1. Introduction
Water is essential for all living beings, and studies concerning the dynamics and structures of water have long been one of the hot topics in the research field [
1,
2]. Water exhibits different structures depending on its temperature [
3,
4,
5]. It has recently been discovered that water possess two different temperature-dependent hydrogen-bonded structures [
6,
7]. When the temperature is closer to its freezing point, water molecules form tetrahedrally bonded clusters that are similar to those in ice [
8], i.e., cold water is anisotropic [
9], Water can form a maximum of four hydrogen bonds [
10] by accepting or donating two electrons. This allows water to have unusual properties that contribute to its high surface tension, maximum density at 4 °C, decrease in density at low temperatures, etc. When temperature increases, more distorted hydrogen bonds form. This results in the loss of enthalpy due to the breaking of hydrogen bonds, but an increase in entropy due to the enhanced van der Waals interactions [
11,
12]. The competition between the enthalpy and entropy factors is the reason water is the densest at 4 °C. When the temperature is greater than 4 °C, the entropy gradually dominates, so fewer directional hydrogen bonds are observed, i.e., warm water is isotropic.
Water is an important intermediate, and solvent, in physical, biological, and chemical processes. We hypothesize that the two structures of water at various temperatures may affect these processes, such as forming different crystal structures, increasing selectivity in chemical and biochemical reactions, and even regulating biofunctions of living systems, etc. Fundamental knowledge of these effects is critical for further exploration into various applications. However, to the best of our knowledge, few reports have demonstrated such an effect. We intended to design a series of experiments to verify this hypothesis. In this work, we first targeted the temperature effect on the surface stress of water, which is defined as the amount of reversible work to stretch the water surface.
The surface stress of water is known to be isotropic, which has been evidenced by various demonstrations on water surface stress. Due to the two structures of water at various temperatures, we posit that the surface stress of water will change to anisotropic when the temperature is closer to 0 °C. To confirm this, droplets of melted candle wax were allowed to fall on the surface of water and the shapes of the solidified candle droplets in contact with the surface of water were studied. The results from this simple yet effective experiment showed that the two temperature-dependent structures of water do affect isotropic property of water surface. To the best of our knowledge, this is the first example of a solidification process that can be affected by the two structures of water (and possibly the first example of a physical process that can be affected by the two structures of water). We used the candle droplet at a millimeter score, but we anticipate the shape of solidified particles at a micro or nano scale can be controlled using the same technique.
2. Materials and Methods
A white candle made of paraffin wax was used. The candle was melted and then transferred, dropwise, using a disposable glass Pasteur pipet into water and other solutions. The tip of the pipet was shortened to ensure the melted candle droplets fell from the pipet drop-by-drop. The pipet was occasionally heated to ensure it was not blocked by solidified wax. The melted candle wax was dripped into tap water, oil, and sodium dodecyl sulfate/water mixture at a height of 1 and 8 cm above the surface of the solution to investigate the shape of the solidified candle droplets.
3. Results and Discussion
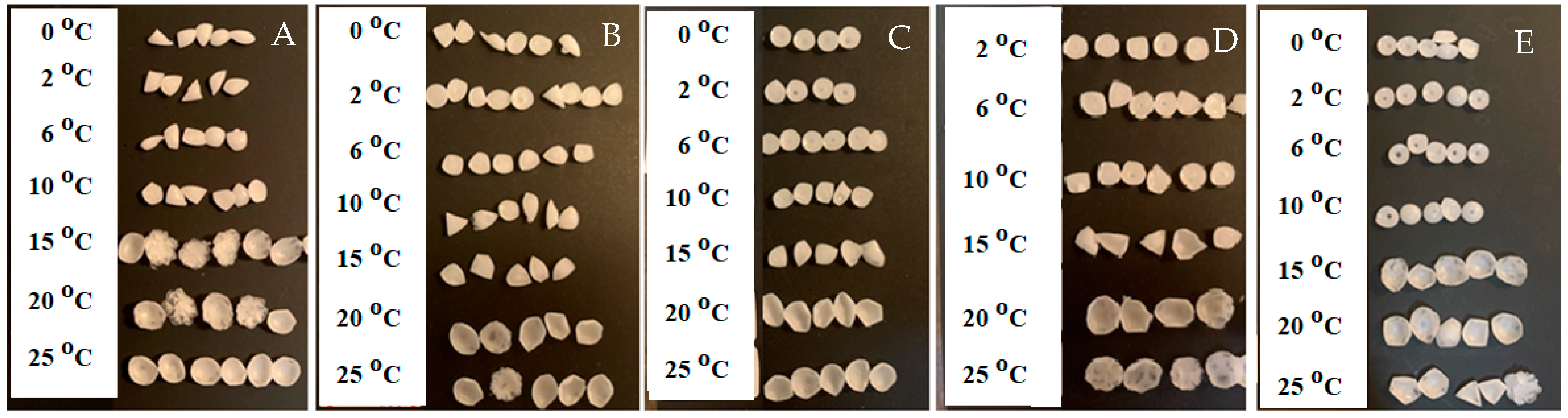
This experiment was performed using pure water, corn oil, and a sodium dodecyl sulfate (SDS)/water mixture of 0.1 mM, 0.25 mM, 0.5 mM, 1 mM, and 3 mM. Melted candle wax was dripped into the aqueous solution, and corn oil, at a height of 1 cm and 8 cm, and at temperatures of 0 °C, 2 °C, 6 °C, 10 °C, 15 °C, 20 °C, and 25 °C.
3.1. Temperature Effect on the Formed Shapes of Candle Drops
The temperature effect on the shape of solidified droplets of melted candle wax dripped into water was first investigated. Candles are typically made of paraffin wax, which has a melting point range of 46–68 °C. When the temperature of the liquid that the melted candle wax was dripped into was changed, different shapes were observed.
When the melted candle wax was added dropwise to corn oil, at any of the above listed temperatures, the solidified candle droplets remained round or oval (
Figure 1 Left). However, when the melted candle wax was added dropwise to water, the temperature of the water had a significant effect on the shape of the solidified candle droplet formed on the surface of the water. When the temperature of the water was
6 °C, all of the solidified candle droplets exhibited sharp edges (
Figure 1 Middle). The shapes formed include triangle, quadrangle, etc. However, as the temperature of the water was increased to ≥10 °C (
Figure 1 Right), most of the solidified candle droplets adapted a more curved, or round, shape. These results indicate that the temperature of water plays a significant role in determining the shape of the solidified candle droplets.
When comparing corn oil and water, the major difference is the much greater surface tension displayed by water. At 25 °C, the surface tension of corn oil is 31.6 mN/m, while the surface tension of water is 72 mN/m [
13,
14]. For water, as the temperature of water is decreased to 2 °C, its surface tension increases to 75 mN/m due to the change in the structure of water [
13]. It is known that surface tension can create sharp edges and corners in either a solidification process or a viscous liquid [
15,
16]. When the temperature is ≤6 °C, the candle droplets solidified with sharp edges or lines, indicating an anisotropic solidification process. This phenomenon can be attributed to the anisotropic breaking of the directional hydrogen bonds present in cold water. When the temperature is >10 °C, the hydrogen bonds are not directional (i.e., water molecules are randomly oriented). Therefore, a majority of the solidified candle droplets were round or oval, a behavior indicative of isotropic solidification of the liquid candle droplets.
3.2. Sufactant Effect
To confirm that the shape of the solidified candle droplets was mostly dictated by the structure of the water molecules, a surfactant, SDS, was added to the water and the shape of the solidified candle droplets was investigated. Surfactants are known to significantly lower the surface tension of water [
17], i.e., weakening both hydrogen bonding and van der Waals interactions between individual water molecules. This results in a change of the structure of the water molecules, such that only distorted hydrogen bonds are observed between the water molecules [
18,
19], i.e., water is isotropic even at low temperature in the presence of surfactants.
Figure 2 shows the solidified candle droplets in the presence of various concentrations of SDS.
Figure 2 demonstrates that the shape of the solidified candle droplet is indeed affected by the presence of a surfactant (in this experiment SDS). When the concentration of SDS is ≥0.5 mM, no matter the temperature, the solidified candle droplets are round or oval. When the SDS concentration is <0.5 mM, and the temperature is <10 °C, all of the solidified candle droplets exhibit sharp edges. The shape of the candle droplets in the presence of SDS concentrations < 0.5 mM, and temperatures < 10 °C, were, therefore, similar to those observed in pure water. It is noteworthy that some triangle shapes of the droplets under 25 °C at 3 mM SDS concentration were observed, which is contradictory to what we observed in other conditions. Since the SDS concentration is close to its critical micelle concentration (cmc) at 8 mM, one possible explanation is that the cmc may be another factor that contributes to the isotropic property of the solution, which will be studied and reported in due time.
In summary, the results suggest that addition of surfactants in water blocks the conversion of surface stress of water from isotropic to anisotropic when the temperature is ≤6 °C.
A concentration of 0.5 mM SDS appears to be critical in these experiments since any concentration equivalent to this, or greater, results in a round shape of the solidified candle droplets. A unique shape was observed when the SDS solutions were >0.5 mM and the temperature was <10 °C. Under these conditions, the candle droplets formed a concave “bowl”.
Figure 3 shows the top (A) and side view (B) of the concave bowl. The bowl always formed with the portion carved out facing upward. This may due to the surface coverage of the SDS molecules on the water-air interface since it is the first thing the droplet comes in contact with when it hits the surface of the water. It is known that SDS self-assembles into a monolayer on the surface of water.
16 Reports show that the surface coverage of the SDS monolayer is around 50% when the concentration is 0.5 mM. A large surface coverage of the SDS monolayer means low thermal conductivity of the water/SDS surface (The thermal conductivity of alkane and water are 0.15 and 0.6 W/(mK), respectively). When the candle droplet encounters the surface of the water/SDS mixture, the outer layer of the candle droplet quickly cools to a solid upon impact. However, the wax in the middle of the droplet is still liquid due to the low thermal conductivity of SDS coverage. Therefore, the liquid wax in the middle of the droplet is pushed to the edges due to the force of impact with the surface of the water/SDS solution. To confirm this hypothesis, melted candle wax was dripped into the water/SDS mixture at a height of 1 cm. Only round drops were formed due to the less forceful impact. This is consistent with our hypothesis.
4. Conclusions
Our experiments demonstrate that the shape of the candle droplets solidified at the air-water interface are affected by the two temperature-dependent structures of water. The results seem to indicate that the two different structures of water that arise at various temperatures should not only be considered for physical processes but also for various chemical and biological processes. Examples of these processes include the shape of particles formed in water at micrometer or nanometer scale, crystallization of organic and inorganic chemicals in water, chemical reactions in water, purification of contaminated water, etc.
Author Contributions
Conceptualization, H.-F.J.; methodology, H.-F.J.; investigation, A.-X.X. and N.R.; data curation, A.-X.X. and N.R.; writing—original draft preparation, A.-X.X., N.R. and H.-F.J.; writing—review and editing, A.-X.X., N.R. and H.-F.J.; supervision, H.-F.J.; project administration, H.-F.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Acknowledgments
We thanks Drexel for support of the students.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brini, E.; Fennell, C.J.; Fernandez-Serra, M.; Hribar-Lee, B.; Lukšič, M.; Dill, K.A. How Water’s Properties Are Encoded in Its Molecular Structure and Energies. Chem. Rev. 2017, 117, 12385–12414. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, L.G.M.; Henchman, R.H.; Nilsson, A. Water—The Most Anomalous Liquid. Chem. Rev. 2016, 116, 7459–7462. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.H.; Sciortino, F.; Essmann, U.; Stanley, H.E. Phase behaviour of metastable water. Nature 1992, 360, 324–328. [Google Scholar] [CrossRef]
- Soper, A.K. Is water one liquid or two? J. Chem. Phys. 2019, 150, 234503. [Google Scholar] [CrossRef] [PubMed]
- Mishima, O.; Stanley, H.E. The relationship between liquid, supercooled and glassy water. Nature 1998, 396, 329–335. [Google Scholar] [CrossRef]
- Pettersson, L.G.M.; Nilsson, A. The structure of water; from ambient to deeply supercooled. J. Non-Crystal. Solids 2015, 407, 399–417. [Google Scholar] [CrossRef]
- Kühne, T.D.; Khaliullin, R.Z. Electronic signature of the instantaneous asymmetry in the first coordination shell of liquid water. Nat. Commun. 2013, 4, 1450. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ross, D.K. Evidence for two kinds of hydrogen bond in ice. Nature 1993, 365, 327–329. [Google Scholar] [CrossRef]
- Nilsson, A.; Pettersson, L.G.M. Perspective on the structure of liquid water. Chem. Phys. 2011, 389, 1–34. [Google Scholar]
- Nilsson, A.; Pettersson, L. The structural origin of anomalous properties of liquid water. Nat. Commun. 2015, 6, 8998. [Google Scholar] [PubMed]
- Huang, C.; Wikfeldt, K.T.; Tokushima, T.; Nordlund, D.; Harada, Y.; Bergmann, U.; Niebuhr, M.; Weiss, T.M.; Horikawa, Y.; Leetmaa, M. The inhomogeneous structure of water at ambient conditions. Proc. Natl. Acad. Sci. USA 2009, 106, 15214–15218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camisasca, G.; Schlesinger, D.; Zhovtobriukh, I.; Pitsevich, G.; Pettersson, L.G.M. A proposal for the structure of high- and low-density fluctuations in liquid water. J. Chem. Phys. 2019, 151, 034508. [Google Scholar] [PubMed]
- Karbalaei, A.; Kumar, R.; Cho, H.J. Thermocapillarity in Microfluidics—A Review. Micromachines 2016, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, S.N.; Rodriguez-Martinez, V.; O’Meara, M.; Farkas, B.E. Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: Measurement and modeling. Int. J. Food Prop. 2017, 20, 1965–1981. [Google Scholar] [CrossRef]
- Pomeau, Y.; Villermaux, E. Two Hundred Years of Capillarity Research. Phys. Today 2006, 59, 39. [Google Scholar] [CrossRef]
- Pomeau, Y. Surface Tension: From Fundamental Principles to Applications in Liquids and in Solids. In 5th Warsaw School of Statistical Kazimierz Dolny, Poland. Physics, 22–29 June 2013; Warsaw University Press: Warsaw, Poland, 2013; pp. 149–202. ISBN 978-83-235-1739-9. [Google Scholar]
- Abraham, M.H.; Chadha, H.S.; Dixon, J.P.; Rafols, C.; Treiner, C. Hydrogen bonding. Part 41.1 Factors that influence the distribution of solutes between water and hexadecylpyridinium chloride micelles. J. Chem. Soc. Perkin Trans. 2 1997, 1, 19–24. [Google Scholar] [CrossRef]
- Wu, N.; Li, X.; Liu, S.; Zhang, M.; Ouyang, S. Effect of Hydrogen Bonding on the Surface Tension Properties of Binary Mixture (Acetone-Water) by Raman Spectroscopy. Appl. Sci. 2019, 9, 1235. [Google Scholar] [CrossRef]
- Menger, F.M.; Rizvi, S.A.A. Relationship between Surface Tension and Surface Coverage. Langmuir 2011, 27, 13975–13977. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).