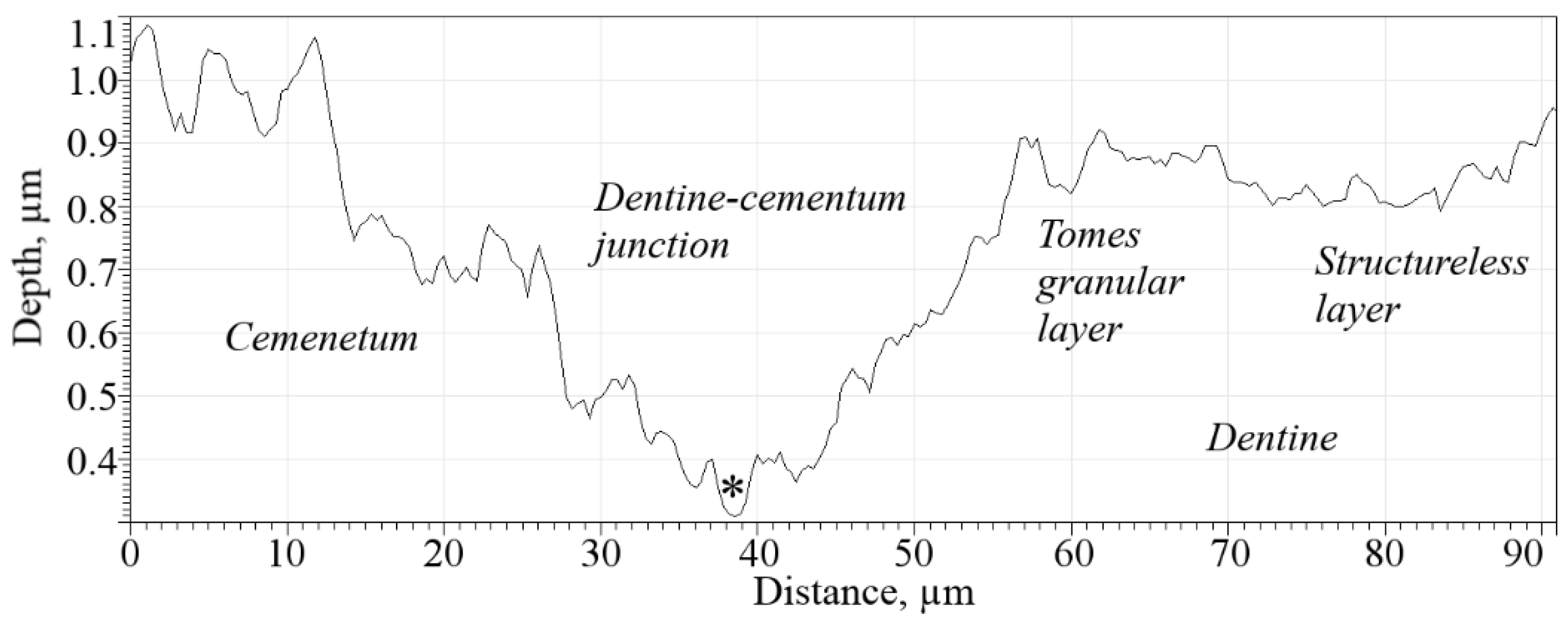
Table 2. The thickness of the acellular cementum layer was ~23.0 μm. The profile of the dentine–cementum junction is shown in
Figure 6.
As additional measurements, a study of the mechanical characteristics of the dentine in the tooth crown (additional areas 1 and 2,
Figure 7) and the dentine of the cervical region near the dentine–enamel junction (in area 1) was made. The results are listed in
Table 3, and they are generally within the range of values from the literature [
44,
45,
46].
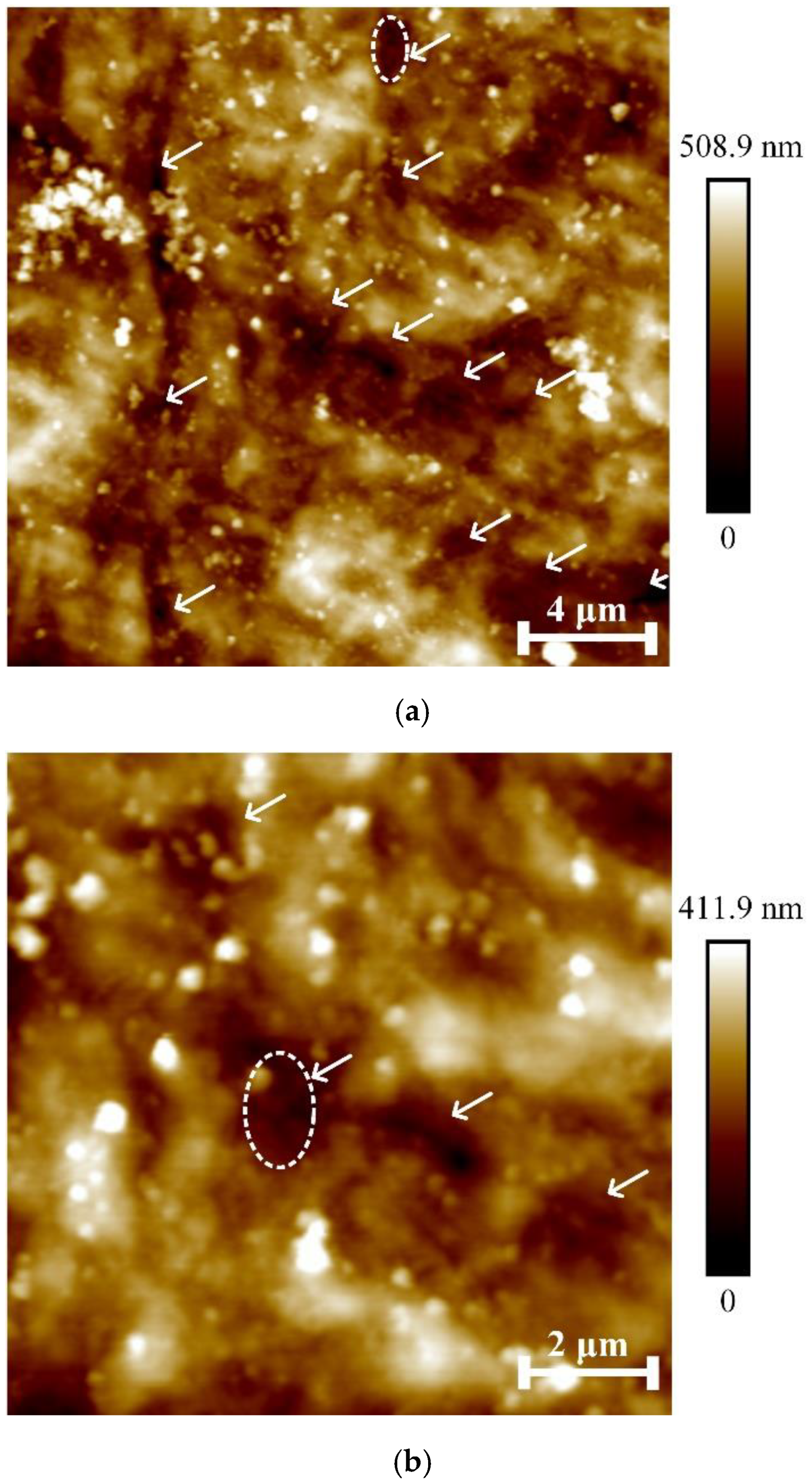
Figure 8 shows the surface of the cellular cementum from
Figure 5 on a smaller scan field (compared to
Figure 5). White arrows indicate depressions empty after the apoptosis of cementocytes lacunae.
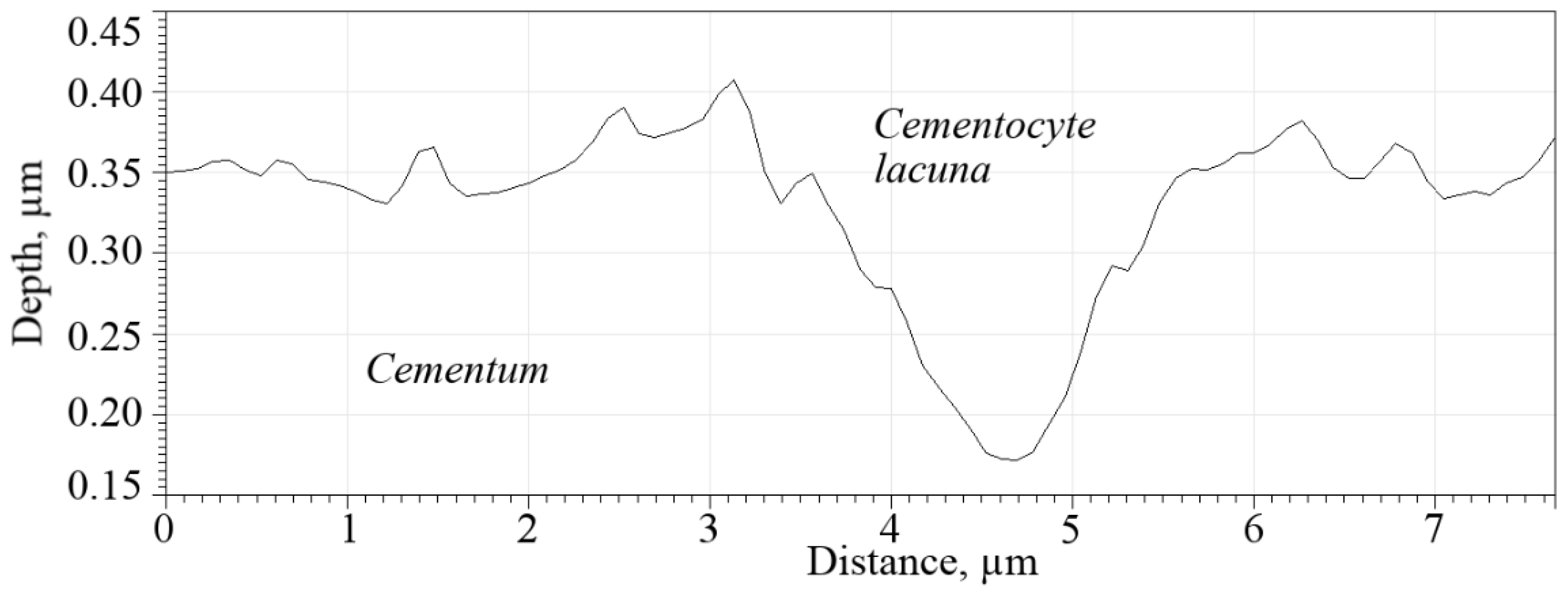
Figure 9 shows the profile of the topmost gap in
Figure 8a (marked with a dotted oval). It can be seen that the bottom of the lacuna is convex without irregularities, which indicates that there are no cementocyte residues left on its bottom. The depth of the lacuna is ~0.20 µm.
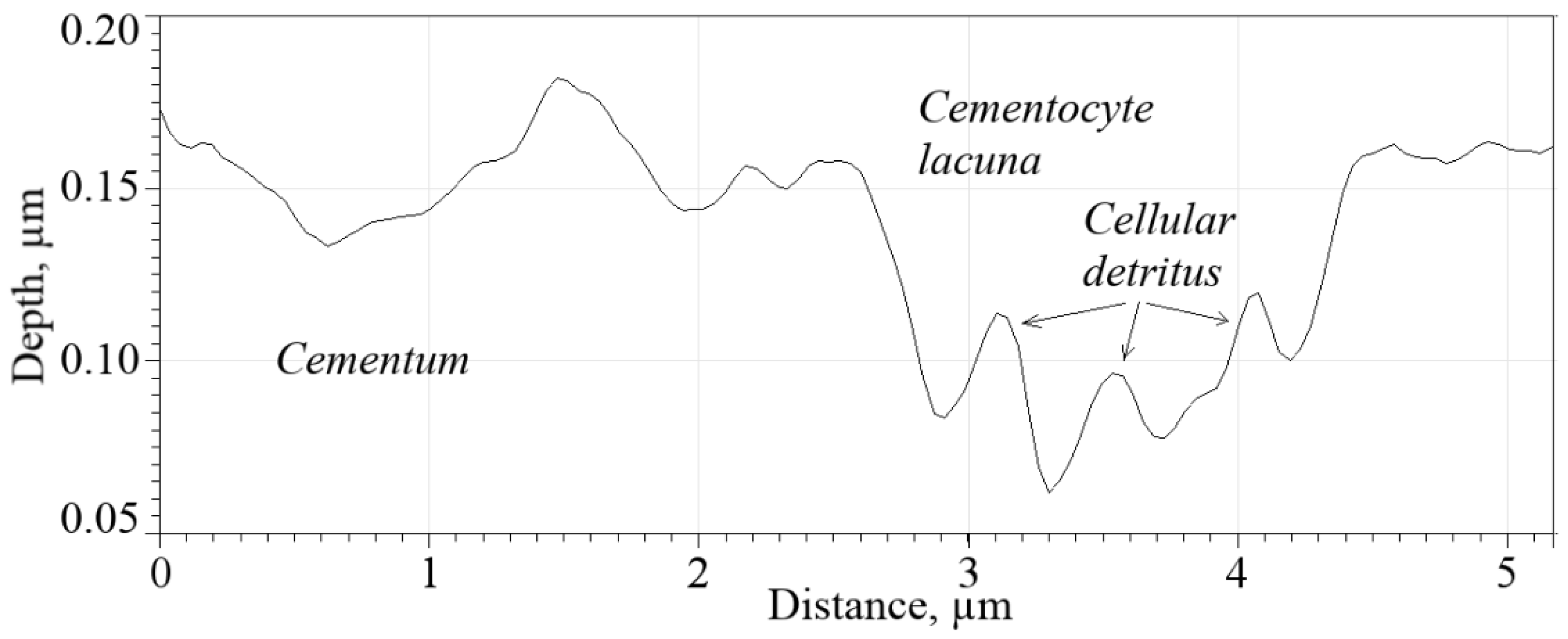
Figure 10 shows the leftmost lacuna from
Figure 8b (marked by a dotted oval); the bottom of the lacuna has an uneven relief, which indicates that cell detritus has accumulated on it (a granular mass formed by necrotic tissue [
6]). The depth of the lacuna is ~0.10 µm.
A similar nature of the profiles was also observed for other lacunae, marked with arrows in
Figure 8. In this regard, in the future it seems promising to develop protocols for the preparation of dental root cementum for micromechanical testing, which allows preservation of the cellular structure of the tissue to a greater extent.
4. Discussion
In the present work, the properties of the tooth root cementum were investigated by applying a load along the dentine–cementum interface. At the same time, the quantitative values of the mechanical properties in each of the areas obtained in this work are close to those values in [
30], where the properties were studied by applying a load along the normal to the dentine–cementum junction and with part of the large set of values obtained in [
25]. Let us also note the closeness of the values of the Young’s modulus of the root cementum with the values obtained by Ho et al. [
29]. The difference in mechanical properties between
Section 2 and
Section 3 is similar to that from [
21]. (However, the quantitative values differ, presumably due to the use of different types of indenters and the methodology for analyzing the results). On the whole, the nature of the difference in properties of the type “growth from
Section 1 to
Section 2, then decrease to
Section 3” is also characteristic of the observations [
30], from which it can be assumed that such a behavior of the mechanical properties of cementum is inherent, not only in its surface, but also throughout the volume. Attention is drawn to the microgeometrical characteristics, which, in contrast to the mechanical properties, practically do not change, amounting to 151.3 ± 3 nm. It is well established that some factors can affect the roughness of cementum, such as acidic beverages [
47] or polishing techniques applied by the dental clinician [
48]. In this regard, the latter observation provides a basis for further ex vivo studies of either cementum alteration under certain conditions or efficacy of dental treatments on recovery of the natural roughness parameters. Besides, the closeness in the roughness of different anatomical parts of root cementum may lead to simplification of the cementum–periodontal ligament interface mathematical models [
49,
50].
A monotonous decrease in
Ra in the direction from the cellular cementum to the dentine by 38.5% was observed. A decrease in
Rt, with the difference that this value for the acellular cementum is higher than that for the cellular cementum by 11.74%, was also demonstrated. This phenomenon is also visible visually in
Figure 5, where acellular cementum forms a ridge along the dentine–cementum junction.
Another interesting effect was noted for the dentine. Thus, by conducting atomic force microscopy on the tooth crown dentine (an additional area, marked with a circular marker in
Figure 2), we obtained the following values for the microgeometrical characteristics:
Ra = 37.0 ± 17 nm, and
Rt = 210, 8 ± 101.3 nm (similar to the previous measurements: 5 vertical and 5 horizontal profiles, excluding the dentinal tubule,
Figure 7). These values are, respectively, 24.8% and 6.4% lower than the values for dentine in the vicinity of the junction with the cementum. Presumably, the relief of dentine becomes more developed as it approaches the cementum.
The dentine–cementum junction is represented as a discernible layer 10–15 µm thick that attaches bulk cementum to root dentine. Structurally, the junction can be considered as a region of interspaced collagen fiber bridges (
cfb on
Figure 5) formed during development after the breakdown of Hertwig’s epithelial root sheath [
51,
52,
53]. The pore-like structures found between bulk cementum and dentine (marked with * on
Figure 5 and
Figure 6) are presumably the remnants of Hertwig’s epithelial root sheath after its breakdown, also known as the epithelial rests of Malassez [
51,
52,
53]. Their measured diameter was 2.71 ± 0.42 µm. Tomes granular layer (marked
tgl on
Figure 5 and also shown in
Figure 6) [
54] appears to be more structurally sound after the sample preparation, which speaks of its higher mechanical properties compared to the structureless layer (marked
sll in
Figure 5 and also shown in
Figure 6). This fact leads to formation of another ridge by the Tomes granular layer although not so pronounced as the one formed by acellular cementum. Understanding the micro- and nanostructure of the epithelial rests of Malassez in further works (presumably with scanning electron microscopy) is crucial due to their participation in the development of radicular cysts [
55,
56].
From the theoretical studies of Shaw et al. [
57], it is known that the stresses distributed across cementum (root surface) are much higher than the stresses distributed across the comparable regions of dentine emerging as a result of such forces as rotation, tipping (labial–lingual, mesial–distal) and extrusive/intrusive loads. However, the model [
57] did not take into account the dentine–cementum junction, which from the results of the current research is represented as a separate region with a rather complex structure. Thus, for the construction of further models, taking account the junction of the finite thickness (namely 9.95 µm measured by the white segment near the
dcj notation in
Figure 5—the thickness was uniform in the observed area) and its mechanical properties would be beneficial for more accurate calculation of stress distribution across the tooth root.
At the same time, there is a decrease in the reduced Young’s modulus by 5.8% and indentation hardness by 29.4% in the cervical region compared to the coronal part, which is presumably due to the proximity of the dentine–cementum junction and a change in the dentine microstructure for a more optimal formation of the junction from the point of view of tooth structure.
5. Conclusions
In the present work, the mechanical and microgeometrical properties of the tooth root cementum were studied on a longitudinal cross-section of a human molar. Analysis of the obtained experimental data allowed us to draw the following conclusions:
- –
mechanical properties increase from the cervical region to the central part of the root, then decrease again towards the apex of the tooth root;
- –
microgeometrical characteristics, in contrast to mechanical properties, practically do not change from the cervical region to the apex of the tooth root, amounting to 151.3 ± 3 nm;
- –
monotonic decrease in Ra in the direction from cellular cementum to dentine was 38.5%;
- –
a decrease in Rt in the direction from cellular cementum to dentine was observed, except that this value for acellular cementum is higher than that for the cellular cementum by 11.74%; acellular cementum forms a ridge along the dentine–cementum junction;
- –
a decrease in the reduced Young’s modulus of dentine by 5.8% and indentation hardness by 29.4% in the cervical area compared to the crown part of the tooth was found;
- –
Ra and Rt of the coronal dentine, respectively, are 24.8% and 6.4% lower than the values of the properties of dentine in the vicinity of the junction with cementum—this change is associated with a change in the microstructure of dentine near its junction with cementum;
- –
AFM investigation of the dentine–cementum junction with high resolution revealed the interspaced collagen fiber bridges and epithelial rests of Malassez, their measured diameter was 2.71 ± 0.42 µm and the thickness of the junction was 9.95 µm;
- –
Tomes granular layer demonstrated higher resistance against the sample preparation procedures compared to the structureless layer of dentine;
- –
the development of the relief of cementocyte lacunae is characterized by the presence or absence of cellular detritus in them.
The results of this work can provide important assistance to researchers and engineers involved in the design of dental implants, as well as practicing dental clinicians, to assess the dynamics of changes in the properties of cementum during pathological changes in this tissue.
Author Contributions
Conceptualization, E.S. and S.A.; methodology, V.L., T.K. and E.S.; software, V.L.; validation, E.S. and D.Y.; formal analysis, V.L. and T.K.; investigation, E.S., V.L., T.K. and D.Y.; resources, S.M.; data curation, E.S.; writing—original draft preparation, E.S.; writing—review and editing, S.A., V.L., T.K. and D.Y.; visualization, V.L., T.K. and E.S.; supervision, S.A.; project administration, S.M.; funding acquisition, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
E.S., S.M. and S.A. were supported by the Government of the Russian Federation, grant number 14.Z50.31.0046. V.L. and T.K. were supported by the grant of the Belarusian Republican Foundation for Fundamental Research BRFFR No. F18R-239.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Local Independent Ethics Committee of Rostov State Medical University (protocol code 13/22, date of approval: 8 September 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ongoing character of the research.
Acknowledgments
The authors thank Michael Vincent Swain for his advice and support. Sample preparation and analysis was conducted in the Nanocenter of Don State Technical University (
http://nano.donstu.ru, accessed date 26 September 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamaguchi, M.; Mishima, H. The Role of RANKL and Involvement of Cementum in Orthodontic Root Resorption. Appl. Sci. 2021, 11, 7244. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Selvig, K.A. Dental cementum: The dynamic tissue covering of the root. Periodontology 2000 1997, 13, 41–75. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.T.; Le, L.H.; Kaipatur, N.R.; Major, P.W. Imaging the cemento-enamel junction using a 20-MHz ultrasonic transducer. Ultrasound Med. Biol. 2016, 42, 333–338. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y. Teeth. In Advanced Ceramics for Dentistry; Butterworth-Heinemann: Oxford, UK, 2014; pp. 5–21. [Google Scholar]
- Ho, S.P.; Kurylo, M.P.; Fong, T.K.; Lee, S.S.; Wagner, H.D.; Ryder, M.I.; Marshall, G.W. The biomechanical characteristics of the bone-periodontal ligament-cementum complex. Biomaterials 2010, 31, 6635–6646. [Google Scholar] [CrossRef]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum proteins: Role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontology 2000 2015, 67, 211–233. [Google Scholar] [CrossRef]
- Yamamoto, T.; Domon, T.; Takahashi, S.; Islam, N.; Suzuki, R. Twisted plywood structure of an alternating lamellar pattern in cellular cementum of human teeth. Anat. Embryol. 2000, 202, 25–30. [Google Scholar] [CrossRef]
- Lee, C.; Darling, C.L.; Fried, D. Polarization-sensitive optical coherence tomographic imaging of artificial demineralization on exposed surfaces of tooth roots. Dent. Mat. 2009, 25, 721–728. [Google Scholar] [CrossRef]
- Feller, L.; Khammissa, R.A.; Thomadakis, G.; Fourie, J.; Lemmer, J. Apical external root resorption and repair in orthodontic tooth movement: Biological events. BioMed Res. Int. 2016, 2016, 4864195. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H. Biomaterial-based approaches for regeneration of periodontal ligament and cementum using 3D platforms. Int. J. Mol. Sci. 2019, 20, 4364. [Google Scholar] [CrossRef]
- Gualdi-Russo, E.; Saguto, I.; Frisoni, P.; Neri, M.; Rinaldo, N. Tooth Cementum Thickness as a Method of Age Estimation in the Forensic Context. Biology 2022, 11, 784. [Google Scholar] [CrossRef]
- Yamamoto, H.; Niimi, T.; Yokota-Ohta, R.; Suzuki, K.; Sakae, T.; Kozawa, Y. Diversity of acellular and cellular cementum distribution in human permanent teeth. J. Hard Tissue Biol. 2009, 18, 40–44. [Google Scholar] [CrossRef]
- Morgenthal, A.; Zaslansky, P.; Fleck, C. Cementum thickening leads to lower whole tooth mobility and reduced root stresses: An in silico study on aging effects during mastication. J. Struct. Biol. 2021, 213, 107726. [Google Scholar] [CrossRef] [PubMed]
- Dastmalchi, R.; Poison, A.; Bouwsma, O.; Proskin, H. Cementum thickness and mesial drift. J. Clin. Periodontol. 1990, 17, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Zaytsev, D. Mechanical properties of human enamel under compression: On the feature of calculations. Mater. Sci. Eng. C 2016, 62, 518–523. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Swain, M.V. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. J. Mech. Behav. Biomed. Mater. 2008, 1, 18–29. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Du, W.; Zhou, X.D.; Yu, H.Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef]
- Sadyrin, E.; Swain, M.; Mitrin, B.; Rzhepakovsky, I.; Nikolaev, A.; Irkha, V.; Yogina, D.; Lyanguzov, N.; Maksyukov, S.; Aizikovich, S. Characterization of enamel and dentine about a white spot lesion: Mechanical properties, mineral density, microstructure and molecular composition. Nanomaterials 2020, 10, 1889. [Google Scholar] [CrossRef]
- Cuy, J.L.; Mann, A.B.; Livi, K.J.; Teaford, M.F.; Weihs, T.P. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 2002, 47, 281–291. [Google Scholar] [CrossRef]
- Sadyrin, E.V. Correlating the Mechanical Properties to the Mineral Density of Brown Spot Lesion in Dentine Using Nanoindentation and X-ray Micro-tomography. In Advanced Materials Modelling for Mechanical, Medical and Biological Applications; Altenbach, H., Eremeyev, V., Galybin, A., Vasiliev, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 389–398. [Google Scholar]
- Sadyrin, E.V.; Yogina, D.V.; Swain, M.V.; Maksyukov, S.Y.; Vasiliev, A.S. Efficacy of dental materials in terms of apparent mineral density restoration: Composite resin, glass ionomer cement and infiltrant. Compos. Part C Open Access 2021, 6, 100192. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Avilov, A.V.; Avilova, N.V.; Tananakina, E.S.; Sadyrin, E.V. Modelling of the stress-strain state of the lower jaw. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1029, 012071. [Google Scholar] [CrossRef]
- Yeo, I.S.L. Modifications of dental implant surfaces at the micro-and nano-level for enhanced osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Hodge, H.C.; McKay, H. The microhardness of teeth. J. Amer. Dent. Assoc. 1933, 20, 227–233. [Google Scholar]
- Poolthong, S. Determination of the Mechanical Properties of Enamel Dentine and Cementum by an Ultra Micro-Indentation System. Ph.D. Thesis, The University of Sydney, Sydney, Australia, 1998. [Google Scholar]
- Ho, S.P.; Senkyrikova, P.; Marshall, G.W.; Yun, W.; Wang, Y.; Karan, K.; Li, C.; Marshall, S.J. Structure, chemical composition and mechanical properties of coronal cementum in human deciduous molars. Dent. Mater. 2009, 25, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.P.; Marshall, S.J.; Ryder, M.I.; Marshall, G.W. The tooth attachment mechanism defined by structure, chemical composition and mechanical properties of collagen fibers in the periodontium. Biomaterials 2007, 28, 5238–5245. [Google Scholar] [CrossRef]
- Ho, S.P.; Yu, B.; Yun, W.; Marshall, G.W.; Ryder, M.I.; Marshall, S.J. Structure, chemical composition and mechanical properties of human and rat cementum and its interface with root dentin. Acta Biomater. 2009, 5, 707–718. [Google Scholar] [CrossRef]
- Foster, B.L. Methods for studying tooth root cementum by light microscopy. Int. J. Oral Sci. 2012, 4, 119–128. [Google Scholar] [CrossRef]
- Jang, A.T.; Lin, J.D.; Choi, R.M.; Choi, E.M.; Seto, M.L.; Ryder, M.I.; Gansky, S.A.; Curtis, D.A.; Ho, S.P. Adaptive properties of human cementum and cementum dentin junction with age. J. Mech. Behav. Biomed. Mater 2014, 39, 184–196. [Google Scholar] [CrossRef]
- Chutimanutskul, W.; Darendeliler, M.; Swain, M.V.; Shen, G.; Petocz, P. Physical properties of human premolar cementum: Hardness and elasticity. Aust. Orthod. J. 2005, 21, 117–121. [Google Scholar]
- Malek, S.; Darendeliler, M.A.; Rex, T.; Kharbanda, O.P.; Srivicharnkul, P.; Swain, M.V.; Petocz, P. Physical properties of root cementum: Part 2. Effect of different storage methods. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 561–570. [Google Scholar] [CrossRef]
- Malek, S.; Darendeliler, M.A.; Swain, M.V. Physical properties of root cementum: Part I. A new method for 3-dimensional evaluation. Amer. J. Orthod. Dentofac. Orthop. 2001, 120, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Srivicharnkul, P.; Kharbanda, O.P.; Swain, M.V.; Petocz, P.; Darendeliler, M.A. Physical properties of root cementum: Part 3. Hardness and elastic modulus after application of light and heavy forces. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Rex, T.; Kharbanda, O.P.; Petocz, P.; Darendeliler, M.A. Physical properties of root cementum: Part 4. Quantitative analysis of the mineral composition of human premolar cementum. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Darendeliler, M.A. Physical properties of root cementum: Part 5. Volumetric analysis of root resorption craters after application of light and heavy orthodontic forces. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 186–195. [Google Scholar] [CrossRef]
- Radermacher, C.; Malyaran, H.; Craveiro, R.B.; Peglow, S.; Behbahani, M.; Pufe, T.; Wolf, M.; Neuss, S. Mechanical Loading on Cementoblasts: A Mini Review. Osteologie 2022, 31, 111–118. [Google Scholar] [CrossRef]
- Hinrichs, C.; Nicklisch, N.; Mardare, C.C.; Orechovski, B.; Hassel, A.W.; Kleber, C.; Alt, K.W. Incremental lines in human acellular tooth cementum–New insights by SEM analysis. Ann. Anat.-Anat. Anz. 2022, 243, 151933. [Google Scholar] [CrossRef] [PubMed]
- Arefnia, B.; Koller, M.; Wimmer, G.; Lussi, A.; Haas, M. In vitro study of surface changes induced on enamel and cementum by different scaling and polishing techniques. Oral Health Prev. Dent. 2021, 19, 85–92. [Google Scholar] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Colard, T.; Falgayrac, G.; Bertrand, B.; Naji, S.; Devos, O.; Balsack, C.; Delannoy, Y.; Penel, G. New insights on the composition and the structure of the acellular extrinsic fiber cementum by Raman analysis. PLoS ONE 2016, 11, e0167316. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Mishima, H. Hardness Variations and Chemical Compositional Changes in Human Cementum. J. Oral Tissue Eng. 2021, 19, 1–9. [Google Scholar] [CrossRef]
- Angker, L.; Swain, M.V.; Kilpatrick, N. Micro-mechanical characterisation of the properties of primary tooth dentine. J. Dent. 2003, 31, 261–267. [Google Scholar] [CrossRef]
- Mahoney, E.; Holt, A.; Swain, M.; Kilpatrick, N. The hardness and modulus of elasticity of primary molar teeth: An ultra-micro-indentation study. J. Dent. 2000, 28, 589–594. [Google Scholar] [CrossRef]
- Senawongse, P.; Otsuki, M.; Tagami, J.; Mjör, I. Age-related changes in hardness and modulus of elasticity of dentine. Arch. Oral Biol. 2006, 51, 457–463. [Google Scholar] [CrossRef]
- Rajeev, G.; Lewis, A.J.; Srikant, N. A time based objective evaluation of the erosive effects of various beverages on enamel and cementum of deciduous and permanent teeth. J. Clin. Exp. Dent. 2020, 12, e1–e8. [Google Scholar] [CrossRef]
- Chowdhary, Z.; Mohan, R. Efficiency of three different polishing methods on enamel and cementum: A scanning electron microscope study. J. Indian Soc. Periodontol. 2018, 22, 18–24. [Google Scholar] [PubMed]
- Fill, T.S.; Toogood, R.W.; Major, P.W.; Carey, J.P. Analytically determined mechanical properties of, and models for the periodontal ligament: Critical review of literature. J. Biomech. 2012, 45, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, Y.; Shi, X.; Zhang, C. Tensile creep mechanical behavior of periodontal ligament: A hyper-viscoelastic constitutive model. Comput. Methods Programs Biomed. 2021, 207, 106224. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Ito, Y.; Diekwisch, T.G. Evolution and development of Hertwig’s epithelial root sheath. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontology 2000 2006, 40, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, W.; Chen, J.; Chen, G.; Tian, W.; Bai, D. Are Hertwig’s epithelial root sheath cells necessary for periodontal formation by dental follicle cells? Arch. Oral Biol. 2018, 94, 1–9. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Chaussain, C.; Osdoby, P.; Brandi, M.L.; Clarke, B.; Thakker, R.V. The role of biomineralization in disorders of skeletal development and tooth formation. Nat. Rev. Endocrinol. 2021, 17, 336–349. [Google Scholar] [CrossRef]
- Lin, L.M.; Ricucci, D.; Kahler, B. Radicular cysts review. JSM Dent. Surg. 2017, 2, 1017.1–1017.3. [Google Scholar]
- Jimson, S.; Sankari, L.; Bhanumurthy, L.; Julius, A. Radicular cyst of jaw: A Review. Indian J. Public Health Res. Dev. 2019, 10, 3229–3232. [Google Scholar] [CrossRef]
- Shaw, A.M.; Sameshima, G.T.; Vu, H.V. Mechanical stress generated by orthodontic forces on apical root cementum: A finite element model. Orthod. Craniofacial Res. 2004, 7, 98–107. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).