Microbial Interactions with Particulate and Floating Pollutants in the Oceans: A Review
Abstract
1. Introduction
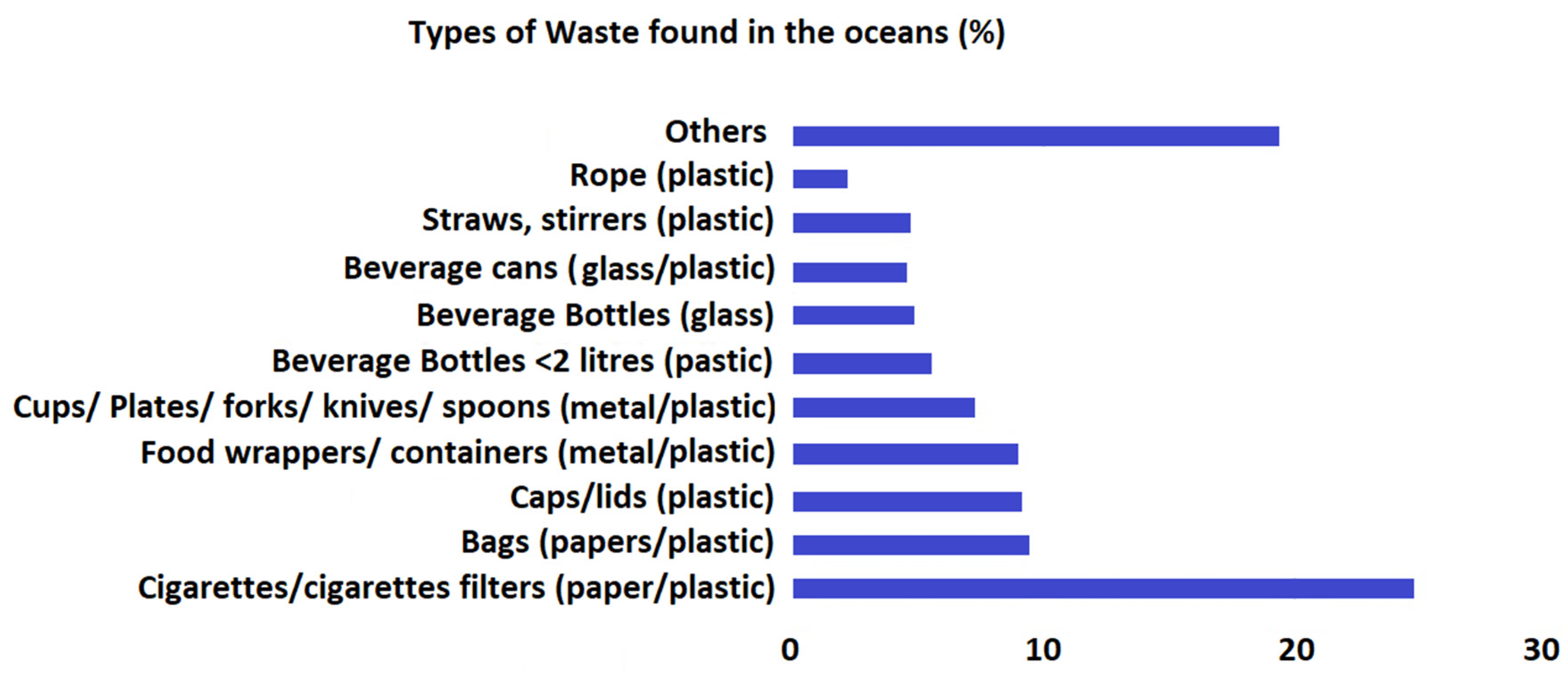
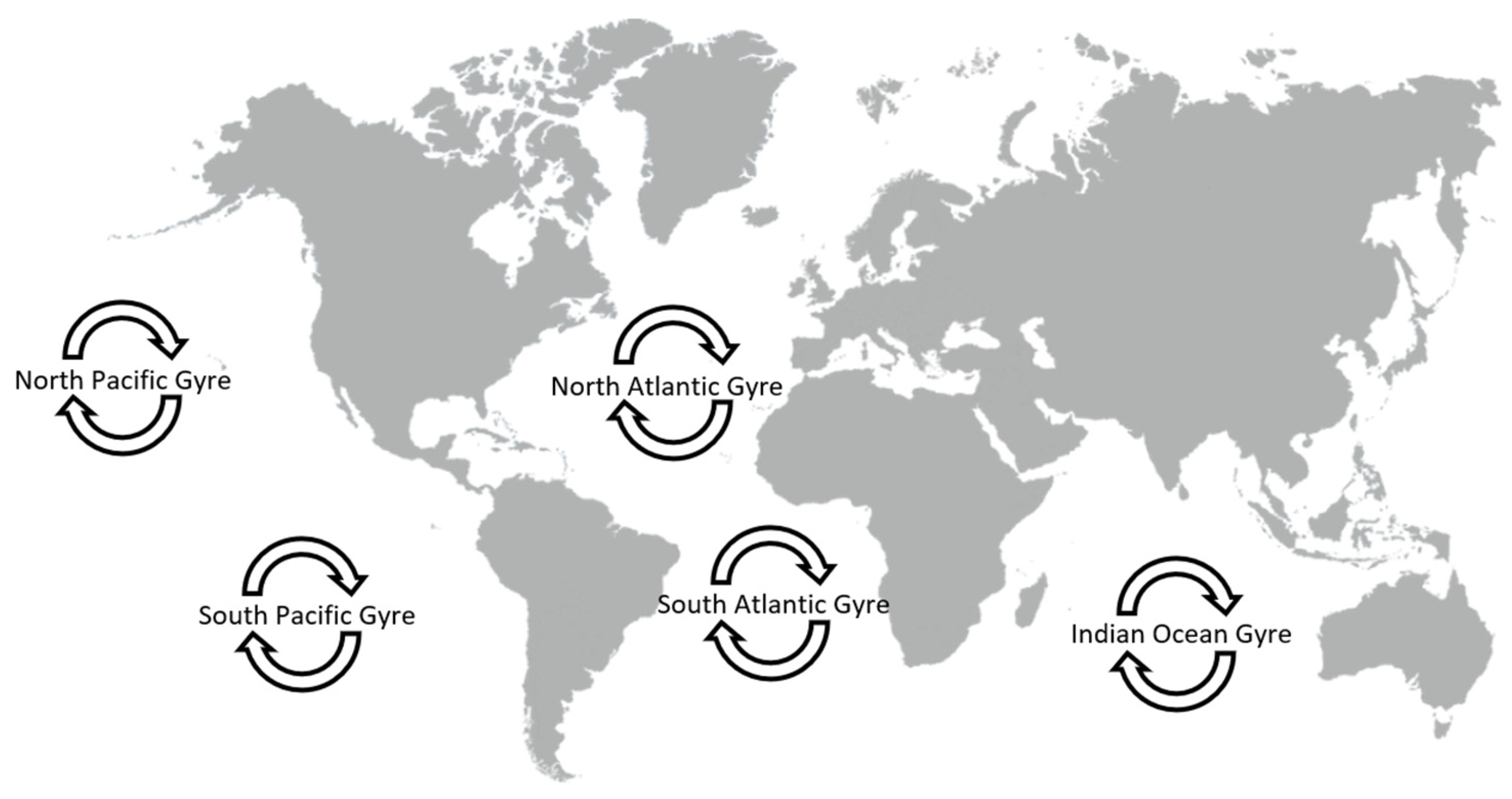
2. Macrodebris
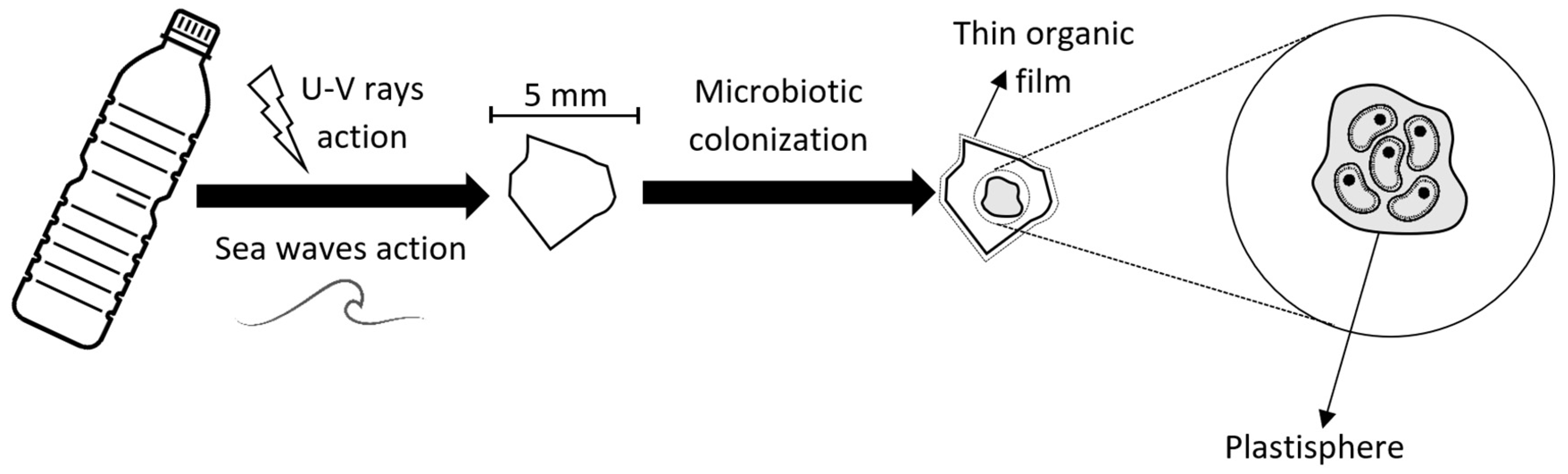
3. Microplastics
4. Engineered Nanoparticles
5. Metallic Particles
6. Sinking Particles (“Marine Snow”) and Pollution
7. Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Coleman, M.A.; Wood, G.; Filbee-Dexter, K.; Minne, A.J.P.; Goold, H.D.; Verges, A.; Marzinelli, E.M.; Steinberg, P.D.; Wernberg, T. Restore or redefine: Future trajectories for restoration. Front. Mar. Sci. 2020, 7, 237. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayami, M.; Qafoku, N.P.; Yang, Y.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Milo, R. The biomass composition of the oceans: A blueprint of our blue planet. Cell 2019, 179, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Ohte, N. Ecological perspectives on microbes involved in N-cycling. Microbes Environ. 2014, 29, 4–16. [Google Scholar] [CrossRef]
- Ando, N.; Barquera, B.; Bartlett, D.H.; Boyd, E.; Burnim, A.A.; Byer, A.S.; Colman, D.; Gillilan, R.E.; Gruebele, M.; Makhatadze, G.; et al. The molecular basis for life in extreme environments. Annu. Rev. Biophys 2021, 50, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial Diversity in Extreme Marine Habitats and Their Biomolecules. Microorganisms 2017, 5, 25. [Google Scholar] [CrossRef]
- Henderson, J.; Salem, H. CHAPTER 1: The atmosphere: Its developmental history and contributions to microbial evolution and habitat. In Aerobiology: The Toxicology of Airborne Pathogens and Toxins; RSC Publishing: London, UK, 2016; pp. 1–41. ISBN 978-1-84973-791-3. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Pierce, E.C.; Dutton, R.J. Putting microbial interactions back into community contexts. Curr. Opin. Microbiol. 2022, 65, 56–63. [Google Scholar] [CrossRef]
- Roukaerts, A.; Deman, F.; Van der Linden, F.; Carnat, G.; Bratkic, A.; Moreau, S.; Dehairs, F.; Delille, B.; Tison, J.-L.; Fripiat, F. The biogeochemical role of a microbial biofilm in sea ice: Antarctic landfast sea ice as a case study. Elem. Sci. Anthr. 2021, 9, 00134. [Google Scholar] [CrossRef]
- Gadkari, J.; Bhattacharya, S.; Shrivastav, A. Importance and applications of biofilm in microbe-assisted bioremediation. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Nertherland, 2022; pp. 153–173. [Google Scholar] [CrossRef]
- Serra, D.O.; Hengge, R. Stress responses go three dimensional—the spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471. [Google Scholar] [CrossRef]
- Tan, D.; Svenningsen, S.L.; Middelboe, M. Quorum sensing determines the choice of antiphage defense strategy in Vibrio anguillarum. mBio 2015, 6, e00627-15. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, S.; Costa, S.; Caeiro, S. Marine litter: A review of educative interventions. Mar. Pollut. Bull. 2021, 168, 112446. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, W.J.; Broad, S.; Caine, J.; Clarke, S.J.; Collins, A.M.; Dicks, L.V.; Doran, H.; Esmail, N.; Fleishman, E.; Frost, N.; et al. A horizon scan of global conservation issues for 2016. Trends Ecol Evol. 2016, 31, 44–53. [Google Scholar] [CrossRef] [PubMed]
- GEF—Secretariat of the Convention on Biological Diversity and the Scientific and Technical Advisory Panel. Impacts of Marine Debris on Biodiversity: Current Status and Potential Solutions; GEF: Montreal, QC, Canada, Technical Series No. 67; 2012; Available online: https://www.cbd.int/doc/publications/cbd-ts-67-en.pdf (accessed on 19 April 2022).
- Jacquin, J.; Cheng, J.; Odobel, C.; Pandin, C.; Conan, P.; Pujo-Pay, M.; Barbe, V.; Meisterzheim, A.-L.; Ghiglione, J.-F. Microbial ecotoxicology of marine plastic debris: A review on colonization and biodegradation by the “plastisphere”. Front. Microbiol. 2019, 10, 865. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment: A Global Assessment; Kershaw, P.J., Ed.; International Maritime Organization: London, UK, 2015; p. 52. Available online: https://ec.europa.eu/environment/marine/good-environmental-status/descriptor-10/pdf/GESAMP_microplastics%20full%20study.pdf (accessed on 19 April 2022).
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Phil. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Ruiz, I.; Abascal, A.J.; Basurko, O.C.; Rubio, A. Modelling the distribution of fishing-related floating marine litter within the Bay of Biscay and its marine protected areas. Environ. Pollut. 2022, 292, 118216. [Google Scholar] [CrossRef]
- McIlgorm, A.; Raubenheimer, K.; McIlgorm, D.E.; Nichols, R. The cost of marine litter damage to the global marine economy: Insights from the Asia-Pacific into prevention and the cost of inaction. Mar. Pollut. Bull. 2022, 174, 113167. [Google Scholar] [CrossRef]
- Camargo, M.; Sandrini-Neto, L.; Carreira, R.; Camargo, M. Effects of hydrocarbon pollution in the structure of macrobenthic assemblages from two large estuaries in Brazil. Mar. Poll. Bull. 2017, 125, 66–76. [Google Scholar] [CrossRef]
- Häder, D.-P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, N.A.; Gonzalez, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef]
- Jadhav, S.; Sharma, S.; Sibi, G. Microbial degradation of petroleum hydrocarbons and factors influencing the degradation process. Bioprocess Eng. 2019, 3, 6–11. [Google Scholar] [CrossRef]
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, L. Microbial degradation of hydrocarbons—Basic Principles for bioremediation: A review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef] [PubMed]
- Mahjoubi, M.; Cappello, S.; Souissi, Y.; Jaouani, A.; Cherif, A. Microbial bioremediation of petroleum hydrocarbon-contaminated marine environments. In Recent Insights in Petroleum Science and Engineering; Zoveidavianpoor, M., Ed.; InTech: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Baptista Neto, J.A.; Gaylarde, C.; da Fonseca, E.M. Microplastics: A pelagic habitat for microorganisms and invertebrates. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Asmutis-Silvia, R.; Drinkwin, J.; Gilardi, K.V.K.; Giskes, I.; Jones, G.; O’Brian, K.; Pragnell-Raasch, H.; Ludwig, L.; Antonelis, K.; et al. Building evidence around ghost gear: Global trends and analysis for sustainable solutions at scale. Mar. Pollut. Bull. 2019, 138, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Angiolillo, M. Debris in Deep Water. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 14; pp. 251–268. [Google Scholar] [CrossRef]
- Angiolillo, M.; Fortibuoni, T. Impacts of marine litter on Mediterranean reef systems: From shallow to deep waters. Front. Mar. Sci. 2020, 7, 826. [Google Scholar] [CrossRef]
- Lee, J.-W.; Nam, J.-H.; Kim, Y.-H.; Lee, K.-H.; Lee, D.-H. Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. J. Microbiol. 2008, 46, 174–182. [Google Scholar] [CrossRef]
- Caruso, G. Microplastics in marine environments: Possible interactions with the microbial assemblage. J. Pollut. Eff. Cont. 2015, 3, e111. [Google Scholar] [CrossRef]
- Lee, D.-I.; Cho, H.-S.; Jeong, S.-B. Distribution characteristics of marine litter on the sea bed of the East China Sea and the South Sea of Korea. Estuar Coast. Shelf Sci. 2006, 70, 187–194. [Google Scholar] [CrossRef]
- Melli, V.; Angiolillo, M.; Ronchi, F.; Canese, S.; Giovanardi, O.; Querin, S.; Fortibuoni, T. The first assessment of marine debris in a Site of Community Importance in the north-western Adriatic Sea (Mediterranean Sea). Mar. Pollut. Bull. 2017, 114, 821–830. [Google Scholar] [CrossRef]
- Watters, D.L.; Yoklavich, M.M.; Love, M.S.; Schroeder, D.M. Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull. 2010, 60, 131–138. [Google Scholar] [CrossRef]
- Sherrington, C. Plastics in the Marine Environment; Eunomia Research & Consulting Ltd.: Bristol, UK, 2016; p. 16. [Google Scholar]
- Wei, C.-L.; Rowe, G.T.; Nunnally, C.C.; Wicksten, M.K. Anthropogenic “Litter” and macrophyte detritus in the deep Northern Gulf of Mexico. Mar. Pollut. Bull. 2012, 64, 966–973. [Google Scholar] [CrossRef]
- Carvalho-Souza, G.F.; Llope, M.; Tinôco, M.S.; Medeiros, D.V.; Maia-Nogueira, R.; Sampaio, C.L.S. Marine litter disrupts ecological processes in reef systems. Mar. Pollut. Bull. 2018, 133, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Fruh, E.L.; Johnson, M.M.; Simon, V.; McGourty, C. Distribution and abundance of anthropogenic marine debris along the shelf and slope of the US West Coast. Mar. Pollut. Bull. 2010, 60, 692–700. [Google Scholar] [CrossRef]
- Carson, H.S.; Nerheim, M.S.; Carroll, K.A.; Eriksen, M. The plastic-associated microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 2013, 75, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Schlundt, C.; Welch, M.J.L.; Knochel, A.M.; Zettler, E.; Amarel-Zettler, L. Spatial structure in the “Plastisphere”: Molecular resources for imaging microscopic communities on plastic marine debris. Mol. Ecol. Resour. 2019, 20, 620–634. [Google Scholar] [CrossRef]
- UNEP. Plastic Debris in the Ocean. UNEP Year Book 2014 Emerging Issues Update. 2014. UN Environment Yearbooks/Global Environmental Outlook Yearbooks|UNEP-UN Environment Programme. Available online: https://www.unep.org/resources/year-books (accessed on 19 April 2022).
- Sherman, D.; Besteiro, S.; Fox, C.; Sherman, K. International Coastal Cleanup Report: 2009; Ocean Conservancy: Washington, DC, USA, 2007. [Google Scholar] [CrossRef]
- Anfuso, G.; Lynch, K.; Williams, A.T.; Perales, J.A.; Silva, C.P.; Nogueira, M.R.; Maanan, M.; Pretti, C.; Pranzini, E.; Winter, C.; et al. Comments on marine litter in oceans, seas and beaches: Characteristics and impacts. Ann. Mar. Biol. Res. 2015, 2, 1008. [Google Scholar]
- Margiotta, F.; Balestra, C.; Buondonno, A.; Casotti, R.; D’Ambra, I.; Di Capua, I.; Gallia, R.; Mazzocchi, M.G.; Merquiol, L.; Pepi, M.; et al. Do plankton reflect the environmental quality status? The case of a post-industrial Mediterranean Bay. Mar. Environ. Res. 2020, 160, 104980. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; Roos, P.J.; Cadée, G.C. Trans-Atlantic rafting by the brooding reef coral Favia fragum on man-made flotsam. Mar. Ecol. Prog. Ser. 2012, 445, 209–218. [Google Scholar] [CrossRef]
- Kiessling, T.; Gutow, L.; Thiel, M. Marine litter as habitat and dispersal vector. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 141–180. [Google Scholar] [CrossRef]
- Woodall, L.C.; Jungblut, A.D.; Hopkins, K.; Hall, A.C.; Robinson, L.; Gwinnett, C.; Paterson, G. Deep-sea anthropogenic macrodebris harbours rich and diverse communities of bacteria and archaea. PLoS ONE 2018, 13, e0206220. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically influenced corrosion. In Wiley Series in Corrosion; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- McBeth, J.M.; Little, B.J.; Ray, R.I.; Farrar, K.; Emerson, D. Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl. Environ. Microbiol. 2011, 77, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Tourova, T.P.; Sokolova, D.S.; Nazina, T.N.; Laptez, A.B. Comparative analysis of the taxonomic composition of bacterial fouling developing on various materials exposed to aqueous environments. Microbiology 2021, 90, 416–427. [Google Scholar] [CrossRef]
- Muthukrishnan, T.; Al Khaburi, M.; Abed, R.M.M. Fouling microbial communities on plastics compared with wood and steel: Are they substrate- or location-specific? Microb. Ecol. 2019, 78, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.; Elshafei, A. Protection of metal surfaces from microbial colonization. Ann. Res. Rev. Biol. 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Castro, Í.B.; Westphal, E.; Fillmann, G. Tintas anti-incrustantes de terceira geração: Novos biocidas no ambiente aquático. [Third generation antifouling paints: New biocides in the aquatic environment]. Quim. Nova 2011, 34, 1021–1031. [Google Scholar] [CrossRef]
- Thomas, K.V.; Brooks, S. The environmental fate and effects of antifouling paint biocides. Biofouling 2010, 26, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Baptista Neto, J.A.; da Fonseca, E. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 2021, 162, 111847. [Google Scholar] [CrossRef]
- Soroldoni, S.; da Silva, S.V.; Castro, Í.B.; Martins, C.M.G.; Pinho, L.; Lopes, G. Antifouling paint particles cause toxicity to benthic organisms: Effects on two species with different feeding modes. Chemosphere 2020, 238, 124610. [Google Scholar] [CrossRef]
- Eklund, B.; Johansson, L.; Ytreberg, E. Contamination of a boatyard for maintenance of pleasure boats. J. Soils Sed. 2014, 14, 955–967. [Google Scholar] [CrossRef]
- Soroldoni, S.; Castro, Í.B.; Abreu, F.; Duarte, F.A.; Choueri, R.B.; Moller, O.O., Jr.; Fillmann, G.; Pinho, G.L.L. Antifouling paint particles: Sources, occurrence, composition and dynamics. Water Res. 2018, 137, 47–56. [Google Scholar] [CrossRef]
- Takahashi, C.K.; Turner, A.; Millward, G.E.; Glegg, G.A. Persistence and metallic composition of paint particles in sediments from a tidal inlet. Mar. Pollut. Bull. 2012, 64, 133–137. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Han, L.; Tam, K.C. Superhydrophobic surfaces from sustainable colloidal systems. Curr. Opin. Colloid Interface Sci. 2022, 57, 101534. [Google Scholar] [CrossRef]
- Boxall, S. From rubber ducks to ocean gyres. Nature 2009, 459, 1058–1059. [Google Scholar] [CrossRef][Green Version]
- Capolupo, M.; Sørensen, L.; Jayasena, K.D.R.; Booth, A.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Shah, N.; Kanwal, N.; Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeterior. Biodegrad. 2013, 83, 145–157. [Google Scholar] [CrossRef]
- Andler, R.; Hiessl, S.; Yücel, O.; Tesch, M.; Steinbuchel, A. Cleavage of poly(cis-1,4-isoprene) rubber as solid substrate by culture of Gordonia polyisoprenivorans. New Biotechnol. 2018, 44, 6–12. [Google Scholar] [CrossRef]
- Imai, S.; Ichikawa, K.; Muramatsu, Y.; Kasai, D.; Masai, E.; Fukuda, M. Isolation and characterisation of Streptomyces, Actinoplanes and Methylibium strains that are involved in degradation of natural rubber and synthetic poly(cis-1,4-isoprene). Enzym. Microb. Technol. 2011, 49, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Linos, A.; Berekaa, M.M.; Reichelt, R.; Keller, U.; Schmitt, J.; Flemming, H.; Koppenstedt, R.; Steinbuchel, A. Biodegradation of cis-1,4-polyisoprene rubbers by distinct actinomycetes: Microbial strategies and detailed surface analysis. Appl. Environ. Microbiol. 2000, 66, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Carrión, O.; Larke-Mejía, N.L.; Gibson, L.; Ul Haque, M.F.; Ramiro-Garcia, J.; McGenity, T.J.; Murrell, J.C. Gene probing reveals the widespread distribution, diversity and abundance of isoprene-degrading bacteria in the environment. Microbiome 2018, 6, 219. [Google Scholar] [CrossRef]
- Johnston, A.; Crombie, A.T.; Khawand, M.E.; Sims, L.; Whited, G.M.; McGenity, T.J.; Murrell, J.C. Identification and characterisation of isoprene-degrading bacteria in an estuarine environment. Environ. Microbiol. 2017, 19, 3526–3537. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Kwak, J.I.; Liu, H.; Wang, D.; Lee, Y.H.; Lee, J.-S.; An, Y. Critical review of environmental impacts of microfibers in different environmental matrices. Comp. Biochem. Physiol. Pt C Toxicol. Pharmacol. 2021, 251, 109196. [Google Scholar] [CrossRef] [PubMed]
- Pirc, U.; Vidmar, M.; Mozer, A.; Kržan, A. Emissions of microplastic fibers from microfiber fleece during domestic washing. Environ. Sci. Pollut. Res. 2016, 23, 22206–22211. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.; Baptista Neto, J.A.; da Fonseca, E. Plastic microfibre pollution: How important is clothes’ laundering? Heliyon 2021, 7, e07105. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.; Laitala, K.; Klepp, I.G. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef]
- Industrievereinigung Chemiefaser; Statista Inc. Worldwide Production Volume of Chemical and Textile Fibers from 1975 to 2018. 2018. Available online: https://www.statista.com/statistics/263154/worldwide-production-volume-of-textile-fibers-since-1975/ (accessed on 13 November 2021).
- Hartline, N.; Bruce, N.; Karba, S.; Ruff, E.O.; Sonar, S.U.; Holden, P.A. Microfiber masses recovered from conventional machine washing of new or aged garments. Environ. Sci. Technol. 2016, 50, 11532–11538. [Google Scholar] [CrossRef]
- Andrady, A. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 8, 1596–1605. [Google Scholar] [CrossRef]
- Guo, H.; Qian, Y.; Qiu, P.; Kong, Z.; Zheng, X.; Tang, Z.; Guo, H. Textile wastewater treatment for water reuse: A case study. Processes 2019, 7, 34. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Health and environmental hazards of synthetic dyes. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar] [CrossRef]
- Ho, H.; Watanabe, T. Distribution and removal of nonylphenol ethoxylates and nonylphenol from textile wastewater—A comparison of a cotton and a synthetic fiber factory in Vietnam. Water 2017, 9, 386. [Google Scholar] [CrossRef]
- Luongo, G. Chemicals in Textiles: A Potential Source for Human Exposure and Environmental Pollution. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2015. [Google Scholar]
- Samchetshabam, G.; Hussan, A. Impact of textile dyes waste on aquatic environments and its treatment. Environ. Ecol. 2017, 35, 2349–2353. [Google Scholar] [CrossRef]
- Almroth, B.M.C.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, M. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Carretero, O.; Filgueiras, A.V.; Viñas, L. Synthetic microfibers in the marine environment: A review on their occurrence in seawater and sediments. Mar. Pollut. Bull. 2018, 127, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, S.M.; Bao, S.; Chow, A.T. Natural fibers: A missing link to chemical pollution dispersion in aquatic environments. Environ. Sci. Technol. 2015, 49, 12609–12610. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.; Windsor, F.M.; Munday, M.; Durance, I. Natural or synthetic–how global trends in textile usage threaten freshwater environments. Sci. Total Environ. 2020, 718, 134689. [Google Scholar] [CrossRef]
- Stanton, T.; Johnson, M.; Nathanail, P.; Macnaughtan, W.; Gomes, R. Freshwater and airborne textile fibre populations are dominated by ‘natural’, not microplastic, fibres. Sci. Total Environ. 2019, 666, 377–389. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Sharabatia, M.A.; Abokwiek, R.; Al-Othman, A.; Tawalbeh, M.; Karaman, C.; Orooji, Y.; Karimi, F. Biodegradable polymers and their nano-composites for the removal of endocrine-disrupting chemicals (EDCs) from wastewater: A review. Environ. Res. 2021, 202, 111694. [Google Scholar] [CrossRef]
- Darbre, P.D. What are endocrine disrupters and where are they found? In Endocrine Disruption and Human Health; Academic Press: Cambridge, MA, USA, 2022; pp. 3–29. [Google Scholar] [CrossRef]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Girard, E.; Kaliwoda, M.; Schmall, W.W.; Woerdeide, G.; Orsi, W. Textile waste and microplastic induce activity and development of unique hydrocarbon-degrading marine bacterial communities. bioRxiv 2020, 2, 939876. [Google Scholar] [CrossRef]
- Halsband, C.; Herzke, D. Plastic litter in the European Arctic: What do we know? Emerg. Contam. 2019, 5, 308–318. [Google Scholar] [CrossRef]
- Setala, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.L.; Carreres-Calabuig, J.A.; Gorokhova, E.; Posth, N.R. Micro-by-micro interactions: How microorganisms influence the fate of marine microplastics. Limnol. Oceanogr. 2020, 5, 18–36. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-Leon, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Montoto-Martínez, T.; Hernández-Brito, J.J.; Gelado-Caballero, M.D. Pump-underway ship intake: An unexploited opportunity for Marine Strategy Framework Directive (MSFD) microplastic monitoring needs on coastal and oceanic waters. PLoS ONE 2020, 15, e0232744. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.S. Life in the ‘plastisphere’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.L.; Cruz, B.N.; Polidoro, B.; Neuer, S. Microbial colonization of microplastics in the Caribbean Sea. Limnol. Oceanogr. Lett. 2020, 5, 5–17. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Wichels, A.; Gullans, E.; Krohne, G.; Gerdts, G. The Plastisphere—Uncovering tightly attached plastic “specific” microorganisms. PLoS ONE 2019, 14, e0215859. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2018, 8, 2709. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR) and marine plastics: Can food packaging litter act as a dispersal mechanism for AMR in oceanic environments? Mar. Pollut. Bull. 2020, 150, 110702. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ. Int. 2019, 123, 79–86. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.-J.; Cai, Z.-H.; Zeng, Y.-H.; Zhu, J.-M.; Dhu, X.-P. Opportunistic bacteria use quorum sensing to disturb coral symbiotic communities and mediate the occurrence of coral bleaching. Environ. Microbiol. 2020, 22, 1944–1962. [Google Scholar] [CrossRef] [PubMed]
- Frère, L.; Maignien, L.; Chalopin, M. Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ. Pollut. 2018, 242 Pt A, 614–625. [Google Scholar] [CrossRef]
- Bryant, J.A.; Clemente, T.M.; Viviani, D.A.; Fong, A.A.; Thomas, K.A.; Kemp, P.; Karl, D.M.; White, A.E.; DeLong, E. Diversity and activity of communities inhabiting plastic debris in the North Pacific Gyre. mSystems 2016, 1, e00016–e00024. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.C.; Carson, H.S.; Eriksen, M. Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar. Biol. 2014, 161, 1441–1453. [Google Scholar] [CrossRef]
- Soares, M.O.; de Lima Xavier, F.R.; Dias, N.M.; da Silva, M.Q.M.; Lima, J.P.; Barroso, C.X.; Vieira, L.M.; Paiva, S.V.; Matthews-Cascon, H.; Bezerra, L.E.A.; et al. Alien hotspot: Benthic marine species introduced in the Brazilian semiarid coast. Mar. Pollut. Bull. 2022, 174, 113250. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.T.; Fileman, T.W.; Hall-Spencer, J.M. Invasive species in the Northeastern and Southwestern Atlantic Ocean: A review. Mar. Pollut. Bull. 2017, 116, 41–47. [Google Scholar] [CrossRef]
- Hughes, K.A.; Pescott, O.L.; Peyton, J.; Adriaens, T.; Cottier-Cook, E.J.; Key, G.; Rabbitsch, W.; Tricarico, E.; Barnes, D.K.A.; Baxter, N.; et al. Invasive non-native species likely to threaten biodiversity and ecosystems in the Antarctic Peninsula region. Glob. Change Biol. 2020, 26, 2702–2716. [Google Scholar] [CrossRef]
- Richard, H.; Carpenter, E.J.; Komada, T.; Palmer, P.T.; Rochman, C.M. Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci. Total Environ. 2019, 683, 600–608. [Google Scholar] [CrossRef]
- Freese, H.M.; Methner, A.; Overmann, J. Adaptation of surface-associated bacteria to the open ocean: A genomically distinct subpopulation of Phaeobacter gallaeciensis colonizes Pacific mesozooplankton. Front. Microbiol. 2017, 8, 1659. [Google Scholar] [CrossRef]
- Debroas, D.; Mone, A.; Halle, A.T. Plastics in the North Atlantic garbage patch: A boat-microbe for hitchhikers and plastic degraders. Sci. Total Environ. 2017, 599–600, 1222–1232. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Hazard. Mat. 2019, 280, 120899. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, S.; Ray, S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. Int. 2013, 20, 4339–4355. [Google Scholar] [CrossRef] [PubMed]
- Baptista Neto, J.A.B.; Gaylarde, C.; Beech, I.; Bastos, A.; Quaresma, V.S.; de Carvalho, D.G. Microplastics and attached microorganisms in sediments of the Vitória Bay estuarine system in SE Brazil. Ocean. Coast. Manag. 2019, 169, 247–253. [Google Scholar] [CrossRef]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Chyc, M.; Ryszka, P.; Latowski, D. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ. Sci. Pollut. Res. 2016, 23, 11349–11356. [Google Scholar] [CrossRef]
- Gilan, O.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar] [CrossRef]
- Hadad, D.; Geresh, S.; Sivan, A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J. Appl. Microbiol. 2005, 98, 1093–1100. [Google Scholar] [CrossRef]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Huang, X.; Cao, L.; Qin, Z.; Li, S.; Kong, W.; Liu, Y. Tat-independent secretion of polyethylene terephthalate hydrolase PETase in Bacillus subtilis 168 mediated by its native signal peptide. J. Agric. Food Chem. 2018, 66, 13217–13227. [Google Scholar] [CrossRef]
- Sudhakar, M.; Doble, M.; Murthy, P.S.; Venkatesan, R. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int. Biodeterior. Biodegrad. 2008, 61, 203–213. [Google Scholar] [CrossRef]
- Roth, C.; Wei, R.; Oeser, T.; Then, J.; Follner, C.; Zimmermann, W.; Strater, N. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl. Microbiol. Biotechnol. 2014, 98, 7815–7823. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, D.; Herrero Acero, E.; Greimel, K.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Rodriguez, R.D.; Steinkellner, G.; Gruber, K.; Schwab, H.; et al. A new esterase from Thermobifida halotolerans hydrolyses polyethylene terephthalate (PET) and polylactic acid (PLA). Polymers 2021, 4, 617. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Loeder, M.G.J.; Gerdts, G.; Osborn, A.M. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microb. Ecol. 2014, 90, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wei, R.; Cui, Q.; Bornscheuer, U.T.; Liu, Y.-J. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum. Microb. Biotechnol. 2021, 14, 374–385. [Google Scholar] [CrossRef]
- Kawai, F.; Oda, M.; Tamashiro, T.; Waku, T.; Tanaka, N.; Yamamoto, M.; Mizushima, H.; Miyakawa, T.; Tanokura, M. A novel Ca2+-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl. Microbiol. Biotechnol. 2014, 98, 10053–10064. [Google Scholar] [CrossRef]
- Yamada-Onodera, K.; Mukumoto, H.; Katsuyaya, Y.; Saiganji, A.; Tani, Y. Degradation of polyethylene by a fungus Penicillium simplicissimus YK. Polym. Degrad. Stab. 2001, 72, 323–327. [Google Scholar] [CrossRef]
- Almeida, E.L.; Carrillo, R.A.F.; Jackson, S.A.; Dobson, A.D.W. In silico screening and heterologous expression of a polyethylene terephthalate hydrolase (PETase)-like enzyme (SM14est) with polycaprolactone (PCL)-degrading activity, from the marine sponge-derived strain Streptomyces sp. SM14. Front. Microbiol 2019, 10, 2187. [Google Scholar] [CrossRef]
- Pometto, A.L.; Lee, B.; Johnson, K.E. Production of an extracellular polyethylene-degrading enzyme by Streptomyces sp. Appl. Environ. Microbiol. 1992, 58, 731–733. [Google Scholar] [CrossRef]
- Shao, H.; Chen, M.; Fei, X.; Zhang, R.; Zhong, Y.-X.; Ni, W.; Tao, X.; He, X.; Zhang, E.; Yong, B.; et al. Complete genome sequence and characterization of a polyethylene biodegradation strain, Streptomyces albogriseolus LBX-2. Microorganisms 2019, 7, 379. [Google Scholar] [CrossRef]
- Devi, R.S.; Kannan, V.R.; Nivas, D. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Ramalingappa, M.; Krishnappa, M.; Thippeswamy, B. Degradation of polyethylene by Penicillium simplicissimum isolated from local dumpsite of Shivamogga district. Environ. Dev. Sustain. 2014, 17, 731–745. [Google Scholar] [CrossRef]
- Pattnaik, P.; Dangayach, G.S.; Bhardwaj, A.K. A review on the sustainability of textile industries wastewater with and without treatment methodologies. Rev. Environ. Health 2018, 33, 163–203. [Google Scholar] [CrossRef] [PubMed]
- Kvale, K.F.; Friederike, P.A.E.; Oschlies, A. A critical examination of the role of marine snow and zooplankton fecal pellets in removing ocean surface microplastic. Front. Mar. Sci. 2020, 6, 808. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassi, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Shimao, M. Biodegradation of plastics. Curr. Opin. Biotechnol. 2001, 12, 242–247. [Google Scholar] [CrossRef]
- Mabrouk, M.M.; Sabry, S.A. Degradation of poly (3hydroxybutyrate) and its copolymer poly (3-hydroxybutyrate-co3-hydroxyvalerate) by a marine Streptomyces sp. SNG9. Microbiol. Res. 2001, 156, 323–335. [Google Scholar] [CrossRef]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.M.; Pereira, R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Saika, A.; Nomura, K.; Watanabe, T.; Watanabe, T.; Fujimoto, Y.; Enoki, M.; Sato, T.; Kato, C.; Kanehiro, H. Biodegradation of aliphatic polyesters soaked in deep seawaters and isolation of poly(ε-caprolactone)-degrading bacteria. Polym. Degrad. Stab. 2011, 96, 1397–1403. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Oba, K.; Takizawa, R.; Kasuya, K.-i. Microbial degradation of poly(ε-caprolactone) in a coastal environment. Polym. Degrad. Stab. 2018, 149, 1–8. [Google Scholar] [CrossRef]
- Chua, T.-K.; Tseng, M.; Yang, M.-K. Degradation of poly (ε-caprolactone) by thermophilic Streptomyces thermoviolaceus subsp. thermoviolaceus 76T-2. AMB Express 2013, 3, 8. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Labrenz, M. Marine microbial assemblages on microplastics: Diversity, adaptation, and role in degradation. Ann. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kuroki, Y.; Nagai, K. Isolation of thermophiles degrading poly (L-lactic acid). J. Biosci. Bioeng. 1999, 87, 752–755. [Google Scholar] [CrossRef]
- Kim, D.Y.; Rhee, Y.H. Biodegradation of microbial and synthetic polyesters by fungi. Appl. Microbiol. Biotechnol. 2003, 61, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Akutsu, Y.; Nakajima-Kambe, T.; Nomura, N.; Nakahara, T. Purification and Properties of a Polyester Polyurethane-Degrading Enzyme from Comamonas acidovorans TB-35. Appl. Environ. Microbiol. 1998, 64, 62–67. [Google Scholar] [CrossRef]
- Howard, G.T.; Ruiz, C.; Hillard, N.P. Growth of Pseudomonas chlororaphis on a polyester-polyurethane and the purification and characterization of a polyurethane-esterase enzyme. Int. Biodeter. Biodegrad. 1999, 43, 7–12. [Google Scholar] [CrossRef]
- Peng, Y.H.; Shih, Y.H.; Lai, Y.C.; Liu, Y.-Z.; Liu, Y.-T.; Lin, N.-C. Degradation of polyurethane by bacterium isolated from soil and assessment of polyurethanolytic activity of a Pseudomonas putida strain. Environ. Sci. Pollut. Res. 2014, 21, 9529–9537. [Google Scholar] [CrossRef]
- Howard, G.T.; Norton, W.N.; Burks, T. Growth of Acinetobacter gerneri P7 on polyurethane and the purification and characterization of a polyurethanase enzyme. Biodegradation 2012, 23, 561–573. [Google Scholar] [CrossRef]
- Nawaz, A.; Hasan, F.; Shah, A.A. Degradation of poly (ε-caprolactone) (PCL) by a newly isolated Brevundimonas sp. strain MRL-AN1 from soil. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Murata, N.; Tanabe, E.; Kubota, K.; Kubo, M. Isolation and characterization of an ether-type polyurethane-degrading micro-organism and analysis of degradation mechanism by Alternaria sp. J. Appl. Microb. 2010, 108, 1946–1953. [Google Scholar] [CrossRef]
- Danko, A.S.; Luo, M.; Bagwell, C.E.; Brigman, R.I.; Freedman, D.L. Involvement of linear plasmids in aerobic biodegradation of vinyl chloride. Appl. Environ. Microbiol. 2014, 70, 6092–6097. [Google Scholar] [CrossRef]
- Pereira, O.; Hochart, C.; Auguet, J.C.; Debroas, D.; Galand, P.E. Genomic ecology of Marine Group II, the most common marine planktonic Archaea across the surface ocean. MicrobiologyOpen 2019, 8, e852. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.S.; Nixon, M.; Eastwood, I.M.; Greenhalgh, M.; Robson, G.D.; Handley, P.S. Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl. Environ. Microbiol. 2000, 66, 3194–3200. [Google Scholar] [CrossRef] [PubMed]
- Mor, R.; Sivan, A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber. Biodegradation 2008, 19, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Nakamiya, K.; Sakasita, G.; Ooi, T.; Kinoshita, S. Enzymatic degradation of polystyrene by hydroquinone peroxidase of Azotobacter beijerinckii HM121. J. Ferm. Bioeng. 1997, 84, 480–482. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Brandon, J.A.; Jones, W.; Ohman, M.D. Multidecadal increase in plastic particles in coastal ocean sediments. Sci. Adv. 2019, 5, eaax0587. [Google Scholar] [CrossRef]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamiltin, J.P.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef]
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef]
- Kane, I.A.; Clare, M.A.; Miramontes, E.; Wogelius, R.; Rothwell, J.J.; Garreau, P.; Pohl, F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science 2020, 368, 1140–1145. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Huang, W.; Li, J.; Wang, C.; Zhang, D.; Zhang, C. Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Poll. 2020, 259, 113948. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef] [PubMed]
- Tagg, A.S.; Oberbeckmann, S.; Fischer, D.; Kreikemeyer, B. Paint particles are distinct and variable substrate for marine bacteria. Mar. Pollut. Bull. 2019, 146, 117–124. [Google Scholar] [CrossRef]
- Gangadoo, S.; Owen, S.; Rajapaksha, P.; Plaisted, K.; Cheeseman, S.; Haddara, H.; Truong, V.K.; Ngo, S.T.; Vu, V.; Cozzolino, D.; et al. Nano-plastics and their analytical characterisation and fate in the marine environment: From source to sea. Sci. Total Environ. 2020, 732, 138792. [Google Scholar] [CrossRef]
- Dedman, C.J.; King, A.M.; Christie-Oleza, J.A.; Davies, G.L. Environmentally relevant concentrations of titanium dioxide nanoparticles pose negligible risk to marine microbes. Environ. Sci. Nano 2021, 8, 1236–1255. [Google Scholar] [CrossRef]
- Abioye, O.P.; Loto, C.A.; Fayomi, O.S.I. Evaluation of Anti-biofouling Progresses in Marine Application. J. Bio-Tribo-Corros. 2019, 5, 22. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.; Yang, J.; Zhou, S. Preparation and synergistic antifouling effect of self-renewable coatings containing quaternary ammonium-functionalized SiO2 nanoparticles. J. Colloid. Interface Sci. 2020, 563, 261–271. [Google Scholar] [CrossRef]
- Reyes-Estebanez, M.; Ortega-Morales, B.O.; Chan-Bacab, M.; Granados-Echegoyer, C.; Camacho-Chab, J.C.; Pereanez-Sacarias, J.E.; Gaylarde, C. Antimicrobial engineered nanoparticles in the built cultural heritage context and their ecotoxicological impact on animals and plants: A brief review. Herit. Sci. 2018, 6, 52. [Google Scholar] [CrossRef]
- Falfushynska, H.; Sokolova, I.; Stoika, R. Uptake, biodistribution, and mechanisms of toxicity of metal-containing nanoparticles in aquatic invertebrates and vertebrates. In Biomedical Nanomaterials; Springer: Cham, Switzerland, 2022; pp. 227–263. [Google Scholar] [CrossRef]
- Zha, S.J.; Rong, J.H.; Guan, X.F.; Tang, Y.; Han, Y.; Liu, G. Immunotoxicity of four nanoparticles to a marine bivalve species, Tegillarca granosa. J. Hazard. Mater. 2019, 377, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.; Patiño-Ruiz, D.; Tejeda-Benitez, L.; Herrera, A. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: A review on the applications, nanotoxicological effects, and risk control strategies. Environ. Sci. Pollut. Res. 2021, 28, 16962–16981. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhai, Y.; Liu, Y.; Hao, L.; Guo, H. Comparison of the in vitro and in vivo toxic effects of three sizes of zinc oxide (ZnO) particles using flounder gill (FG) cells and zebrafish embryos. J. Ocean Univ. China 2017, 16, 93–106. [Google Scholar] [CrossRef]
- Ferry, J.; Craig, P.; Hexel, C.; Sisco, P.; Frey, R.; Pennington, P.L.; Fulton, M.H.; Scott, I.G.; Decho, A.W.; Kashiwada, S.; et al. Transfer of gold nanoparticles from the water column to the estuarine food web. Nat. Nanotechnol. 2009, 4, 441–444. [Google Scholar] [CrossRef]
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the risk of engineered nanomaterials in the environment: Development and application of the nanoFate model. Environ. Sci. Technol. 2017, 51, 5541–5551. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Grimmelpont, M.V.; Corsi, I.; Bergami, E.; Curzi, D.; Burini, D.; Bouchet, V.M.P.; Ambrogini, P.; Gobbi, P.; Ujiie, Y.; et al. Nanoparticle-biological interactions in a marine benthic foraminifer. Sci. Rep. 2019, 9, 19441. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.G.M.; Baalousha, M.; Chen, J.; Chaudry, Q.; von der Kammer, F.; Kuhlbusch, T.A.J.; Nickel, C.; Quick, J.T.K.; Renkerg, M.; Koelmans, A.A. A Review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2084–2134. [Google Scholar] [CrossRef]
- Chiu, M.; Khan, Z.A.; Garcia, S.G. Effect of engineered nanoparticles on exopolymeric substances release from marine phytoplankton. Nanoscale Res. Lett. 2017, 12, 620. [Google Scholar] [CrossRef]
- Sendra, M.; Moreno, I.; Blasco, J. Toxicity of metal and metal oxide engineered nanoparticles to phytoplankton. In Ecotoxicity of Nanoparticles in Aquatic Systems; Blasco, J., Corsi, I., Eds.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 1351657550/9781351657556. [Google Scholar]
- Tsiola, A.; Toncelli, C.; Fodelianakis, S.; Michaud, G.; Bucheli, T.; Gavriilidou, A.; Kagiorgi, M.; Kalantzi, I.; Knauer, K.; Kotulas, G.; et al. Low-dose addition of silver nanoparticles stresses marine plankton communities. Environ. Sci. Nano 2018, 5, 1965–1980. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, M.; Wang, Z.; Cao, X.; Xing, B. Engineered nanomaterials in the environment: Are they safe? Crit. Rev. Environ. Sci. Technol. 2021, 51, 1443–1478. [Google Scholar] [CrossRef]
- Gillard, B.; Chatzievangelou, D.; Thomsen, L.; Ullrich, M.S. Heavy-metal-resistant microorganisms in deep-sea sediments disturbed by mining activity: An application toward the development of experimental in vitro systems. Front. Mar. Sci. 2019, 6, 432. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.; Son, S.K.; Le, A.D.; Kagiri, A.; Ramos, J.; Tsai, S.M.; Drobenaire, H.W.; Santschi, P.H.; Quigg, A. Fine-scale microbial communities associated with manganese nodules in deep-sea sediment of the Korea Deep Ocean Study Area in the Northeast Equatorial Pacific. Ocean Sci. J. 2018, 53, 337–353. [Google Scholar] [CrossRef]
- Lemaitre, N.; de Souza, G.F.; Archer, C.; Wang, R.-M.; Planquette, H.; Sarthou, G.; Vance, D. Pervasive sources of isotopically light zinc in the North Atlantic Ocean. Earth Planet Sci. Lett. 2020, 539, 116216. [Google Scholar] [CrossRef]
- Conway, T.M.; Hamilton, D.S.; Shelley, R.U.; Aguilar-Islas, A.M.; Landing, W.M.; Mahowald, M.N.; John, S.G. Tracing and constraining anthropogenic aerosol iron fluxes to the North Atlantic Ocean using iron isotopes. Nat. Commun. 2019, 10, 2628. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.S.; Moore, J.K.; Arneth, A.; Bond, T.C.; Carslaw, K.S.; Hantson, S.; Ito, A.; Kaplan, J.O.; Lindsay, K.; Nieradzik, L.P.; et al. Impact of changes to the atmospheric soluble iron deposition flux on ocean biogeochemical cycles in the Anthropocene. Glob. Biogeochem. Cycles 2020, 34, e2019GB006448. [Google Scholar] [CrossRef]
- Chuang, C.-Y.; Santschi, P.H.; Ho, Y.-F.; Conte, M.; Guo, L.; Schumann, D.; Ayranov, M.; Li, Y.-h. Biopolymers as major carrier phases and redox regulators of Th, Pa, Pb, Po, and Be in settling particles from the Atlantic Ocean. Mar. Chem. 2013, 15, 131–143. [Google Scholar] [CrossRef]
- Boyd, P.; Ellwood, M.; Tagliabue, A.; Twining, B.S. Biotic and abiotic retention, recycling and remineralization of metals in the ocean. Nat. Geosci. 2017, 10, 167–173. [Google Scholar] [CrossRef]
- Hoffman, C.L.; Nicholas, S.L.; Ohnemus, D.C.; Fitzsimmons, J.; Sherrell, R.; German, C.; Heller, M.; Lee, J.-M.; Lam, P.; Toner, B.M.; et al. Near-field iron and carbon chemistry of non-buoyant hydrothermal plume particles, Southern East Pacific Rise 15°S. Mar. Chem. 2018, 201, 183–197. [Google Scholar] [CrossRef]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef]
- Arctic Monitoring and Assessment Programme (AMAP). United Nations Environment Programme (UNEP) Global Mercury Assessment: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013. [Google Scholar]
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in marine and oceanic waters—A review. Water Air Soil Pollut. 2016, 227, 371. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Zhao, L.; Liu, X.; Pierce, E.M.; Gu, B. Mercury sorption and desorption on organo-mineral particulates as a source for microbial methylation. Environ. Sci. Technol. 2019, 53, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ruiz, F.; Paytan, A.; Gonzalez-Muñoz, M.T.; Jroundi, F.; Abad, M.M.; Lam, P.J.; Kastner, M. Barite formation in the ocean: Origin of amorphous and crystalline precipitates. Chem. Geol. 2019, 511, 441–451. [Google Scholar] [CrossRef]
- Zamanillo, M.; Ortega-Retuerta, E.; Nunes, S.; Rodriguez-Ros, P.; Dall’Osto, M.; Estrada, M.; Sala, M.M.; Simo, R. Main drivers of transparent exopolymer particle distribution across the surface Atlantic Ocean. Biogeosciences 2019, 16, 733–749. [Google Scholar] [CrossRef]
- Lundgreen, R.B.C.; Jaspers, C.; Traving, S.J.; Ayala, D.J.; Lombard, F.; Grossart, H.-P.; Nielsen, T.G.; Munk, P.; Riemann, L. Eukaryotic and cyanobacterial communities associated with marine snow particles in the oligotrophic Sargasso Sea. Sci. Rep. 2019, 9, 8891. [Google Scholar] [CrossRef]
- Arnosti, C.; Ziervogel, K.; Yang, T.; Teske, A. Oil-derived marine 817 aggregates–hot spots of polysaccharide degradation by specialized bacterial 818 communities. Deep-Sea Res. PT II 2016, 129, 179–186. [Google Scholar] [CrossRef]
- Duran Suja, L.; Summers, S.; Gutierrez, T. Role of EPS, Dispersant and Nutrients on the Microbial Response and MOS Formation in the Subarctic Northeast Atlantic. Front. Microbiol. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Capo, E.; Bravo, A.G.; Soerensen, A.L.; Bertilsson, S.; Pinhassi, J.; Feng, C.; Andersson, A.F.; Buck, M.; Bjorn, E. Marine snow as a habitat for microbial mercury methylators in the Baltic Sea. bioRxiv 2020, 3, 975987. [Google Scholar] [CrossRef]
- Achberger, A.M.; Doyle, S.M.; Mills, M.I.; Holmes II, C.P.; Quigg, A.; Sylvan, J.B. Bacteria-Oil Microaggregates Are an Important Mechanism for Hydrocarbon Degradation in the Marine Water Column. mSystems 2021, 6, e01105-21. [Google Scholar] [CrossRef]
- Duret, M.T.; Lampitt, R.S.; Lam, P. Prokaryotic niche partitioning between suspended and sinking marine particles. Environ. Microbiol. Rep. 2019, 11, 386–400. [Google Scholar] [CrossRef]
- Datta, M.; Sliwerska, E.; Gore, J.; Polz, M.F.; Cordero, O.X. Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat. Commun. 2016, 7, 11965. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, J.; Jia, Z.; Chen, S.; Zhang, L.; Gao, W. DNA stable-isotope probing reveals potential key players for microbial decomposition and degradation of diatom-derived marine particulate matter. MicrobiologyOpen 2020, 9, e1013. [Google Scholar] [CrossRef] [PubMed]
- Alcolombri, U.; Peaudecerf, F.J.; Fernandez, V.I.; Behrendt, L.; Lee, K.S.; Stocker, R. Sinking enhances the degradation of organic particles by marine bacteria. Nat. Geosci. 2021, 14, 775–780. [Google Scholar] [CrossRef]
- Bochdansky, A.B.; Clouse, M.A.; Herndl, G.J. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 2017, 11, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.; Zühlke, D.; Bernhardt, J.; Francis, T.B.; Albrecht, D.; Hirschfeld, C.; Markert, S.; Riedel, K. An optimized metaproteomics protocol for a holistic taxonomic and functional characterization of microbial communities from marine particles. Environ. Microbiol. Rep. 2020, 12, 367–376. [Google Scholar] [CrossRef]
- López-Pérez, M.; Kimes, N.E.; Haro-Moreno, J.M.; Rodriguez-Valera, F. Not all particles are equal: The selective enrichment of particle-associated bacteria from the Mediterranean sea. Front. Microbiol. 2016, 7, 996. [Google Scholar] [CrossRef]
- Gregson, B.H.; McKew, B.A.; Holland, R.D.; Nedwed, T.; Prince, R.; McGenity, T. Marine oil snow, a microbial perspective. Front. Mar. Sci. 2021, 28, 11. [Google Scholar] [CrossRef]
| Plastic | Microorganism | Reference |
|---|---|---|
| PE/PET | Achromobacter xylosoxidans Rhodococcus ruber Brevibacillus borstelensis Bacillus spp. Thermobifida fusca Thermobifida halotolerans Alcanivorax Marinobacter Arenibacter Ideonella sakaiensis Kocuria palustris Clostridium thermocellum Saccharomonospora viridis Thermomonospora curvata Streptomyces sp. Streptomyces albogriseolus Aspergillus sp. Penicillium simplicissimum Zalerion maritimu Anabaena spiroides | [120] [42,121] [122] [123,124,125] [126] [127] [119,128] [119] [119] [100] [78] [129] [130] [131] [132,133] [134] [135] [105,136] [137] [138] |
| PHA | Pseudomonas stutzeri Alkaligenes faecalis Streptomyces sp. | [139] [140] [141] |
| PCL | Alkaligenes faecalis Alcanivorax Tenacibaculum Pseudomonas spp. Clostridium botulinum Streptomyces thermoviolaceus Fusarium sp. Brevundimonas sp. | [142] [143] [143] [143,144] [139] [145] [53,102] [146] |
| PLA | Bacillus brevis Fusarium moniliforme Penicillium roqueforti | [147] [148] [139] |
| Polyurethane/PE-PU | Comamonas acidovorans Pseudomonas chlororaphis Pseudomonas putida Acinetobacter gerneri Aureobasidium pullulans Fusarium solani Alternaria sp. | [149] [150] [151] [152] [153] [140] [154] |
| PVC | Pseudomonas putida | [155,156,157] |
| Polystyrene | Rhodococcus ruber Azotobacter beijerinckii | [158] [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonseca, E.M.; Gaylarde, C.; Baptista Neto, J.A.; Camacho Chab, J.C.; Ortega-Morales, O. Microbial Interactions with Particulate and Floating Pollutants in the Oceans: A Review. Micro 2022, 2, 257-276. https://doi.org/10.3390/micro2020017
da Fonseca EM, Gaylarde C, Baptista Neto JA, Camacho Chab JC, Ortega-Morales O. Microbial Interactions with Particulate and Floating Pollutants in the Oceans: A Review. Micro. 2022; 2(2):257-276. https://doi.org/10.3390/micro2020017
Chicago/Turabian Styleda Fonseca, Estefan Monteiro, Christine Gaylarde, José Antônio Baptista Neto, Juan Carlos Camacho Chab, and Otto Ortega-Morales. 2022. "Microbial Interactions with Particulate and Floating Pollutants in the Oceans: A Review" Micro 2, no. 2: 257-276. https://doi.org/10.3390/micro2020017
APA Styleda Fonseca, E. M., Gaylarde, C., Baptista Neto, J. A., Camacho Chab, J. C., & Ortega-Morales, O. (2022). Microbial Interactions with Particulate and Floating Pollutants in the Oceans: A Review. Micro, 2(2), 257-276. https://doi.org/10.3390/micro2020017






