Abstract
A dense artificial neural network, ESE-ΔH-DNN, with two hidden layers for calculating both solvation free energies ΔG°solv and enthalpies ΔH°solv for neutral solutes in organic solvents is proposed. The input features are generalized-Born-type monatomic and pair electrostatic terms, the molecular volume, and atomic surface areas of the solute, as well as five easily available properties of the solvent. ESE-ΔH-DNN is quite accurate for ΔG°solv, with an RMSE (root mean square error) below 0.6 kcal/mol and an MAE (mean absolute error) well below 0.4 kcal/mol. It performs particularly well for alkane, aromatic, ester, and ketone solvents. ESE-ΔH-DNN also exhibits a fairly good accuracy for ΔH°solv prediction, with an RMSE below 1 kcal/mol and an MAE of about 0.6 kcal/mol.
1. Introduction
Solvation is a major effect in chemistry and has to be accounted for in chemical computations. Typically, in high demand is the solvation free energy ΔG°solv, since it makes a significant contribution to the total free energy of chemical reactions in solution and is also crucially important for the estimation of partition coefficients. Nevertheless, solvation enthalpy ΔH°solv also plays a significant role, as it gives a direct measure of the heat of solvation and provides, in conjunction with ΔG°solv, access to the solvation entropy ΔS°solv. Hence, the development of reliable and efficient computational tools for prediction ΔG°solv and ΔH°solv is an important task in computational chemistry.
While highly accurate explicit methods for solvation energy evaluation, such as umbrella sampling [1,2] and free energy perturbation [3], are available, they are quite costly. Consequently, most practical calculations of solvation energy rely on the continuum solvation (CS) model, in which the electrostatic Eelst and nonelectrostatic contributions to ΔG°solv are evaluated separately. The two most prominent CS approaches are the polarizable continuum model (PCM) [4,5,6,7,8,9,10,11,12,13,14,15] and the generalized Born (GB) method [16,17], including SMx [18,19,20,21]. In the PCM family of methods, the solute placed in a cavity interacts with the solvent, represented by a continuum. The cavity surface needs to be constructed and induced charges placed on it have to be computed.
In contrast, in the GB method, Eelst is calculated directly from atomic charges {QI}:
where ε is the dielectric constant of the solvent and fIJ is a function of the atomic radii RI (in this context referred to as the Born radii) and the interatomic distance rIJ. The monatomic terms (self-terms) EIself = (1 − 1/ε)QI2/RI in Equation (1) correspond to the solvation energy in the Born theory [22] for spherical ions. The function fIJ in the pair term EIJpair = (1 − 1/ε)QIQJ/fIJ is designed to interpolate between the Coulombic limit (1 − 1/ε)QIQJ/rIJ at large rIJ and the Born or Onsager limits at small rIJ, when the diatomic fragment IJ collapses either into an single ion of charge QI + QJ or into a neutral.
The choice of effective Born radii RI in the traditional GB methods is crucial for achieving an acceptable accuracy [23]. A typical form of fIJ is the following [16]:
although alternative expressions for fIJ are also in use [18,23,24,25,26]. The GB approach has been implemented within the framework of numerous solvation energy schemes [17,18,19,21]. The GB-type methods are generally more efficient than the PCM-type schemes, since they avoid an explicit cavity construction and induced-charge calculation. However, the GB methods are in general less accurate than PCM.
Nonelectrostatic correction is necessary for both PCM- and GB-type methods in order to achieve reasonably accurate results. Typically, it is made of linear or nonlinear terms depending on atomic surfaces SI, with the simplest form of the correction being ∑IκISI [16]. In addition, the correction term often involves the molecular volume, induced charges, and possibly other characteristics of the solute and typically contains some adjustable parameters that must be fitted on a suitable database. Thus, the CS methods inevitably have some degree of empiricity.
In our previous works [27,28,29,30,31,32], we developed a family of non-iterative methods for evaluating ΔG°solv (uESE—universal Easy Solvation Energy). The electrostatics is included by means of COSMO (COnductor-like Screening MOdel) [14,15], while the correction term depends on induced charges and surfaces. The atomic charges necessary for the COSMO calculation can be evaluated by means of various techniques [27,33,34,35,36], including ab initio or DFT-based, semiempirical, and even electronegativity-equalization (EE) [31] charges. However, changing the charge scheme requires re-parameterization of the correction term. Although the semiempirical and EE charges provide ΔG°solv estimates of reasonable quality, the accuracy of the EE-based method (ESE-EE) [31] is not as high as that of the uESE method [29] employing DFT charges. In order to improve the quality of an EE-based scheme, artificial neural networks (ANN) were introduced as a computational framework [37,38]. In the ESE-EE-DNN method [37,39], the ANN input features are the COSMO electrostatic energy, atomic cavity surface areas, total cavity volume, and induced surface charges. In contrast, the ESE-GB-DNN method [38,40] is based on GB-style input features. In particular, the number of atoms in the solute molecule, the total charge, the molecular volume, the atomic surface areas as well as GB-type self-terms and pair terms (EIself and EIJpair summed over the elements) are used to represent the solute, while the solvent is described by just three parameters: the dielectric constant ε, the boiling point (BP), and the number of non-hydrogen atoms. The full list of solvents and their properties is given in the Supporting Information (Table S1). Since no cavity construction is needed, the ESE-GB-DNN method is substantially faster than ESE-EE-DNN (and dramatically more efficient than any DFT-based approach), allowing for a virtually instantaneous ΔG°solv calculation for solutes up to 100 atoms in size.
In the present paper, I further develop the GB-based approach proposed in [38]. The purpose of the present work is to create a consistent ANN-based predictor of both ΔG°solv and ΔH°solv at 298 K for organic solvents.
Neural networks for ΔG°solv evaluation were developed previously. Chen et al. [41] presented a graph ANN with atomistic embedding. Vermeire and Green’s approach [42] employed a directed message-passing ANN with SMILES (Simplified Molecular-Input Line-Entry System) and InChI (International Chemical Identifier) as input features. Another successful, but sophisticated, message-passing ANN for neutral solutes was presented by Low et al. [43]. Lim and Jung [44] developed a recurrent ANN and a graph convolutional ANN based on atomic vectors. Alibakhshi and Hartke [45] achieved quite accurate results within their ANN that uses a self-consistent C-PCM input. The works by Bernazzani et al. [46], Hutchinson and Kobayashi [47], Wang et al. [48], Jaquis et al. [49], and Chung et al. [50] are also worth mentioning. The latter two address approaches that target solvation enthalpy in addition to Gibbs free energy.
2. Methods
The atomic charges used for the calculations are computed using an EE-based scheme described in detail in my previous paper [38]. This is similar but not identical to the version by Svobodová Vařeková et al. [51].
The original input features used to describe a solute closely follow the approach used in my previous paper [38]. They are as follows:
- (1) the number of atoms in the solute molecule N;
- (2) the molecular volume Vtot: Vtot= ∑IVI;
- (3) the total surface area Stot composed of atomic surfaces: Stot= ∑ISI;
- (4–12) atomic surface areas summed over all the atoms of a given element L: SL = ∑IϵLSI for L = H, C, N, O, F, S, Cl, Br, and I. The atomic volumes VI and surfaces SI are efficiently calculated by simple formulas based on geometric considerations. The details are given in [38];
- (13–21) the Born-type self-terms, also summed over all the atoms of a given element L:
E1Born(L) = ∑IϵLEIself = (1 − 1/ε)∑IϵLQI2/RI
- (22–51) the Born-type pair terms:
E2Born(L1,L2) = ∑IϵL1∑JϵL2EIJpair = (1 − 1/ε)∑IϵL1∑JϵL2QIQJ/fIJ
The thirty L1–L2 pair terms are as follows: H–H, C–C, C–H, N–N, N–H, N–C, O–O, O–H, O–C, O–N, F–F, F–H, F–C, F–O, S–H, S–C, S–N, S–O, Cl–H, Cl–C, Cl–N, Cl–O, Cl–F, Br–H, Br–C, Br–N, Br–O, Br–Cl, I–H, and I–C.
The radii RI used in Equations (2)–(4) are unmodified Bondi [52] radii;
- (52–56) five solvent-related input features: in addition to the dielectric constant, boiling point, and the number of nonhydrogen atoms employed in my previous work [38], in this paper, the molar volume and the number of hydrogen-bond centers (the sum of the donor and acceptor centers) are also used.
Thus, the initial input feature set consistes of 56 parameters. Some of them exhibit a strong correlation on the training dataset used (vide infra). For instance, Vtot and Stot have a correlation coefficient of 0.99; Stot and N have a correlation coefficient of 0.97; E1Born(Cl) and E2Born(Cl,H) have a correlation coefficient of −0.99, etc. In total, 16 pairs of features with correlation coefficients greater than 0.96 were identified. To reduce the number of ANN parameters, principal component analysis using the sklearn.decomposition.PCA class from the Python Scikit-learn package [53] was applied. This process allowed truncating the 16 most correlated features, resulting in a 56 × 40 transformation matrix. Consequently, a vector of 40 input features is produced and fed into the ANN.
I employed a dense ANN with 40 input neurons and 2 hidden layers with 14 and 6 neurons, respectively, and an output layer with two neurons (corresponding to ΔG°solv and ΔH°solv). The ReLU (Rectified Linear Unit) activation function for the hidden layers and the linear activation for the output layer were used. Other ANN configurations were also tested, including an ANN with a single hidden layer, but the abovementioned 40 × 14 × 6 × 2 network (Figure 1) turned out to be the most accurate. It contains 678 adjustable parameters (40 × 14 + 14 × 6 + 6 × 2 = 656 weights plus 14 + 6 + 2 = 22 biases). The input data are min-max scaled and fed into the dense ANN described above. The ANN fitting was performed on a suitable database (vide infra) using the Nesterov-accelerated [54] Adaptive Moment Estimation algorithm [55] as implemented in the tensorflow.keras.optimizers.Nadam class [56], with mean squared error as the loss function and L2 regularization with a strength λ = 0.01.
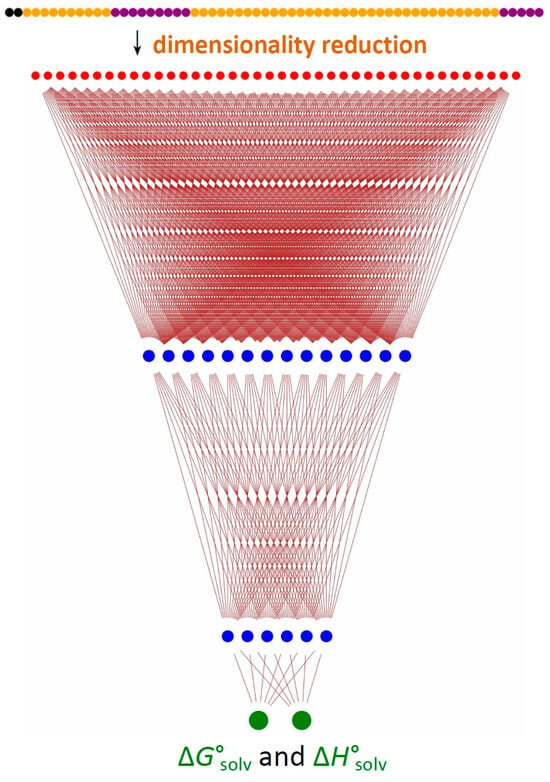
Figure 1.
DNN architecture used in the present work. The first row (various colors) denotes the original 56 input features. The dimensionality reduction is achieved via a 56 × 40 linear transformation. The second row (40 red circles) represents the DNN input layer (40 linear combinations of the 56 initial features). The following blue circles denote two hidden layers (14 and 6 neurons, respectively). The green circles at the bottom are neurons in the output layer, corresponding to ΔG°solv and ΔH°solv.
3. Results and Discussion
3.1. Database
The datasets used for the training and testing of ESE-ΔH-DNN are based on the database of ΔH°solv and partition coefficients at T = 298 K by Prof. W. E. Acree [57,58]. The logarithms of partition coefficients log10p were converted to solvation free energies: ΔG°solv = −RTln(10)log10p. Subsequently, chemical names of the solutes were automatically translated into SMILES codes using the website of the National Institutes of Health [59]. The resulting SMILES codes were then automatically converted to Cartesian coordinates, and the geometries were optimized with MMFF94 (Merck Molecular Force Field) [60] using the rdkit.Chem.AllChem module [61]. Subsequently, the geometries were manually controlled and corrected when necessary. Atomic charges, volumes, and surfaces were computed based on these geometries. The solvent parameters (boiling points, dielectric constants, molar volume, and the number of hydrogen-bond centers) were retrieved semi-automatically from other public databases [62,63]. Finally, the resulting database contained 5201 ΔH°solv values and 3789 ΔG°solv values. It was randomly divided into a training/validation set (80%) and testing set (20%). The training/validation set was further split into a training set and a validation set, with 20% assigned for validation. Once trained, the optimized ESE-ΔH-DNN parameters (neuron weights and biases) were incorporated into a user-friendly Fortran code that reads the molecular geometry, computes the EE charges and input features, and finally evaluates ΔG°solv and ΔH°solv via ESE-ΔH-DNN.
3.2. Training
The Cartesian coordinates of the solutes obtained as described above were used to calculate the EE charges and atomic surfaces and volumes as explained in the Section 2. Along with the five solvent-related features (52–56), they were submitted first to the dimension-reducing 56 × 40 linear transformation and subsequently to the ANN training. The mean-square error was used as the loss function. The learning rate was initially set to 1 × 10−3 mol2/kcal2 and then reduced to 1 × 10−4 mol2/kcal2 within the same training run. Since ESE-ΔH-DNN was trained simultaneously for both ΔH°solv and ΔG°solv, the loss function was defined as the sum of two residual sums of squares calculated from the predicted and reference ΔH°solv and ΔG°solv values. After a number of training runs, ESE-ΔH-DNN with the fitted parameters was evaluated on the testing sets that include various classes of solvents.
3.3. Performance of ESE-ΔH-DNN
The statistical data for predicted ΔH°solv and ΔG°solv values for various classes of solvents are reported in Table 1 and Table 2. Table 1 shows errors in predicted values, while Table 2 contains information about the correlation of predicted and reference values. Individual data for the training, validation, and testing sets are given in the Supporting Information (Tables S2 and S3).

Table 1.
Mean signed error (MSE), mean absolute error (MAE), and root mean square error (RMSE) of the predicted solvation free energy and solvation enthalpy for various testing subsets of ESE-ΔH-DNN.

Table 2.
Slope, intercept (in kcal/mol), and coefficient of determination R2 of the predicted solvation free energy and solvation enthalpy for various testing subsets of the ESE-ΔH-DNN.
3.3.1. Performance for Solvation Gibbs Free Energies
The overall quality of ESE-ΔH-DNN for ΔG°solv over the entire testing set is characterized by an RMSE (root mean square error) below 0.6 kcal/mol and an MAE (mean absolute error) well below 0.4 kcal/mol. This performance is very convincing: even DFT-based methods often yield an RMSE between 0.6 and 1.5 kcal/mol. For instance, for nonaqueous solvents, our uESE [29] yields an RMSE between 0.6 and 1 kcal/mol depending on the solvent class, while the SCCS model by Hille et al. [12] yields an average RMSE of about 0.8 kcal/mol. The recent methods from W. H. Green’s group [50] exhibit an RMSE range from about 0.7 to 1.5 kcal/mol. It should be noted, however, that the databases used are not identical and the comparison is therefore illustrative only. Considering the ESE-ΔH-DNN results separately for various solvent classes (Table 1), very good results (with RMSE < 0.5 kcal/mol, MAE < 0.3 kcal/mol) were obtained for nonpolar solvents (alkanes and aromatic solvents), as well as for esters and ketone solvents. For other solvent classes, the results are also compelling, with an RMSE mostly below 0.7 kcal/mol. The only clearly unfavorable exception is alkoxyalcohols, for which a significant error (RMSE > 1.5 kcal/mol) is detected. The failure with alkoxyalcohols is also reflected by poor slope and R2 values (Table 2).
The ESE-ΔH-DNN results for the entire testing set are illustrated in Figure 2a. There are 420 entries (46%) with an error below 0.2 kcal/mol and 564 entries (61%) with an error below 0.3 kcal/mol, thus confirming the overall good performance of ESE-ΔH-DNN. Still, there are some 60 (6.5%) outliers with a deviation ΔΔG°solv > 1 kcal/mol. The worst failure is benzene dissolved in 2-butoxyethanol and pentane in anisole, for which ESE-ΔH-DNN fails to reproduce experimental positive ΔG°solv. For nitromethane in triethylene glycol and SO2F2 in tributyl phosphate, the predicted ΔG°solv values are much too negative. On the contrary, for C3F8 dissolved in hexafluorobenzene, a too positive ΔG°solv is incorrectly predicted.
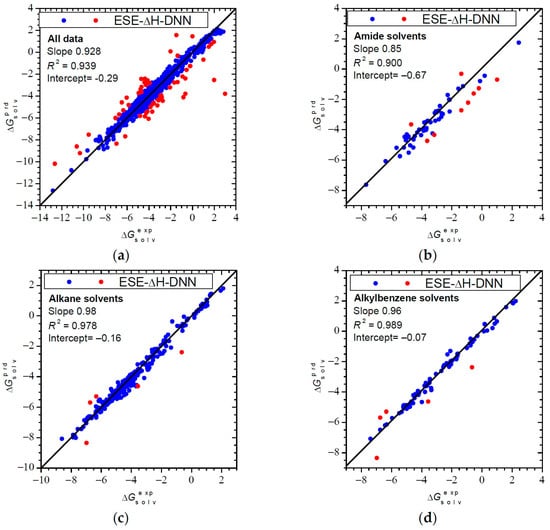
Figure 2.
Solvation free energies (in kcal/mol) calculated by ESE-ΔH-DNN: (a) the entire testing set (922 entries); (b) amide solvents (56 entries); (c) alkane solvents (245 entries); (d) aromatic solvents (81 entries) versus reference values. Red points denote outliers with a deviation greater than 1 kcal/mol. The slanting line represents the identity line.
The results for amide solvents (Figure 2b) may also appear somewhat disturbing based on a slope value of 0.84. Indeed, ESE-ΔH-DNN tends to slightly overestimate |ΔG°solv| for amides, but in fact the accuracy is still quite good: even for the worst-case amide solvent, methylformamide, the RMSE is just 0.81 kcal/mol and there are only 9 (16%) outliers (ΔΔG°solv > 1 kcal/mol) out of 56 entries. Other examples shown in Figure 2 are alkane and aromatic solvents. Figure 2c,d demonstrate a very good agreement between the predicted and reference data for these solvent classes. There are only five (6%) outliers in each case.
The statistical ΔG°solv data for all the solvents tested are summarized in Table 3. ESE-ΔH-DNN performs consistently well for all the alkane, aromatic, and ketone solvents. The results for the ethers are also very encouraging, except for anisole, where RMSE is about 1.2 kcal/mol. Ether and amide solvents are discussed above. The results for haloalkanes are also very good. Only chloroform and methylene iodide exhibit slightly larger deviations (RMSE about 0.7 kcal/mol). The haloaromatics are mostly very good (RMSE around 0.5 kcal/mol), with the only exception being perfluorobenzene. The problematic solvents within the alkoxyalcohol class are butoxyethanol, diethylene glycol, and triethylene glycol, the former being the worst case with an RMSE of almost 2.9 kcal/mol. The broad class of various solvents that are grouped together in Table 3 as Miscellaneous demonstrate a reliable accuracy, with an RMSE ranging from 0.2 to 1 kcal/mol. Eleven of the 24 solvents exhibit an RMSE below 0.4 kcal/mol, and eight more exhibit an RMSE below 0.8 kcal/mol. Only tributyl phosphate is slightly problematic, with an RSME of about 1 kcal/mol, which is still an acceptable accuracy for many applications.

Table 3.
MSE, MAE, and RMSE of the predicted solvation free energy and solvation enthalpy for various solvents (testing subsets).
3.3.2. Performance for Solvation Enthalpies
Table 1, Table 2 and Table 3 also provide statistical data for predicted solvation enthalpies ΔH°solv. Overall, ESE-ΔH-DNN yields somewhat larger errors for ΔH°solv than for ΔG°solv, with a global RMSE of 0.96 kcal/mol and an MAE of 0.62 kcal/mol. While it is difficult to pinpoint the exact reasons of the lower absolute accuracy of ESE-ΔH-DNN for ΔH°solv, it is tempting to assume that a large total range of ΔH°solv contributes to it. In fact, ΔH°solv is virtually always more negative than ΔG°solv due to negative solvation entropy. An inspection of Figure 3 shows that larger deviations often occur for more negative ΔH°solv. The worst result is obtained for benzo-15-crown-5 in propanol, with |ΔH°solv| overestimated by 6.7 kcal/mol and for glycerol in methanol and tert-butanol, for which |ΔH°solv| is underestimated by 5.5 and 4.9 kcal/mol, respectively. Nevertheless, it is encouraging that 859 of 1036 values exhibit a deviation within 1 kcal/mol and 603 within 0.5 kcal/mol.
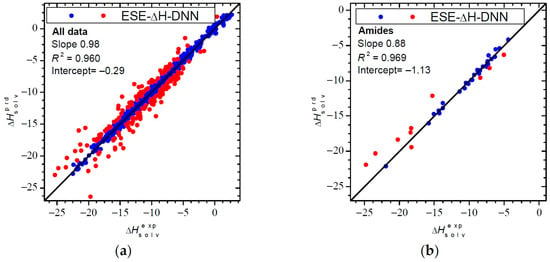
Figure 3.
Solvation enthalpies (in kcal/mol) calculated by ESE-ΔH-DNN: (a) the entire testing set (1036 entries); (b) amide solvents (39 entries) versus reference values. Red points denote outliers with a deviation greater than 1 kcal/mol. The slanting line represents the identity line.
Various classes of solvent demonstrate a fairly homogeneous distribution of errors for ΔH°solv, with RSME ranging from approximately 0.4 kcal/mol for alkoxyalkohols to about 1.1 kcal/mol for amides (see the rightmost column of Table 1). There are no solvent classes with notably large errors. It is worth noting that the performance of ESE-ΔH-DNN for ΔH°solv does not correlate with that for ΔG°solv: the lowest ΔH°solv RSME (0.43 kcal/mol) is observed for alkoxyalkohols, which is the most problematic in terms of ΔG°solv. The largest RMSE observed is for amides, but it does not remarkably deviate from the global average.
As for individual solvents, relatively large errors are detected for some amides and amines: formamide, pyridine, triethylamine, methylformamide, and N-methylpyrrolidone. While methylformamide is also problematic for ΔG°solv, pyridine and N-methylpyrrolidone show quite accurate results in terms of ΔG°solv,
In addition to the tests conducted on our randomly selected testing dataset, I performed evaluations using the structures retrieved from the Minnesota Solvation Database (MNSol) [64]. The statistical results for nonpolar solvents are presented in Table 4. Individual data are given in the Supporting Information (Table S4). Overall, these data confirm a good performance of ESE-ΔH-DNN, with a global RMSE of about 0.7 kcal/mol. An inferior performance was observed only for ethyl acetate, tributyl phosphate, and diethyl ether solvents.

Table 4.
MSE, MAE, and RMSE of the solvation free energy predicted by ESE-ΔH-DNN for various nonpolar solvents (MNSol database).
4. Conclusions
In this paper, I proposed ESE-ΔH-DNN, a dense neural network for calculating the ΔG°solv and ΔH°solv based on physically sound input features such as generalized-Born-type electrostatic terms, the molecular volume, and the atomic surface area of the solute as well as five characteristics of the solvent. ESE-ΔH-DNN is defined for neutral solutes in a wide range of organic solvents. It requires only the molecular geometry as the input and provides accurate results, with an average RMSE below 0.6 kcal/mol. It is particularly accurate for alkane, aromatic, ester, and ketone solvents. The limitations (which also present opportunities for further development) are that ESE-ΔH-DNN is trained only on neutral solutes and is thus not automatically applicable to ions. It also has lower accuracy for specific alkoxyalcohol solvents (butoxyethanol, triethylene glycol, and diethylene glycol), as well as for hexafluorobenzene.
ESE-ΔH-DNN also demonstrates a fairly good accuracy for ΔH°solv prediction, although the errors are slightly larger, with an average RMSE still below 1 kcal/mol. One positive aspect is that the error is quite homogeneous, with the RMSE showing small variance among diverse solvent classes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/liquids4030030/s1: Part 1: ESE-ΔH-DNN neural network buildup: 56 initial input vector components; 56 × 40 dimensionality reduction linear transformation; ESE-ΔH-DNN weights and biases; Part 2: Solvents and their properties. Part 3: Predicted and reference solvation free energies and solvation enthalpies for the training, validation, and testing sets; predicted and reference solvation free energies for nonpolar solvents from MNSol database. Reference [64] appear in the Supplementary material.
Funding
This research received no external funding.
Data Availability Statement
The executable ESE-ΔH-DNN program and a user guide are openly available for download: https://github.com/vyboishchikov/ESE-DeltaH-DNN. Accessed on 3 July 2024.
Acknowledgments
I am very grateful to W. E. Acree for kindly providing me with the large database of experimental ΔG°solv and ΔH°solv used in this work.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Torrie, G.; Valleau, J. Nonphysical sampling distributions in Monte Carlo free-energy estimation: Umbrella sampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar] [CrossRef]
- You, W.; Tang, Z.; Chang, C.A. Potential mean force from umbrella sampling simulations: What can we learn and what is missed? J. Chem. Theory Comput. 2019, 15, 2433–2443. [Google Scholar] [CrossRef] [PubMed]
- Chipot, C.; Pohorille, A. Calculating free energy differences using perturbation theory. In Free Energy Calculations: Theory and Applications in Chemistry and Biology; Chipot, C., Pohorille, A., Eds.; Springer Series in Chemical Physics; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; Volume 86, p. 33. ISBN 540-38447-2. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Skyner, R.E.; McDonagh, J.L.; Groom, C.R.; van Mourik, T.; Mitchell, J.B.O. A review of methods for the calculation of solution free energies and the modelling of systems in solution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M.; Tomasi, J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anistropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Mennucci, B.; Cancès, E.; Tomasi, J. Evaluation of solvent effects in isotropic and anisotropic dielectrics, and in ionic solutions with a unified integral equation method: Theoretical bases, computational implementation and numerical applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Robb, M.A. A direct procedure for the evaluation of solvent effects in MC-SCF calculations. J. Chem. Phys. 1999, 111, 5295–5302. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. (Theochem) 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Pomogaeva, A.; Chipman, D.M. Hydration energy from a composite method for implicit representation of solvent. J. Chem. Theory Comput. 2014, 10, 211–219. [Google Scholar] [CrossRef]
- Hille, C.; Ringe, S.; Deimel, M.; Kunkel, C.; Acree, W.E.; Reuter, K.; Oberhofer, H. Generalized molecular solvation in non-aqueous solutions by a single parameter implicit solvation scheme. J. Chem. Phys. 2019, 150, 041710. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Klamt, A. The COSMO and COSMO-RS solvation models. WIREs Comput. Mol. Sci. 2011, 1, 699–709. [Google Scholar] [CrossRef]
- Still, W.C.; Tempczyk, A.; Hawley, R.C.; Hendrickson, T.J. Semianalytical treatment of solvation for molecular mechanics and dynamics. J. Am. Chem. Soc. 1990, 112, 6127–6129. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Generalized Born solvation model SM12. J. Chem. Theory Comput. 2013, 9, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.J.; Truhlar, D.G. General parameterized SCF model for free energies of solvation in aqueous solution. J. Am. Chem. Soc. 1991, 113, 8305–8311. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. An SCF solvation model for the hydrophobic effect and absolute free energies of aqueous solvation. Science 1992, 256, 213–217. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. A universal approach to solvation modeling. Acc. Chem. Res. 2008, 41, 760–768. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Uniform treatment of solute–solvent dispersion in the ground and excited electronic states of the solute based on a solvation model with state-specific polarizability. J. Chem. Theory Comput. 2013, 9, 3649–3659. [Google Scholar] [CrossRef]
- Born, M. Volumen und Hydratationswärme der Ionen. Z. Physik 1920, 1, 45–48. (In German) [Google Scholar] [CrossRef]
- Onufriev, A.V.; Case, D.A. Generalized Born implicit solvent models for biomolecules. Annu. Rev. Biophys. 2019, 48, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Grycuk, T. Deficiency of the Coulomb-field approximation in the generalized Born model: An improved formula for Born radii evaluation. J. Chem. Phys. 2003, 119, 4817–4826. [Google Scholar] [CrossRef]
- Grant, J.; Pickup, B.; Sykes, M.; Kitchen, C.; Nicholls, A. The Gaussian Generalized Born model: Application to small molecules. Phys. Chem. Chem. Phys. 2007, 9, 4913–4922. [Google Scholar] [CrossRef] [PubMed]
- Lange, A.W.; Herbert, J.M. Improving Generalized Born models by exploiting connections to Polarizable Continuum Models. I. An improved effective Coulomb operator. J. Chem. Theory Comput. 2012, 8, 1999–2011. [Google Scholar] [CrossRef] [PubMed]
- Voityuk, A.A.; Vyboishchikov, S.F. A simple COSMO-based method for calculation of hydration energies of neutral molecules. Phys. Chem. Chem. Phys. 2019, 21, 18706–18713. [Google Scholar] [CrossRef] [PubMed]
- Voityuk, A.A.; Vyboishchikov, S.F. Fast and accurate calculation of hydration energies of molecules and ions. Phys. Chem. Chem. Phys. 2020, 22, 14591–14598. [Google Scholar] [CrossRef]
- Vyboishchikov, S.F.; Voityuk, A.A. Fast non-iterative calculation of solvation energies for water and nonaqueous solvents. J. Comput. Chem. 2021, 42, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Vyboishchikov, S.F.; Voityuk, A.A. Solvation free energies for aqueous and nonaqueous solutions computed using PM7 atomic charges. J. Chem. Inf. Model. 2021, 61, 4544–4553. [Google Scholar] [CrossRef]
- Vyboishchikov, S.F. A quick solvation energy estimator based on electronegativity equalization. J. Comput. Chem. 2023, 44, 307–318. [Google Scholar] [CrossRef]
- Vyboishchikov, S.F.; Voityuk, A.A. Noniterative solvation energy method based on atomic charges. In Chemical Reactivity: Approaches and applications; Kaya, S., von Szentpály, L., Serdaroğlu, G., Guo, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 399–427. [Google Scholar] [CrossRef]
- Voityuk, A.A.; Stasyuk, A.J.; Vyboishchikov, S.F. A simple model for calculating atomic charges in molecules. Phys. Chem. Chem. Phys. 2018, 20, 23328–23337. [Google Scholar] [CrossRef] [PubMed]
- Vyboishchikov, S.F.; Voityuk, A.A. Iterative atomic-charge partitioning of valence electron density. J. Comp. Chem. 2019, 40, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Jerome, S.V.; Cramer, C.J.; Truhlar, D.G. Charge Model 5: An extension of Hirshfeld population analysis for the accurate description of molecular interactions in gaseous and condensed phases. J. Chem. Theory Comput. 2012, 8, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Kříž, K.; Řezáč, J. Reparametrization of the COSMO solvent model for semiempirical methods PM6 and PM7. J. Chem. Inf. Model. 2019, 59, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Vyboishchikov, S.F. Dense neural network for calculating solvation free energies from electronegativity-equalization atomic charges. J. Chem. Inf. Model. 2023, 63, 6283–6292. [Google Scholar] [CrossRef] [PubMed]
- Vyboishchikov, S.F. Predicting solvation free energies using electronegativity-equalization atomic charges and a dense neural network: A generalized-Born approach. J. Chem. Theory Comput. 2023, 19, 8340–8350. [Google Scholar] [CrossRef]
- The Program Executable Is Available Free of Charge. Available online: https://github.com/vyboishchikov/ESE-EE-DNN (accessed on 3 July 2024).
- The Program Executable Is Available Free of Charge. Available online: https://github.com/vyboishchikov/ESE-GB-DNN (accessed on 3 July 2024).
- Chen, Y.; Krämer, A.; Charron, N.E.; Husic, B.E.; Clementi, C.; Noé, F. Machine learning implicit solvation for molecular dynamics. J. Chem. Phys. 2021, 155, 084101. [Google Scholar] [CrossRef]
- Vermeire, F.H.; Green, W.H. Transfer learning for solvation free energies: From quantum chemistry to experiments. Chem. Engin. J. 2021, 418, 129307. [Google Scholar] [CrossRef]
- Low, K.; Coote, M.L.; Izgorodina, E.I. Explainable solvation free energy prediction combining graph neural networks with chemical intuition. J. Chem. Inf. Model. 2022, 62, 5457–5470. [Google Scholar] [CrossRef]
- Lim, H.; Jung, I. MLSolvA: Solvation free energy prediction from pairwise atomistic interactions by machine learning. J. Cheminform. 2021, 13, 56. [Google Scholar] [CrossRef]
- Alibakhshi, A.; Hartke, B. Improved prediction of solvation free energies by machine-learning polarizable continuum solvation model. Nat. Commun. 2021, 12, 3584. [Google Scholar] [CrossRef] [PubMed]
- Bernazzani, L.; Duce, C.; Micheli, A.; Mollica, V.; Tiné, M.R. Quantitative structure–property relationship (QSPR) prediction of solvation Gibbs energy of bifunctional compounds by recursive neural networks. J. Chem. Eng. Data 2010, 55, 5425–5428. [Google Scholar] [CrossRef]
- Hutchinson, S.T.; Kobayashi, R. Solvent-specific featurization for predicting free energies of solvation through machine learning. J. Chem. Inf. Model. 2019, 59, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, C.; Wu, K.; Wei, G.W. Breaking the polar–non-polar division in solvation free energy prediction. J. Comput. Chem. 2018, 39, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Jaquis, B.J.; Li, A.; Monnier, N.D.; Sisk, R.G.; Acree, W.E.; Lang, A.S.I.D. Using machine learning to predict enthalpy of solvation. J. Solut. Chem. 2019, 48, 564–573. [Google Scholar] [CrossRef]
- Chung, Y.; Vermeire, F.H.; Wu, H.; Walker, P.J.; Abraham, M.H.; Green, W.H. Group contribution and machine learning approaches to predict Abraham solute parameters, solvation free energy, and solvation enthalpy. J. Chem. Inf. Model. 2022, 62, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Svobodová Vařeková, R.; Jiroušková, Z.; Vaněk, J.; Suchomel, Š.; Koča, J. Electronegativity equalization method: Parameterization and validation for large sets of organic, organohalogene and organometal molecule. Int. J. Mol. Sci. 2007, 8, 572–582. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. Available online: https://scikit-learn.org/stable (accessed on 1 March 2024).
- Nesterov, Y. A method of solving a convex programming problem with convergence rate O(1/k2). Sov. Math. Dokl. 1983, 27, 372–376. [Google Scholar]
- Dozat, T. Incorporating Nesterov Momentum into Adam. In International Conference on Learning Representations. 2016. Available online: https://openreview.net/pdf/OM0jvwB8jIp57ZJjtNEZ.pdf (accessed on 8 November 2023).
- Martín, A.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-Scale Machine Learning on Heterogeneous Systems. 2015. Available online: http://tensorflow.org (accessed on 30 April 2023).
- Lu, J.Z.; Acree, W.E.; Abraham, M.H. Updated Abraham model correlations for enthalpies of solvation of organic solutes dissolved in benzene and acetonitrile. Phys. Chem. Liquids 2019, 57, 84–99. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; (University of North Texas, Denton, TX, USA). Personal communication, 2024.
- Available online: http://cactus.nci.nih.gov/chemical/structure (accessed on 1 March 2024).
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Landrum, G. Rdkit Documentation, Release 1 September 2019. 2019. Available online: https://buildmedia.readthedocs.org/media/pdf/rdkit/latest/rdkit.pdf (accessed on 1 March 2024).
- Available online: https://webbook.nist.gov (accessed on 1 March 2024).
- Available online: https://www.stenutz.eu/chem (accessed on 1 March 2024).
- Marenich, A.V.; Kelly, C.P.; Thompson, J.D.; Hawkins, G.D.; Chambers, C.C.; Giesen, D.J.; Winget, P.; Cramer, C.J.; Truhlar, D.G. Minnesota Solvation Database—Version 2012, University of Minnesota, 26 November 2012. Available online: https://conservancy.umn.edu/bitstream/handle/11299/213300/MNSolDatabase_v2012.zip (accessed on 10 June 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).