Ab Initio Investigation of the Hydration of the Tetrahedral d0 Transition Metal Oxoanions NbO43−, TaO43−, CrO42−, MoO42−, WO42−, MnO4−, TcO4−, ReO4−, and of FeO4, RuO4, and OsO4
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Permanganate
3.2. Pertechnetate
3.3. Perrhenate
3.4. Iron, Ruthenium, and Osmium Tetroxide
3.5. Chromate
3.6. Molybdate
3.7. Tungstate
3.8. Niobates
3.8.1. Niobium Oxides
3.8.2. Lithium Niobates
3.8.3. Sodium Niobates
3.8.4. Potassium Niobates
3.8.5. Rubidium Niobates
3.8.6. Cesium Niobates
3.8.7. Calculations on Orthoniobate
3.9. Tantalates
3.9.1. Tantalum Oxide
3.9.2. Lithium Tantalates
3.9.3. Sodium Tantalates
3.9.4. Potassium Tantalates
3.9.5. Rubidium Tantalates
3.9.6. Cesium Tantalates
3.9.7. Calculations on Orthotantalate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richens, D.T. The Chemistry of Aqua Ions; Wiley: Chichester, UK, 1997. [Google Scholar]
- Applegarth, L.M.S.G.A.; Corbeil, C.R.; Mercer, D.J.W.; Pye, C.C.; Tremaine, P.R. Raman and ab Initio Investigation of Aqueous Cu(I) Chloride Complexes from 25 to 80 °C. J. Phys. Chem. B 2014, 118, 204–214, and references 41, 42, 44 therein. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Thallium(III) and Mercury(II). J. Sol. Chem. 2020, 49, 1419–1429. [Google Scholar] [CrossRef]
- Pye, C.C.; Whynot, D.C.M.; Corbeil, C.R.; Mercer, D.J.W. Desymmetrization in geometry optimization: Application to an ab initio study of copper(I) hydration. Pure Appl. Chem. 2020, 92, 1643–1654. [Google Scholar] [CrossRef]
- Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Lead(II). Liquids 2022, 2, 39–49. [Google Scholar] [CrossRef]
- Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Tin(II). Liquids 2022, 2, 465–473. [Google Scholar] [CrossRef]
- Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Antimony(III). Liquids 2024, 4, 322–331. [Google Scholar] [CrossRef]
- Pye, C.C.; Walker, V.E.J. Ab Initio Investigation of the Hydration of the Tetrahedral Perchlorate, Perbromate, Selenate, Arsenate, and Vanadate Anions. J. Phys. Chem. A 2011, 115, 13007–13015, and references 9, 10 therein. [Google Scholar] [CrossRef] [PubMed]
- Pye, C.C.; Michels, M.R. An ab Initio Investigation of Hydrogen Phosphate Ion Hydration. Can. J. Anal. Sci. Spectrosc. 2004, 49, 175–184. [Google Scholar]
- Pye, C.C.; Michels, M.R. An ab Initio Investigation of Dihydrogen Phosphate Ion Hydration. Can. J. Anal. Sci. Spectrosc. 2005, 50, 70–86. [Google Scholar]
- Pye, C.C. An ab initio study of the effect of hydration on the vibrational spectrum of hydrogen sulfate. Comp. Theor. Chem. 2020, 1176, 112749. [Google Scholar] [CrossRef]
- Pye, C.C.; Akbari, P.; Goodall, B.L.; Shah Alam, M.Y. An ab initio study of the effect of hydration on the vibrational spectrum of hydrogen selenate ion. J. Mol. Liq. 2022, 359, 119383. [Google Scholar] [CrossRef]
- Pye, C.C.; Chiome, T.; Goodall, B.L. An ab initio study of the effect of hydration on the vibrational spectrum of hydrogen arsenate ion. Comp. Theor. Chem. 2022, 1215, 113838. [Google Scholar] [CrossRef]
- Pye, C.C.; Berryman, V.E.J.; Goodall, B.L. An Ab Initio Study of the Effect of Hydration on the Vibrational Spectrum of Hydrogen Vanadate Ion. J. Solution Chem. 2024, 53, 626–641. [Google Scholar] [CrossRef]
- Applegarth, L.M.S.G.A.; Pye, C.C.; Cox, J.S.; Tremaine, P.R. Raman Spectroscopic and ab Initio Investigation of Aqueous Boric Acid, Borate, and Polyborate Speciation from 25 to 80 °C. Ind. Eng. Chem. Res. 2017, 56, 13983–13996. [Google Scholar] [CrossRef]
- Pye, C.C. A Crystallographic Review of Alkali Borate Salts and Ab Initio Study of Borate Ions/Molecules. In Concepts, Methods and Applications of Quantum Systems in Chemistry and Physics, Progress in Theoretical Chemistry and Physics; Wang, Y.A., Thachuk, M., Krems, R., Maruani, J., Eds.; Springer: Cham, Switzerland, 2018; Volume 31, pp. 107–142. [Google Scholar]
- Pye, C.C. An Ab Initio Study of Boric Acid, Borate, and their Interconversion. In Concepts, Methods and Applications of Quantum Systems in Chemistry and Physics, Progress in Theoretical Chemistry and Physics; Wang, Y.A., Thachuk, M., Krems, R., Maruani, J., Eds.; Springer: Cham, Switzerland, 2018; Volume 31, pp. 143–177. [Google Scholar]
- Sasidharanpillai, S.; Cox, J.S.; Pye, C.C.; Tremaine, P.R. A Raman spectroscopic and ab initio investigation of aqueous boron speciation under alkaline hydrothermal conditions: Evidence for the structure and thermodynamic stability of the diborate ion. Dalton Trans. 2023, 52, 18391–18406. [Google Scholar] [CrossRef] [PubMed]
- Goodall, B.L. An Ab Initio Investigation of the Hydration of the d0 Transition Metal Tetraoxo Complexes: CrO42−, MnO4−, FeO4. Honours Thesis, Saint Mary’s University, Halifax, UK, 2012. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.02; Gaussian, Inc.: Wallingford, CT, USA, 2004.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019.
- Mooney, R.C.L. The Crystal Structure of Potassium Permanganate. Phys. Rev. 1931, 37, 1306–1310. [Google Scholar] [CrossRef]
- Ramaseshan, S.; Venkatesan, K.; Mani, N.V. The use of anomalous scattering for the determination of crystal structures—KMnO4. Proc. Indian Acad. Sci. A 1957, 46, 95–111. [Google Scholar] [CrossRef]
- Palenik, G.J. Crystal Structure of Potassium Permanganate. Inorg. Chem. 1967, 6, 503–507. [Google Scholar] [CrossRef]
- Hoppe, R.; Fischer, D.; Schneider, J. Zur Kenntnis von Oxyden A[MO4]: Über LiMnO4, KMnO4, RbMnO4, CsMnO4 sowie RbIO4 und CsIO4. (-Was heißt eigenlich „Die Kristallstruktur von...“?-) [On the Knowledge of Oxides A[MO4]: On LiMnO4, KMnO4, RbMnO4, CsMnO4 as well as RbIO4, CsIO4. (– What does ”The Crystal Structure of...“ mean? –)]. Z. Anorg. Allg. Chem. 1999, 625, 1135–1142. (In German) [Google Scholar]
- Marabello, D.; Bianchi, R.; Gervasio, G.; Cargnoni, F. An experimental (120 K) and theoretical electron-density study of KMnO4 and KClO4. Acta Crystallogr. A 2004, 60, 494–501. [Google Scholar] [CrossRef]
- Fischer, D.; Hoppe, R.; Schaefer, W.; Knight, K.S. Koordinationszahl 4 oder 6 für Lithium?: Die Kristallstruktur von Wasserfreiem Lithiumpermanganat, Li[MnO4] [Coordination number 4 or 6 for Lithium?: The Crystal Structure of Lithium Permanganate Li[MnO4]]. Z. Anorg. Allg. Chem. 1993, 619, 1419–1425. (In German) [Google Scholar] [CrossRef]
- Bauchert, J.M.; Henning, H.; Schleid, T. Synthesis and crystal structure of anhydrous Na[MnO4]. Z. Naturforsch. B 2016, 71, 993–995. [Google Scholar] [CrossRef]
- Ketelaar, J.A.A. Die Kristallstruktur von K-, Rb-, Cs-, und Tl-Silicofluorid und von LiMnO4·3H2O [The crystal structure of K-, Rb-, Cs-, and Tl-hexafluorosilicate, and of LiMnO4·3H2O]. Z. Krist. 1935, 92, 155–156. (In German) [Google Scholar]
- Fischer, D.; Hoppe, R. Uber den Aufbau von LiMnO4·3H2O = [Li(OH2)6/2][MnO4] [On the structure of LiMnO4·3H2O = [Li(OH2)6/2][MnO4]]. Z. Anorg. Allg. Chem. 1990, 590, 18–22. (In German) [Google Scholar] [CrossRef]
- Kiefer, W.; Bernstein, H.J. Resonance Raman effect in MnO4− with 5145 Å laser excitation. Chem. Phys. Lett. 1971, 8, 381–383. [Google Scholar] [CrossRef]
- Kiefer, W.; Bernstein, H.J. The resonance Raman effect of the permanganate and chromate ions. Mol. Phys. 1972, 23, 835–851. [Google Scholar] [CrossRef]
- Weinstock, N.; Schulze, H.; Müller, A. Assignment of ν2(E) and ν4(F2) of tetrahedral species by the calculation of the relative Raman intensities: The vibrational spectra of VO43−, CrO42−, MoO42−, WO42−, MnO4−, TcO4−, ReO4−, RuO4, and OsO4. J. Chem. Phys. 1973, 59, 5063–5067. [Google Scholar] [CrossRef]
- Dong, J.; Wang, Y.; Zhou, M. The vibrational spectrum of the MnO2− and MnO4− anions in solid argon. Chem. Phys. Lett. 2002, 364, 511–516. [Google Scholar] [CrossRef]
- Wolfsberg, M.; Helmholz, L. The Spectra and Electronic Structure of the Tetrahedral Ions MnO4−, CrO42−, and ClO4−. J. Chem. Phys. 1952, 20, 837–843. [Google Scholar] [CrossRef]
- Ballhausen, C.J. A vibrational analysis of the MnO4− bands. Theor. Chim. Acta 1963, 1, 285–293. [Google Scholar] [CrossRef]
- Holt, S.L.; Ballhausen, C.J. Low temperature absorption spectra of KMnO4 in KClO4. Theor. Chim. Acta 1967, 7, 313–320. [Google Scholar] [CrossRef]
- Hillier, I.H.; Saunders, V.R. Ab initio Molecular Orbital Calculations of the Ground and Excited States of the Permanganate and Chromate Ions. Proc. Roy. Soc. London A 1970, 320, 161–173. [Google Scholar]
- Wood, M.H. An ab initio study of the ground and low-lying excited states of the permanganate ion. Theor. Chim. Acta 1975, 36, 309–315. [Google Scholar] [CrossRef]
- Dickson, R.M.; Ziegler, T. A Density Functional Study of the Electronic Spectrum of Permanganate. Int. J. Quant. Chem. 1996, 58, 681–687. [Google Scholar] [CrossRef]
- Stuckl, A.C.; Daul, C.A.; Gudel, H.U. Excited-state energies and distortions of d transition metal tetraoxo complexes: A density functional study. J. Chem. Phys. 1997, 107, 4606–4617. [Google Scholar] [CrossRef]
- Buhl, M. Structure, Dynamics, and Magnetic Shieldings of Permanganate Ion in Aqueous Solution. A Density Functional Study. J. Phys. Chem. A 2002, 106, 10505–10509. [Google Scholar] [CrossRef]
- Carbonniere, P.; Ciofini, I.; Adamo, C.; Pouchan, C. Vibrational behavior of tetrahedral d0 oxo-compounds: A theoretical study. Chem. Phys. Lett. 2006, 429, 52–57. [Google Scholar] [CrossRef]
- Jose, L.; Seth, M.; Ziegler, T. Molecular and Vibrational Structure of Tetroxo d0 Metal Complexes in their Excited States. A Study Based on Time-Dependent Density Functional Calculations and Franck-Condon Theory. J. Phys. Chem. A 2012, 116, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Xu, W.-H.; Xu, C.-F.; Schwarz, W.H.E.; Li, J. Theoretical Studies on the Photoelectron and Absorption Spectra of MnO4− and TcO4−. Inorg. Chem. 2013, 52, 9867–9874. [Google Scholar] [CrossRef]
- Almeida, N.M.S.; McKinlay, R.G.; Paterson, M.J. Excited electronic states of MnO4−: Challenges for wavefunction and density functional response theories. Chem. Phys. 2015, 446, 86–91. [Google Scholar] [CrossRef]
- Olsen, J.M.H.; Hedegard, E.D. Modeling the absorption spectrum of the permanganate ion in vacuum and in aqueous solution. Phys. Chem. Chem. Phys. 2017, 19, 15870–15875. [Google Scholar] [CrossRef] [PubMed]
- Smiechowski, M.; Persson, I. Hydration of Oxometallate Ions in Aqueous Solution. Inorg. Chem. 2020, 59, 8231–8239. [Google Scholar] [CrossRef] [PubMed]
- Krebs, B.; Hasse, K.-D. Refinements of the Crystal Structures of KTcO4, KReO4 and OsO4. The Bond Lengths in Tetrahedral Oxo-Anions and Oxides of d0 Transition Metals. Acta Crystallogr. B 1976, 32, 1334–1337. [Google Scholar] [CrossRef]
- German, K.F.; Grigoriev, M.S.; Garashchenko, B.L.; Kopytin, A.V.; Tyupina, E.A. Redetermination of the crystal structure of NaTcO4 at 100 and 296 K based on single-crystal X-ray data. Acta Crystallogr. E 2017, 73, 1037–1040. [Google Scholar] [CrossRef]
- Weaver, J.; Soderquist, C.Z.; Washton, N.M.; Lipton, A.D.; Gassman, P.L.; Lukens, W.W.; Kruger, A.A.; Wall, N.A.; McCloy, J.S. Chemical Trends in Solid Alkali Pertechnetates. Inorg. Chem. 2017, 56, 2533–2544. [Google Scholar] [CrossRef]
- Meyer, G.; Hoppe, R. Die Kristallstruktur von CsTcO4 [The crystal structure of CsTcO4]. Z. Anorg. Allg. Chem. 1976, 420, 40–50. (In German) [Google Scholar] [CrossRef]
- Maruk, A.Y.; Grigorev, M.S.; German, K.E. Lithium Pertechnetate Trihydrate LiTcO4·3H2O: Synthesis and Crystal Structure. Russ. J. Coord. Chem. 2011, 37, 444–446. [Google Scholar]
- Busey, R.H.; Keller, O.L., Jr. Structure of the Aqueous Pertechnetate Ion by Raman and Infrared Spectroscopy. Raman and Infrared Spectra of Crystalline KTcO4, KReO4, Na2MoO4, Na2WO4, Na2MoO4·2H2O, and Na2WO4·2H2O. J. Chem. Phys. 1964, 41, 215–225. [Google Scholar] [CrossRef]
- Morrow, J.C. The crystal structure of KReO4. Acta Crystallogr. 1960, 13, 443–445. [Google Scholar] [CrossRef]
- Lock, C.J.L.; Turner, G. A Reinvestigation of the Crystal Structure of Potassium Perrhenate. Acta Crystallogr. B 1975, 31, 1764–1765. [Google Scholar]
- Brown, R.J.C.; Powell, B.M.; Stuart, S.N. Thermal Effects in the Structure of Potassium Perrhenate. Acta Crystallogr. C 1993, 49, 214–216. [Google Scholar] [CrossRef]
- Atzesdorfer, A.; Range, K.-J. Sodium Metaperrhenate, NaReO4: High Pressure Synthesis of Single Crystals and Structure Refinement. Z. Naturforsch. B 1995, 50, 1417–1418. [Google Scholar] [CrossRef]
- Beintema, J. Die Kristallstruktur der Alkaliperrhenate und -perjodate [The crystal structure of alkali perrhenate and periodate]. Z. Kristallogr. 1937, 97, 300–322. (In German) [Google Scholar] [CrossRef]
- Rogner, P.; Range, K.-J. Strukturverfeinerung von Rubidiumperrhenat bei 297 und 159 K [Structure Refinement of Rubidium Perrhenate at 297 and 159 K]. Z. Naturforsch. B 1993, 48, 233–234. (In German) [Google Scholar] [CrossRef]
- Jaeger, F.M.; Beintema, J. The Crystalstructure of Cesium-, Thallium- and Rubidium-Perrhenates. Proc. K. Ned. Akad. Wet. 1933, 36, 523–528. [Google Scholar]
- Rogner, P.; Range, K.-J. The Crystal Structure of β-CsReO4, the Room-Temperature Modification of Cesium Perrhenate. Z. Naturforsch. B 1993, 48, 685–687. [Google Scholar] [CrossRef]
- Range, K.-J.; Rogner, P.; Heyns, A.M.; Prinsloo, L.C. An X-Ray, Raman and IR Study of α-CsReO4, the High-Temperature Modification of Cesium Perrhenate. Z. Naturforsch. B 1992, 47, 1513–1520. [Google Scholar] [CrossRef]
- Betz, T.; Hoppe, R. Über Perrhenate.1. Zur Kenntnis von LiReO4 [About Perrhenates. 1. On LiReO4]. Z. Anorg. Allg. Chem. 1983, 500, 23–30. (In German) [Google Scholar] [CrossRef]
- Abakumov, A.M.; Rozova, M.G.; Shpanchenko, R.V.; Mironov, A.V.; Antipov, E.V.; Bramnik, K.G. Synthesis and crystal structure of the lithium perrhenate monohydrate LiReO4·H2O. Solid State Sci. 2001, 3, 581–586. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Varfolomeev, M.B.; Shamrai, N.B.; Lindeman, S.V.; Struchkov, Y.T. The Crystal Structure of Lithium Perrhenate Sesquihydrate. Russ. J. Inorg. Chem. 1995, 40, 197–201, Translation of Zh. Neorg. Khim. pp. 208–213. [Google Scholar]
- Pley, M.; Wickleder, M.S. Two crystalline modifications of RuO4. J. Solid State Chem. 2005, 178, 3206–3209. [Google Scholar] [CrossRef]
- Trehoux, J.; Thomas, D.; Nowogrocki, G.; Tridot, G. Étude cristallographique du tétroxyde de ruthenium, du nitrosopentachlorure d’ammonium et du dichlorure de ruthenium chloropentammine [Crystallographic study of ruthenium tetroxide, ammonium nitrosopentachlororuthenide(III) and chloropentammineruthenium(III) dichloride]. Compt. Rend. C 1969, 268, 246–249. (In French) [Google Scholar]
- Ueki, T.; Zalkin, A.; Templeton, D.H. The Crystal Structure of Osmium Tetroxide. Acta Crystallogr. 1965, 19, 157–160. [Google Scholar] [CrossRef]
- Seip, H.M.; Stolevik, R. Studies on the Failure of the First Born Approximation In Electron Diffraction. II. Osmium Tetroxide. Acta Chem. Scand. 1966, 20, 385–394. [Google Scholar] [CrossRef]
- Dodd, R.E. Infra-red Spectra of Ruthenium and Osmium Tetroxides. Trans. Faraday Soc. 1959, 55, 1480–1483. [Google Scholar] [CrossRef]
- Ortner, M.H. Infrared Spectrum and Thermodynamic Properties of Ruthenium Tetroxide. J. Chem. Phys. 1961, 34, 556–558. [Google Scholar] [CrossRef]
- Griffith, W.P. Raman spectra of ruthenium tetroxide and related species. J. Chem. Soc. A 1968, 1663–1664. [Google Scholar] [CrossRef]
- Woodward, L.A.; Roberts, H.L. The Raman and Infra-red Absorption Spectra of Osmium Tetroxide: Relation to the Structure of the Perrhenate and Tungstate Ions in Aqueous Solution. Trans. Faraday Soc. 1956, 52, 615–619. [Google Scholar] [CrossRef]
- Huston, J.L.; Claassen, H.H. Raman Spectra and Force Constants for OsO4 and XeO4. J. Chem. Phys 1970, 52, 5646–5648. [Google Scholar] [CrossRef]
- McDowell, R.S.; Goldblatt, M. The Vibrational Spectrum and Force Field of Osmium Tetroxide. Inorg. Chem. 1971, 10, 625–630. [Google Scholar] [CrossRef]
- Huang, W.; Xing, D.-H.; Lu, J.-B.; Long, B.; Schwarz, W.H.E.; Li, J. How Much Can Density Functional Approximations (DFA) Fail? The Extreme Case of the FeO4 Species. J. Chem. Theory Comput. 2016, 12, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J. The Crystal Structure of Anhydrous Sodium Chromate, Na2CrO4. Z. Kristallogr. 1936, 94, 131–136. [Google Scholar] [CrossRef]
- Zachariasen, W.H.; Ziegler, G.E. The Crystal Structure of Potassium Chromate, K2CrO4. Z. Kristallogr. 1931, 80, 164–173. [Google Scholar] [CrossRef]
- Smith, H.W., Jr.; Colby, M.Y. The Crystal Structure of Rubidium Chromate, Rb2CrO4. Z. Kristallogr. 1941, 103, 90–95. [Google Scholar] [CrossRef]
- Miller, J.J. The Crystal Structure of Caesium Chromate, Cs2CrO4. Z. Kristallogr. 1938, 99, 32–37. [Google Scholar] [CrossRef]
- McGinnety, J.A. Redetermination of the Structures of Potassium Sulphate and Potassium Chromate: The Effect of Electrostatic Crystal Forces upon Observed Bond Lengths. Acta Crystallogr. B 1972, 28, 2845–2852. [Google Scholar] [CrossRef]
- Toriumi, K.; Saito, Y. Electron-Density Distribution in Crystals of α-K2CrO4. Acta Crystallogr. B 1978, 34, 3149–3156. [Google Scholar] [CrossRef]
- Brown, I.D.; Faggiani, R. Refinement of the Crystal Structure of Lithium Chromate. Acta Crystallogr. B 1975, 31, 2364–2365. [Google Scholar] [CrossRef]
- Nimmo, J.K. Sodium Chromate (II) at 296 K (Neutron). Acta Crystallogr. B 1981, 37, 431–433. [Google Scholar] [CrossRef]
- Aleksovska, S.; Nyburg, S.C.; Pejov, L.; Petrusevski, V.M. β-K2SO4-Type Isomorphs: Prediction of Structures and Refinement of Rb2CrO4. Acta Crystallogr. B 1998, 54, 115–120. [Google Scholar] [CrossRef]
- Morris, A.J.; Kennard, C.H.L.; Moore, F.H.; Smith, G.; Montgomery, H. Cesium Chromate, CrCs2O4 (Neutron). Cryst. Struct. Comm. 1981, 10, 529–532. [Google Scholar]
- Ruben, H.; Olovsson, I.; Zalkin, A.; Templeton, D.H. Sodium Chromate Tetrahydrate. Acta Crystallogr. B 1973, 29, 2963–2964. [Google Scholar] [CrossRef]
- Kahlenberg, V. Crystal structures of two hydrated sodium chromates: Na2CrO4 × 10H2O and Na2CrO4 × 1.5H2O. Z. Kristallogr. 2012, 227, 621–628. [Google Scholar] [CrossRef]
- Cadbury, W.E., Jr.; Meldrum, W.B.; Lucasse, W.W. Transition Phenomena Involving Decahydrated Mixed Crystals of Sodium Sulfate and Sodium Chromate. J. Am. Chem. Soc. 1941, 63, 2262–2267. [Google Scholar] [CrossRef]
- Richards, T.W.; Kelley, G.L. The Transition Temperatures of Sodium Chromate as Convenient Fixed Points in Thermometry. J. Am. Chem. Soc. 1911, 33, 847–863. [Google Scholar] [CrossRef]
- Venkateswaran, C.S. The Raman Spectra of Some Inorganic Compounds. Proc. Indian Acad. Sci. 1938, 7, 144–155. [Google Scholar] [CrossRef]
- Stammreich, H.; Bassi, D.; Sala, O. Raman spectrum and force constants of the chromate ion. Spectrochim. Acta 1958, 12, 403–405. [Google Scholar] [CrossRef]
- Michel, G.; Machiroux, R. Raman Spectroscopic Investigations of the CrO42−/Cr2O72− Equilibrium in Aqueous Solution. J. Raman. Spectrosc. 1983, 14, 22–27. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Darab, J.G.; Fulton, J.L. An Infrared and X-ray Absorption Study of the Equilibria and Structures of Chromate, Bichromate, and Dichromate in Ambient Aqueous Solutions. J. Phys. Chem. A 2001, 105, 1772–1782. [Google Scholar] [CrossRef]
- Barinova, A.V.; Rastsvetaeva, R.K.; Nekrasov, Y.V.; Pushcharovskii, D.Y. Crystal structure of Li2MoO4. Dokl. Chem. 2001, 376, 16–19, translation of Dokl. Akad. Nauk 2001, 376, 343–346. [Google Scholar] [CrossRef]
- Yip, T.W.S.; Cussen, E.J.; Wilson, C. Spontaneous formation of crystalline lithium molybdate from solid reagents at room temperature. Dalton Trans. 2010, 39, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kolitsch, U. The crystal structures of phenacite-type Li2(MoO4), and scheelite-type LiY(MoO4)2 and LiNd(MoO4)2. Z. Kristallogr. 2001, 216, 449–454. [Google Scholar] [CrossRef]
- Zachariasen, W. Notiz Über die Kristallstruktur von Phenacit, Willemit und Verwandten Verbindungen [Note about the crystal structure of phenacite, willemite, and related compounds]. Nor. Geol. Tidsskr. 1926, 9, 65–73. (In German) [Google Scholar]
- Bramnik, K.G.; Ehrenberg, H. Study of the Na2O-MoO3 System. Na5Mo11O36—A New Oxide with Anatase-related Structure, and the Crystal Structures of Na2MoO4. Z. Anorg. Allg. Chem. 2004, 630, 1336–1341. [Google Scholar] [CrossRef]
- Fortes, A.D. Crystal structures of spinel-type Na2MoO4 and Na2WO4 revisited using neutron powder diffraction. Acta Crystallogr. E 2015, 71, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, B.M.; Leverett, P. Crystal structure of potassium molybdate, K2MoO4. J. Chem. Soc. A 1969, 849–854. [Google Scholar] [CrossRef]
- Kools, F.X.N.M.; Koster, A.S.; Rieck, G.D. The Structures of Potassium, Rubidum and Cesium Molybdate and Tungstate. Acta Crystallogr. B 1970, 26, 1974–1977. [Google Scholar] [CrossRef]
- Shigematsu, H.; Nomura, K.; Nishiyama, K.; Tojo, T.; Kawaji, H.; Atake, T.; Kawamura, Y.; Miyoshi, T.; Matsushita, Y.; Tanaka, M.; et al. Structures and Phase Transitions in Rb2MoO4 and Rb2WO4. Ferroelectrics 2011, 414, 195–200. [Google Scholar] [CrossRef][Green Version]
- Gonschorek, W.; Hahn, T. Die Kristallstruktur des Caesiummolybdats, Cs2MoO4 [The Crystal Structure of Cesium Molybdate, Cs2MoO4]. Z. Kristallogr. 1973, 138, 167–176. (In German) [Google Scholar] [CrossRef]
- Mitra, R.P.; Verma, H.K.L. Crystal Structures of Dihydrates of Sodium Tungstate & Sodium Molybdate. Indian J. Chem. 1969, 7, 598–602. [Google Scholar]
- Atovmyan, L.O.; Dyachenko, O.A. X-ray Structural Investigation of Na2MoO4·2H2O Crystals. J. Struct. Chem. 1969, 10, 416–418, translation of Zh. Strukt. Khim. 1969, 10, 504–507. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kobayashi, A.; Sasaki, Y. The Crystal Structure of Sodium Molybdate Dihydrate, Na2MoO4·2H2O. Bull. Chem. Soc. Jpn. 1975, 48, 1009–1013. [Google Scholar] [CrossRef]
- Capitelli, F.; Selim, M.; Mukherjea, K.K. Synthesis and Crystal Structure Determination of Sodium Molybdate Dihydrate. Asian J. Chem. 2006, 18, 2856–2860. [Google Scholar]
- Fortes, A.D. Crystal structures of deuterated sodium molybdate dihydrate and sodium tungstate dihydrate from time-of-flight neutron powder diffraction. Acta Crystallogr. E 2015, 71, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Dean, K.J.; Wilkinson, G.R. Precision Raman Investigation of the ν1 Mode of Vibration of SO42−, WO42− and MoO42− in Aqueous Solutions of Different Concentrations. J. Raman. Spectrosc. 1983, 14, 130–134. [Google Scholar] [CrossRef]
- Johansson, G.; Caminiti, R. The Hydration of Tungstate and Molybdate Ions in Aqueous Solution. Z. Naturforsch. A 1986, 41, 1325–1329. [Google Scholar] [CrossRef]
- Zachariasen, W.H.; Plettinger, H.A. The Crystal Structure of Lithium Tungstate. Acta Crystallogr. 1961, 14, 229–230. [Google Scholar] [CrossRef]
- Okada, K.; Morikawa, H.; Marumo, F.; Iwai, S. Sodium Tungstate. Acta Crystallogr. B 1974, 30, 1872–1873. [Google Scholar] [CrossRef]
- Koster, A.S.; Kools, F.X.N.M.; Rieck, G.D. The Crystal Structure of Potassium Tungstate, K2WO4. Acta Crystallogr. B 1969, 25, 1704–1708. [Google Scholar] [CrossRef]
- Holtzberg, F.; Reisman, A.; Berry, M.; Berkenblit, M. Chemistry of the Group VB Pentoxides. VI. The Polymorphism of Nb2O5. J. Am. Chem. Soc. 1957, 79, 2039–2043, Correction, p. 6579. [Google Scholar] [CrossRef]
- Reisman, A.; Holtzberg, F. Further Comments on the Polymorphism of Nb2O5. The High Temperature Metastable Phase. J. Am. Chem. Soc. 1959, 81, 3182–3184. [Google Scholar] [CrossRef]
- Reisman, A.; Holtzberg, F. Heterogeneous Equilibria in the Systems Li2O-, Ag2O-Nb2O5 and Oxide-Models. J. Am. Chem. Soc. 1958, 80, 6503–6507. [Google Scholar] [CrossRef]
- Reisman, A.; Holtzberg, F.; Banks, E. Reactions of the Group VB Pentoxides with Alkali Oxides and Carbonates. VII. Heterogeneous Equilibria in the system Na2O or Na2CO3-Nb2O5. J. Am. Chem. Soc. 1958, 80, 37–42. [Google Scholar] [CrossRef]
- Reisman, A.; Holtzberg, F. Phase Equilibria in the System K2CO3-Nb2O5 by the Method of Differential Thermal Analysis. J. Am. Chem. Soc. 1955, 77, 2115–2119. [Google Scholar] [CrossRef]
- Reisman, A.; Holtzberg, F. Equilibria in the System Rb2O-Nb2O5 and Sequential Trends in Oxide-Oxide Interactions: The Prediction of Compound Retention. J. Phys. Chem. 1960, 64, 748–753. [Google Scholar] [CrossRef]
- Reisman, A.; Mineo, J. Compound Repetition in Oxide-Oxide Interactions. The System Cs2O-Nb2O5. J. Phys. Chem. 1961, 65, 996–998. [Google Scholar] [CrossRef]
- Reisman, A.; Holtzberg, F.; Berkenblit, M. Metastability in Niobate Systems. J. Am. Chem. Soc. 1959, 81, 1292–1295. [Google Scholar] [CrossRef]
- Brauer, G. Die Oxyde des Niobs [The Oxide of Niobium]. Z. Anorg. Allg. Chem. 1941, 248, 1–31. (In German) [Google Scholar] [CrossRef]
- Gatehouse, B.M.; Wadsley, A.D. The Crystal Structure of the High Temperature form of Niobium Pentoxide. Acta Crystallogr. 1964, 17, 1545–1553. [Google Scholar] [CrossRef]
- Kato, K. Structure Refinement of H-Nb2O5. Acta Crystallogr. B 1976, 32, 764–767. [Google Scholar] [CrossRef]
- Hahn, R.B. Phosphates of Niobium and Tantalum. J. Am. Chem. Soc. 1951, 73, 5091–5093. [Google Scholar] [CrossRef]
- Frevel, L.K.; Rinn, H.W. Powder Diffraction Standards for Niobium Pentoxide and Tantalum Pentoxide. Anal. Chem. 1955, 27, 1329–1330. [Google Scholar] [CrossRef]
- Nolander, B.; Norin, R. Note on the Crystal Structure of T-Nb2O5 and of an Isostructural High-Temperature Zr-Nb-oxide. Acta Chem. Scand. 1972, 26, 3814–3816. [Google Scholar] [CrossRef][Green Version]
- Kato, K.; Tamura, S. Die Kristallstruktur von T-Nb2O5. [The Crystal Structure of T-Nb2O5]. Acta Crystallogr. B 1975, 31, 673–677. (In German) [Google Scholar] [CrossRef]
- Mertin, W.; Andersson, S.; Gruehn, R. Über die Kristallstructur von M-Nb2O5 [On the Crystal Structure of M-Nb2O5]. J. Solid State Chem. 1970, 1, 419–424. (In German) [Google Scholar] [CrossRef]
- Laves, F.; Petter, W.; Wulf, H. Die Kristalstruktur von ζ-Nb2O5 [The Crystal Structure of ζ-Nb2O5]. Naturwissenschaften 1964, 51, 633–634. [Google Scholar] [CrossRef]
- Andersson, S. The Crystal Structure of N-Nb2O5, Prepared in the Presence of Small Amounts of LiF. Z. Anorg. Allg. Chem. 1967, 351, 106–112. [Google Scholar] [CrossRef]
- Norin, R.; Nolander, B. Note on the Crystal Structures of a Niobium-rich Phase in the Li2O-Nb2O5 System and of the Isostructural Compound N-Nb2O5. Acta Chem. Scand. 1971, 25, 741–743. [Google Scholar] [CrossRef][Green Version]
- Blasse, G. New Types of Cation-Order in the Rocksalt Lattice: The Structure of Li3SbO4 and Li3NbO4. Z. Anorg. Allg. Chem. 1963, 326, 44–46. [Google Scholar] [CrossRef]
- Grenier, J.-C.; Martin, C.; Durif, A. Étude cristallographique des orthoniobates et orthotantalates de lithium [Crystallographic study of the orthoniobates and orthotantalates of lithium]. Bull Soc. Fr. Min. Crist. 1964, 87, 316–320. (In French) [Google Scholar]
- Grenier, J.-C.; Bassi, G. Affinement de la structure de NbLi3O4 [Refinement of the structure of NbLi3O4]. Bull Soc. Fr. Min. Crist. 1965, 88, 345–346. (In French) [Google Scholar]
- Whiston, C.D.; Smith, A.J. Double Oxides Containing Niobium or Tantalum. I. Systems Including Alkali Metals. Acta Crystallogr. 1965, 19, 169–173. [Google Scholar] [CrossRef]
- Ukei, K.; Suzuki, H.; Shishido, T.; Fukuda, T. Li3NbO4. Acta Crystallogr. C 1994, 50, 655–656. [Google Scholar] [CrossRef]
- Bailey, P. An X-ray Diffraction Investigation of the Structures of Anti-Ferroelectric Calcium Titanate and Ferroelectric Lithium Columbate. Ph.D. Thesis, University of Bristol, Bristol, UK, 1952. [Google Scholar]
- Megaw, H.D. Ferroelectricity and Crystal Structure. II. Acta Crystallogr. 1954, 7, 187–194. [Google Scholar] [CrossRef]
- Shiozaki, Y.; Mitsui, T. Powder Neutron Diffraction Study of LiNbO3. J. Phys. Chem. Solids 1963, 24, 1057–1061. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Reddy, J.M.; Bernstein, J.L. Ferroelectric Lithium Niobate. 3. Single Crystal X-ray Diffraction Study at 24 °C. J. Phys. Chem. Solids 1966, 27, 997–1012. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Hamilton, W.C.; Reddy, J.M. Ferroelectric Lithium Niobate. 4. Single Crystal Neutron Diffraction Study at 24 °C. J. Phys. Chem. Solids 1966, 27, 1013–1018. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Levinstein, H.J.; Reddy, J.M. Ferroelectric Lithium Niobate. 5. Polycrystal X-ray Diffraction Study between 24 °C and 1200 °C. J. Phys. Chem. Solids 1966, 27, 1019–1026. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Marsh, P. Defect Structure Dependence on Composition in Lithium Niobate. Acta Crystallogr. B 1986, 42, 61–68. [Google Scholar] [CrossRef]
- Oghaki, M.; Tanaka, K.; Marumo, F. Structure Refinement of lithium (I) niobium (V) trioxide, LiNbO3, with anharmonic thermal vibration model. Miner. J. 1992, 16, 150–160. [Google Scholar]
- Kumada, N.; Kinomura, N.; Muto, F. イルメナイト型 LiNbO3 及び NaNbO3 の結晶構造 [Crystal Structures of Ilmenite Type LiNbO3 and NaNbO3]. J. Ceramic Soc. Jpn. 1990, 98, 384–388. (In Japanese) [Google Scholar] [CrossRef]
- Boysen, H.; Altorfer, F. A Neutron Powder Investigation of the High-Temperature Structure and Phase Transition in LiNbO3. Acta Crystallogr. B 1994, 50, 405–414. [Google Scholar] [CrossRef]
- Hsu, R.; Maslen, E.N.; Du Boulay, D.; Ishizawa, N. Synchrotron X-ray Studies of LiNbO3 and LiTaO3. Acta Crystallogr. B 1997, 53, 420–428. [Google Scholar] [CrossRef]
- Etschmann, B.; Ishikawa, N.; Streltsov, V.; Oishi, S. A Synchrotron X-ray diffraction analysis of near-stoichiometric LiNbO3. Z. Kristallogr. 2001, 216, 455–461. [Google Scholar] [CrossRef]
- Lundberg, M. The Crystal Structure of LiNb3O8. Acta Chem. Scand. 1971, 25, 3337–3346. [Google Scholar] [CrossRef][Green Version]
- Gatehouse, B.M.; Leverett, P. Lithium Triniobate(V), LiNb3O8. Cryst. Struct. Commun. 1972, 1, 83–85. [Google Scholar]
- Scholder, R.; Glaser, H. Über Lithium- und Natriumuranate(V) und über strukturelle Beziehungem zwischen den Verbindungstypen Li7AO6 und Li8AO6 [About lithium and sodium uranates (V) and about structural relationships between the compound types Li7AO6 and Li8AO6]. Z. Anorg. Allg. Chem. 1964, 327, 15–27. (In German) [Google Scholar] [CrossRef]
- Muhle, C.; Dinnebier, R.E.; van Wullen, L.; Schwering, G.; Jansen, M. New Insights into the Structural and Dynamical Features of Lithium Hexaoxometalates Li7MO6 (M = Nb, Ta, Sb, Bi). Inorg. Chem. 2004, 43, 874–881. [Google Scholar] [CrossRef]
- Braun, R.M.; Hoppe, R. Zur Kenntnis von Li16Nb4O18 [On the knowledge of Li16Nb4O18]. Z. Anorg. Allg. Chem. 1982, 493, 7–16. (In German) [Google Scholar] [CrossRef]
- Bouillaud, Y. Polymorphisme de l’orthoniobate de sodium [Polymorphism of sodium orthoniobate]. Bull. Soc. Chim. Fr. 1967, 34, 3879–3880. (In French) [Google Scholar]
- Barker, M.G.; Wood, D.J. Preparation and Characterisation of the Compounds Sodium Tetraoxoniobate(V) Na3NbO4 and Sodium Tetraoxotantalate(V) Na3TaO4. J. Chem. Soc. Dalton Trans. 1972, 9–13. [Google Scholar] [CrossRef]
- Meyer, G.; Hoppe, R. Na3NbO4, das erste Orthioniobat mit Inselstruktur: Na12[Nb4O16] [Na3NbO4, the first orthoniobate with an island structure: Na12[Nb4O16]]. Naturwissenschaften 1974, 61, 501. (In German) [Google Scholar] [CrossRef]
- Barth, T. Die Kristallstruktur von Perowskit und Verwandten Verbindungen [The crystal structure of perovskite and related compounds]. Norsk. Geolog. Tidsskr. 1925, 8, 201–216. [Google Scholar]
- Wood, E.A. Polymorphism in Potassium Niobate, Sodium Niobate, and other ABO3 Compounds. Acta Crystallogr. 1951, 4, 353–362. [Google Scholar] [CrossRef]
- Vousden, P. A Study of the Unit-cell Dimensions and Symmetry of certain Ferroelectric Compounds of Niobium and Tantalum at Room Temperature. Acta Crystallogr. 1951, 4, 373–376. [Google Scholar] [CrossRef]
- Vousden, P. The Structure of Ferroelectric Sodium Niobate at Room Temperature. Acta Crystallogr. 1951, 4, 545–551. [Google Scholar] [CrossRef]
- Pepinsky, R. Remarks on Vousden’s structure of ferroelectric sodium niobate. Acta Crystallogr. 1952, 5, 288. [Google Scholar] [CrossRef]
- Vousden, R. The non-polarity of sodium niobate. Acta Crystallogr. 1952, 5, 690. [Google Scholar] [CrossRef]
- Shirane, G.; Newnham, R.; Pepinsky, R. Dielectric Properties and Phase Transitions of NaNbO3 and (Na,K)NbO3. Phys. Rev. 1954, 96, 581–588. [Google Scholar] [CrossRef]
- Francombe, M.H. High-Temperature Structure Transitions in Sodium Niobate. Acta Crystallogr. 1956, 9, 256–259. [Google Scholar] [CrossRef]
- Solovev, S.P.; Venevtsev, Y.N.; Zhdanov, G.S. An X-ray Study of Phase Transitions in NaNbO3. Sov. Phys. Crystallogr. 1961, 6, 171–175, translated from Kristallographiya, 218–224. [Google Scholar]
- Bouillaud, Y. Métaniobate de sodium non stœchiométrique [Nonstoichiometric sodium metaniobate]. Bull. Soc. Fr. Miner. Crist. 1969, 92, 347–351. (In French) [Google Scholar]
- Sakowski-Cowley, A.C.; Lukaszewicz, K.; Megaw, H.D. The Structure of Sodium Niobate at Room Temperature, and the Problem of Reliability in Pseudosymmetric Structures. Acta Crystallogr. B 1969, 25, 851–865. [Google Scholar] [CrossRef]
- Hewat, A.W. Neutron powder profile refinement of ferroelectric and antiferroelectric crystal structures: Sodium niobate at 22 °C. Ferroelectrics 1974, 7, 83–85. [Google Scholar] [CrossRef]
- Darlington, C.N.; Megaw, H.D. The Low-Temperature Phase Transition of Sodium Niobate and the Structure of the Low-Temperature Phase. N. Acta Crystallogr. B 1973, 29, 2171–2185. [Google Scholar] [CrossRef]
- Seidel, P.; Hoffmann, W. Verfeinerung der Kristallstruktur von NaNbO3 N. Bestimmung der absoluten Konfiguration und des Zwillingsgesetzes [Refinement of the crystal structure of NaNbO3 N. Determination of the absolute configuration and twin’s law]. Z. Kristallogr. 1976, 143, 444–459. (In German) [Google Scholar] [CrossRef]
- Glazer, A.M.; Megaw, H.D. Studies of the Lattice Parameters and Domains in the Phase Transitions of NaNbO3. Acta Crystallogr. A 1973, 29, 489–495. [Google Scholar] [CrossRef]
- Glazer, A.M.; Megaw, H.D. The structure of sodium niobate (T2) at 600 °C, and the cubic-tetragonal transition in relation to soft-phonon modes. Phil. Mag. 1972, 25, 1119–1135. [Google Scholar] [CrossRef]
- Ishida, K.; Honjo, G. Soft modes and Superlattice Structures in NaNbO3. J. Phys. Soc. Jpn. 1973, 34, 1279–1288. [Google Scholar] [CrossRef]
- Darlington, C.N.W.; Knight, K.S. High-temperature phases of NaNbO3 and NaTaO3. Acta Crystallogr. B 1999, 55, 24–30. [Google Scholar] [CrossRef]
- Ahtee, M.; Glazer, A.M.; Megaw, H.D. The structures of sodium niobate between 480 ° and 575 °C, and their relevance to soft-phonon modes. Philos. Mag. 1972, 25, 995–1014. [Google Scholar] [CrossRef]
- Shanker, V.; Samal, S.L.; Pradhan, G.K.; Narayana, C.; Ganguli, A.K. Nanocrystalline NaNbO3 and NaTaO3: Rietveld studies, Raman spectroscopy and dielectric properties. Solid State Sci. 2009, 11, 562–569. [Google Scholar] [CrossRef]
- Johnston, K.E.; Tang, C.C.; Parker, J.E.; Knight, K.S.; Lightfoot, P.; Ashbrook, S.E. The Polar Phase of NaNbO3: A Combined Study by Powder Diffraction, Solid-State NMR, and First-Principles Calculations. J. Am. Chem. Soc. 2010, 132, 8732–8746. [Google Scholar] [CrossRef]
- Darlington, C.N.W.; Knight, K.S. On the lattice parameters of sodium niobate at room temperature and above. Physica B 1999, 266, 368–372. [Google Scholar] [CrossRef]
- Koruza, J.; Tellier, J.; Malic, B.; Bobnar, V.; Kosec, M. Phase transitions of sodium niobate powder and ceramics, prepared by solid state synthesis. J. Appl. Phys. 2010, 108, 113509-1–113509-9. [Google Scholar] [CrossRef]
- Mishra, S.K.; Mittal, R.; Pomjakushin, V.Y.; Chaplot, S.L. Phase stability and structural temperature dependence in sodium niobate: A high-resolution powder neutron diffraction study. Phys. Rev. B 2011, 83, 134105-1–1134105-9. [Google Scholar] [CrossRef]
- Peel, M.D.; Thompson, S.P.; Daoud-Aladine, A.; Ashbrook, S.E.; Lightfoot, S. New Twists on the Perovskite Theme: Crystal structures of the Elusive Phases R and S of NaNbO3. Inorg. Chem. 2012, 51, 6876–6889. [Google Scholar] [CrossRef]
- Darriet, J.; Maazaz, A.; Bouloux, J.C.; Delman, C. Les Phases Na5NbO5 et Na5TaO5: Structure Cristalline de Na5NbO5 [The Phases Na5NbO5 and Na5TaO5: Crystal Structure of Na5NbO5]. Z. Anorg. Allg. Chem. 1982, 485, 115–121. (In French) [Google Scholar] [CrossRef]
- Jahnberg, L. Crystal Structures of Na2Nb4O11 and CaTa4O11. J. Solid State Chem. 1970, 1, 454–462. [Google Scholar] [CrossRef]
- Andersson, S. Phase Analysis Studies on the NaNbO3-Nb2O5, NaF-Nb2O5, and NaNbO3-Nb2O5-H2O Systems. Acta Chem. Scand. 1967, 21, 1777–1782. [Google Scholar] [CrossRef]
- Masó, N.; West, A.R. A new family of ferroelectric materials: Me2Nb4O11 (Me = Na and Ag). J. Mater. Chem. 2010, 20, 2082–2084. [Google Scholar] [CrossRef]
- Bouillaud, Y. Mise en evidence et étude cristallographique d’une solution solide entre les composés NaNb3O8 et Nb2W3O14 [Demonstration and crystallographic study of a solid solution between the compounds NaNb3O8 and Nb2W3O14]. Bull. Soc. Fr. Mineral. Crist. 1968, 91, 289–291. (In French) [Google Scholar]
- Nedjar, R.; Borel, M.M.; Leclaire, A.; Raveau, B. The Sodium Niobate NaNb3O8: A Novel Lamellar Oxide Synthesized by Soft Chemistry. J. Solid State Chem. 1987, 71, 182–188. [Google Scholar] [CrossRef]
- Range, K.-J.; Wildenauer, M.; Heyns, A.M. Extremely short non-Bonding Oxygen-Oxygen Distances: The Crystal Structures of NbBO4, NaNb3O8, and NaTa3O8. Angew. Chem. Int. Ed. 1988, 27, 969–971. [Google Scholar] [CrossRef]
- Andersson, S. The Crystal Structure of NaNb13O33 and the Geometrical Principles of the Homologous Series NaM3n+1O8n+1. Acta Chem. Scand. 1965, 19, 557–563. [Google Scholar] [CrossRef]
- Guerchais, J.-E. Réactions entre l’oxyde de niobium et le carbonate de potassium par voie sèche; hydrolyse des produits obtenus [Reactions between niobium oxide and potassium carbonate by dry method: Hydrolysis of the products obtained]. Bull. Soc. Chim. Fr. 1962, 82, 103–107. [Google Scholar]
- Addison, C.C.; Barker, M.G.; Lintonbon, R.M. Reactions of the Oxides of Niobium with Liquid Potassium. J. Chem. Soc. A 1970, 1465–1468. [Google Scholar] [CrossRef]
- Stecura, S. Recovery and Determination of Crystallographic Modifications of K3TaO4 and K3NbO4. J. Less-Common Met. 1971, 25, 1–10. [Google Scholar] [CrossRef]
- Meyer, G.; Hoppe, R. Zur Kenntnis der Strukturfamilie A3MO4 (A = K, Rb, Cs): Darstellung, kristallographische und magnetische Eigenschaften [Knowledge of the structure family A3MO4 (A = K, Rb, Cs): Representation, crystallographic and magnetic properties]. Rev. Chim. Miner. 1975, 12, 454–465. (In German) [Google Scholar]
- Reisman, A.; Holtzberg, F.; Triebwasser, S.; Berkenblit, M. Preparation of Pure Potassium Metaniobate. J. Am. Chem. Soc. 1956, 78, 719–720. [Google Scholar] [CrossRef]
- Katz, L.; Megaw, H.D. The Structure of Potassium Niobate at Room Temperature: The Solution of a Pseudosymmetric Structure by Fourier Methods. Acta Crystallogr. 1967, 22, 639–648. [Google Scholar] [CrossRef]
- Hewat, A.W. Soft modes and the structure, spontaneous polarization and Curie constants of perovskite ferroelectrics: Tetragonal potassium niobate. J. Phys. C Solid State Phys. 1973, 6, 1074–1084. [Google Scholar] [CrossRef]
- Hewat, A.W. Cubic-tetragonal-orthorhombic-rhombohedral ferroelectric transitions in perovskite potassium niobate: Neutron powder profile refinement of the structures. J. Phys. C Solid State Phys. 1973, 6, 2559–2572. [Google Scholar] [CrossRef]
- Sugimoto, W.; Mimuro, K.; Sugahara, Y.; Kuroda, K. Synthesis and Structural Study of the KNb4O6-Type Compound. J. Ceramic Soc. Jpn. 1999, 107, 318–321. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Lee, J.-H.; Lee, J.; Kim, S.-W.; Kim, M.H.; Park, S.; Chung, H.; Kim, Y.-I.; Kim, W. Synthesis of Monoclinic Potassium Niobate Nanowires That Are Stable at Room Temperature. J. Am. Chem. Soc. 2012, 135, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Gasperin, M.; Le Bihan, M.T. Mecanisme d’hydratation des niobates alcalins lamellaires de formule A3Nb4O17 (A = K, Rb, Cs) [Mechanism of hydration of lamellar alkaline niobates of formula A3Nb4O17 (A = K, Rb, Cs)]. J. Solid State Chem. 1982, 43, 346–353. (In French) [Google Scholar] [CrossRef]
- Becker, P.; Held, P. Crystal structure of potassium niobate, K6Nb10.80O30, a partially filled tetragonal tungsten bronze-type structure. Z. Kristallogr. NCS 2000, 215, 319–320. [Google Scholar] [CrossRef]
- Fallon, G.D.; Gatehouse, B.M.; Guddat, L. Crystal Structures of Some Niobium and Tantalum Oxides. IX. K3Nb7O19: A New Potassium Niobium Oxide Tunnel Structure. J. Solid State Chem. 1986, 61, 181–187. [Google Scholar] [CrossRef]
- Gasperin, M. Structure du Triniobate(V) de Potassium KNb3O8, un Niobate Lamellaire [Structure of Potassium Triniobate(V) KNb3O8, a Lamellar Niobate]. Acta Crystallogr. B 1982, 38, 2024–2026. (In French) [Google Scholar] [CrossRef]
- Kwak, J.; Yun, H.; Chae, H.K. A new potassium niobate, KNb5O13. Acta Crystallogr. E 2005, 61, i132–i134. [Google Scholar] [CrossRef]
- Hu, J.J.; Li, F.H.; Fan, H.F. Crystal structure determination of K2O·7Nb2O5 by combining high-resolution electron microscopy and electron diffraction. Ultramicroscopy 1992, 41, 387–397. [Google Scholar] [CrossRef]
- Serafin, M.; Hoppe, R. Die Koordinationszahl 5 bei RbNbO3: Eine Pyrgomstruktur [The Coordination Number 5 for RbNbO3: A tower structure]. Naturwissenschaften 1979, 66, 50–51. (In German) [Google Scholar] [CrossRef]
- Serafin, M.; Hoppe, R. Zur Kenntnis von RbNbO3—Ein Metaniobat mit Pyrgomstruktur [About RbNbO3—A metaniobate with a tower structure]. J. Less Common Met. 1980, 76, 299–316. (In German) [Google Scholar] [CrossRef]
- Kafalas, J.A. Influence of Madelung Energy and Covalency on the Structure of A+B5+O3 Compounds. In Proceedings of the 5th Materials Research Symposium Sponsored by the Institute for Materials Research, National Bureau of Standards, Gaithersburg, Maryland, 18–21 October 1972; pp. 287–293. [Google Scholar]
- Iyer, P.N.; Smith, A.J. Double Oxides Containing Niobium, Tantalum, or Protactinium. IV. Further Systems Involving Alkali Metals. Acta Crystallogr. B 1971, 27, 731–734. [Google Scholar] [CrossRef]
- Gasperin, M.; Le Bihan, M.T. Un niobate de rubidium d’un type structural nouveau: Rb4Nb6O17·3H2O [A rubidium niobate of a new structural type: Rb4Nb6O17·3H2O]. J. Solid State Chem. 1980, 33, 83–89. (In French) [Google Scholar] [CrossRef]
- Fallon, G.D.; Gatehouse, B.M. Crystal Structures of Some Niobium and Tantalum Oxides. II. The 4Rb2O:11Nb2O5 Phase—A Tunnel Structure. J. Solid. State Chem. 1977, 22, 405–409. [Google Scholar] [CrossRef]
- Dewan, J.C.; Edwards, A.J.; Jones, G.R. Crystal Structures of Octacesium and Octarubidium Docosaniobates. J. Chem. Soc. Dalton Trans. 1978, 968–972. [Google Scholar] [CrossRef]
- Jones, G.R.; Robertson, D.S. The Growth and Properties of Crystalline Rubidium and Cesium Niobates. J. Crystal Growth 1978, 43, 115–119. [Google Scholar] [CrossRef]
- Voronkova, V.I.; Yanovskii, V.K.; Leontyeva, I.N.; Kharitonova, E.P.; Sorokina, N.I.; Simonov, V.I. Growth and Properties of RM3O8 (R = Rb, Cs; M = Nb, Ta) Crystals. Cryst. Rep. 1998, 43, 345–347, translated from Kristallographiya, 379–381. [Google Scholar]
- Minor, D.B.; Roth, R.S.; Parker, H.S.; Brower, W.S. Phase Equilibria and Crystal Chemistry of Rubidium Niobates and Rubidium Tantalates. J. Res. Nat. Bur. Stds. 1977, 82, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, B.M.; Lloyd, D.J.; Miskin, B.K. The Single Crystal X-ray Structure Determination of Some Alkali Metal Molybdates and Niobates. In Proceedings of the 5th Materials Research Symposium Sponsored by the Institute for Materials Research, National Bureau of Standards, Gaithersburg, Maryland, 18–21 October 1972; pp. 15–27. [Google Scholar]
- Meyer, G.; Hoppe, R. Über Oxoniobate(V): Die Kristallstructur von CsNbO3 [On Oxoniobate(V): The Crystal Structure of CsNbO3]. Z. Anorg. Allg.Chem. 1977, 436, 75–86. (In German) [Google Scholar] [CrossRef]
- Gasperin, M. Structure du Niobate de Césium Cs2Nb4O11 [Structure of Cesium Niobate Cs2Nb4O11]. Acta Crystallogr. B 1982, 37, 641–643. (In French) [Google Scholar] [CrossRef]
- Smith, R.W.; Luo, G.; Mei, W.-M. High-temperature crystal structure and electronic properties of cesium niobate Cs2Nb4O11. J. Phys. Chem. Solids 2010, 71, 1357–1361. [Google Scholar] [CrossRef]
- King, B.W.; Schultz, J.; Durbin, E.A.; Duckworth, W.H. Some Properties of Tantala Systems; Report #BMI-1106, Metallurgy and Ceramics (TID-4500, 11th Ed.); Battelle Memorial Institute, U.S. Government: Columbus, OH, USA, 1956; pp. 1–39.
- Reisman, A. Compound Repetition in Oxide Systems. Solid Phases in the System Li2O-Ta2O5 and Na2O-Ta2O5. J. Phys. Chem. 1962, 66, 15–21. [Google Scholar] [CrossRef]
- Reisman, A.; Holtzberg, F.; Berkenblit, M.; Berry, M. Reactions of the Group VB Pentoxides with Alkali Oxides and Carbonates. III. Thermal and X-ray Phase Diagrams of the System K2O or K2CO3 with Ta2O5. J. Am Chem. Soc. 1956, 78, 4514–4520. [Google Scholar] [CrossRef]
- Lagergren, S.; Magneli, A. On the Tantalum—Oxygen System. Acta Chem. Scand. 1952, 6, 444. [Google Scholar] [CrossRef]
- Blasse, G. On the Structure of some Compounds Li3Me5+O4 and some other Mixed Metal Oxides Containing Lithium. Z. Anorg. Allg. Chem. 1964, 331, 44–50. [Google Scholar] [CrossRef]
- Zocchi, M.; Gatti, M.; Santoro, A.; Roth, R.S. Neutron and X-ray Diffraction Study on Polymorphism in Lithium Orthotantalate, Li3TaO4. J. Solid State Chem. 1983, 48, 420–430, Correction, Ibid. 1984, 53, 277–278. [Google Scholar] [CrossRef]
- Du Boulay, D.; Sakaguchi, A.; Suda, K.; Ishizawa, N. Reinvestigation of β-Li3TaO4. Acta Crystallogr. E 2003, 59, i80–i82. [Google Scholar] [CrossRef]
- Lapitskii, A.V.; Simanov, Y.P. METAHИOБAT И METATAHTAЛAT ЛИTИЯ [Lithium Metaniobate and Metatantalate]. Zh. Fiz. Khim. (Russ. J. Phys. Chem.) 1955, 29, 1201. (In Russian) [Google Scholar]
- Abrahams, S.C.; Bernstein, J.L. Ferroelectric Lithium Tantalate-2. Single Crystal X-ray Diffraction Study at 24 °C. J. Phys. Chem. Solids 1967, 28, 1685–1692. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Hamilton, W.C.; Sequeira, A. Ferroelectric Lithium Tantalate-2. Single Crystal Neutron Diffraction Study at 24 °C. J. Phys. Chem. Solids 1967, 28, 1693–1698. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Buehler, E.; Hamilton, W.C.; Laplaca, S.J. Ferroelectric Lithium Tantalate-III. Temperature Dependence of the Structure in the Ferroelectric Phase and the Paraelectric Structure at 940 °K. J. Phys. Chem. Solids 1973, 34, 521–532. [Google Scholar] [CrossRef]
- Roth, R.S.; Parker, H.S.; Brewer, W.S.; Minor, D. Alkali Oxide-Tantalum Oxide and Alkali Oxide-Niobium Oxide Ionic Conductors; NASA Report CR-134599; National Bureau of Standards: Washington, DC, USA, 1974; p. 54.
- Gatehouse, B.M.; Negas, T.; Roth, R.S. The Crystal Structure of M-LiTa3O8 and Its Relationship to the Mineral Wodginite. J. Solid State Chem. 1976, 18, 1–7. [Google Scholar] [CrossRef]
- Santoro, A.; Roth, R.S.; Minor, D. Neutron Powder Diffraction Study of the Intermediate-Temperature Form of Lithium Tantalate. Acta Crystallogr. B 1977, 33, 3945–3947. [Google Scholar] [CrossRef]
- Nord, A.G.; Thomas, J.O. Structural Studies of the Solid Electrolyte High-LiTa3O8. Acta Chem. Scand. A 1978, 32, 539–544. [Google Scholar] [CrossRef]
- Werner, P.-E.; Marinder, B.-O.; Magneli, A. Structural Studies on Li1−xTa3O8−xFx Solid Solutions by Full-profile Refinements of Guinier-Hagg X-ray Film Data. Mat. Res. Bull. 1978, 13, 1371–1378. [Google Scholar] [CrossRef]
- Fallon, G.D.; Gatehouse, B.M.; Roth, R.S.; Roth, S.A. Crystal Structures of Some Niobium and Tantalum Oxides. Part VI. The Structure of H-LiTa3O8. J. Solid State Chem. 1979, 27, 255–259. [Google Scholar] [CrossRef]
- Hodeau, J.L.; Marezio, M.; Santoro, A.; Roth, R.S. Neutron Diffraction Structure Determination of the High-Temperature Form of Lithium Tritantalate, H-LiTa3O8. J. Solid State Chem. 1984, 51, 275–292. [Google Scholar] [CrossRef]
- Kay, H.F.; Miles, J.L. The Structure of Cadmium Titanate and Sodium Tantalate. Acta Crystallogr. 1957, 10, 213–218. [Google Scholar] [CrossRef]
- Ismailzade, I.G. An X-ray Diffraction Study of Phase Transitions in Sodium Tantalate. Sov. Phys. Cryst. 1963, 7, 584–587, Translation of Kristallografiya 1962, 7, 718–723. [Google Scholar]
- Ahtee, M.; Unonius, L. The Structure of NaTaO3 by X-ray Powder Diffraction. Acta Crystallogr. A 1977, 33, 150–154. [Google Scholar] [CrossRef]
- Ahtee, M.; Darlington, C.N.W. Structures of NaTaO3 by Neutron Powder Diffraction. Acta Crystallogr. B 1980, 36, 1007–1014. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Prodjosantoso, A.K.; Howard, C.J. Powder neutron diffraction study of the high temperature phase transitions in NaTaO3. J. Phys. Condens. Matter 1999, 11, 6319–6327. [Google Scholar] [CrossRef]
- Chaminade, J.-P.; Pouchard, M.; Hagenmuller, P. Tantalates et oxyfluorotantalates de sodium [Tantalates and oxyfluorotantalates of sodium]. Rev. Chim. Miner. 1972, 9, 381–402. (In French) [Google Scholar]
- Ercit, T.S.; Hawthorne, F.C.; Cerny, P. The crystal structure of synthetic natrotantite. Bull. Mineral. 1985, 108, 541–549. [Google Scholar] [CrossRef]
- Mattes, R.; Schaper, J. Die Struktur von Na2Ta4O11 [The Structure of Na2Ta4O11]. Rev. Chim. Miner. 1985, 22, 817–820. (In German) [Google Scholar]
- Hickam, C.W., Jr. Corrosion Product of the Tantalum-Interstitial Oxygen-Potassium System at 1800 °F (982 °C). J. Less-Common Met. 1968, 14, 315–322. [Google Scholar] [CrossRef]
- Zhurova, E.A.; Ivanov, Y.; Zavodnik, V.; Tsirelson, V. Electron density and atomic displacements in KTaO3. Acta Crystallogr. B 2000, 56, 594–600. [Google Scholar] [CrossRef]
- Sawaguchi, E.; Kikuchi, A. Crystal Structure and the Dielectric Properties of K2O·2Ta2O5. J. Phys. Soc. Jpn. 1964, 19, 579. [Google Scholar] [CrossRef]
- Awadalla, A.A.; Gatehouse, B.M. Crystal structure of Some Niobium and Tantalum Oxides. IV. The Structure of KTa5O13 and its relationship to the α-PbO2 structure. J. Solid State Chem. 1978, 24, 183–187. [Google Scholar] [CrossRef]
- Hartl, H.; Pickhard, F.; Emmerling, F.; Rohr, C. Rubidium- und Caesium-Verbindungen mit dem Isopolyanion [Ta6O19]8−—Synthesen, Kristallstructuren, thermische und schwingungensspektroskopische Untersuchungen der Oxotantalate A8[Ta6O19]·nH2O (A = Rb, Cs; n = 0, 4, 14) [Rubidium and Caesium Compounds with the Isopolyanion [Ta6O19]8−—Synthesis, Crystal Structures, Thermogravimetric and Vibrational Spectroscopic Analys of the Oxotantalates A8[Ta6O19]·nH2O (A = Rb, Cs; n = 0, 4, 14)]. Z. Anorg. Allg. Chem. 2001, 627, 2630–2638. (In German) [Google Scholar]
- Serafin, M.; Hoppe, R. Coordination Number 5 and 6 in RbTaO3: Rb4∞2[Ta4O12]. Angew. Chem. Int. Ed. 1978, 17, 354–355. [Google Scholar] [CrossRef]
- Du Boulay, D.; Yamashita, R.; Ishizawa, N. Synchrotron X-ray study of orthorhombic Rb3Ta5O14 with a modified pyrochlore structure. Acta Crystallogr. C 2002, 58, i40–i44. [Google Scholar] [CrossRef]
- Serafin, M.; Hoppe, R. Ein Oxotantalat mit Gerüststruktur: Cs3Ta5O14. [A scaffolded oxotantalate: Cs3Ta5O14.]. Z. Anorg. Allg. Chem. 1982, 493, 77–92. (In German) [Google Scholar] [CrossRef]
- Du Boulay, D.; Oono, A.; Ishizawa, N. A reinvestigation of the structure of Cs3Ta5O14. Acta Crystallogr. E 2003, 59, i86–i88. [Google Scholar] [CrossRef]
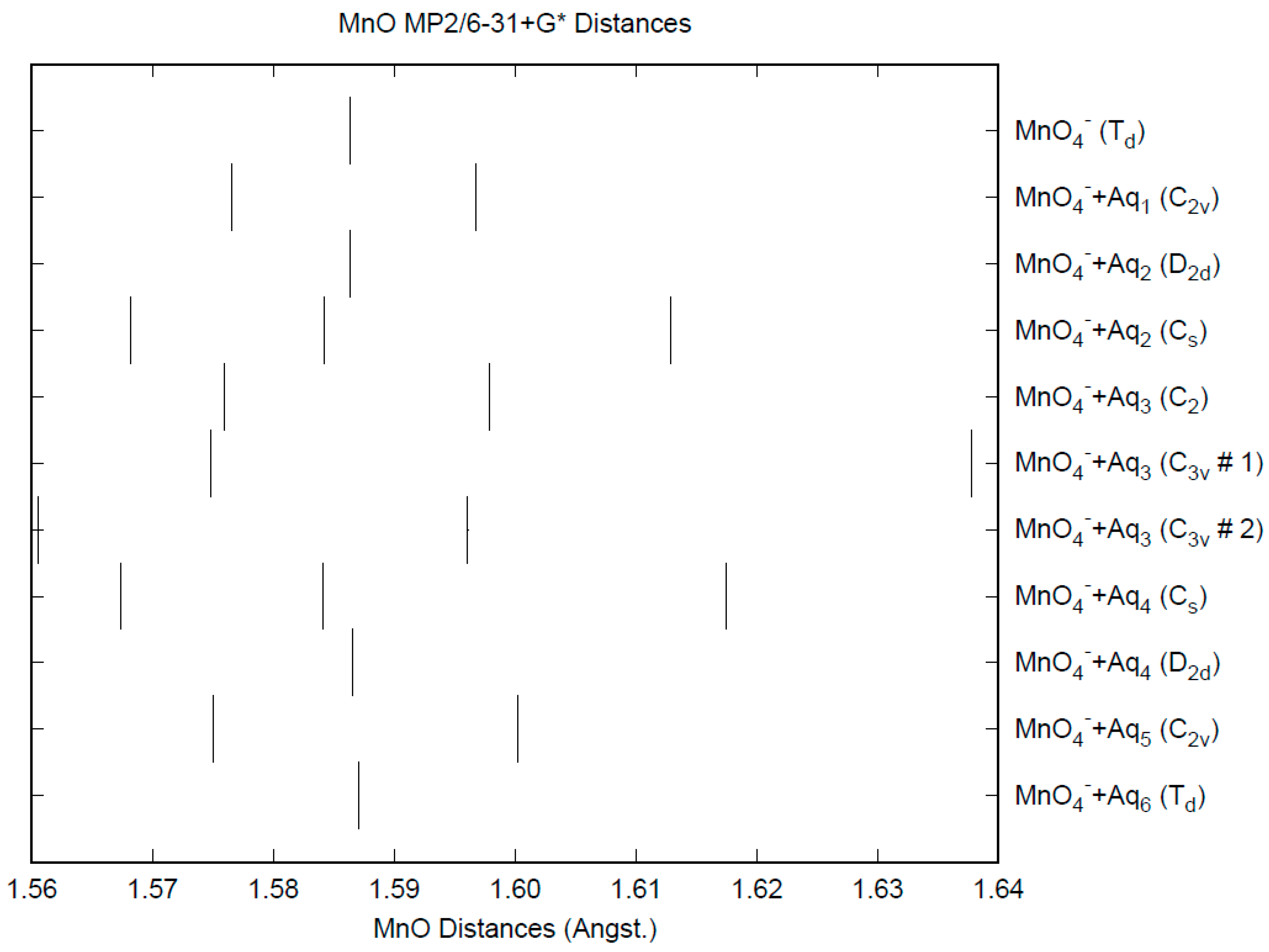
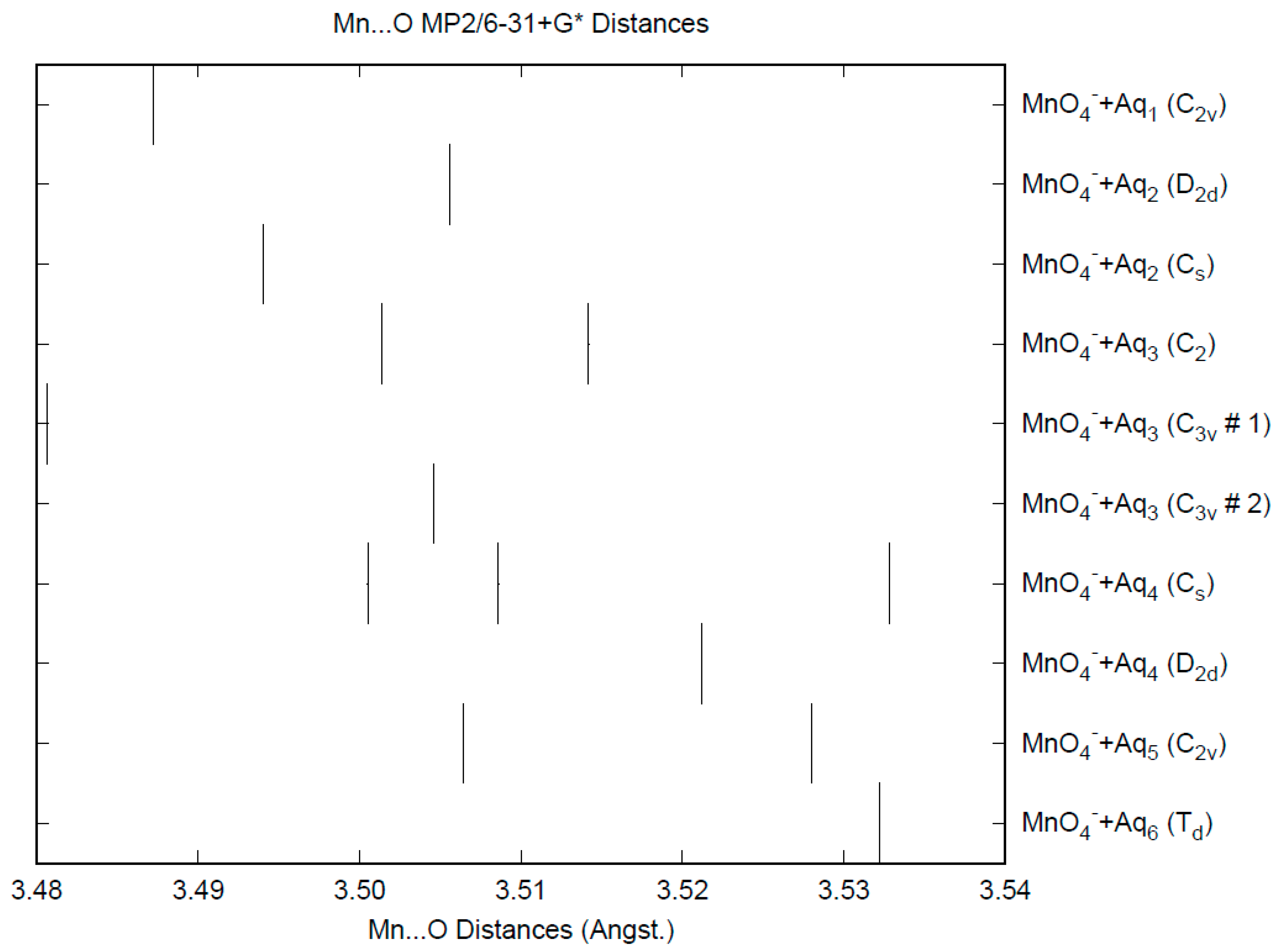
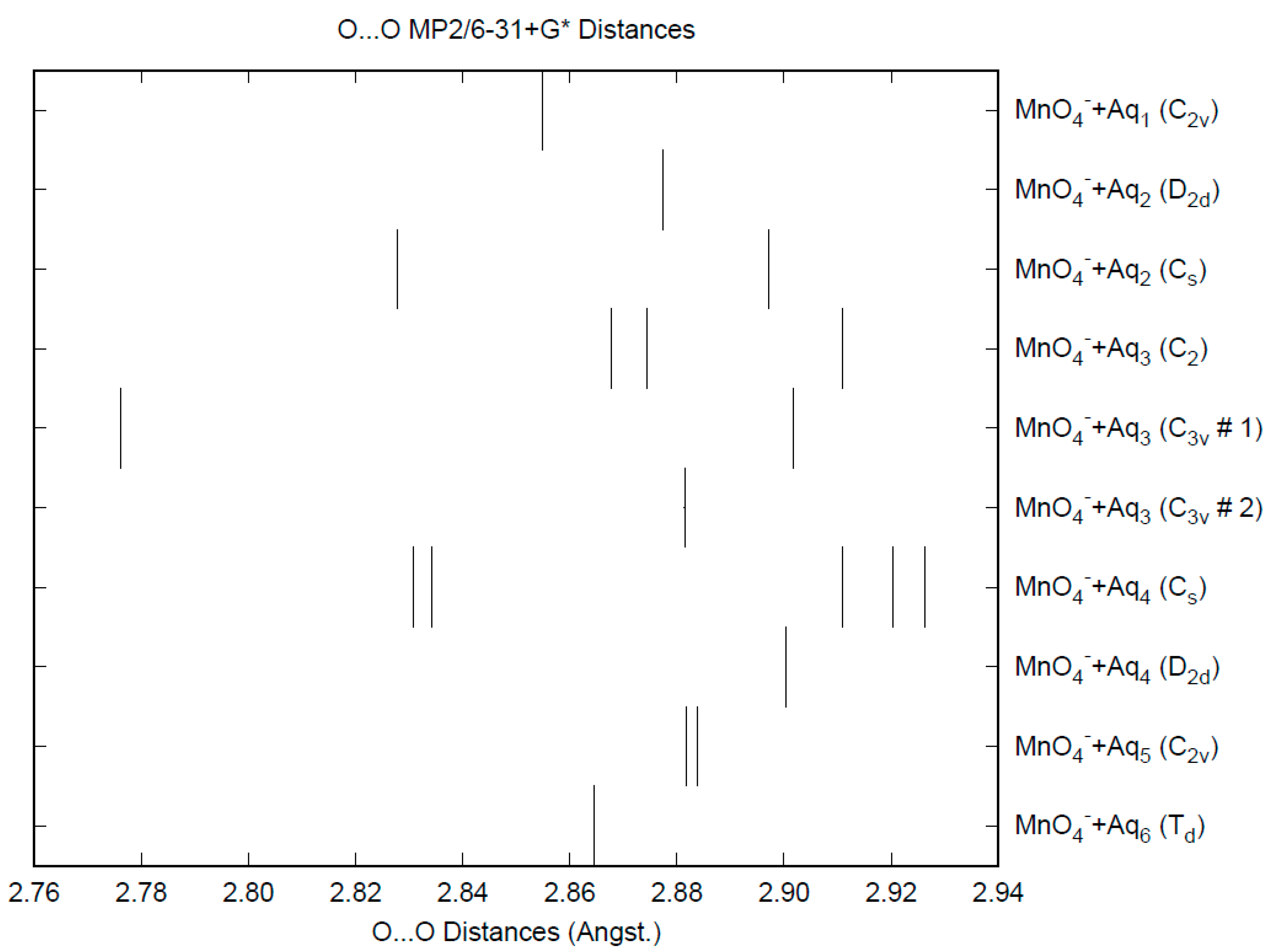

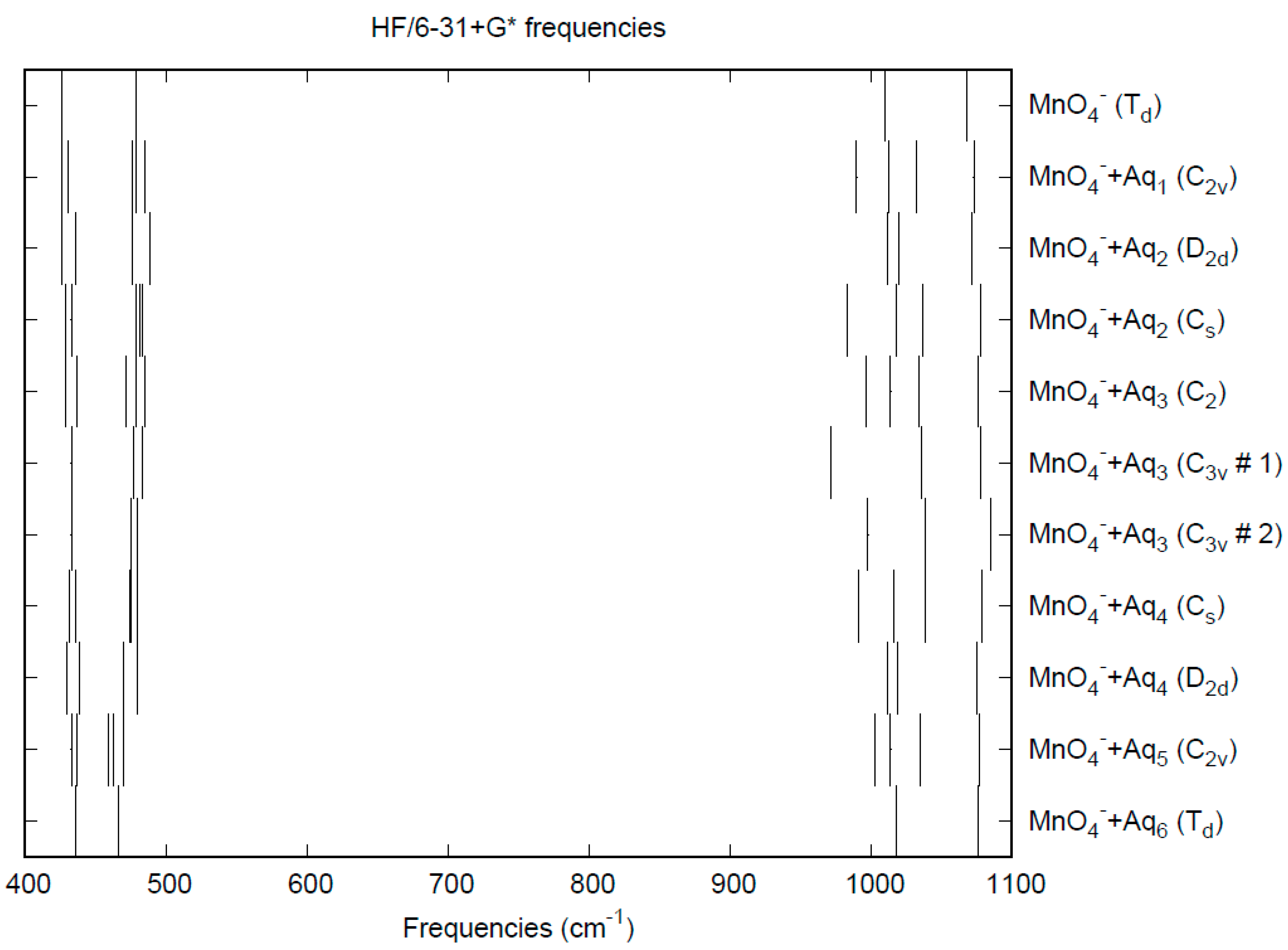
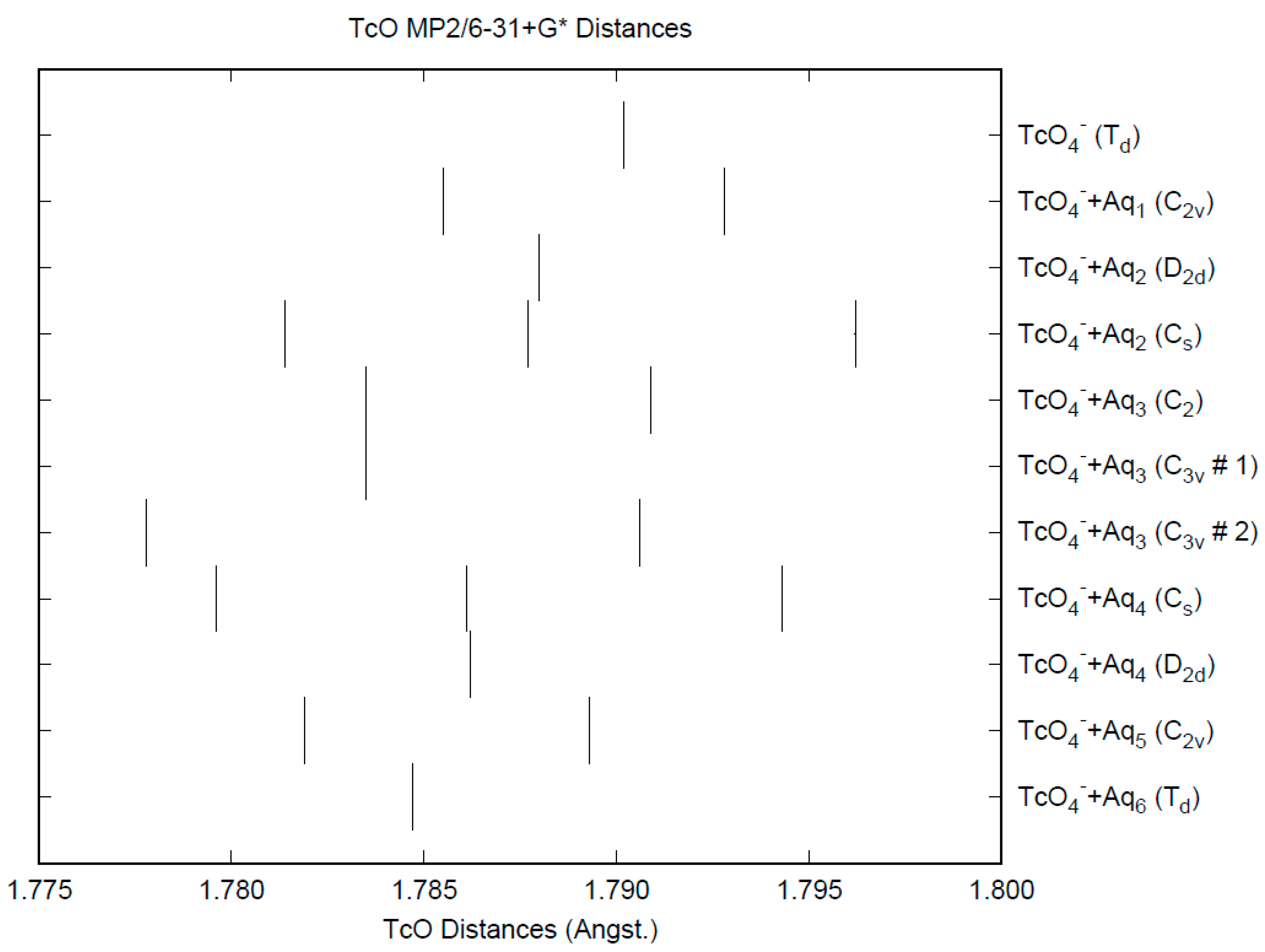
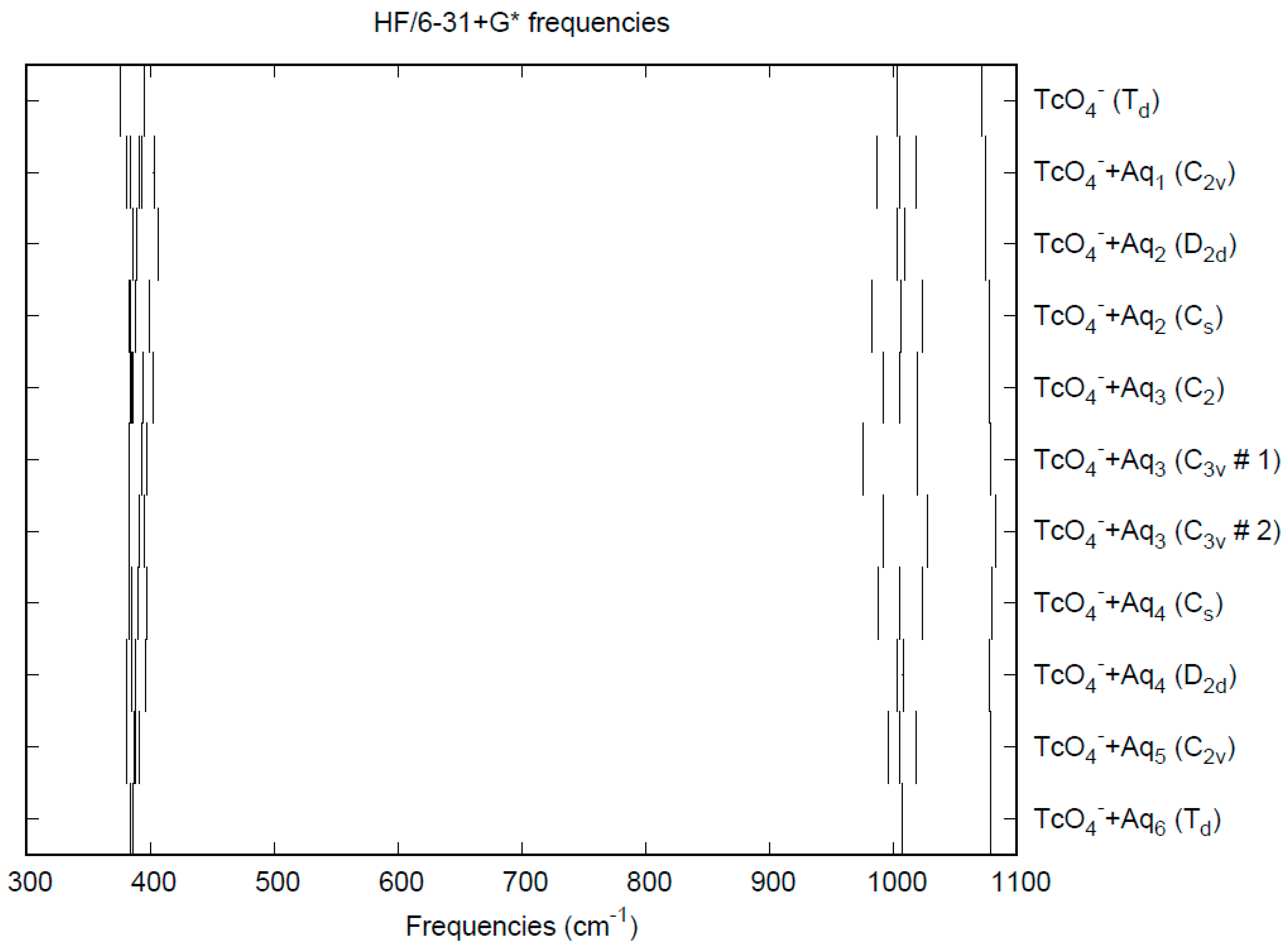
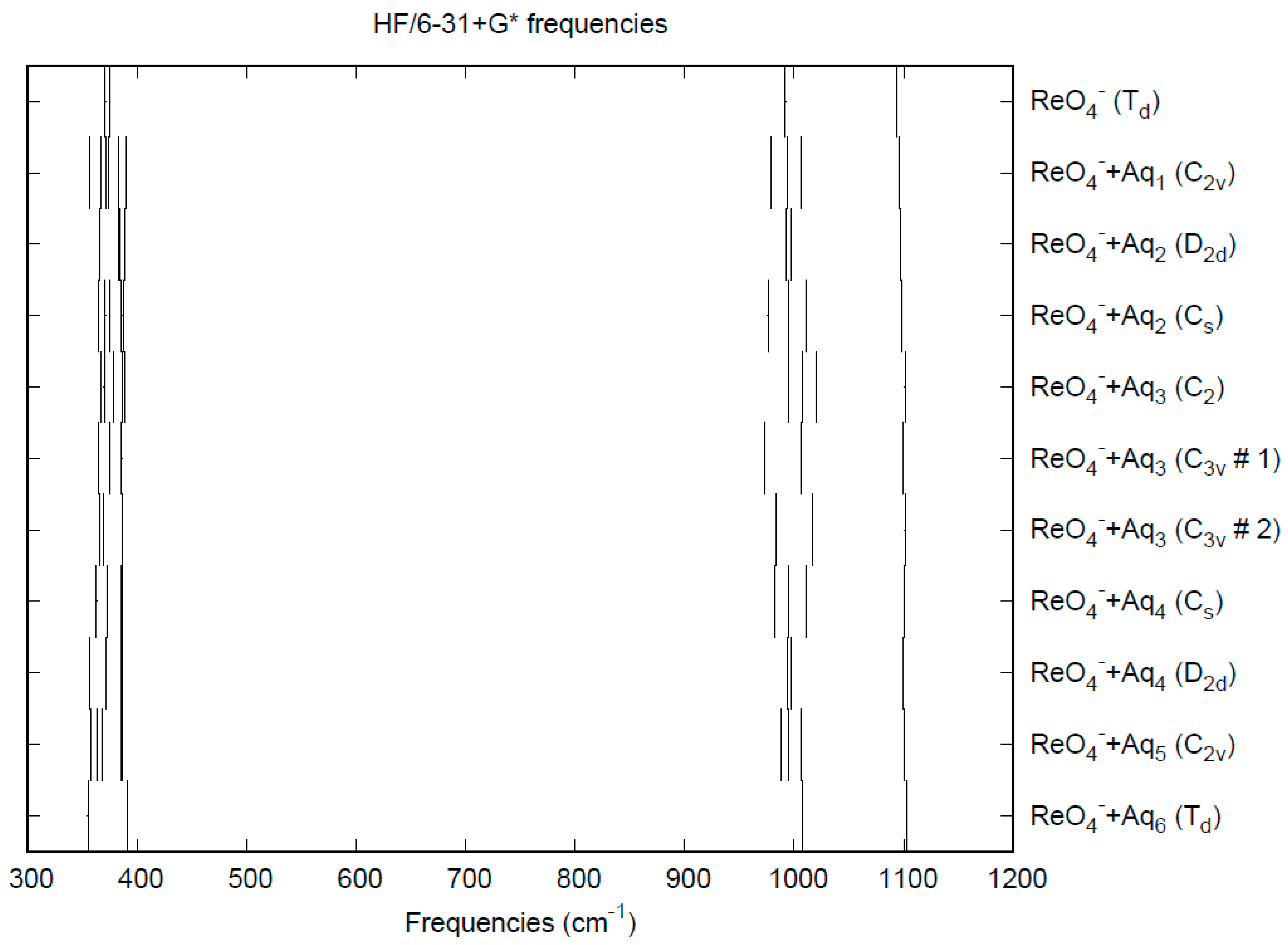

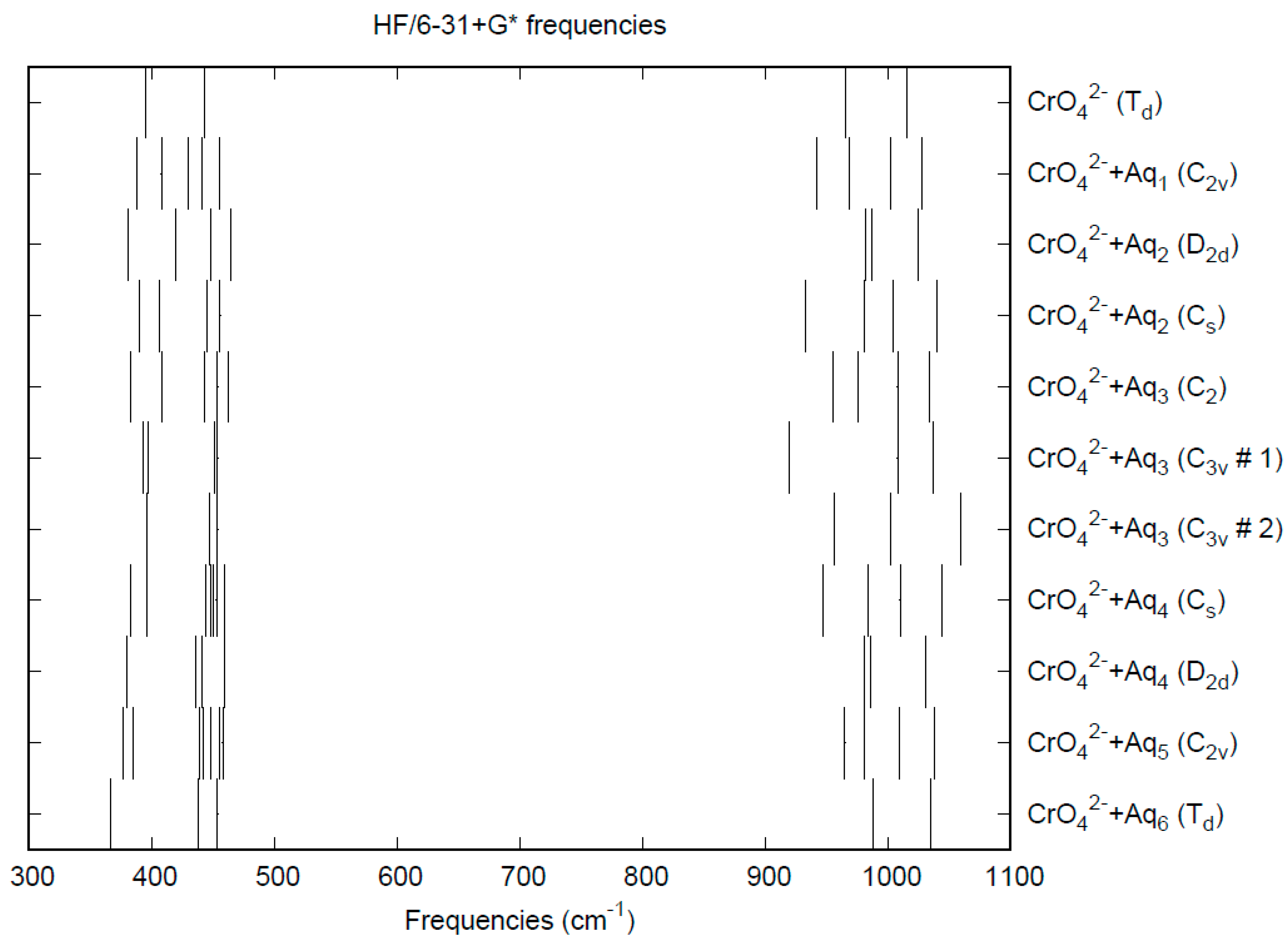
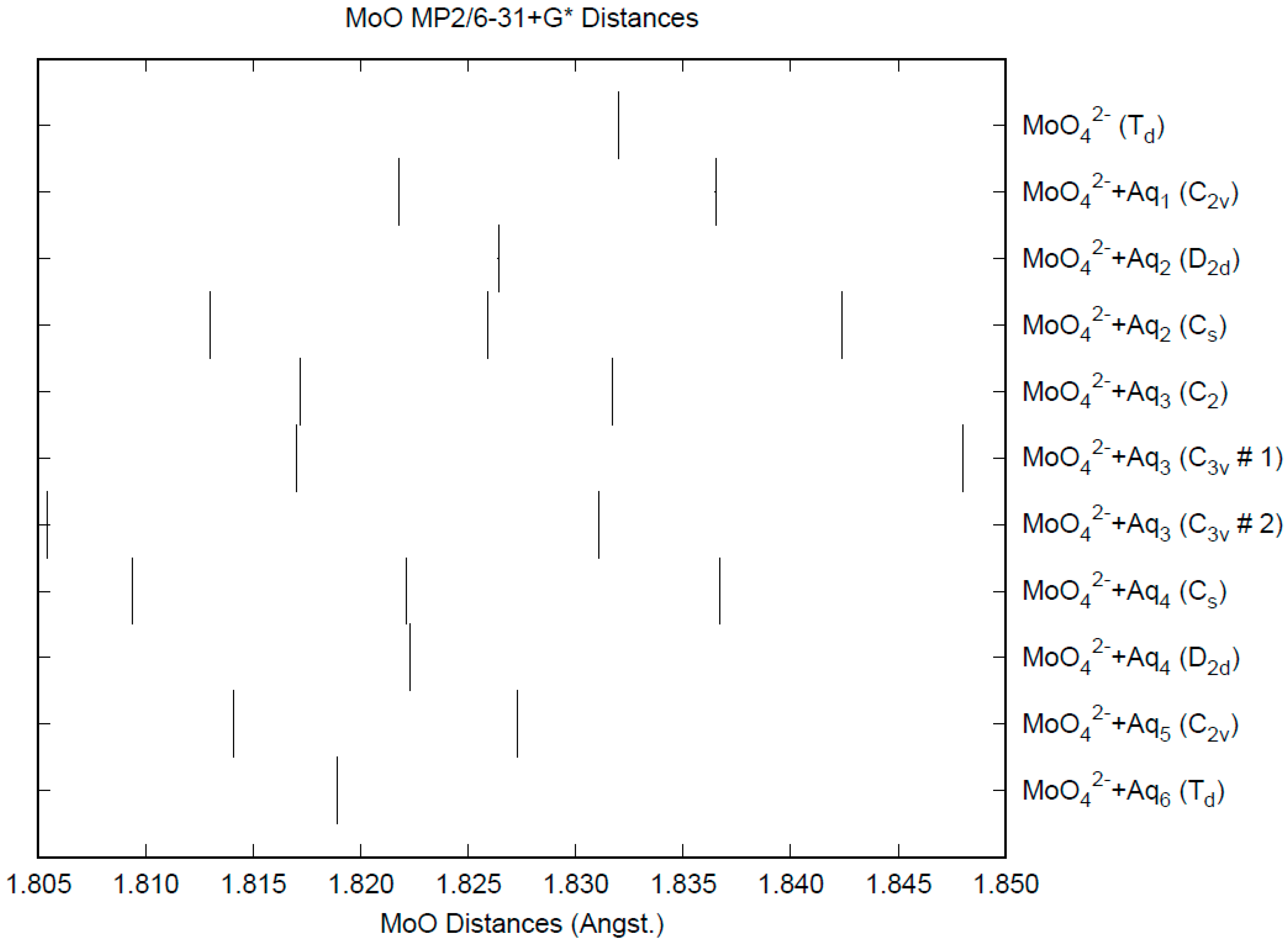
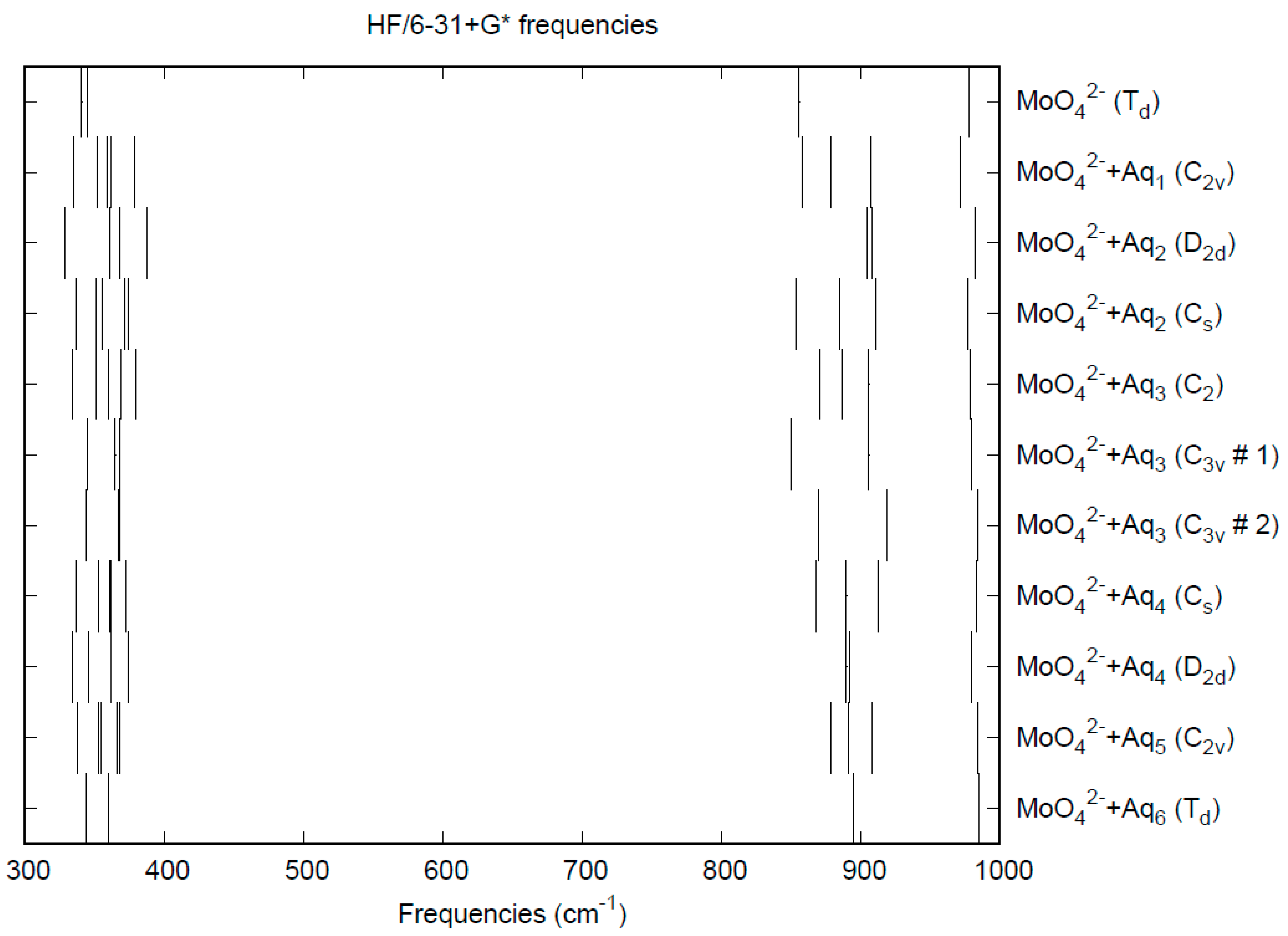

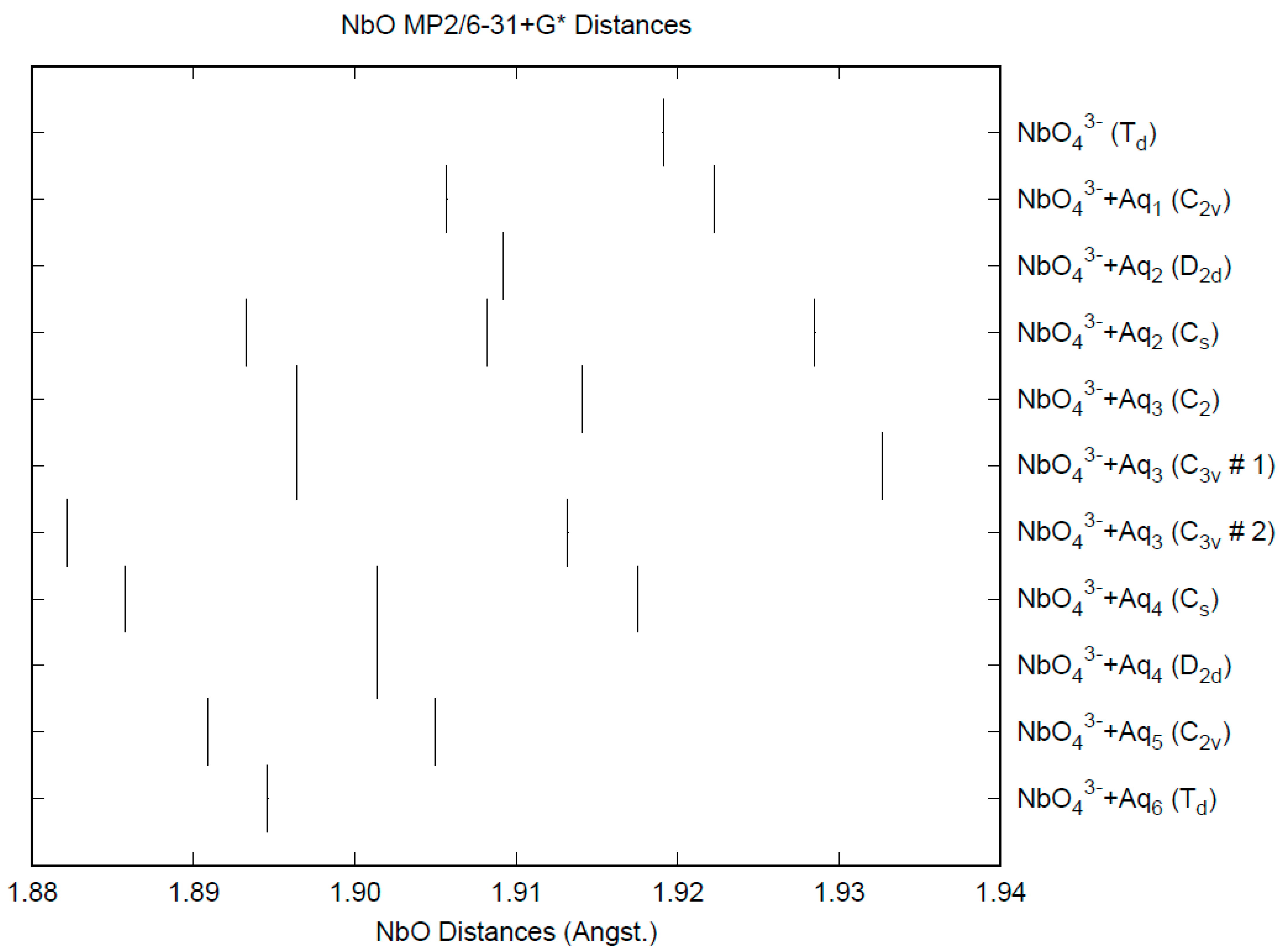
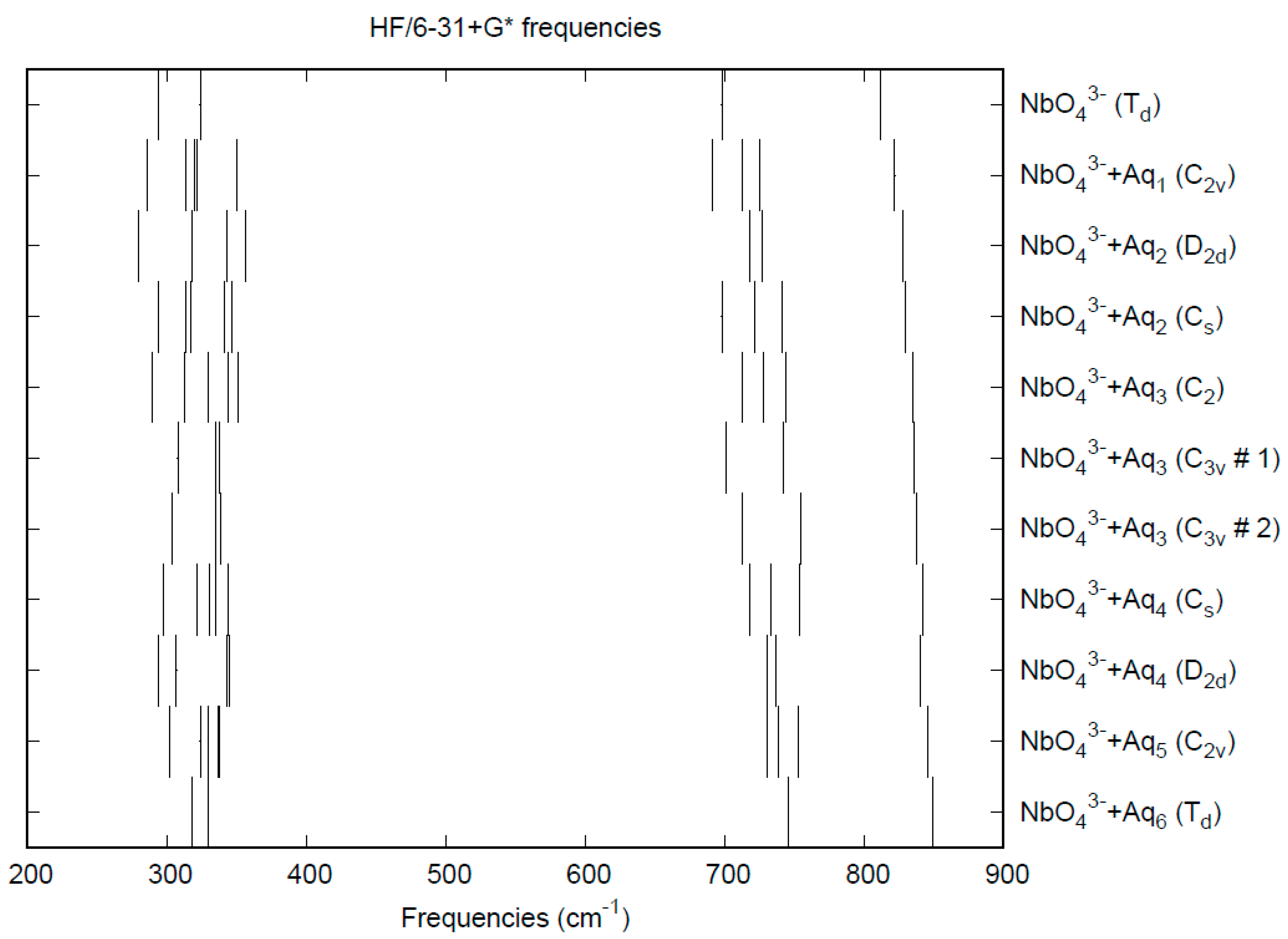
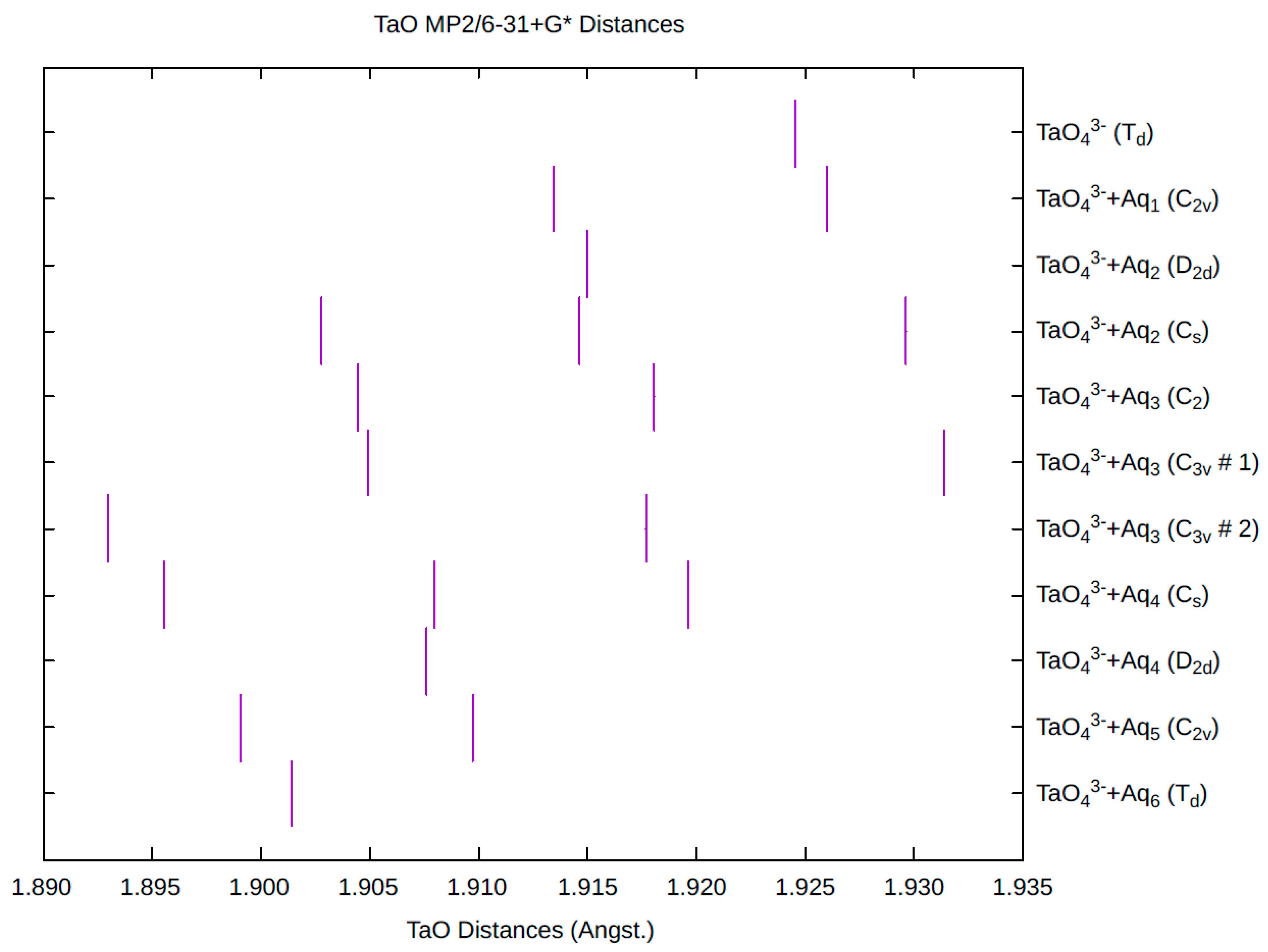
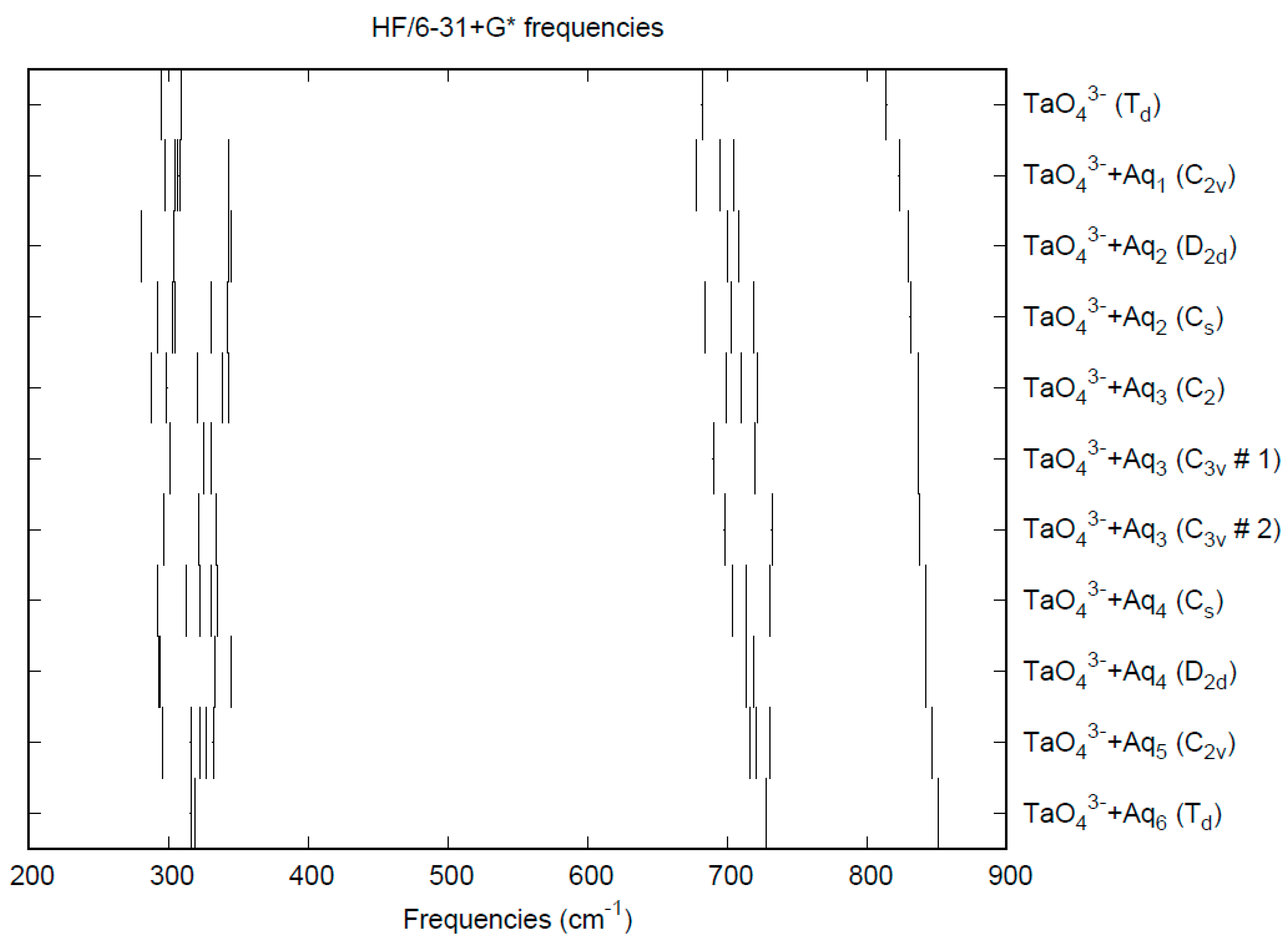
| d(Mn-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.5399 | 1082 | 438 | 1039 | 490 |
| HF/6-31+G(d) | 1.5449 | 1068 | 426 | 1011 | 480 |
| MP2/6-31G(d) | 1.5797 | 1695 | 438 | 1761 | 446 |
| MP2/6-31+G(d) | 1.5864 | 1694 | 438 | 1710 | 447 |
| B3LYP/6-31G(d) | 1.5947 | 957 | 379 | 1018 | 426 |
| B3LYP/6-31+G(d) | 1.6018 | 936 | 368 | 986 | 417 |
| BLYP/6-31G(d) | 1.6232 | 888 | 354 | 959 | 401 |
| BLYP/6-31+G(d) | 1.6315 | 865 | 344 | 927 | 392 |
| PBE/6-31G(d) | 1.6116 | 909 | 360 | 982 | 406 |
| PBE/6-31+G(d) | 1.6194 | 886 | 350 | 949 | 396 |
| CPCM-HF/6-31G(d) | 1.5386 | 1084 | 437 | 1008 | 480 |
| CPCM-HF/6-31+G(d) | 1.5438 | 1070 | 426 | 972 | 469 |
| CPCM-MP2/6-31G(d) | 1.5780 | 1691 | 439 | 1644 | 454 |
| CPCM-MP2/6-31+G(d) | 1.5847 | 1688 | 438 | 1562 | 453 |
| CPCM-B3LYP/6-31G(d) | 1.5927 | 962 | 377 | 1006 | 419 |
| CPCM-B3LYP/6-31+G(d) | 1.5998 | 941 | 366 | 967 | 409 |
| CPCM-BLYP/6-31G(d) | 1.6210 | 890 | 352 | 952 | 392 |
| CPCM-BLYP/6-31+G(d) | 1.6294 | 866 | 342 | 914 | 382 |
| CPCM-PBE/6-31G(d) | 1.6094 | 912 | 358 | 975 | 398 |
| CPCM-PBE/6-31+G(d) | 1.6173 | 889 | 348 | 936 | 389 |
| d(Tc-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.6822 | 1077 | 376 | 1021 | 400 |
| HF/6-31+G(d) | 1.6835 | 1072 | 377 | 1003 | 396 |
| MP2/6-31G(d) | 1.7847 | 824 | 291 | 978 | 304 |
| MP2/6-31+G(d) | 1.7902 | 813 | 295 | 962 | 304 |
| B3LYP/6-31G(d) | 1.7305 | 935 | 322 | 935 | 342 |
| B3LYP/6-31+G(d) | 1.7333 | 927 | 325 | 917 | 340 |
| BLYP/6-31G(d) | 1.7558 | 875 | 304 | 886 | 325 |
| BLYP/6-31+G(d) | 1.7593 | 865 | 308 | 867 | 324 |
| PBE/6-31G(d) | 1.7442 | 895 | 310 | 904 | 329 |
| PBE/6-31+G(d) | 1.7470 | 886 | 313 | 887 | 328 |
| CPCM-HF/6-31G(d) | 1.6831 | 1068 | 370 | 981 | 345 |
| CPCM-HF/6-31+G(d) | 1.6845 | 1062 | 371 | 955 | 339 |
| CPCM-MP2/6-31G(d) | 1.7863 | 833 | 291 | 964 | 289 |
| CPCM-MP2/6-31+G(d) | 1.7929 | 823 | 297 | 941 | 290 |
| CPCM-B3LYP/6-31G(d) | 1.7315 | 912 | 284 | 910 | 297 |
| CPCM-B3LYP/6-31+G(d) | 1.7346 | 900 | 280 | 885 | 290 |
| CPCM-BLYP/6-31G(d) | 1.7575 | 858 | 274 | 869 | 285 |
| CPCM-BLYP/6-31+G(d) | 1.7615 | 846 | 276 | 845 | 281 |
| CPCM-PBE/6-31G(d) | 1.7451 | 877 | 277 | 885 | 288 |
| CPCM-PBE/6-31+G(d) | 1.7484 | 865 | 277 | 863 | 283 |
| d(Re-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.7089 | 1098 | 375 | 1009 | 374 |
| HF/6-31+G(d) | 1.7101 | 1094 | 275 | 993 | 372 |
| MP2/6-31G(d) | 1.7885 | 844 | 304 | 906 | 297 |
| MP2/6-31+G(d) | 1.7927 | 833 | 308 | 887 | 299 |
| B3LYP/6-31G(d) | 1.7511 | 963 | 325 | 922 | 321 |
| B3LYP/6-31+G(d) | 1.7535 | 956 | 327 | 905 | 321 |
| BLYP/6-31G(d) | 1.7740 | 905 | 308 | 875 | 306 |
| BLYP/6-31+G(d) | 1.7770 | 896 | 312 | 858 | 306 |
| PBE/6-31G(d) | 1.7635 | 924 | 314 | 892 | 309 |
| PBE/6-31+G(d) | 1.7659 | 916 | 317 | 876 | 309 |
| CPCM-HF/6-31G(d) | 1.7117 | 1057 | 322 | 964 | 284 |
| CPCM-HF/6-31+G(d) | 1.7132 | 1049 | 314 | 940 | 276 |
| CPCM-MP2/6-31G(d) | 1.7890 | 852 | 299 | 907 | 264 |
| CPCM-MP2/6-31+G(d) | 1.7939 | 841 | 306 | 884 | 268 |
| CPCM-B3LYP/6-31G(d) | 1.7548 | 933 | 277 | 899 | 260 |
| CPCM-B3LYP/6-31+G(d) | 1.7578 | 923 | 277 | 876 | 256 |
| CPCM-BLYP/6-31G(d) | 1.7780 | 889 | 284 | 864 | 257 |
| CPCM-BLYP/6-31+G(d) | 1.7817 | 879 | 288 | 843 | 256 |
| CPCM-PBE/6-31G(d) | 1.7667 | 904 | 282 | 878 | 256 |
| CPCM-PBE/6-31+G(d) | 1.7697 | 894 | 284 | 857 | 254 |
| ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | Type | Ref. |
|---|---|---|---|---|---|
| RuO4 | |||||
| 920 | IR, gas | [71] | |||
| (880) 1 | 914 | IR, CCl4 sol. | [71] | ||
| (879) | 913 | 330 | IR, liquid | [72] | |
| 882 | 323 | 914 | 334 | R, liquid | [73] |
| 883 | 318 | 921 | 332 | R, sat. aq. | [73] |
| 881 | obscured | 916 | obscured | R, CCl4 sol. | [73] |
| OsO4 | |||||
| 965 | 335 | 954 | 335 | R, liquid | [74] |
| 953 | 325 | IR, solid | [74] | ||
| (968) 2 | 960 | IR, gas | [71] | ||
| (967) 3 | 954 | IR, CCl4 sol. | [71] | ||
| 964 | 338 | 953 | 334 | R, liquid | [73] |
| 962 | 333 | 952 | 333 | R, sat. aq. | [73] |
| 965.5 | 333.1 | 960.1 | 322.7 | R, gas | [75] |
| 964.5 | 335.2 | 954 | R, CCl4 sol. | [76] | |
| 960.5 | 329.0 | IR, gas | [76] | ||
| 955.0 | 326.0 | IR, CCl4 sol. | [76] | ||
| d(Fe-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.5199 | 927 | 426 | 513 | 446 |
| HF/6-31+G(d) | 1.5222 | 932 | 419 | 508 | 456 |
| MP2/6-31G(d) | 1.5023 | 2493 | 578 | 3136 | 524 |
| MP2/6-31+G(d) | 1.5069 | 2514 | 575 | 3143 | 528 |
| B3LYP/6-31G(d) | 1.5711 | 968 | 386 | 1038 | 437 |
| B3LYP/6-31+G(d) | 1.5768 | 950 | 377 | 1015 | 439 |
| BLYP/6-31G(d) | 1.6009 | 895 | 361 | 978 | 411 |
| BLYP/6-31+G(d) | 1.6078 | 875 | 352 | 953 | 402 |
| PBE/6-31G(d) | 1.5891 | 917 | 368 | 1003 | 418 |
| PBE/6-31+G(d) | 1.5955 | 898 | 359 | 978 | 409 |
| CPCM-HF/6-31G(d) | 1.5206 | 913 | 413 | 481 | 397 |
| CPCM-HF/6-31+G(d) | 1.5229 | 917 | 408 | 472 | 407 |
| CPCM-MP2/6-31G(d) | 1.5026 | 2510 | 572 | 3032 | 517 |
| CPCM-MP2/6-31+G(d) | 1.5070 | 2531 | 570 | 3012 | 522 |
| CPCM-B3LYP/6-31G(d) | 1.5722 | 966 | 380 | 1023 | 422 |
| CPCM-B3LYP/6-31+G(d) | 1.5776 | 951 | 371 | 999 | 414 |
| CPCM-BLYP/6-31G(d) | 1.6020 | 892 | 347 | 966 | 395 |
| CPCM-BLYP/6-31+G(d) | 1.6087 | 869 | 335 | 939 | 385 |
| CPCM-PBE/6-31G(d) | 1.5901 | 918 | 360 | 992 | 404 |
| CPCM-PBE/6-31+G(d) | 1.5963 | 898 | 349 | 966 | 395 |
| d(Ru-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
| HF/6-31G(d) | 1.6394 | 1116 | 393 | 1060 | 405 |
| HF/6-31+G(d) | 1.6396 | 1115 | 392 | 1052 | 403 |
| MP2/6-31G(d) | 1.7631 | 1080 | 312 | 1683 | 350 |
| MP2/6-31+G(d) | 1.7654 | 1082 | 315 | 1698 | 352 |
| B3LYP/6-31G(d) | 1.6977 | 951 | 333 | 974 | 350 |
| B3LYP/6-31+G(d) | 1.6989 | 947 | 334 | 964 | 348 |
| BLYP/6-31G(d) | 1.7259 | 883 | 313 | 916 | 332 |
| BLYP/6-31+G(d) | 1.7277 | 877 | 314 | 905 | 331 |
| PBE/6-31G(d) | 1.7132 | 907 | 320 | 940 | 338 |
| PBE/6-31+G(d) | 1.7145 | 903 | 321 | 930 | 337 |
| CPCM-HF/6-31G(d) | 1.6422 | 1089 | 348 | 1014 | 334 |
| CPCM-HF/6-31+G(d) | 1.6425 | 1087 | 348 | 1001 | 331 |
| CPCM-MP2/6-31G(d) | 1.7674 | 1085 | 286 | 1660 | 322 |
| CPCM-MP2/6-31+G(d) | 1.7705 | 1090 | 291 | 1675 | 324 |
| CPCM-B3LYP/6-31G(d) | 1.7010 | 935 | 328 | 952 | 295 |
| CPCM-B3LYP/6-31+G(d) | 1.7026 | 926 | 319 | 937 | 289 |
| CPCM-BLYP/6-31G(d) | 1.7308 | 842 | 227 | 891 | 266 |
| CPCM-BLYP/6-31+G(d) | 1.7333 | 833 | 224 | 875 | 262 |
| CPCM-PBE/6-31G(d) | 1.7168 | 873 | 257 | 916 | 274 |
| CPCM-PBE/6-31+G(d) | 1.7186 | 865 | 251 | 902 | 270 |
| d(Os-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
| HF/6-31G(d) | 1.6672 | 1152 | 391 | 1075 | 383 |
| HF/6-31+G(d) | 1.6668 | 1154 | 390 | 1070 | 382 |
| MP2/6-31G(d) | 1.7863 | 861 | 310 | 1126 | 311 |
| MP2/6-31+G(d) | 1.7867 | 859 | 314 | 1124 | 314 |
| B3LYP/6-31G(d) | 1.7192 | 991 | 335 | 975 | 330 |
| B3LYP/6-31+G(d) | 1.7197 | 988 | 335 | 966 | 329 |
| BLYP/6-31G(d) | 1.7449 | 925 | 317 | 921 | 313 |
| BLYP/6-31+G(d) | 1.7459 | 920 | 317 | 910 | 313 |
| PBE/6-31G(d) | 1.7334 | 947 | 324 | 942 | 319 |
| PBE/6-31+G(d) | 1.7340 | 944 | 324 | 933 | 318 |
| CPCM-HF/6-31G(d) | 1.6715 | 1131 | 359 | 1039 | 302 |
| CPCM-HF/6-31+G(d) | 1.6712 | 1132 | 358 | 1029 | 299 |
| CPCM-MP2/6-31G(d) | 1.7873 | 870 | 295 | 1120 | 320 |
| CPCM-MP2/6-31+G(d) | 1.7885 | 867 | 299 | 1117 | 324 |
| CPCM-B3LYP/6-31G(d) | 1.7228 | 965 | 282 | 957 | 280 |
| CPCM-B3LYP/6-31+G(d) | 1.7238 | 959 | 278 | 943 | 277 |
| CPCM-BLYP/6-31G(d) | 1.7494 | 896 | 264 | 903 | 292 |
| CPCM-BLYP/6-31+G(d) | 1.7511 | 889 | 264 | 888 | 292 |
| CPCM-PBE/6-31G(d) | 1.7370 | 920 | 269 | 925 | 282 |
| CPCM-PBE/6-31+G(d) | 1.7380 | 914 | 267 | 913 | 281 |
| d(Cr-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.5968 | 1043 | 410 | 1010 | 455 |
| HF/6-31+G(d) | 1.6056 | 1017 | 395 | 967 | 443 |
| MP2/6-31G(d) | 1.6868 | 1029 | 327 | 1100 | 377 |
| MP2/6-31+G(d) | 1.6996 | 998 | 319 | 1018 | 369 |
| B3LYP/6-31G(d) | 1.6479 | 897 | 348 | 925 | 391 |
| B3LYP/6-31+G(d) | 1.6572 | 872 | 338 | 887 | 384 |
| BLYP/6-31G(d) | 1.6756 | 830 | 323 | 865 | 364 |
| BLYP/6-31+G(d) | 1.6859 | 804 | 316 | 832 | 361 |
| PBE/6-31G(d) | 1.6642 | 849 | 328 | 885 | 368 |
| PBE/6-31+G(d) | 1.6737 | 823 | 321 | 851 | 364 |
| CPCM-HF/6-31G(d) | 1.5896 | 1065 | 410 | 993 | 452 |
| CPCM-HF/6-31+G(d) | 1.5984 | 1035 | 391 | 934 | 434 |
| CPCM-MP2/6-31G(d) | 1.6802 | 1023 | 322 | 1028 | 380 |
| CPCM-MP2/6-31+G(d) | 1.6920 | 993 | 319 | 916 | 375 |
| CPCM-B3LYP/6-31G(d) | 1.6397 | 902 | 334 | 924 | 378 |
| CPCM-B3LYP/6-31+G(d) | 1.6488 | 881 | 328 | 873 | 372 |
| CPCM-BLYP/6-31G(d) | 1.6672 | 842 | 317 | 874 | 359 |
| CPCM-BLYP/6-31+G(d) | 1.6769 | 822 | 316 | 828 | 356 |
| CPCM-PBE/6-31G(d) | 1.6558 | 856 | 315 | 892 | 358 |
| CPCM-PBE/6-31+G(d) | 1.6650 | 837 | 315 | 845 | 357 |
| d(Mo-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.7569 | 977 | 341 | 901 | 370 |
| HF/6-31+G(d) | 1.7601 | 966 | 342 | 874 | 365 |
| MP2/6-31G(d) | 1.8241 | 784 | 280 | 803 | 295 |
| MP2/6-31+G(d) | 1.8320 | 766 | 286 | 775 | 296 |
| B3LYP/6-31G(d) | 1.7951 | 863 | 291 | 825 | 314 |
| B3LYP/6-31+G(d) | 1.8005 | 849 | 298 | 801 | 314 |
| BLYP/6-31G(d) | 1.8182 | 812 | 276 | 782 | 298 |
| BLYP/6-31+G(d) | 1.8248 | 797 | 285 | 760 | 300 |
| PBE/6-31G(d) | 1.8070 | 828 | 280 | 798 | 301 |
| PBE/6-31+G(d) | 1.8126 | 814 | 288 | 776 | 302 |
| CPCM-HF/6-31G(d) | 1.7534 | 968 | 315 | 876 | 318 |
| CPCM-HF/6-31+G(d) | 1.7569 | 953 | 313 | 838 | 310 |
| CPCM-MP2/6-31G(d) | 1.8188 | 810 | 291 | 808 | 297 |
| CPCM-MP2/6-31+G(d) | 1.8271 | 787 | 295 | 764 | 291 |
| CPCM-B3LYP/6-31G(d) | 1.7898 | 881 | 298 | 829 | 309 |
| CPCM-B3LYP/6-31+G(d) | 1.7950 | 867 | 303 | 795 | 308 |
| CPCM-BLYP/6-31G(d) | 1.8117 | 835 | 286 | 794 | 297 |
| CPCM-BLYP/6-31+G(d) | 1.8178 | 819 | 292 | 762 | 295 |
| CPCM-PBE/6-31G(d) | 1.8007 | 850 | 289 | 807 | 299 |
| CPCM-PBE/6-31+G(d) | 1.8059 | 836 | 295 | 776 | 298 |
| d(W-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.7781 | 989 | 341 | 881 | 349 |
| HF/6-31+G(d) | 1.7806 | 979 | 341 | 857 | 345 |
| MP2/6-31G(d) | 1.8305 | 828 | 293 | 791 | 289 |
| MP2/6-31+G(d) | 1.8349 | 815 | 298 | 769 | 290 |
| B3LYP/6-31G(d) | 1.8107 | 883 | 297 | 807 | 298 |
| B3LYP/6-31+G(d) | 1.8150 | 870 | 301 | 786 | 297 |
| BLYP/6-31G(d) | 1.8315 | 835 | 282 | 768 | 283 |
| BLYP/6-31+G(d) | 1.8367 | 821 | 288 | 748 | 284 |
| PBE/6-31G(d) | 1.8212 | 849 | 287 | 782 | 284 |
| PBE/6-31+G(d) | 1.8255 | 837 | 292 | 763 | 286 |
| CPCM-HF/6-31G(d) | 1.7762 | 985 | 324 | 868 | 315 |
| CPCM-HF/6-31+G(d) | 1.7792 | 974 | 323 | 834 | 313 |
| CPCM-MP2/6-31G(d) | 1.8221 | 859 | 305 | 805 | 288 |
| CPCM-MP2/6-31+G(d) | 1.8267 | 843 | 309 | 771 | 287 |
| CPCM-B3LYP/6-31G(d) | 1.8060 | 903 | 306 | 816 | 296 |
| CPCM-B3LYP/6-31+G(d) | 1.8105 | 889 | 308 | 785 | 294 |
| CPCM-BLYP/6-31G(d) | 1.8257 | 854 | 290 | 779 | 274 |
| CPCM-BLYP/6-31+G(d) | 1.8309 | 838 | 293 | 749 | 271 |
| CPCM-PBE/6-31G(d) | 1.8156 | 870 | 295 | 793 | 281 |
| CPCM-PBE/6-31+G(d) | 1.8199 | 856 | 298 | 765 | 278 |
| d(Nb-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.8669 | 832 | 289 | 726 | 328 |
| HF/6-31+G(d) | 1.8754 | 812 | 294 | 699 | 325 |
| MP2/6-31G(d) | 1.9054 | 716 | 237 | 614 | 257 |
| MP2/6-31+G(d) | 1.9191 | 694 | 253 | 610 | 266 |
| B3LYP/6-31G(d) | 1.8796 | 736 | 248 | 642 | 278 |
| B3LYP/6-31+G(d) | 1.8966 | 699 | 256 | 624 | 278 |
| BLYP/6-31G(d) | 1.8956 | 739 | 246–257 | 556–605 | 208–237 |
| BLYP/6-31+G(d) | 1.9044 | 719 | 235–252 | 568–573 | 223–229 |
| PBE/6-31G(d) | 1.8851 | 752 | 212–235 | 593–602 | 184 |
| PBE/6-31+G(d) | 1.8929 | 730 | 241–262 | 547–592 | 217–240 |
| CPCM-HF/6-31G(d) | 1.8490 | 859 | 285 | 757 | 292 |
| CPCM-HF/6-31+G(d) | 1.8570 | 834 | 287 | 705 | 284 |
| CPCM-MP2/6-31G(d) | 1.8911 | 764 | 258 | 694 | 266 |
| CPCM-MP2/6-31+G(d) | 1.9030 | 735 | 263 | 641 | 258 |
| CPCM-B3LYP/6-31G(d) | 1.8736 | 786 | 254 | 708 | 262 |
| CPCM-B3LYP/6-31+G(d) | 1.8830 | 766 | 265 | 667 | 261 |
| CPCM-BLYP/6-31G(d) | 1.8934 | 752 | 244 | 678 | 253 |
| CPCM-BLYP/6-31+G(d) | 1.9035 | 729 | 250 | 641 | 253 |
| CPCM-PBE/6-31G(d) | 1.8830 | 760 | 245 | 687 | 253 |
| CPCM-PBE/6-31+G(d) | 1.8916 | 742 | 257 | 652 | 253 |
| d(Ta-O) | ν1 (A1) | ν2 (E) | ν3 (T2) | ν4 (T2) | |
|---|---|---|---|---|---|
| HF/6-31G(d) | 1.8807 | 835 | 292 | 709 | 313 |
| HF/6-31+G(d) | 1.8894 | 814 | 296 | 682 | 309 |
| MP2/6-31G(d) | 1.9118 | 737 | 245 | 611 | 250 |
| MP2/6-31+G(d) | 1.9245 | 714 | 258 | 604 | 257 |
| B3LYP/6-31G(d) | 1.8870 | 784 | 208 | 575–612 | 235–244 |
| B3LYP/6-31+G(d) | 1.8960 | 766 | 218–220 | 551–589 | 243–252 |
| BLYP/6-31G(d) | 1.9030 | 753 | 209 | 597–599 | 220–232 |
| BLYP/6-31+G(d) | 1.9117 | 730 | 258 | 548–583 | 213–229 |
| PBE/6-31G(d) | 1.8933 | 762 | 208 | 604–607 | 221–233 |
| PBE/6-31+G(d) | 1.9009 | 741 | 259–261 | 559–593 | 212–228 |
| CPCM-HF/6-31G(d) | 1.8634 | 858 | 288 | 744 | 2872 |
| CPCM-HF/6-31+G(d) | 1.8721 | 833 | 288 | 698 | 264 |
| CPCM-MP2/6-31G(d) | 1.8907 | 789 | 266 | 697 | 252 |
| CPCM-MP2/6-31+G(d) | 1.9012 | 762 | 269 | 650 | 247 |
| CPCM-B3LYP/6-31G(d) | 1.8831 | 798 | 261 | 696 | 248 |
| CPCM-B3LYP/6-31+G(d) | 1.8925 | 775 | 269 | 659 | 246 |
| CPCM-BLYP/6-31G(d) | 1.9009 | 765 | 248 | 667 | 236 |
| CPCM-BLYP/6-31+G(d) | 1.9109 | 740 | 255 | 632 | 234 |
| CPCM-PBE/6-31G(d) | 1.8912 | 773 | 252 | 675 | 238 |
| CPCM-PBE/6-31+G(d) | 1.8998 | 752 | 261 | 642 | 237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodall, B.L.; Ferguson, J.P.; Pye, C.C. Ab Initio Investigation of the Hydration of the Tetrahedral d0 Transition Metal Oxoanions NbO43−, TaO43−, CrO42−, MoO42−, WO42−, MnO4−, TcO4−, ReO4−, and of FeO4, RuO4, and OsO4. Liquids 2024, 4, 539-580. https://doi.org/10.3390/liquids4030031
Goodall BL, Ferguson JP, Pye CC. Ab Initio Investigation of the Hydration of the Tetrahedral d0 Transition Metal Oxoanions NbO43−, TaO43−, CrO42−, MoO42−, WO42−, MnO4−, TcO4−, ReO4−, and of FeO4, RuO4, and OsO4. Liquids. 2024; 4(3):539-580. https://doi.org/10.3390/liquids4030031
Chicago/Turabian StyleGoodall, Barbara L., Jane P. Ferguson, and Cory C. Pye. 2024. "Ab Initio Investigation of the Hydration of the Tetrahedral d0 Transition Metal Oxoanions NbO43−, TaO43−, CrO42−, MoO42−, WO42−, MnO4−, TcO4−, ReO4−, and of FeO4, RuO4, and OsO4" Liquids 4, no. 3: 539-580. https://doi.org/10.3390/liquids4030031
APA StyleGoodall, B. L., Ferguson, J. P., & Pye, C. C. (2024). Ab Initio Investigation of the Hydration of the Tetrahedral d0 Transition Metal Oxoanions NbO43−, TaO43−, CrO42−, MoO42−, WO42−, MnO4−, TcO4−, ReO4−, and of FeO4, RuO4, and OsO4. Liquids, 4(3), 539-580. https://doi.org/10.3390/liquids4030031







