Vaporisation Thermodynamics: Are Triazolium Ionic Liquids a Real Alternative to Popular Imidazolium-Based Ionic Liquids?
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Absolute Vapour Pressure Measurements
3. Results and Discussion
3.1. Absolute Vapour Pressures and Vaporisation Thermodynamics of Ionic Liquids
3.2. Absolute Vapour Pressures and Vaporisation Thermodynamics of Molecular Liquids: 1,2,4-Triazoles
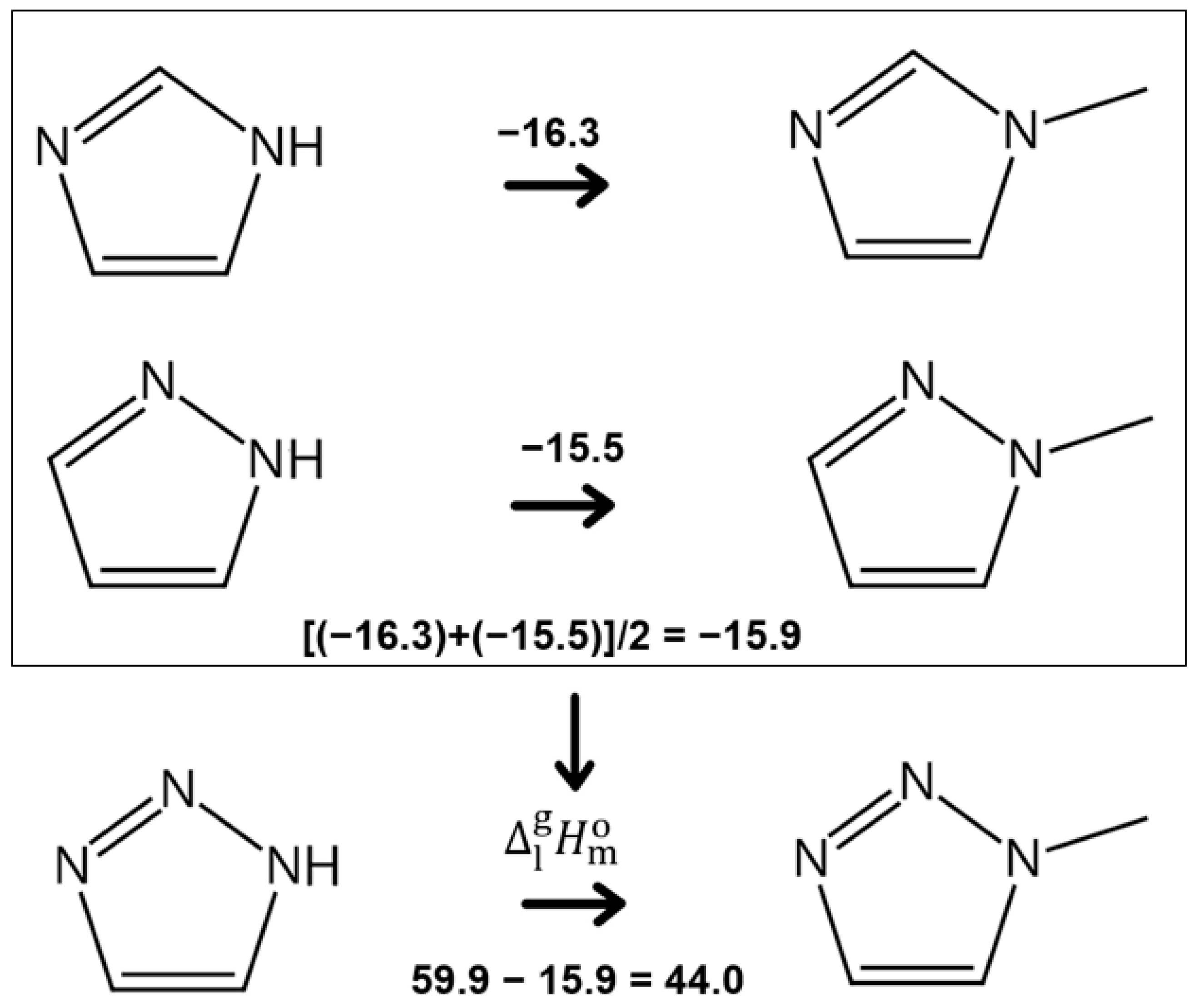
3.3. Vaporisation Thermodynamics of Molecular Liquids: 1,2,3-Triazoles
3.4. Molecular versus Ionic Liquids: Structure–Property Relationships for Predicting Vaporisation Thermodynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brehm, M.; Pulst, M.; Kressler, J.; Sebastiani, D. Triazolium-Based Ionic Liquids: A Novel Class of Cellulose Solvents. J. Phys. Chem. B 2019, 123, 3994–4003. [Google Scholar] [CrossRef] [PubMed]
- Daily, L.A.; Miller, K.M. Correlating structure with thermal properties for a series of 1-alkyl-4-methyl-1,2,4-triazolium ionic liquids. J. Org. Chem. 2013, 78, 4196–4201. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Chen, Z.; Zhang, J.; Yu, M.; Kong, J.; Wu, Z.; Cao, J.; Zhang, J. Thiazolium-based ionic liquids: Synthesis, characterization and physicochemical properties. J. Mol. Liq. 2021, 342, 117553. [Google Scholar] [CrossRef]
- Chand, D.; Wilk-Kozubek, M.; Smetana, V.; Mudring, A.-V. Alternative to the Popular Imidazolium Ionic Liquids: 1,2,4-Triazolium Ionic Liquids with Enhanced Thermal and Chemical Stability. ACS Sustain. Chem. Eng. 2019, 7, 15995–16006. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.; Yermalayeu, A.V.; Schick, C.; Liu, H.; Maginn, E.J.; Bulut, S.; Krossing, I.; Kalb, R.; et al. Making sense of enthalpy of vaporization trends for ionic liquids: New experimental and simulation data show a simple linear relationship and help reconcile previous data. J. Phys. Chem. B 2013, 117, 6473–6486. [Google Scholar] [CrossRef] [PubMed]
- Zaitsau, D.H.; Yermalayeu, A.V.; Schubert, T.J.S.; Verevkin, S.P. Alkyl-imidazolium tetrafluoroborates: Vapor pressure, thermodynamics of vaporization, and enthalpies of formation. J. Mol. Liq. 2017, 242, 951–957. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Yermalayeu, A.V.; Emel’yanenko, V.N.; Butler, S.; Schubert, T.; Verevkin, S.P. Thermodynamics of imidazolium-based ionic liquids containing PF6 anions. J. Phys. Chem. B 2016, 120, 7949–7957. [Google Scholar] [CrossRef]
- Zaitsau, D.H.D.H.; Verevkin, S.P.S.P. Imidazolium-based ionic liquids containing FAP anion: Thermodynamic study. J. Mol. Liq. 2019, 287, 110959. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Zherikova, K.V. Molecular liquids versus ionic liquids: Thermodynamic insights into the interplay between inter-molecular and intra-molecular hydrogen bonding. J. Mol. Liq. 2023, 382, 121938. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Ludwig, R. Molecular Liquids versus Ionic Liquids: The Interplay between Inter-Molecular and Intra-Molecular Hydrogen Bonding as Seen by Vaporisation Thermodynamics. Molecules 2023, 28, 539. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zherikova, K.V.; Martynenko, E.A. Molecular versus ionic liquids: Development of a thermodynamic framework for predicting vaporization thermodynamics. J. Mol. Liq. 2022, 350, 118576. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.; Heintz, A.; Emel’yanenko, V.N.; Heintz, A. A new method for the determination of vaporization enthalpies of ionic liquids at low temperatures. J. Phys. Chem. B 2011, 115, 12889–12895. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Emel’yanenko, V.N. Transpiration method: Vapor pressures and enthalpies of vaporization of some low-boiling esters. Fluid Phase Equilib. 2008, 266, 64–75. [Google Scholar] [CrossRef]
- Kernchen, U.; Etzold, B.; Korth, W.; Jess, A. Solid catalyst with ionic liquid layer (SCILL)—A new concept to improve selectivity illustrated by hydrogenation of cyclooctadiene. Chem. Eng. Technol. 2007, 30, 985–994. [Google Scholar] [CrossRef]
- Fehrmann, R.; Riisager, A.; Haumann, M. Supported Ionic Liquids: Fundamentals and Applications; Fehrmann, R., Riisager, A., Haumann, M., Eds.; WILEY-VCH Verlag: Weinheim, Germany, 2014; ISBN 9783527324293. [Google Scholar]
- Zaitsau, D.H.; Yermalayeu, A.V.; Emel’yanenko, V.N.; Verevkin, S.P. Thermodynamics of Imidazolium-Based Ionic Liquids Containing the Trifluoromethanesulfonate Anion. Chem. Eng. Technol. 2018, 41, 1604–1612. [Google Scholar] [CrossRef]
- Zimmermann, H.; Geisenfelder, H. Über die Mesomerieenergie von Azolen. Z. Elektrochem. Berichte Bunsenges. Phys. Chem. 1961, 65, 368–371. [Google Scholar] [CrossRef]
- Faour, M.; Akasheh, T.S. Heat of combustion of some N-heterocycle compounds. Part 1. J. Chem. Soc. Perkin Trans. 2 1985, 6, 811–813. [Google Scholar] [CrossRef]
- Jiménez, P.; Roux, M.V.; Turrión, C. Thermochemical properties of N-heterocyclic compounds II. Enthalpies of combustion, vapour pressures, enthalpies of sublimation, and enthalpies of formation of 1,2,4-triazole and benzotriazole. J. Chem. Thermodyn. 1989, 21, 759–764. [Google Scholar] [CrossRef]
- Sabbah, R.; Perez, L. Energetics of Intramolecular Bonds in 1H-1,2,4-Triazole and 1H-Benzotriazole. Aust. J. Chem. 1999, 52, 235. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Sazonova, A.Y.; Emel’yanenko, V.N.; Zaitsau, D.H.; Varfolomeev, M.A.; Solomonov, B.N.; Zherikova, K. V Thermochemistry of halogen-substituted methylbenzenes. J. Chem. Eng. Data 2015, 60, 89–103. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Verevkin, S.P. Benchmark thermodynamic properties of 1,3-propanediol: Comprehensive experimental and theoretical study. J. Chem. Thermodyn. 2015, 85, 111–119. [Google Scholar] [CrossRef]
- Rodriguez Castillo, A.S.; Guihéneuf, S.; Biard, P.; Paquin, L.; Amrane, A.; Couvert, A. Physicochemical properties of some hydrophobic room-temperature ionic liquids applied to volatile organic compounds biodegradation processes. J. Chem. Technol. Biotechnol. 2018, 93, 215–223. [Google Scholar] [CrossRef]
- Yacob, Z.; Liebscher, J. 1,2,3-Triazolium Salts as a Versatile New Class of Ionic Liquids. In Ionic Liquids—Classes and Properties; InTech: London, UK, 2011. [Google Scholar]
- Khan, S.S.; Hanelt, S.; Liebscher, J. Versatile synthesis of 1,2,3-triazolium-based ionic liquids. Arkivoc 2009, 2009, 193–208. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Clarke, E.C.W.; Glew, D.N. Evaluation of thermodynamic functions from equilibrium constants. Trans. Faraday Soc. 1966, 62, 539–547. [Google Scholar] [CrossRef]
- Kulikov, D.; Verevkin, S.P.; Heintz, A. Determination of vapor pressures and vaporization enthalpies of the aliphatic branched C5 and C6 alcohols. J. Chem. Eng. Data 2001, 46, 1593–1600. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hosseini, S.; Hesse, D.G.; Liebman, J.F. Heat capacity corrections to a standard state: A comparison of new and some literature methods for organic liquids and solids. Struct. Chem. 1993, 4, 271–278. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Chickos, J.S. Phase transition enthalpy measurements of organic and organometallic compounds and ionic liquids. Sublimation, vaporization, and fusion enthalpies from 1880 to 2015. Part 2. C11–C192. J. Phys. Chem. Ref. Data 2017, 46, 13104. [Google Scholar] [CrossRef]
- Ahmadi, A.; Haghbakhsh, R.; Raeissi, S.; Hemmati, V. A simple group contribution correlation for the prediction of ionic liquid heat capacities at different temperatures. Fluid Phase Equilib. 2015, 403, 95–103. [Google Scholar] [CrossRef]
- Ribeiro da Silva, M.A.; Ribeiro da Silva, M.d.D.M.; Matos, M.A.R.; Jiménez, P.; Victoria Roux, M.; Martin-Luengo, M.; Elguero, J.; Claramunt, R.; Cabildo, P. Enthalpies of combustion, heat capacities, and enthalpies of vaporisation of 1-phenylimidazole and 1-phenylpyrazole. J. Chem. Thermodyn. 2000, 32, 237–245. [Google Scholar] [CrossRef]
- Almeida, A.R.R.P.; Monte, M.J.S. Thermodynamic study of phase transitions of imidazoles and 1-methylimidazoles. J. Chem. Thermodyn. 2012, 44, 163–168. [Google Scholar] [CrossRef]
- Safronov, S.P.; Nagrimanov, R.N.; Samatov, A.A.; Emel’yanenko, V.N.; Zaitsau, D.H.; Pimerzin, A.A.; Skrzypczak, A.; Verevkin, S.P. Benchmark properties of pyrazole derivatives as a potential liquid organic hydrogen carrier: Evaluation of thermochemical data with complementary experimental and computational methods. J. Chem. Thermodyn. 2019, 128, 173–186. [Google Scholar] [CrossRef]
- Verevkin, S.S.P.; Zaitsau, D.H.D.; Emel’yanenko, V.N.; Paulechka, Y.U.; Blokhin, A.V.A.; Bazyleva, A.A.B.; Kabo, G.G.J.; Emel’yanenko, V.; Paulechka, E.; Blokhin, A.V.A.; et al. Thermodynamics of ionic liquids precursors: 1-methylimidazole. J. Phys. Chem. B 2011, 115, 4404–4411. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, J.; Agapito, F.; Bernardes, C.E.S.; Minas da Piedade, M.E. Thermochemistry of 1-alkylimidazoles. J. Chem. Thermodyn. 2015, 80, 59–64. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Portnova, S.V.; Verevkin, S.P.; Skrzypczak, A.; Schubert, T. Building blocks for ionic liquids: Vapor pressures and vaporization enthalpies of 1-(n-alkyl)-imidazoles. J. Chem. Thermodyn. 2011, 43, 1500–1505. [Google Scholar] [CrossRef]



| Cation | psat × 106 [Pa] | psat × 103 [Pa] |
|---|---|---|
| 373 K | 473 K | |
| [2-C1-4-C1-1,2,4-T] | 20 | 70.8 |
| [2-C1-4-C2-1,2,4-T] | 36 | 134.8 |
| [2-C2-4-C1-1,2,4-T] | 35 | 121.5 |
| IL Triazole Cation | T-Range | Tav | (Tav) b | (Tav) c | d | (298 K) e |
|---|---|---|---|---|---|---|
| K | kJ·mol−1 | J·K−1·mol−1 | kJ·mol−1 | |||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| [2-C1-4-C1-1,2,4-T] | 348-395 | 371.0 | 122.2 ± 1.0 | 69.5 ± 1.0 | −49 | 125.7 ± 1.2 |
| [2-C1-4-C2-1,2,4-T] | 345-393 | 368.4 | 123.7 ± 1.0 | 68.1 ± 1.0 | −57 | 127.7 ± 1.3 |
| [2-C2-4-C1-1,2,4-T] | 343-390 | 365.9 | 122.4 ± 1.0 | 68.5 ± 1.0 | −57 | 126.3 ± 1.3 |
| Compound/CAS | M a | T-Range | (Tav) | (298 K) b | Ref. |
|---|---|---|---|---|---|
| K | kJ·mol−1 | kJ·mol−1 | |||
| 1H-1,2,4-triazole (cr) | K | 322–350 | 84.1 ± 1.0 | 84.6 ± 1.0 | [17] |
| n/a | (80.6 ± 0.5) | [18] | |||
| K | 281.5–295.7 | 84.1 ± 1.3 | 84.0 ± 1.3 | [19] | |
| C | 306.0 | 80.5 ± 0.9 | (80.6 ± 0.9) | [20] | |
| T | 322.2–367.5 | 83.7 ± 0.6 | 84.3 ± 0.7 | Table S4 | |
| 84.3 ± 0.5 c | average | ||||
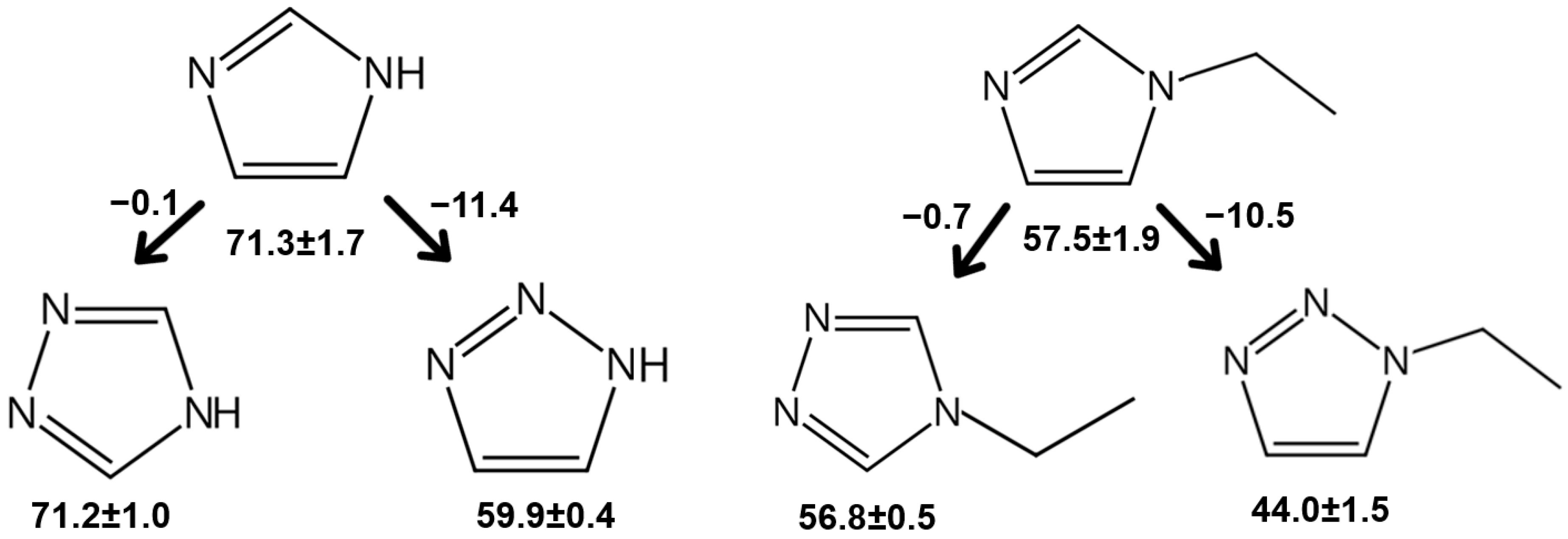
| 1H-1,2,4-triazole (liq) | 71.2 ± 1.0 | Table S5 | |||
| 1-methyl-1,2,4-triazole (liq) | T | 275.0–337.8 | 53.1 ± 0.3 | 53.4 ± 0.4 | Table S4 |
| 1-ethyl-1,2,4-triazole (liq) | T | 280.6–313.1 | 57.0 ± 0.4 | 56.8 ± 0.5 | Table S4 |
| 1H-1,2,3-triazole (liq) | T | 297.2–348.7 | 59.9 ± 0.4 | 59.9 ± 0.4 | Table S4 |
| 1-methyl-1,2,3-triazole (liq) | 44.0 ± 1.5 | Figure 4 | |||
| 1-ethyl-1,2,3-triazole (liq) | 47.0 ± 1.5 | Figure 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verevkin, S.P.; Zaitsau, D.H. Vaporisation Thermodynamics: Are Triazolium Ionic Liquids a Real Alternative to Popular Imidazolium-Based Ionic Liquids? Liquids 2024, 4, 581-591. https://doi.org/10.3390/liquids4030032
Verevkin SP, Zaitsau DH. Vaporisation Thermodynamics: Are Triazolium Ionic Liquids a Real Alternative to Popular Imidazolium-Based Ionic Liquids? Liquids. 2024; 4(3):581-591. https://doi.org/10.3390/liquids4030032
Chicago/Turabian StyleVerevkin, Sergey P., and Dzmitry H. Zaitsau. 2024. "Vaporisation Thermodynamics: Are Triazolium Ionic Liquids a Real Alternative to Popular Imidazolium-Based Ionic Liquids?" Liquids 4, no. 3: 581-591. https://doi.org/10.3390/liquids4030032
APA StyleVerevkin, S. P., & Zaitsau, D. H. (2024). Vaporisation Thermodynamics: Are Triazolium Ionic Liquids a Real Alternative to Popular Imidazolium-Based Ionic Liquids? Liquids, 4(3), 581-591. https://doi.org/10.3390/liquids4030032











