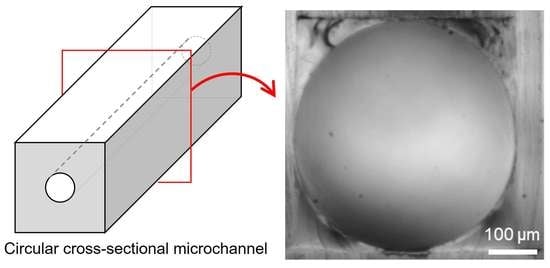
Fabrication of Irregularity-Free, Highly Circular Cross-Sectional Microchannel
Abstract
:1. Introduction
2. Fabrication of Circular Cross-Sectional Microchannel
2.1. Working Principle
2.2. Design of Microchannel
2.3. Fabrication Process of Circular Cross-Sectional Microchannel
3. Fabrication of Circular Cross-Sectional Microchannel
3.1. Experimental Setup
3.2. Parameters for Circular Cross-Section
3.3. Analysis of Microchannel Circularity
4. Results
4.1. Formation of Irregularity-Free Circular Cross-Sectional Microchannel
4.2. Circularity of Circular Cross-Section along Microchannel
4.3. Cross-Sectional Images
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef] [PubMed]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Duncombe, T.A.; Tentori, A.M.; Herr, A.E. Microfluidics: Reframing biological enquiry. Nat. Rev. Mol. Cell Biol. 2015, 16, 554–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weibel, D.B.; Whitesides, G.M. Applications of microfluidics in chemical biology. Curr. Opin. Chem. Biol. 2006, 10, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F. Microfluidic systems for diagnostic applications: A review. J. Lab. Autom. 2012, 17, 330–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preetam, S.; Nahak, B.K.; Patra, S.; Toncu, D.C.; Park, S.; Syväjärvi, M.; Orive, G.; Tiwari, A. Emergence of microfluidics for next generation biomedical devices. Biosens. Bioelectron. 2022, 10, 200206. [Google Scholar] [CrossRef]
- Lima, R.; Wada, S.; Tanaka, S.; Takeda, M.; Ishikawa, T.; Tsubota, K.; Imai, Y.; Yamaguchi, T. In vitro blood flow in a rectangular PDMS microchannel: Experimental observations using a confocal micro-PIV system. Biomed. Microdevices 2008, 10, 153–167. [Google Scholar] [CrossRef] [Green Version]
- Takeishi, N.; Ito, H.; Kaneko, M.; Wada, S. Deformation of a red blood cell in a narrow rectangular microchannel. Micromachines 2019, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Giannetto, M.; Decourcey, J.; Kang, H.; Kang, N.; Li, Y.; Zheng, S.; Zhao, H.; Simmons, W.R.; Wei, H.S.; et al. Oxygen tension-mediated erythrocyte membrane interactions regulate cerebral capillary hyperemia. Sci. Adv. 2019, 5, eaaw4466. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Shimamoto, T.; Ozaki, N.; Takaki, S.; Kuchimaru, T.; Kizaka-Kondoh, S.; Omata, T. Investigation of the influence of glucose concentration on cancer cells by using a microfluidic gradient generator without the induction of large shear stress. Micromachines 2016, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Grist, S.M.; Nasseri, S.S.; Laplatine, L.; Schmok, J.C.; Yao, D.; Hua, J.; Chrostowski, L.; Cheung, K.C. Long-term monitoring in a microfluidic system to study tumour spheroid response to chronic and cycling hypoxia. Sci. Rep. 2019, 9, 17782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Tithof, J.; Kelley, D.H.; Mestre, H.; Nedergaard, M.; Thomas, J.H. Hydraulic resistance of periarterial spaces in the brain. Fluids Barriers CNS 2019, 16, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, N.A.; Okkels, F.; Bruus, H. Reexamination of Hagen-Poiseuille flow: Shape dependence of the hydraulic resistance in microchannels. Phys. Rev. E 2005, 71, 057301. [Google Scholar] [CrossRef] [Green Version]
- Pivlin, I.V.; Peng, Z.; Karniadakis, G.E.; Buffet, P.A.; Dao, M.; Suresh, S. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 7804–7809. [Google Scholar]
- Ciftlik, A.T.; Ettori, M.; Gijs, M.A.M. High Throughput-Per-Footprint Inertial Focusing. Small 2013, 9, 2764–2773. [Google Scholar] [CrossRef]
- Mori, D.; Yano, K.; Tsubota, K.; Ishikawa, T.; Wada, S.; Yamaguchi, T. Simulation of platelet adhesion and aggregation regulated by fibrinogen and von Willebrand factor. Thromb. Haemost. 2008, 99, 108–115. [Google Scholar]
- He, F.; Xu, H.; Cheng, Y.; Ni, J.; Xiong, H.; Xu, Z.; Sugioka, K.; Midorikawa, K. Fabrication of microfluidic channels with a circular cross section using spatiotemporally focused femtosecond laser pulses. Opt. Lett. 2010, 35, 1106–1108. [Google Scholar] [CrossRef]
- Gallab, M.; Tomita, K.; Omata, S.; Arai, F. Fabrication of 3D capillary vessel models with circulatory connection ports. Micromachines 2018, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.-J.; Ho, K.-H.; Hsu, S.-H.; Wang, K.-P. Microvessel scaffold with circular microchannels by photoresist melting. Biomed. Microdevices 2007, 9, 657–663. [Google Scholar] [CrossRef]
- Nakano, T.; Itoyama, T.; Yoshida, K.; Sawada, Y.; Ikeda, S.; Fukuda, T.; Matsuda, T.; Neguro, M.; Arai, F. Multiscale fabrication of a transparent circulation type blood vessel simulator. Biomicrofluidics 2010, 4, 046505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelgawad, M.; Wu, C.; Chien, W.-Y.; Geddie, W.R.; Jewett, M.A.S.; Sun, Y. A fast and simple method to fabricate circular microchannels in polydimethylsiloxane (PDMS). Lab Chip 2011, 11, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, S.S. Shrinkage ratio of PDMS and its alignment method for the wafer level process. Microsyst. Technol. 2008, 14, 205–208. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inagaki, S.; Ishida, T. Fabrication of Irregularity-Free, Highly Circular Cross-Sectional Microchannel. Micro 2022, 2, 325-333. https://doi.org/10.3390/micro2020021
Inagaki S, Ishida T. Fabrication of Irregularity-Free, Highly Circular Cross-Sectional Microchannel. Micro. 2022; 2(2):325-333. https://doi.org/10.3390/micro2020021
Chicago/Turabian StyleInagaki, Satoru, and Tadashi Ishida. 2022. "Fabrication of Irregularity-Free, Highly Circular Cross-Sectional Microchannel" Micro 2, no. 2: 325-333. https://doi.org/10.3390/micro2020021
APA StyleInagaki, S., & Ishida, T. (2022). Fabrication of Irregularity-Free, Highly Circular Cross-Sectional Microchannel. Micro, 2(2), 325-333. https://doi.org/10.3390/micro2020021