Biomass Waste Carbonization in Piranha Solution: A Route to Hypergolic Carbons?
Abstract
1. Introduction
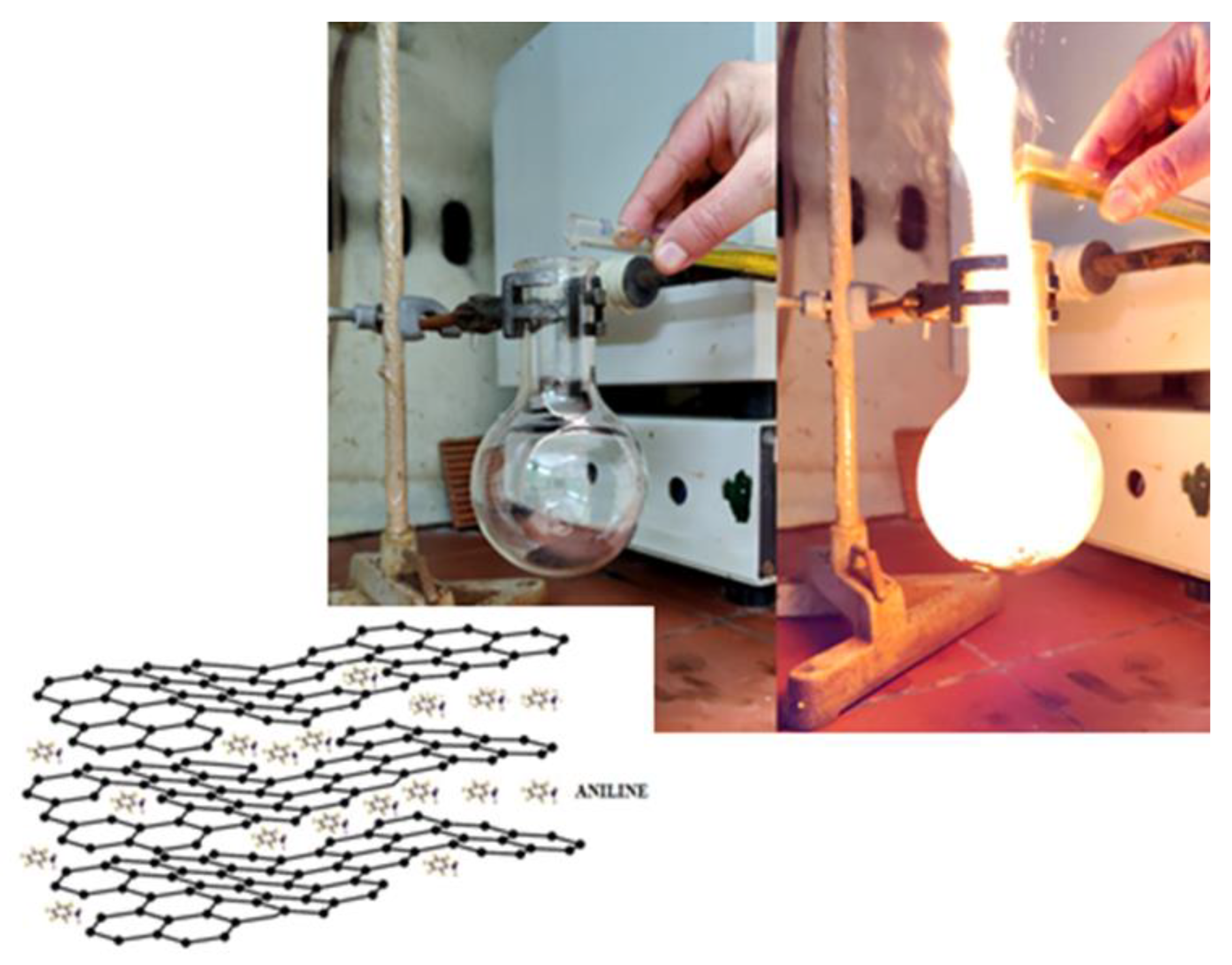
2. Materials and Methods
3. Results
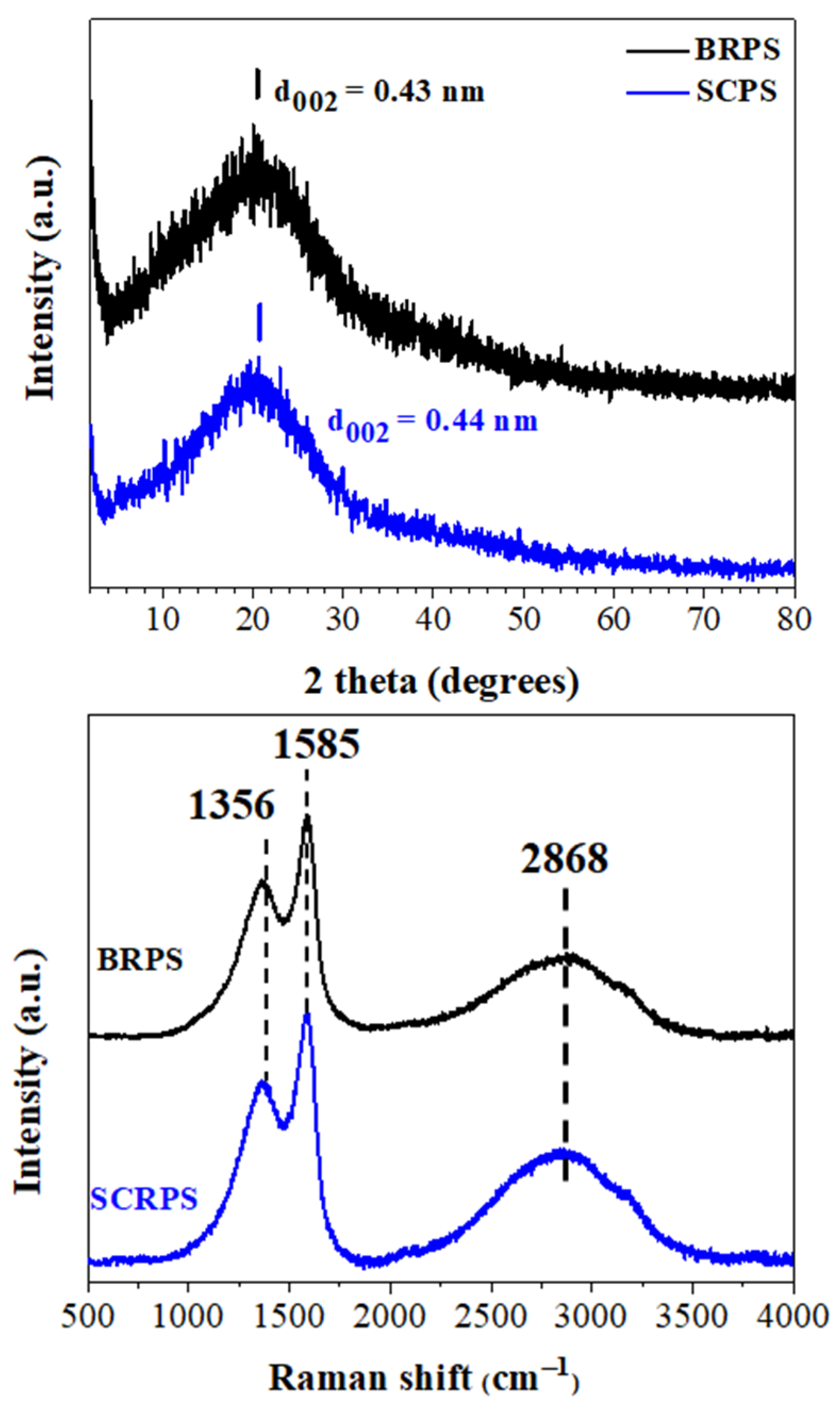
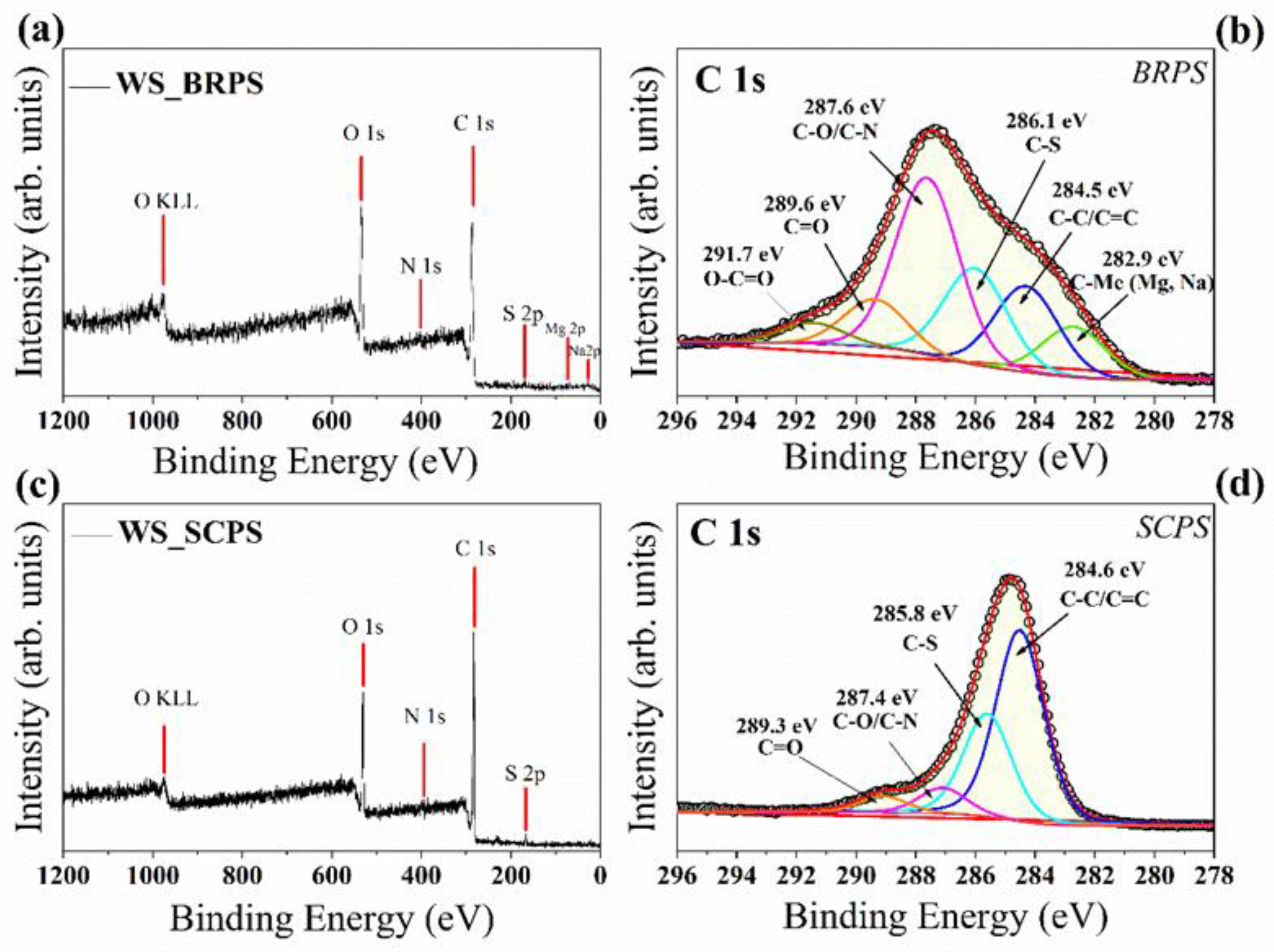
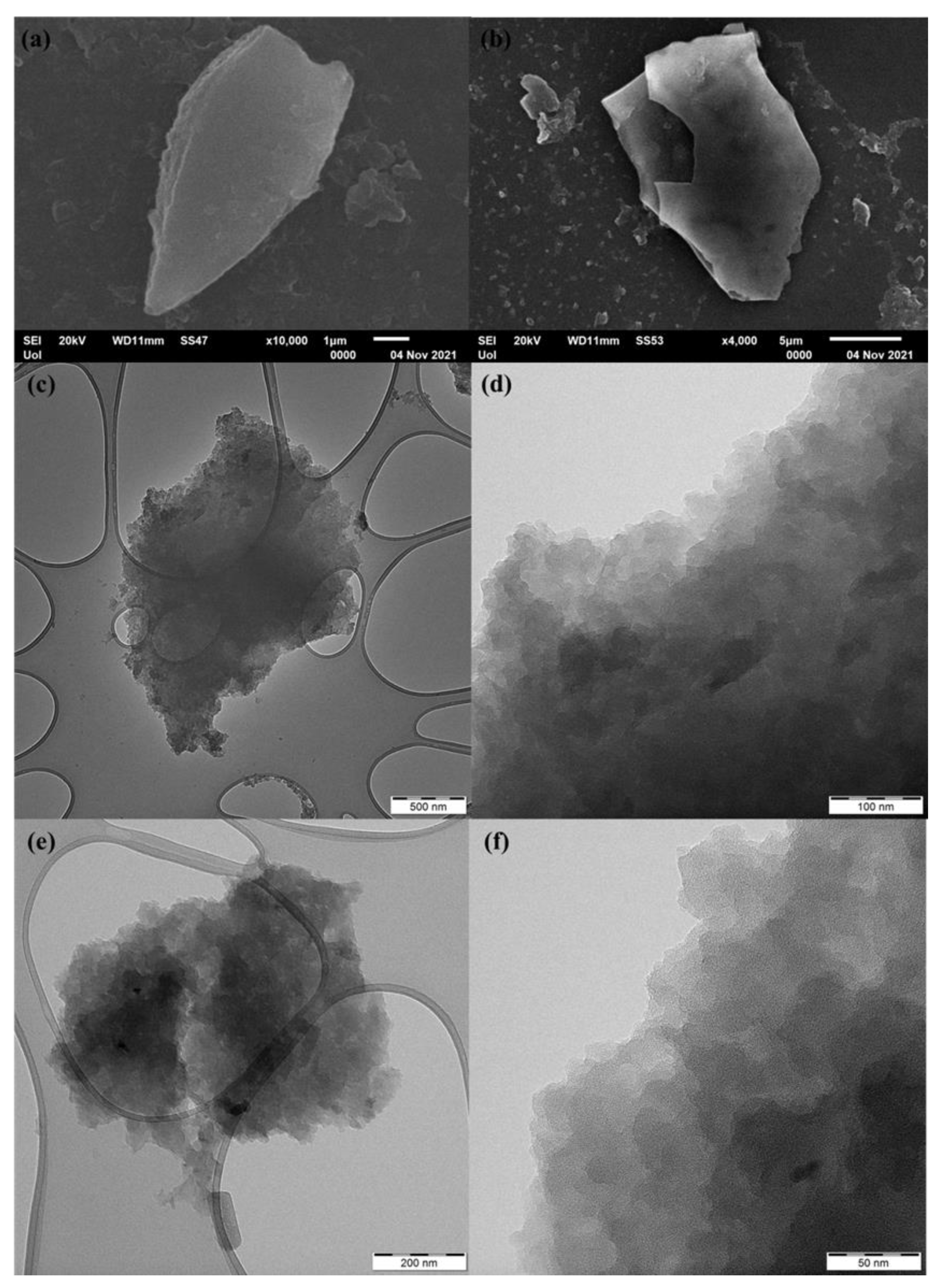
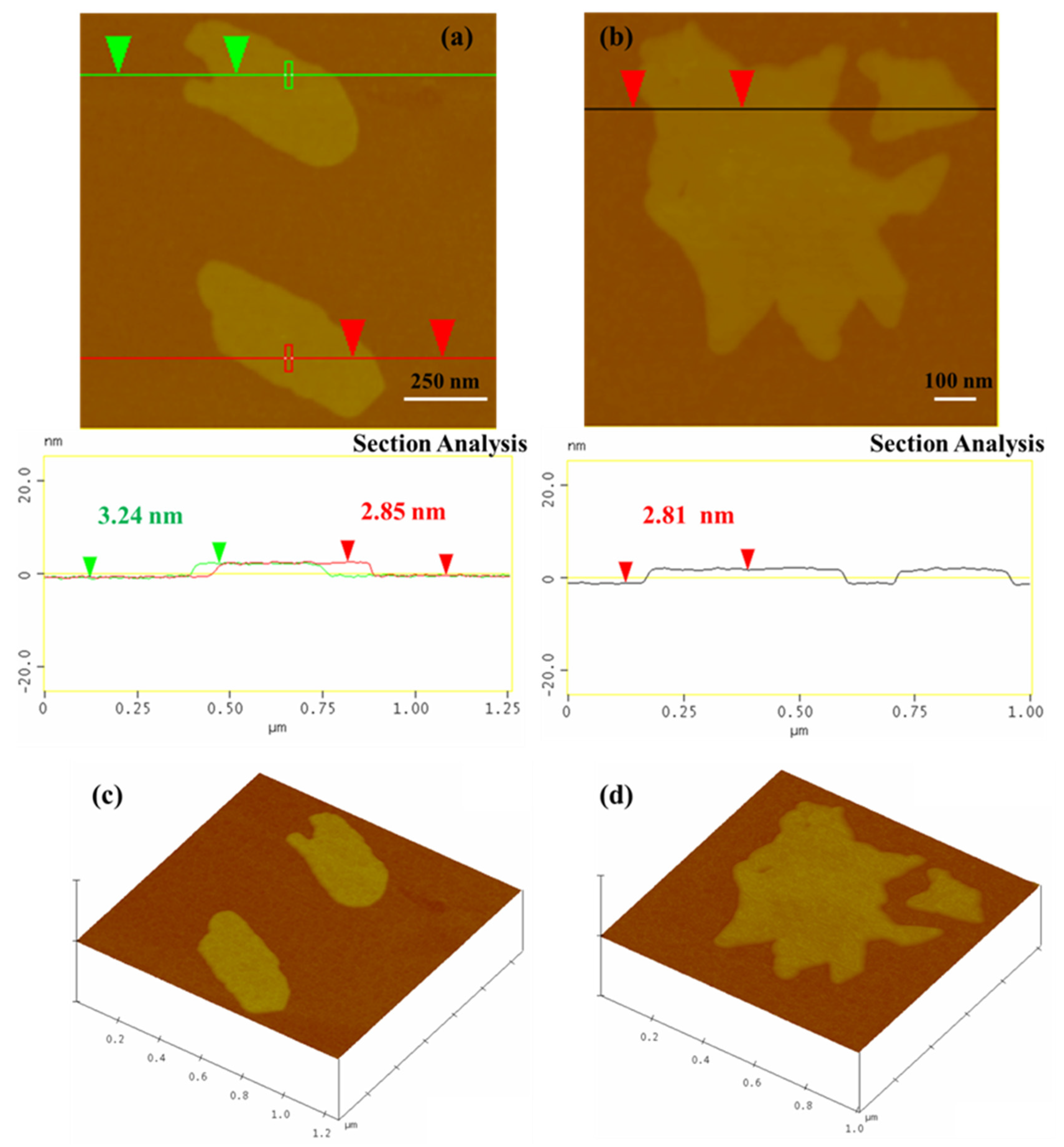
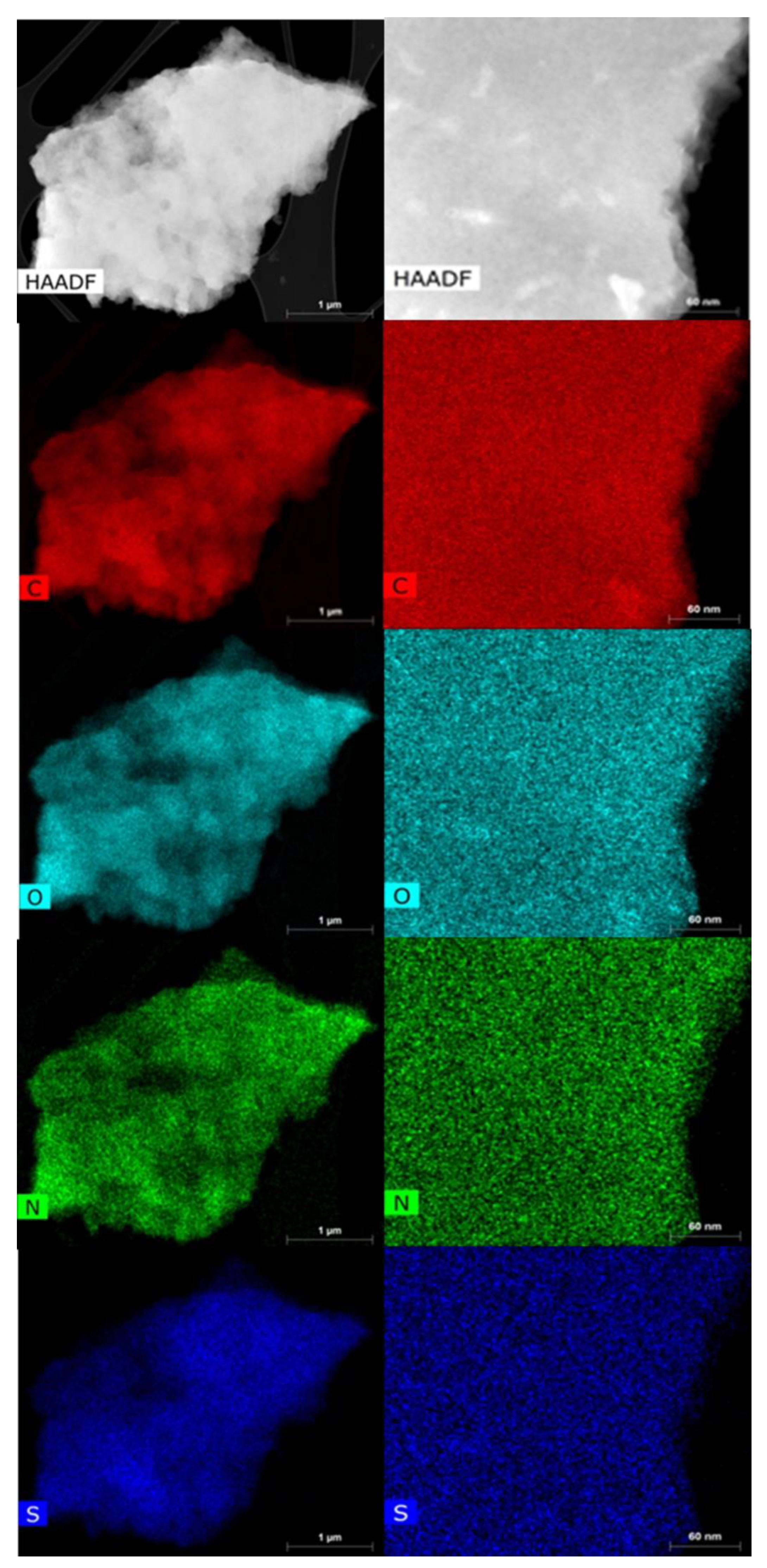
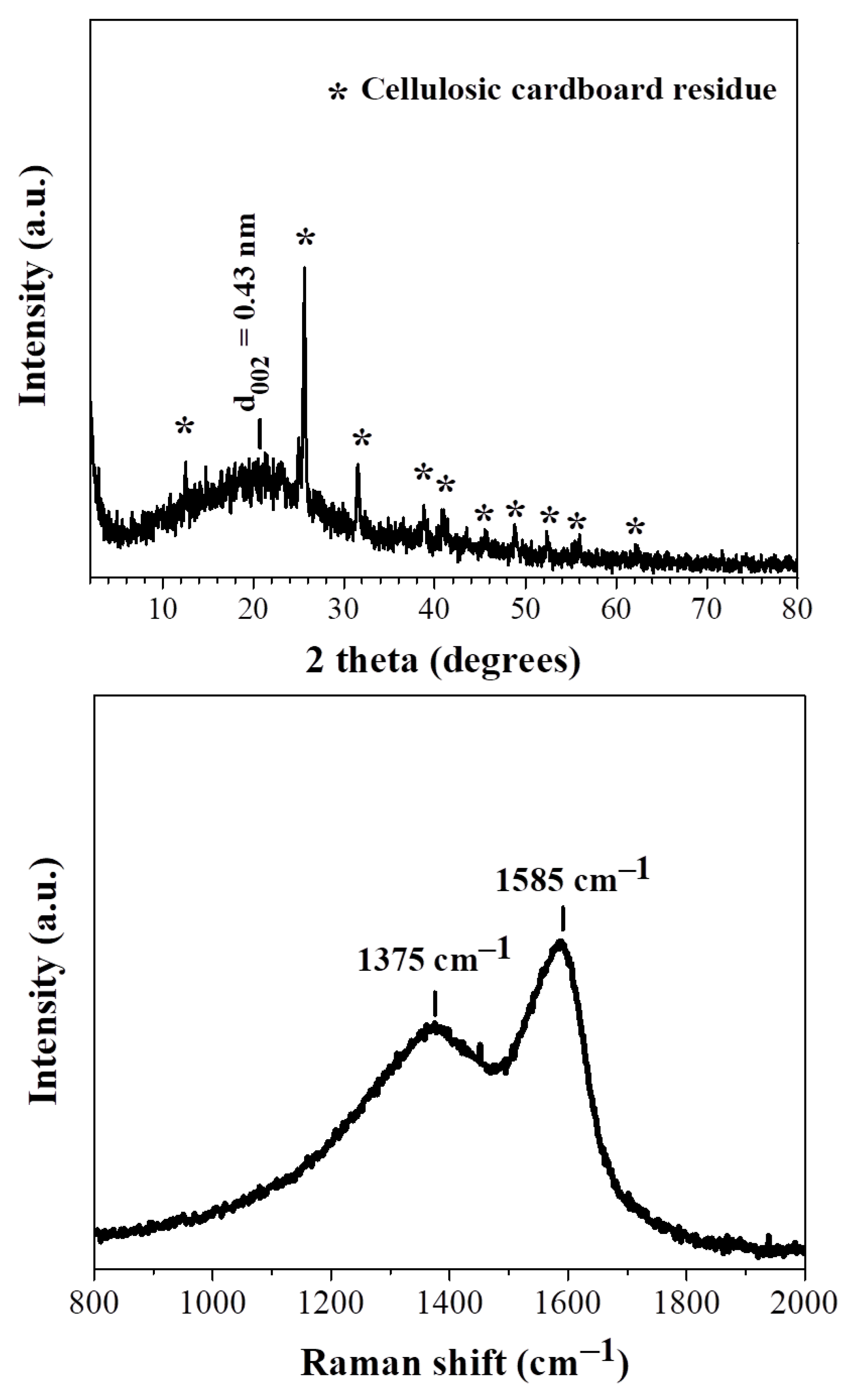
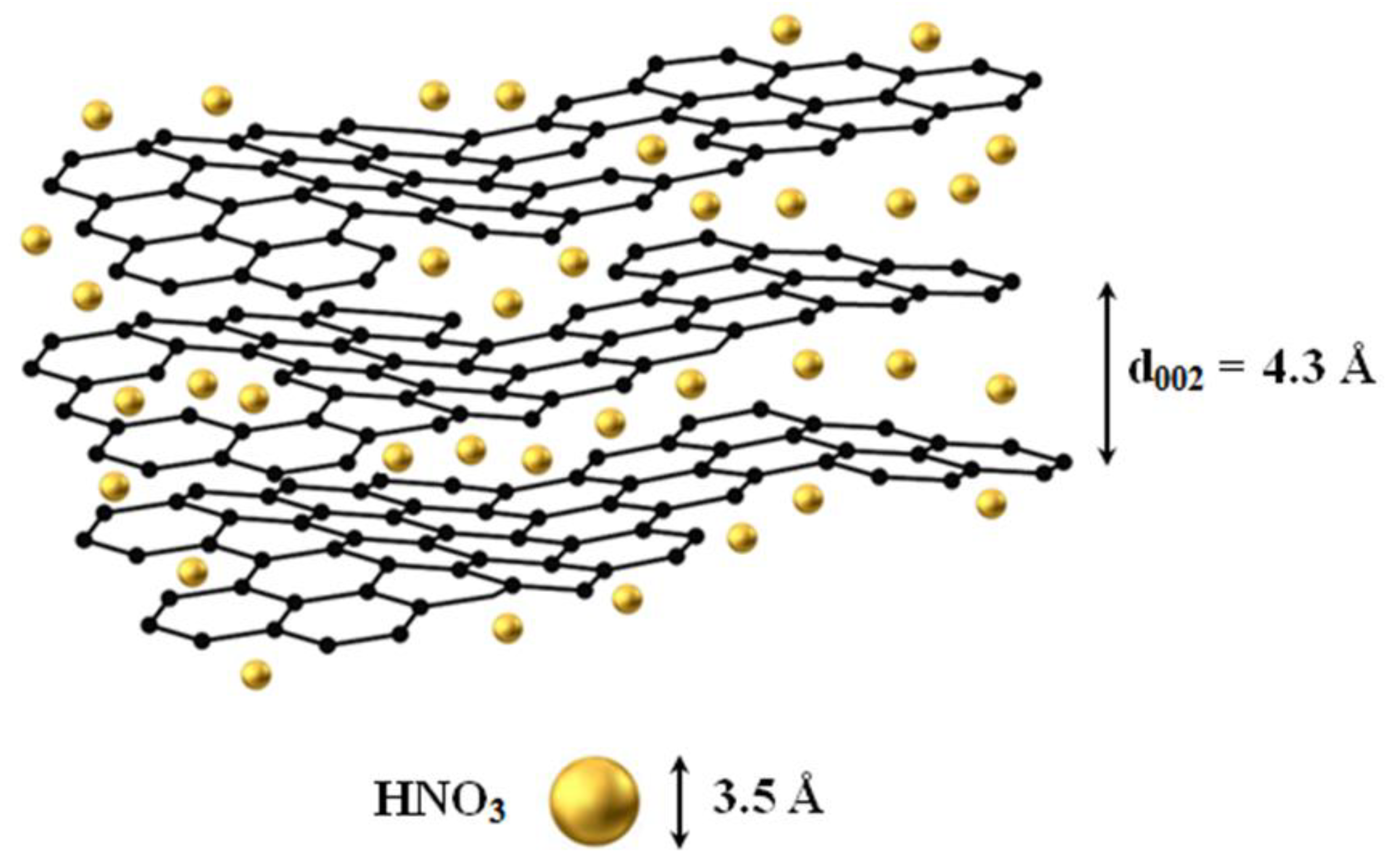
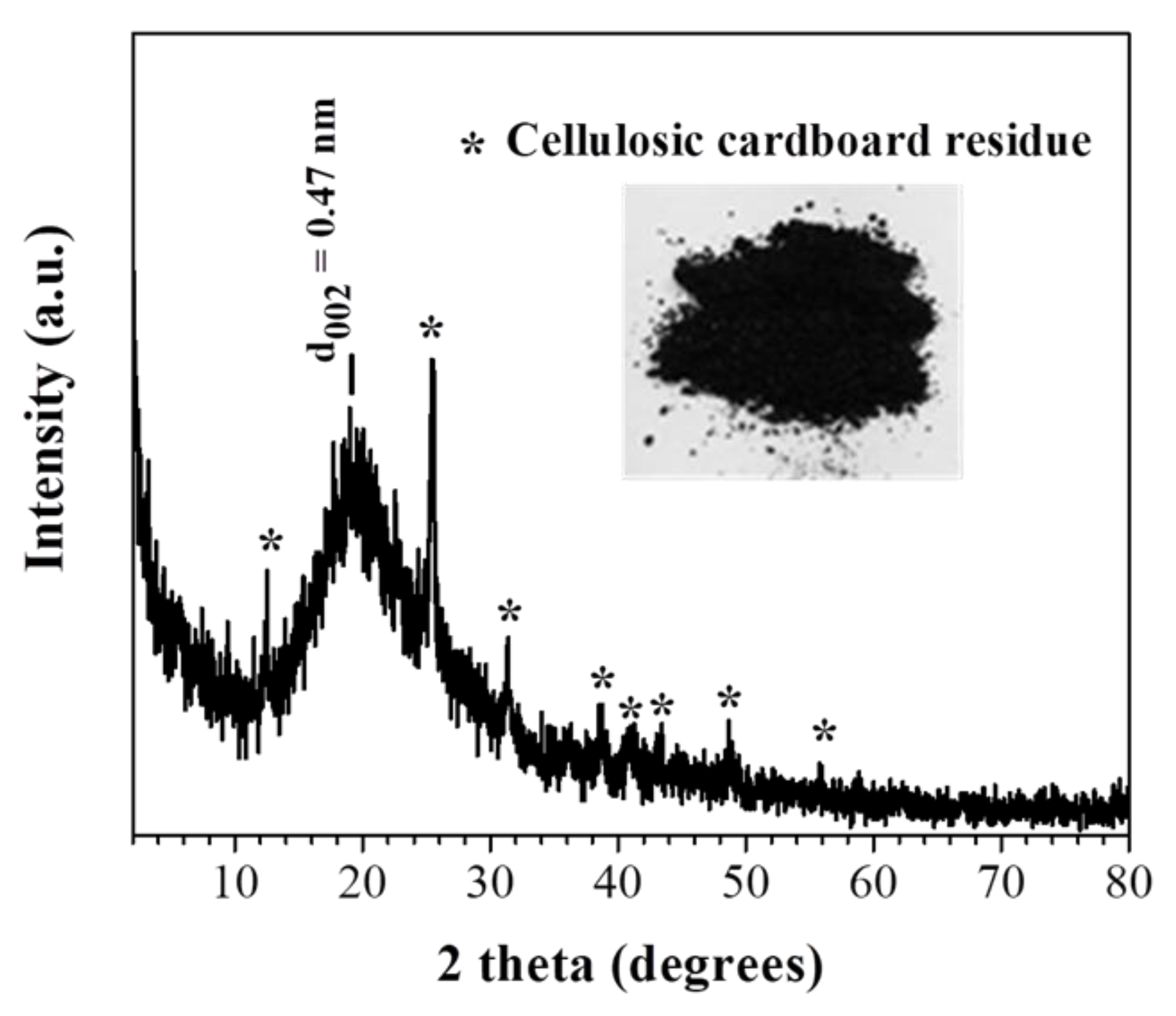
3.1. Structural Characterization and Morphology
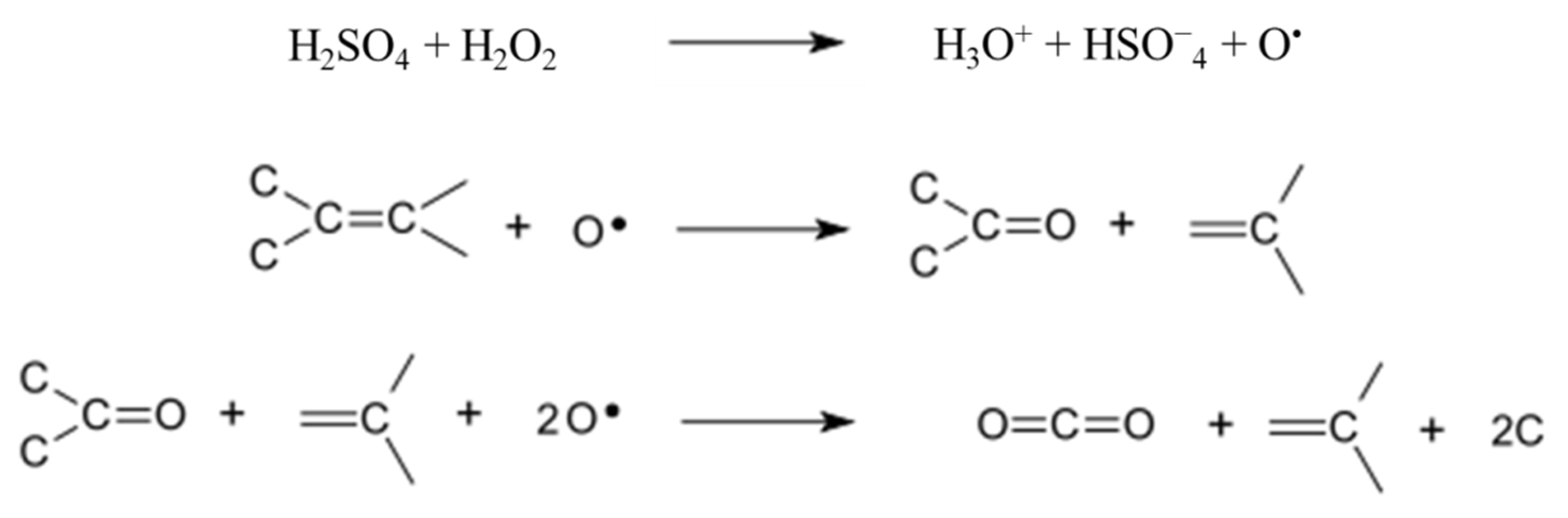
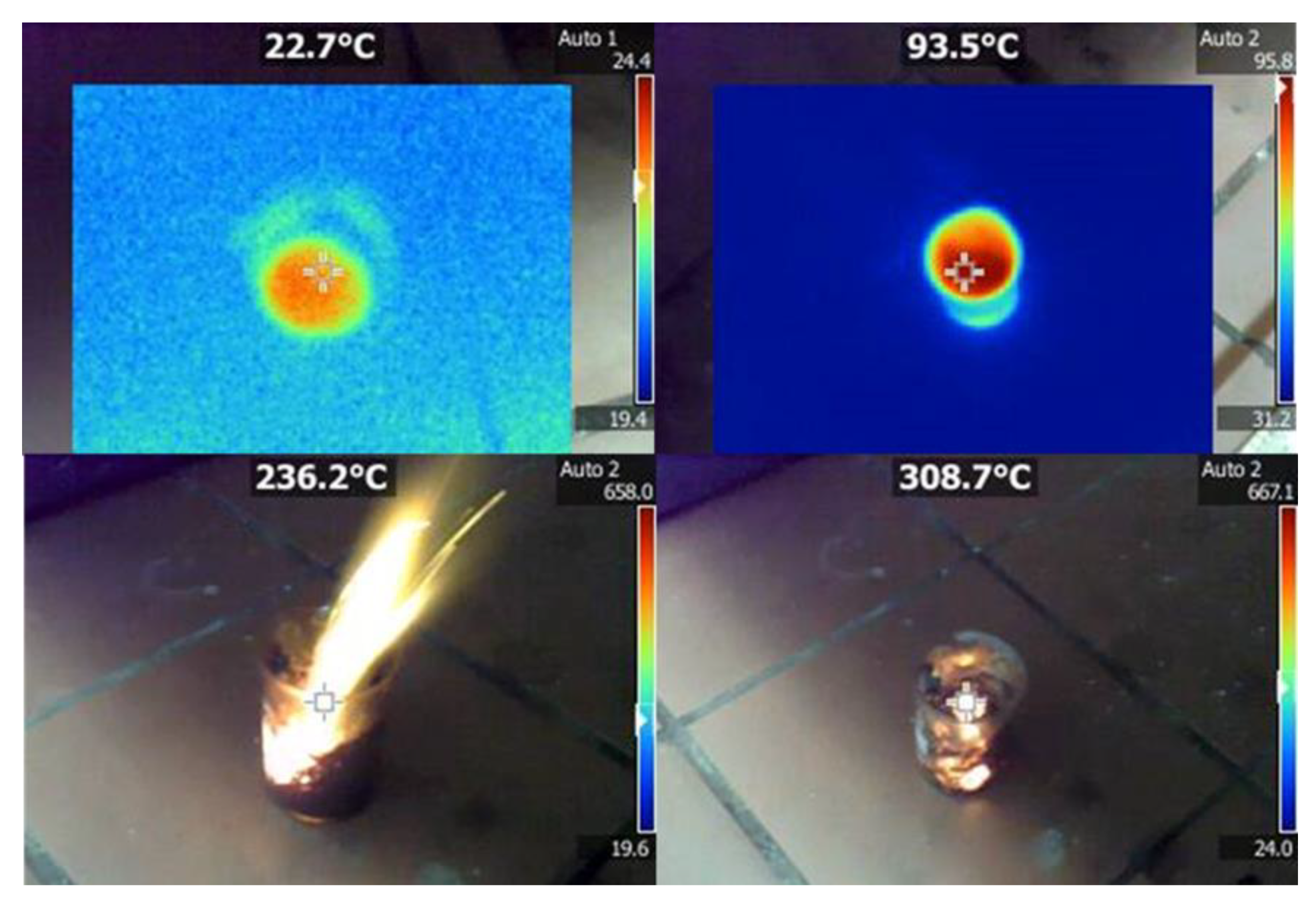
3.2. Carbon Hypergolicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savaram, K.; Kalyanikar, M.; Patel, M.; Brukh, R.; Flach, C.R.; Huang, R.; Khoshi, M.R.; Mendelsohn, R.; Wang, A.; Garfunkel, E.; et al. Synergy of oxygen and a piranha solution for eco-friendly production of highly conductive graphene dispersions. Green Chem. 2015, 17, 869–881. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Vasudevan, D.; Narayan, R.; Raju, K.V.S.N. Controllable synthesis of biosourced blue-green fluorescent carbon dots from camphor for the detection of heavy metal ions in water. RSC Adv. 2014, 4, 57137–57143. [Google Scholar] [CrossRef]
- Mei, Q.; Chen, J.; Zhao, J.; Yang, L.; Liu, B.; Liu, R.; Zhang, Z. Atomic oxygen tailored graphene oxide nanosheets emissions for multicolor cellular imaging. ACS Appl. Mater. Interfaces 2016, 8, 7390–7395. [Google Scholar] [CrossRef]
- Ziegler, K.J.; Gu, Z.; Peng, H.; Flor, E.L.; Hauge, R.H.; Smalley, R.E. Controlled oxidative cutting of single-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Akinwolemiwa, B.; Wang, Q.; Guan, L.; Xia, L.; Hu, D.; Tang, B.; Yu, L.; Chen, G.Z. Mechano-fenton–piranha oxidation of carbon nanotubes for energy application. Adv. Sustain. Syst. 2019, 3, 1900065. [Google Scholar] [CrossRef]
- Lavagna, L.; Musso, S.; Ferro, G.; Pavese, M. Cement-based composites containing functionalized carbon fibers. Cem. Concr. Compos. 2018, 88, 165–171. [Google Scholar] [CrossRef]
- Konkol, I.; Zwierczek, L.; Cenian, A. Biogas production from bakery wastes dynamics, retention time and biogas potential. J. Res. Appl. Agric. Eng. 2018, 63, 32–34. [Google Scholar]
- Narisetty, V.; Cox, R.; Willoughby, N.; Aktas, E.; Tiwari, B.; Matharu, A.S.; Salonitis, K.; Kumar, V. Recycling bread waste into chemical building blocks using a circular biorefining approach. Sustain. Energy Fuels 2021, 5, 4842–4849. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, Y.; Wang, C.; Xu, F.; Lin, Z.; Qin, Y.; Li, Y.; Yang, M.; He, X.; Peng, Q.; et al. Multifunctional stiff carbon foam derived from bread. ACS Appl. Mater. Interfaces 2016, 8, 16852–16861. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Gao, Y.; Ma, Y. Facile preparation of activated carbon foam via pyrolysis of waste bread under CO2 atmosphere. Biomass Convers. Biorefinery 2019, 9, 521–529. [Google Scholar] [CrossRef]
- Bhandari, S.; Mondal, D.; Nataraj, S.K.; Balakrishna, R.G. Biomolecule-derived quantum dots for sustainable optoelectronics. Nanoscale Adv. 2019, 1, 913–936. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar obtained by carbonization of spent coffee grounds and its application in the construction of an energy storage device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Massaya, J.; Pickens, G.; Mills-Lamptey, B.; Chuck, C.J. Enhanced hydrothermal carbonization of spent coffee grounds for the efficient production of solid fuel with lower nitrogen content. Energy Fuels 2021, 35, 9462–9473. [Google Scholar] [CrossRef]
- Asimakopoulos, G.; Baikousi, M.; Kostas, V.; Papantoniou, M.; Bourlinos, A.B.; Zbořil, R.; Karakassides, M.A.; Salmas, C.E. Nanoporous activated carbon derived via pyrolysis process of spent coffee: Structural characterization. Investigation of its use for hexavalent chromium removal. Appl. Sci. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Torrelio Martos, A.G.; López, E.P. Chemical composition, percent of dietary reference intake, and acceptability of gluten-free bread made from Prosopis nigra flour, added with hydrocolloids. Food Sci. Technol. 2018, 38, 619–624. [Google Scholar] [CrossRef]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Synthesis of highly crystalline graphite from spontaneous ignition of in situ derived acetylene and chlorine at ambient conditions. Molecules 2020, 25, 297. [Google Scholar] [CrossRef]
- Roh, J.-S. Structural study of the activated carbon fiber using laser raman spectroscopy. Carbon Lett. 2008, 9, 127–130. [Google Scholar] [CrossRef]
- Saikia, B.K.; Boruah, R.K.; Gogoi, P.K. A X-ray diffraction analysis on graphene layers of Assam coal. J. Chem. Sci. 2009, 121, 103–106. [Google Scholar] [CrossRef]
- Puech, P.; Kandara, M.; Paredes, G.; Moulin, L.; Weiss-Hortala, E.; Kundu, A.; Ratel-Ramond, N.; Plewa, J.-M.; Pellenq, R.; Monthioux, M. Analyzing the raman spectra of graphenic carbon materials from kerogens to nanotubes: What type of information can be extracted from defect bands? C 2019, 5, 69. [Google Scholar] [CrossRef]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of graphene oxide and single layer graphene flakes—defects annealing and healing. Front. Mater. 2018, 5, 37. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Giannelis, E.P.; Sanakis, Y.; Bakandritsos, A.; Karakassides, M.; Gjoka, M.; Petridis, D. A graphite oxide-like carbogenic material derived from a molecular precursor. Carbon 2006, 44, 1906–1912. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Tantis, I.; Bourlinos, A.B.; Bakandritsos, A.; Fotiadou, R.; Patila, M.; Stamatis, H.; Avgeropoulos, A.; Karakassides, M.A.; et al. Carbon nanostructures derived through hypergolic reaction of conductive polymers with fuming nitric acid at ambient conditions. Molecules 2021, 26, 1595. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Vourdas, N.; Zygouri, P.; Chalmpes, N.; Potsi, G.; Kostas, V.; Spyrou, K.; Stathopoulos, V.N.; Gournis, D.; Rudolf, P. Controlled deposition of fullerene derivatives within a graphene template by means of a modified Langmuir-Schaefer method. J. Colloid Interface Sci. 2018, 524, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dai, Y.; Wang, S.; Cheng, H.; Yu, J. In situ incorporation of a S, N doped carbon/sulfur composite for lithium sulfur batteries. RSC Adv. 2015, 5, 78017–78025. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Ferreira, E.; Heleno, S.; Rodrigues, P.; Barros, L.; Ferreira, I.C.F.R. Comparison of different bread types: Chemical and physical parameters. Food Chem. 2020, 310, 125954. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Huang, H.; Lu, Y.-C.; Wang, A.-J.; Liu, J.-H.; Chen, J.-R.; Feng, J.-J. A facile, green, and solvent-free route to nitrogen–sulfur-codoped fluorescent carbon nanoparticles for cellular imaging. RSC Adv. 2014, 4, 11872–11875. [Google Scholar] [CrossRef]
- Jiao, C.; Zhang, Z.; Tao, J.; Zhang, D.; Chen, Y.; Lin, H. Synthesis of a poly(amidoxime-hydroxamic acid) cellulose derivative and its application in heavy metal ion removal. RSC Adv. 2017, 7, 27787–27795. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Kouloumpis, A.; Zygouri, P.; Karouta, N.; Spyrou, K.; Stathi, P.; Tsoufis, T.; Georgakilas, V.; Gournis, D.; Rudolf, P. Layer-by-layer assembly of clay–carbon nanotube hybrid superstructures. ACS Omega 2019, 4, 18100–18107. [Google Scholar] [CrossRef] [PubMed]
- Munjal, N.L. Ignition catalysts for furfuryl alcohol—Red fuming nitric acid bipropellant. AIAA J. 1970, 8, 980–981. [Google Scholar] [CrossRef]
- Schneider, S.; Hawkins, T.; Rosander, M.; Vaghjiani, G.; Chambreau, S.; Drake, G. Ionic liquids as hypergolic fuels. Energy Fuels 2008, 22, 2871–2872. [Google Scholar] [CrossRef]
- Kulkarni, S.G.; Bagalkote, V.S.; Patil, S.S.; Kumar, U.P.; Kumar, V.A. Theoretical evaluation and experimental validation of performance parameters of new hypergolic liquid fuel blends with red fuming nitric acid as oxidizer. Propellants Explos. Pyrotech. 2009, 34, 520–525. [Google Scholar] [CrossRef]
- Kulkarni, S.G.; Bagalkote, V.S. Studies on pre-ognition reactions of hydrocarbon-based rocket fuels hypergolic with red fuming nitric acid as oxidizer. J. Energ. Mater. 2010, 28, 173–188. [Google Scholar] [CrossRef]
- Da Silva, G.; Iha, K. Hypergolic Systems: A review in patents. J. Aerosp. Technol. Manag. 2012, 4, 407–412. [Google Scholar] [CrossRef][Green Version]
- Bhosale, M.V.K.; Kulkarni, S.G.; Kulkarni, P.S. Ionic liquid and biofuel blend: A low–cost and high performance hypergolic fuel for propulsion application. ChemistrySelect 2016, 1, 1921–1925. [Google Scholar] [CrossRef]
- Titi, H.M.; Marrett, J.M.; Dayaker, G.; Arhangelskis, M.; Mottillo, C.; Morris, A.J.; Rachiero, G.P.; Friščić, T.; Rogers, R.D. Hypergolic zeolitic imidazolate frameworks (ZIFs) as next-generation solid fuels: Unlocking the latent energetic behavior of ZIFs. Sci. Adv. 2019, 5, eaav9044. [Google Scholar] [CrossRef]
- Yuan, W.-L.; Zhang, L.; Tao, G.-H.; Wang, S.-L.; Wang, Y.; Zhu, Q.-H.; Zhang, G.-H.; Zhang, Z.; Xue, Y.; Qin, S.; et al. Designing high-performance hypergolic propellants based on materials genome. Sci. Adv. 2020, 6, eabb1899. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Zadra, F. Combustion of nanoaluminum and magnesium in fuel-rich propellants. Propellants Explos. Pyrotech. 2020, 45, 724–729. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Zhong, Y.; Lei, G.; Li, Z.; Zhang, J.; Zhang, T. High-energy metal–organic frameworks with a dicyanamide linker for hypergolic fuels. Inorg. Chem. 2021, 60, 5100–5106. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Bourlinos, A.B.; Talande, S.; Bakandritsos, A.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Nanocarbon from rocket fuel waste: The case of furfuryl alcohol-fuming nitric acid hypergolic pair. Nanomaterials 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Moschovas, D.; Bourlinos, A.B.; Spyrou, K.; Vasilopoulos, K.C.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic ignition of 1,3-cyclodienes by fuming nitric acid toward the fast and spontaneous formation of carbon nanosheets at ambient conditions. Micro 2021, 1, 15–27. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Šedajová, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic materials synthesis through reaction of fuming nitric acid with certain cyclopentadienyl compounds. C 2020, 6, 61. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Gozin, M.; Zhao, F.-Q.; Cohen, A.; Pang, S.-P. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 2016, 8, 4799–4851. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in carbon nanomaterials synthesis: New paradigms and perspectives. Molecules 2020, 25, 2207. [Google Scholar] [CrossRef]
- Turkevich, L.A.; Fernback, J.; Dastidar, A.G.; Osterberg, P. Potential explosion hazard of carbonaceous nanoparticles: Screening of allotropes. Combust. Flame 2016, 167, 218–227. [Google Scholar] [CrossRef]
- Huston, J.L.; Scott, R.G.; Studier, M.H. Reaction of fluorine gas with coal and the aromaticity of coal. Fuel 1976, 55, 281–286. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Terrones, M.; Guardia, A.d.l.; Huc, V.; Grobert, N.; Wei, B.Q.; Lezec, H.; Ramanath, G.; Ebbesen, T.W. Nanotubes in a flash-ignition and reconstruction. Science 2002, 296, 705. [Google Scholar] [CrossRef] [PubMed]
- Cuzzillo, B.R.; Pagni, P. The myth of pyrophoric carbon. Fire Saf. Sci. 2000, 6, 301–312. [Google Scholar] [CrossRef]
- Baikousi, M.; Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Direct production of carbon nanosheets by self-ignition of pyrophoric lithium dialkylamides in air. Mater. Lett. 2019, 254, 58–61. [Google Scholar] [CrossRef]
- Spyrou, K.; Kang, L.; Diamanti, E.K.; Gengler, R.Y.; Gournis, D.; Prato, M.; Rudolf, P. A novel route towards high quality fullerene-pillared graphene. Carbon 2013, 61, 313–320. [Google Scholar] [CrossRef][Green Version]
- Rastogi, R.P.; Munjal, N.L. Mechanism and kinetics of pre-ignition reactions: Part I-aniline-red fuming nitric acid propellants. Indian J. Chem. 1966, 4, 463–468. [Google Scholar]
- Gune, S.G.; Kulkarni, S.G.; Panda, S.P. Synergistic hypergolic ignition of solid substituted anilines mixed with magnesium powder and red fuming nitric acid. Combust. Flame 1985, 61, 189–193. [Google Scholar] [CrossRef]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional carbon materials derived through hypergolic reactions at ambient conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Karakassides, M.A.; Simopoulos, A.; Petridis, D. Synthesis and characterization of magnetically modified clay composites. Chem. Mater. 2000, 12, 2640–2645. [Google Scholar] [CrossRef]
- Dallas, P.; Bourlinos, A.B.; Petridis, D.; Boukos, N.; Papadokostaki, K.; Niarchos, D.; Guskos, N. Synthesis and characterization of 2-D and 3-D covalent networks derived from triazine central cores and bridging aromatic diamines. Polymer 2008, 49, 1137–1144. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmpes, N.; Baikousi, M.; Giousis, T.; Rudolf, P.; Salmas, C.E.; Moschovas, D.; Avgeropoulos, A.; Bourlinos, A.B.; Tantis, I.; Bakandritsos, A.; et al. Biomass Waste Carbonization in Piranha Solution: A Route to Hypergolic Carbons? Micro 2022, 2, 137-153. https://doi.org/10.3390/micro2010009
Chalmpes N, Baikousi M, Giousis T, Rudolf P, Salmas CE, Moschovas D, Avgeropoulos A, Bourlinos AB, Tantis I, Bakandritsos A, et al. Biomass Waste Carbonization in Piranha Solution: A Route to Hypergolic Carbons? Micro. 2022; 2(1):137-153. https://doi.org/10.3390/micro2010009
Chicago/Turabian StyleChalmpes, Nikolaos, Maria Baikousi, Theodosis Giousis, Petra Rudolf, Constantinos E. Salmas, Dimitrios Moschovas, Apostolos Avgeropoulos, Athanasios B. Bourlinos, Iosif Tantis, Aristides Bakandritsos, and et al. 2022. "Biomass Waste Carbonization in Piranha Solution: A Route to Hypergolic Carbons?" Micro 2, no. 1: 137-153. https://doi.org/10.3390/micro2010009
APA StyleChalmpes, N., Baikousi, M., Giousis, T., Rudolf, P., Salmas, C. E., Moschovas, D., Avgeropoulos, A., Bourlinos, A. B., Tantis, I., Bakandritsos, A., Gournis, D., & Karakassides, M. A. (2022). Biomass Waste Carbonization in Piranha Solution: A Route to Hypergolic Carbons? Micro, 2(1), 137-153. https://doi.org/10.3390/micro2010009











