Effect of Viscosity on High-Throughput Deterministic Lateral Displacement (DLD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Various Viscosity Sample Preparation
2.2. Viscometer Measurement
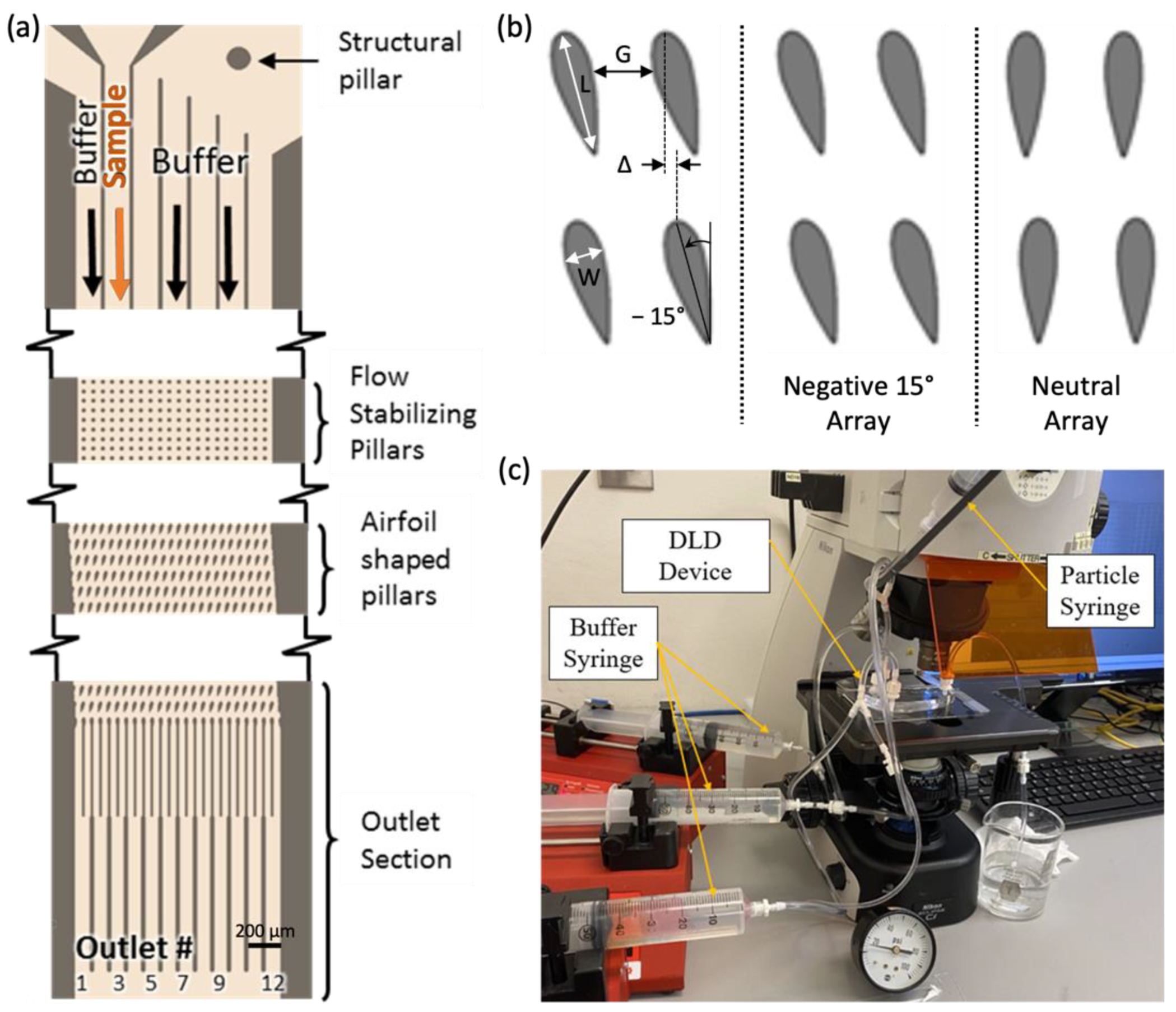
2.3. Airfoil DLD Device Design and Fabrication
2.4. DLD Testing Methods
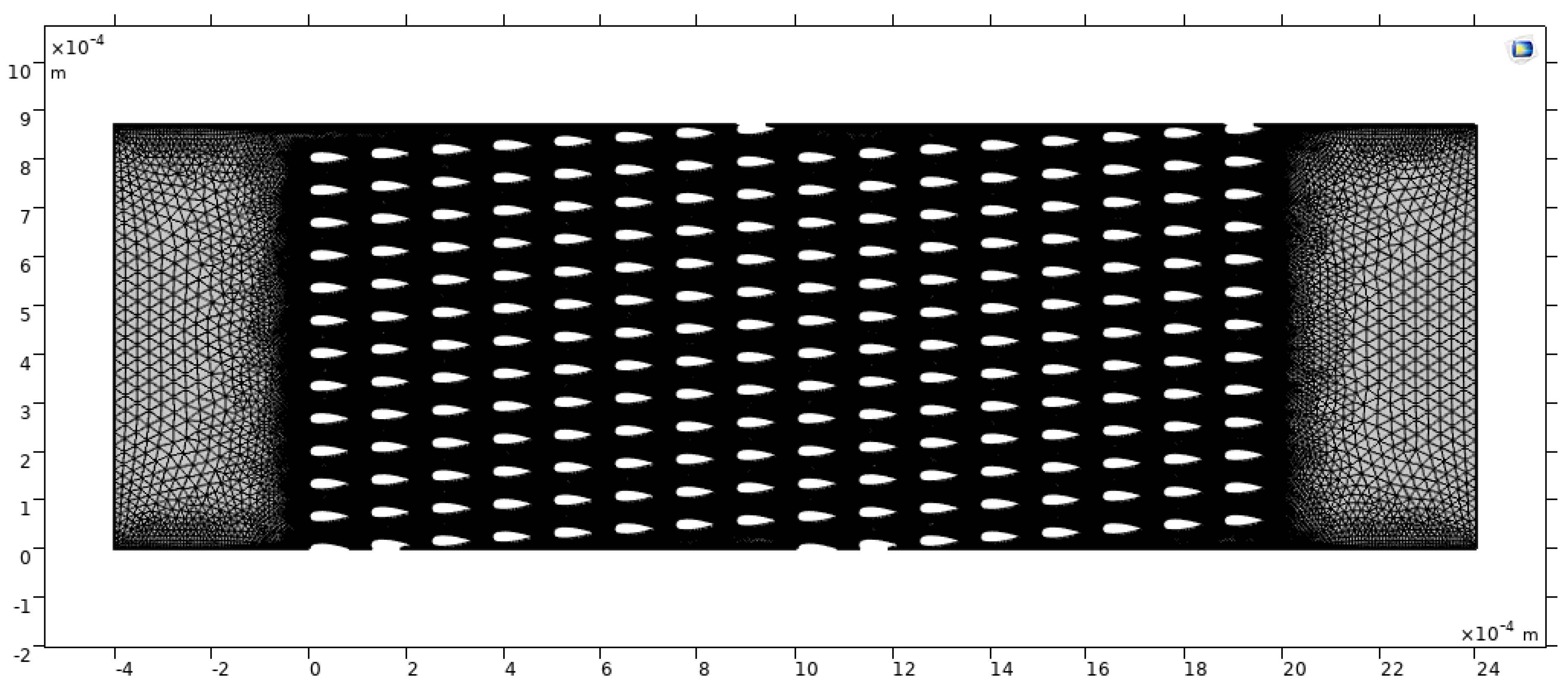
2.5. Computational Study
3. Results and Discussion
3.1. Viscosity Measurement
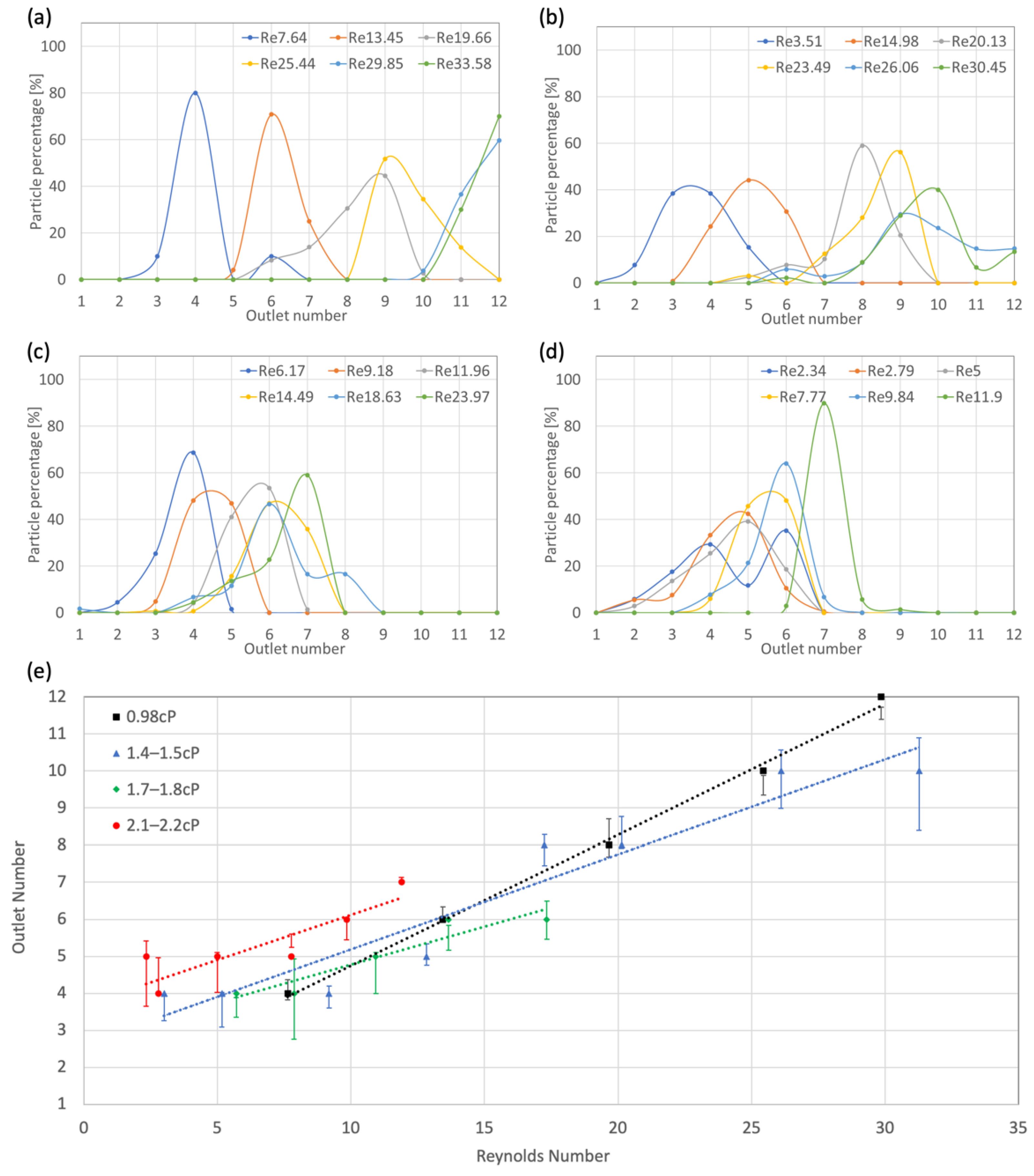
3.2. DLD Testing Results
3.2.1. Flow Rate Linearity
3.2.2. Neutral AoA
3.2.3. Negative 15° AoA
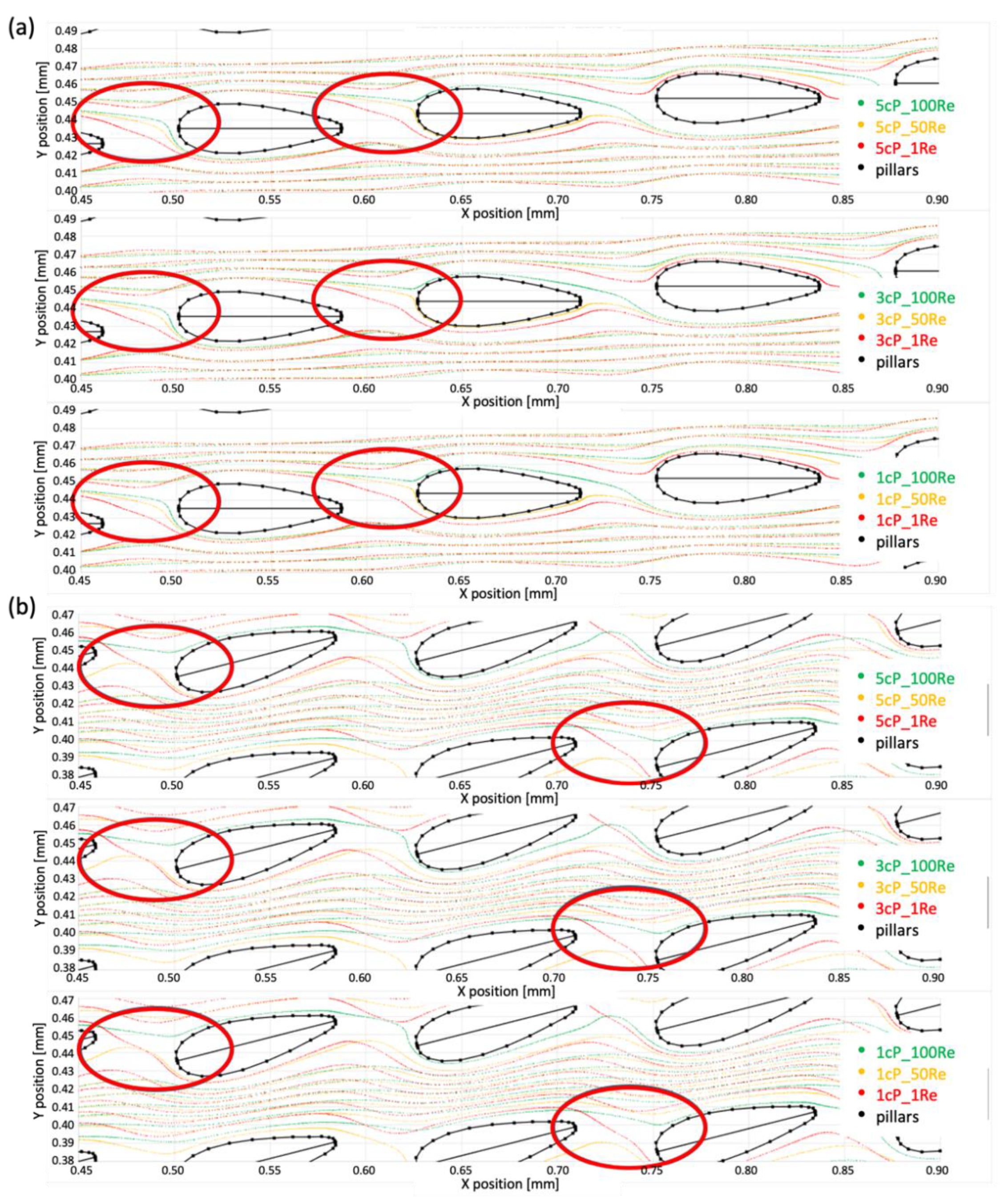
3.3. Computational Study Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, Z.; Zhang, W.; Yu, C.; Zhang, J.; Wen, Y. Recent advances in biological sample preparation methods coupled with chromatography, spectrometry and electrochemistry analysis techniques. TrAC Trends Anal. Chem. 2018, 102, 123–146. [Google Scholar] [CrossRef]
- Amini, H.; Lee, W.; Di Carlo, D. Inertial microfluidic physics. Lab A Chip 2014, 14, 2739–2761. [Google Scholar] [CrossRef]
- Alexander, D.E. Nature’s Machines: An Introduction to Organismal Biomechanics; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar]
- Zhang, Y.; Xu, C.; Guo, T.; Hong, L. An automated bacterial concentration and recovery system for pre-enrichment required in rapid escherichia coli detection. Sci. Rep. 2018, 8, 17808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Henry, E.; Gompper, G.; Fedosov, D.A. Behavior of rigid and deformable particles in deterministic lateral displacement devices with different post shapes. J. Chem. Phys. 2015, 143, 243145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincau, B.M.; Lee, Y.; Kim, J.-H.; Yeo, W.-H. Recent advances in nanoparticle concentration and their application in viral detection using integrated sensors. Sensors 2017, 17, 2316. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab A Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef]
- Aran, K.; Fok, A.; Sasso, L.A.; Kamdar, N.; Guan, Y.; Sun, Q.; Ündar, A.; Zahn, J.D. Microfiltration platform for continuous blood plasma protein extraction from whole blood during cardiac surgery. Lab A Chip 2011, 11, 2858–2868. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.T.F.; Aw Yong, K.M.; Fu, J. Microfluidic blood cell sorting: Now and beyond. Small 2014, 10, 1687–1703. [Google Scholar] [CrossRef] [Green Version]
- Wilding, P.; Pfahler, J.; Bau, H.H.; Zemel, J.N.; Kricka, L.J. Manipulation and flow of biological fluids in straight channels micromachined in silicon. Clin. Chem. 1994, 40, 43–47. [Google Scholar] [CrossRef]
- Weigl, B.H.; Yager, P. Microfluidic diffusion-based separation and detection. Science 1999, 283, 346–347. [Google Scholar] [CrossRef]
- Huang, L.R.; Cox, E.C.; Austin, R.H.; Sturm, J.C. Continuous particle separation through deterministic lateral displacement. Science 2004, 304, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.W.; Davis, J.A.; Austin, R.H.; Sturm, J.C. Critical particle size for fractionation by deterministic lateral displacement. Lab A Chip 2006, 6, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Tottori, N.; Nisisako, T. High-throughput production of satellite-free droplets through a parallelized microfluidic deterministic lateral displacement device. Sens. Actuators B Chem. 2018, 260, 918–926. [Google Scholar] [CrossRef]
- Loutherback, K.; Puchalla, J.; Austin, R.H.; Sturm, J.C. Deterministic microfluidic ratchet. Phys. Rev. Lett. 2009, 102, 045301. [Google Scholar] [CrossRef] [Green Version]
- Holm, S.H.; Beech, J.P.; Barrett, M.P.; Tegenfeldt, J.O. Simplifying microfluidic separation devices towards field-detection of blood parasites. Anal. Methods 2016, 8, 3291–3300. [Google Scholar] [CrossRef] [Green Version]
- Beech, J.P.; Jönsson, P.; Tegenfeldt, J.O. Tipping the balance of deterministic lateral displacement devices using dielectrophoresis. Lab A Chip 2009, 9, 2698–2706. [Google Scholar] [CrossRef] [PubMed]
- Devendra, R.; Drazer, G. Gravity driven deterministic lateral displacement for particle separation in microfluidic devices. Anal. Chem. 2012, 84, 10621–10627. [Google Scholar] [CrossRef]
- Beech, J.P.; Tegenfeldt, J.O. Tuneable separation in elastomeric microfluidics devices. Lab A Chip 2008, 8, 657–659. [Google Scholar] [CrossRef]
- Dincau, B.M.; Aghilinejad, A.; Hammersley, T.; Chen, X.; Kim, J.-H. Deterministic lateral displacement (dld) in the high reynolds number regime: High-throughput and dynamic separation characteristics. Microfluid. Nanofluidics 2018, 22, 59. [Google Scholar] [CrossRef]
- Dincau, B.M.; Aghilinejad, A.; Chen, X.; Moon, S.Y.; Kim, J.-H. Vortex-free high-reynolds deterministic lateral displacement (dld) via airfoil pillars. Microfluid. Nanofluidics 2018, 22, 137. [Google Scholar] [CrossRef]
- Ahasan, K.; Landry, C.M.; Chen, X.; Kim, J.-H. Effect of angle-of-attacks on deterministic lateral displacement (dld) with symmetric airfoil pillars. Biomed. Microdevices 2020, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Choi, S. 3d-printed capillary circuits for calibration-free viscosity measurement of newtonian and non-newtonian fluids. Micromachines 2018, 9, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, H.; Li, Y.; Li, X.; Wu, J.; Qian, S.; Li, F. Dynamic control of particle separation in deterministic lateral displacement separator with viscoelastic fluids. Sci. Rep. 2018, 8, 3618. [Google Scholar] [CrossRef] [PubMed]
- Takamura, K.; Fischer, H.; Morrow, N.R. Physical properties of aqueous glycerol solutions. J. Pet. Sci. Eng. 2012, 98–99, 50–60. [Google Scholar] [CrossRef]
- Cheng, N.-S. Formula for the viscosity of a glycerol−water mixture. Ind. Eng. Chem. Res. 2008, 47, 3285–3288. [Google Scholar] [CrossRef]
- Segur, J.B.; Oberstar, H.E. Viscosity of glycerol and its aqueous solutions. Ind. Eng.Chem. 1951, 43, 2117–2120. [Google Scholar] [CrossRef]
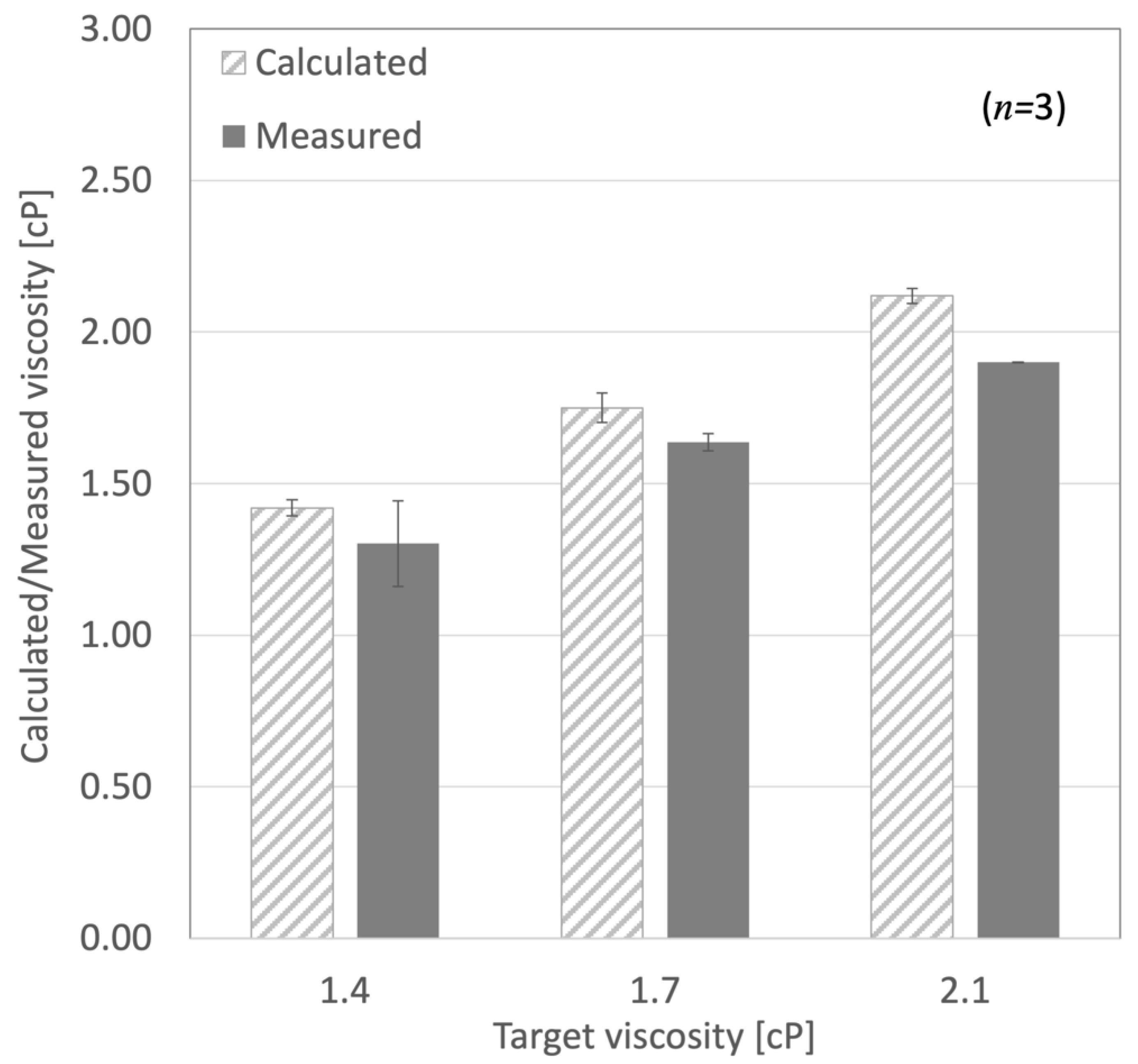


| Viscosity (cP) | Weight Glycerol (g) | Weight Water (g) | Temperature (°C) |
|---|---|---|---|
| 1.40 | 48.42 | 256.65 | 23.5 |
| 1.41 | 48.76 | 255.00 | 23.5 |
| 1.41 | 77.21 | 436.09 | 21.0 |
| 1.42 | 45.07 | 255.00 | 22.0 |
| 1.45 | 49.75 | 262.48 | 21.0 |
| 1.70 | 65.00 | 235.23 | 22.8 |
| 1.72 | 66.19 | 247.52 | 21.7 |
| 1.73 | 78.58 | 271.73 | 23.1 |
| 1.75 | 69.87 | 240.10 | 22.8 |
| 1.76 | 66.19 | 247.52 | 21.0 |
| 1.80 | 72.21 | 243.21 | 22.2 |
| 2.10 | 142.50 | 357.50 | 23.5 |
| 2.11 | 86.11 | 219.78 | 22.9 |
| 2.15 | 87.02 | 213.25 | 23.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senf, B.; Kim, J.-H. Effect of Viscosity on High-Throughput Deterministic Lateral Displacement (DLD). Micro 2022, 2, 100-112. https://doi.org/10.3390/micro2010006
Senf B, Kim J-H. Effect of Viscosity on High-Throughput Deterministic Lateral Displacement (DLD). Micro. 2022; 2(1):100-112. https://doi.org/10.3390/micro2010006
Chicago/Turabian StyleSenf, Brian, and Jong-Hoon Kim. 2022. "Effect of Viscosity on High-Throughput Deterministic Lateral Displacement (DLD)" Micro 2, no. 1: 100-112. https://doi.org/10.3390/micro2010006
APA StyleSenf, B., & Kim, J.-H. (2022). Effect of Viscosity on High-Throughput Deterministic Lateral Displacement (DLD). Micro, 2(1), 100-112. https://doi.org/10.3390/micro2010006






