Use of DFT Calculations as a Tool for Designing New Solvatochromic Probes for Biological Applications
Abstract
1. Introduction
1.1. Desirable Properties in a Solvatochromic Probe
1.2. The Problem of Design
2. Methods
2.1. DFT Calculations
2.2. Determination of ET30 and Lippert–Mataga Slopes
3. Results
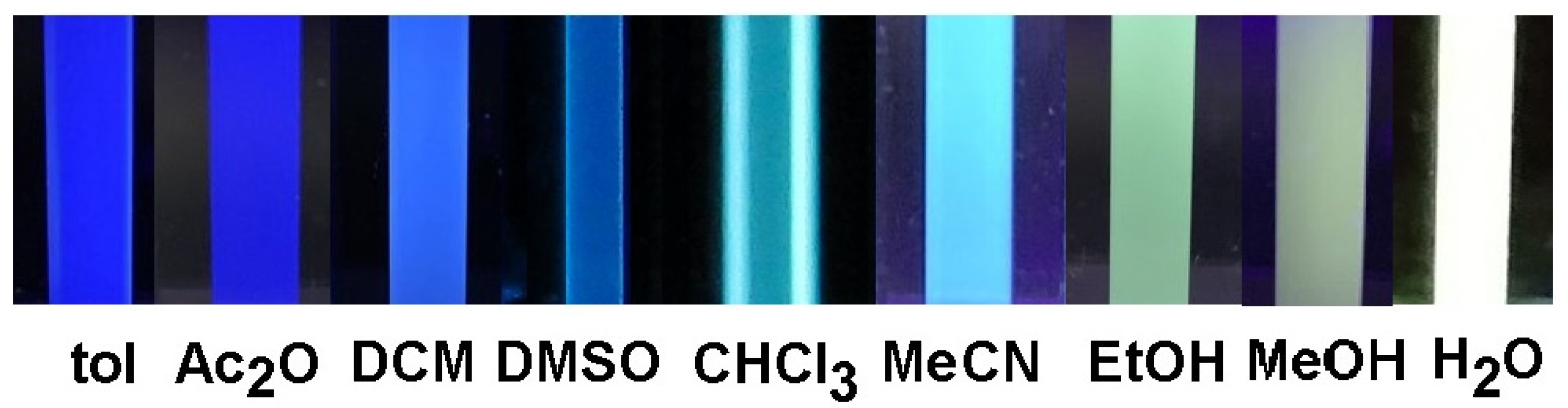
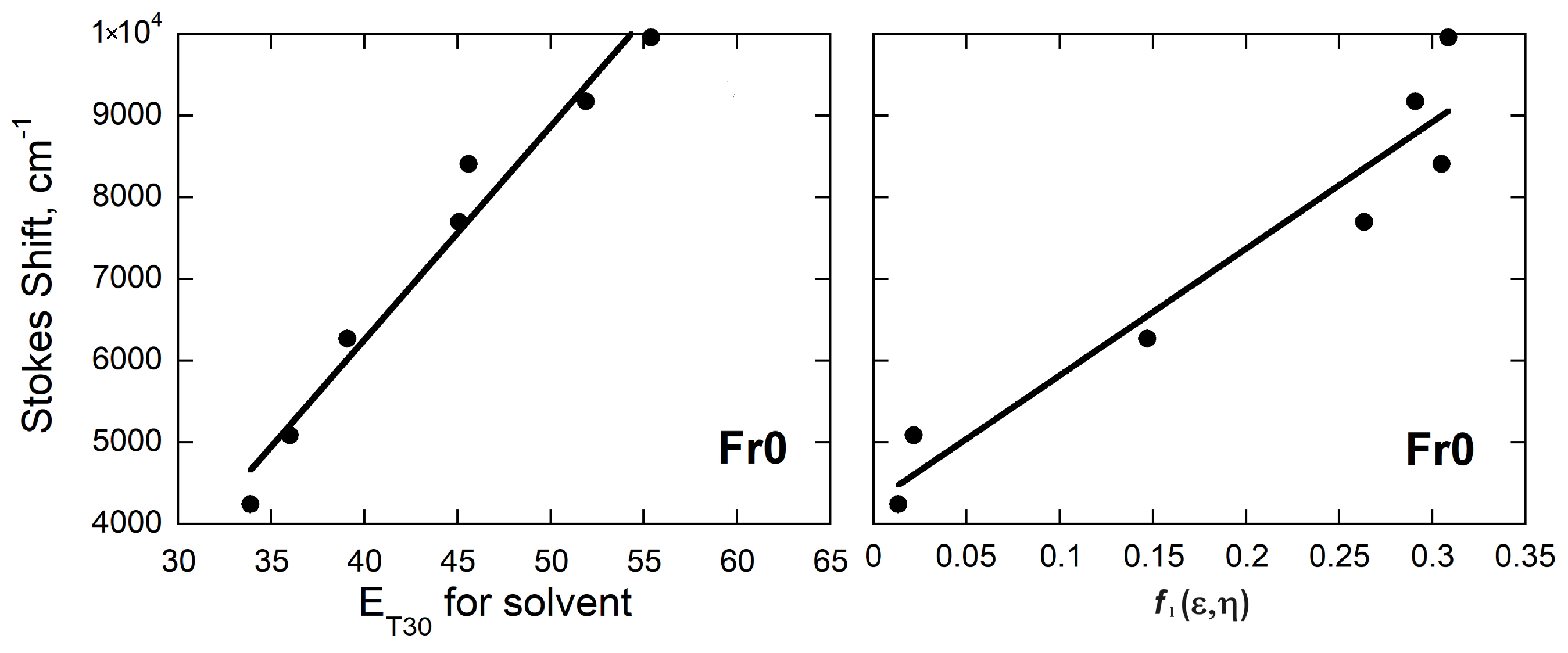
3.1. Experimental Assessments of Solvatochromism
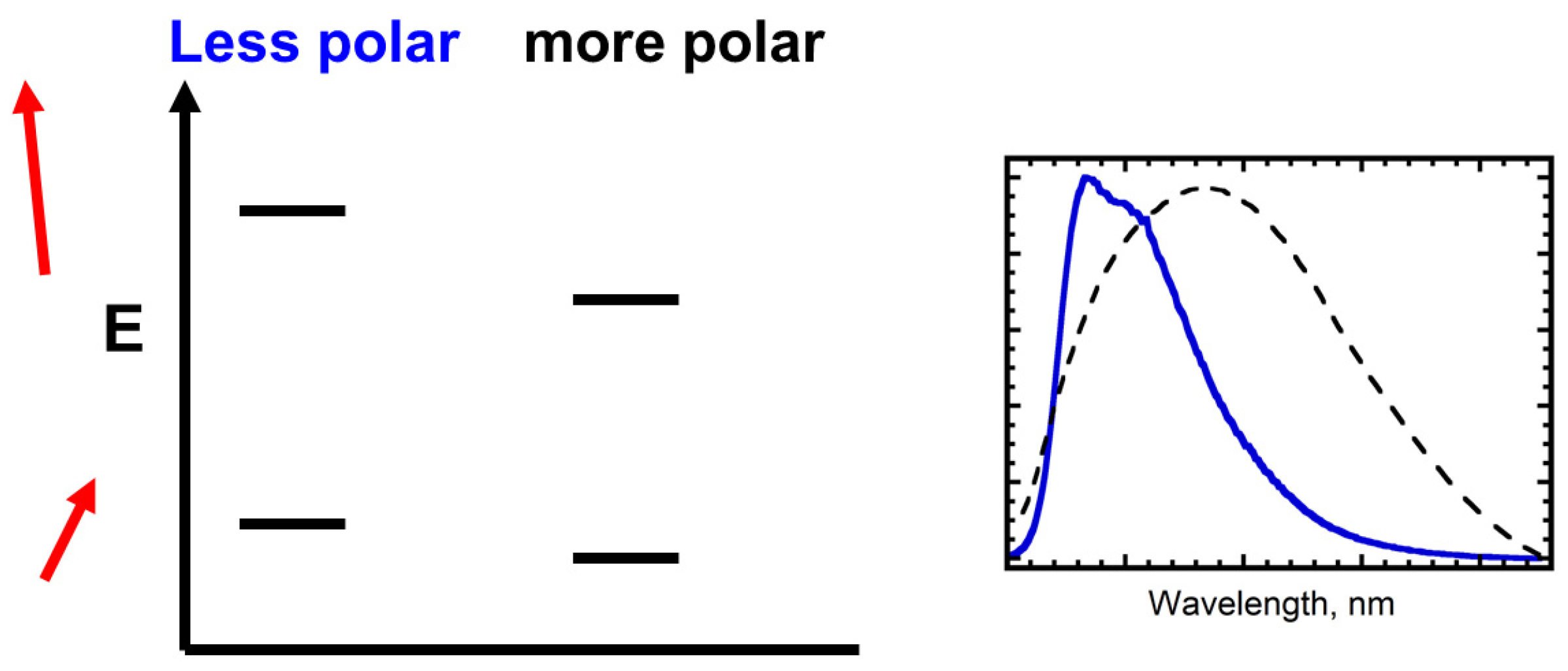
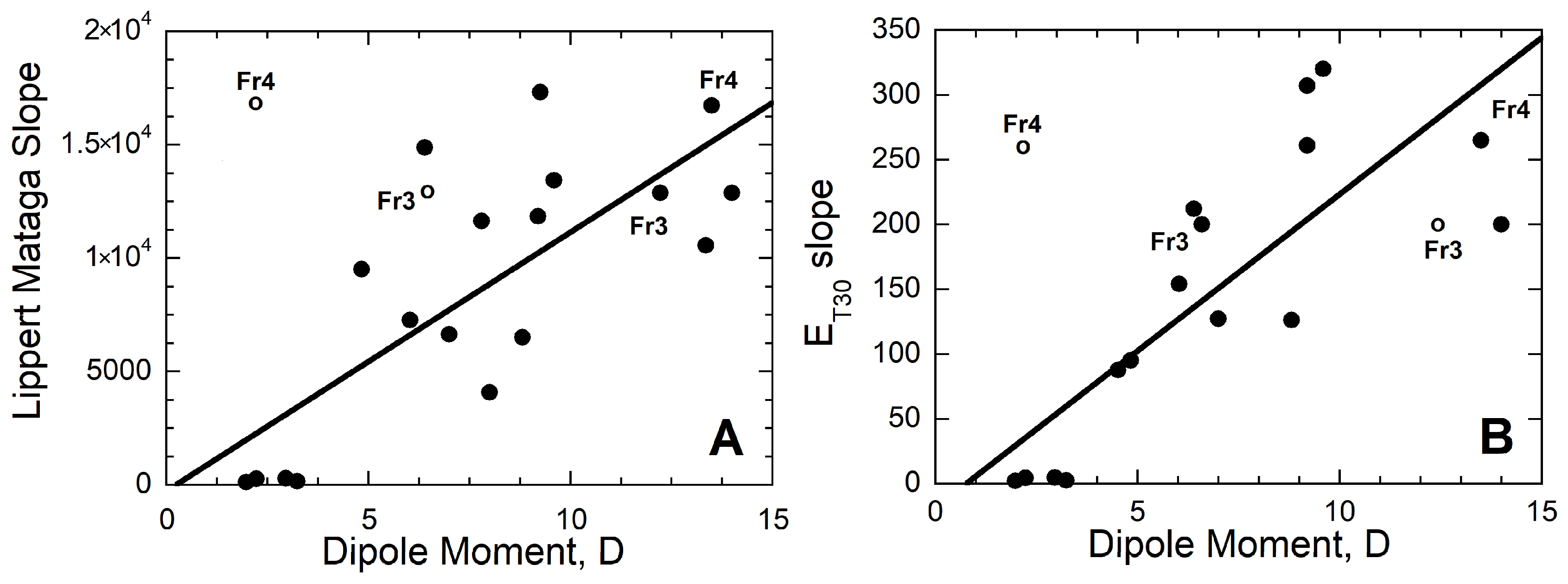
3.2. Solvatochromic Behavior vs. Dipole Moment
3.3. Assessing a Correlation between Experimental Spectral Data and Computed Dipole Moments
3.4. Effects of Probe Modifications and Conformation on Dipole Moment and Electrostatic Potential
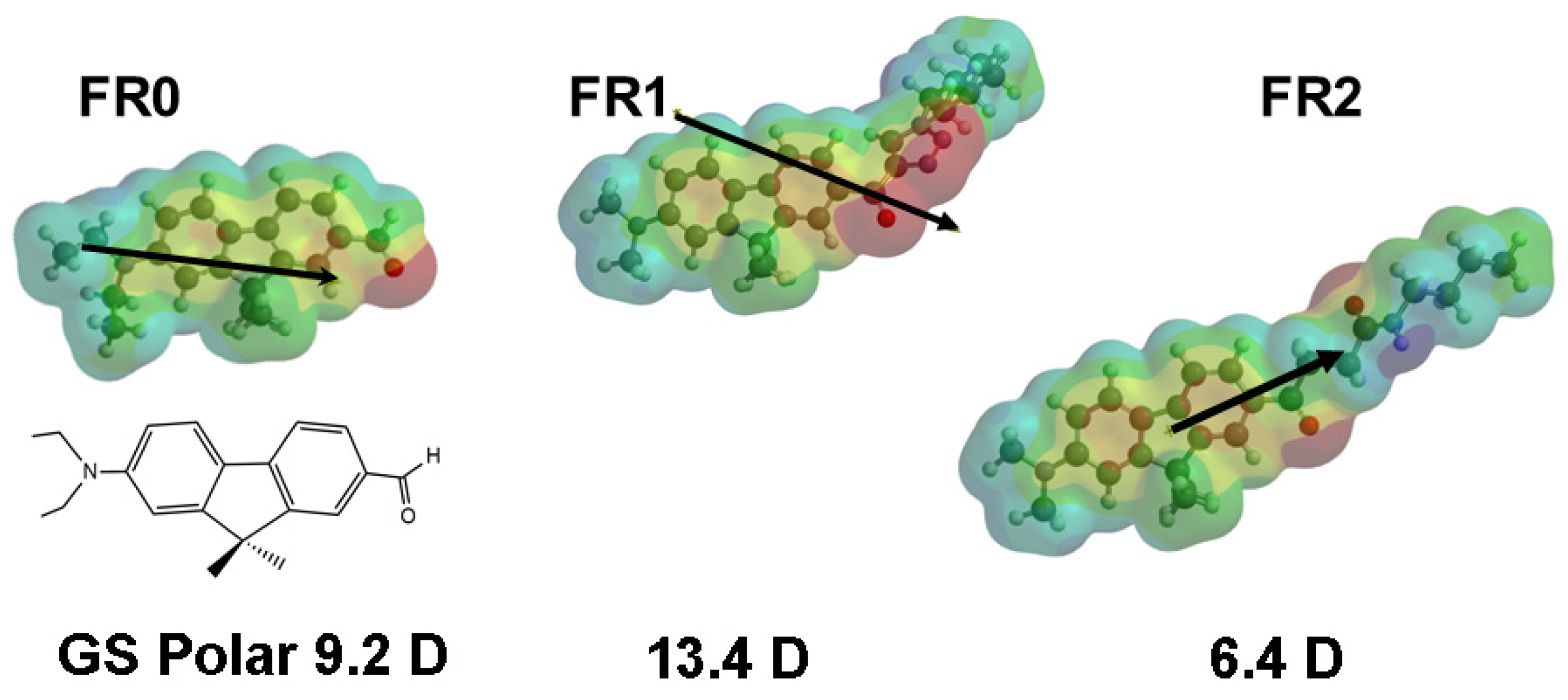
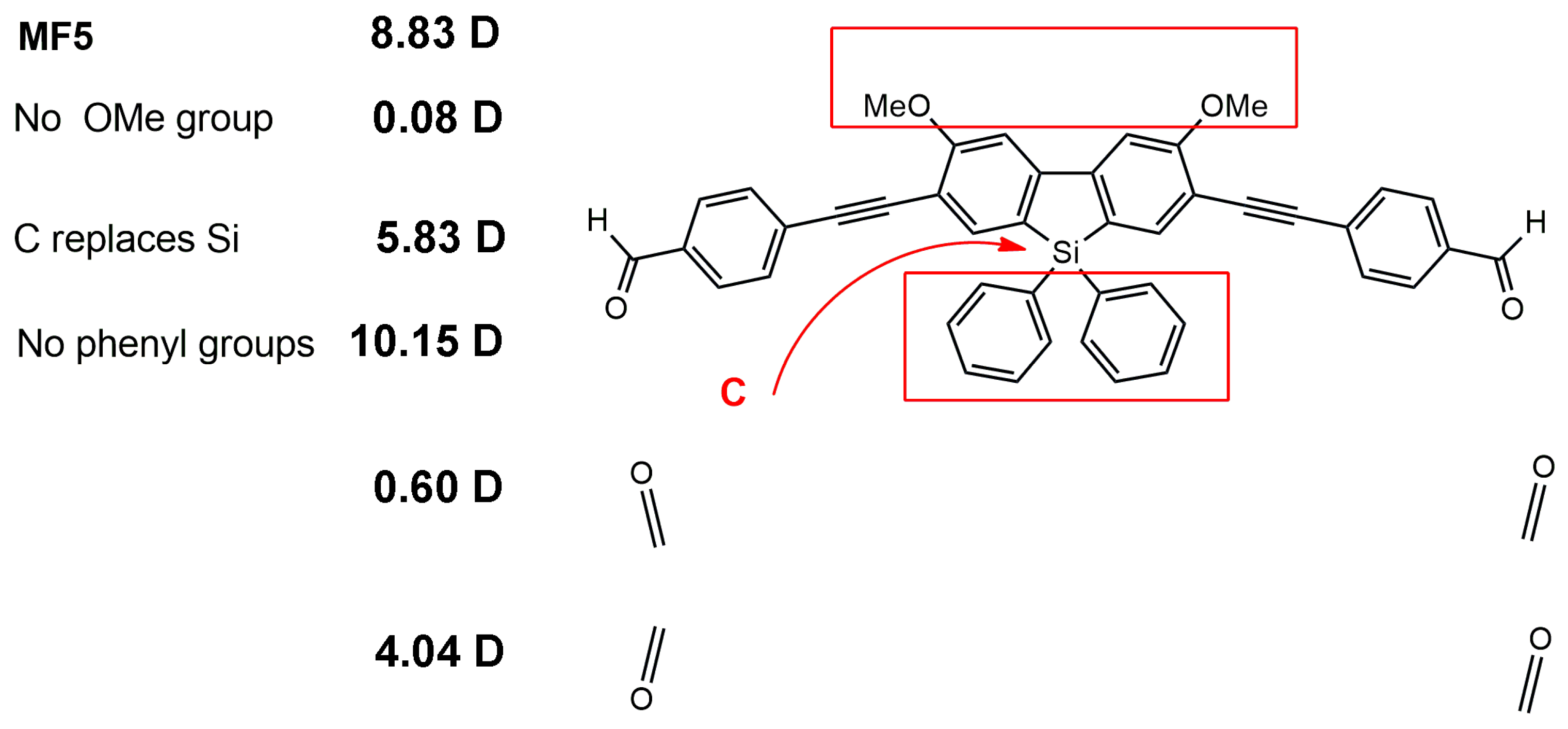
3.4.1. Impact of Probe Modification: Fluorene Series
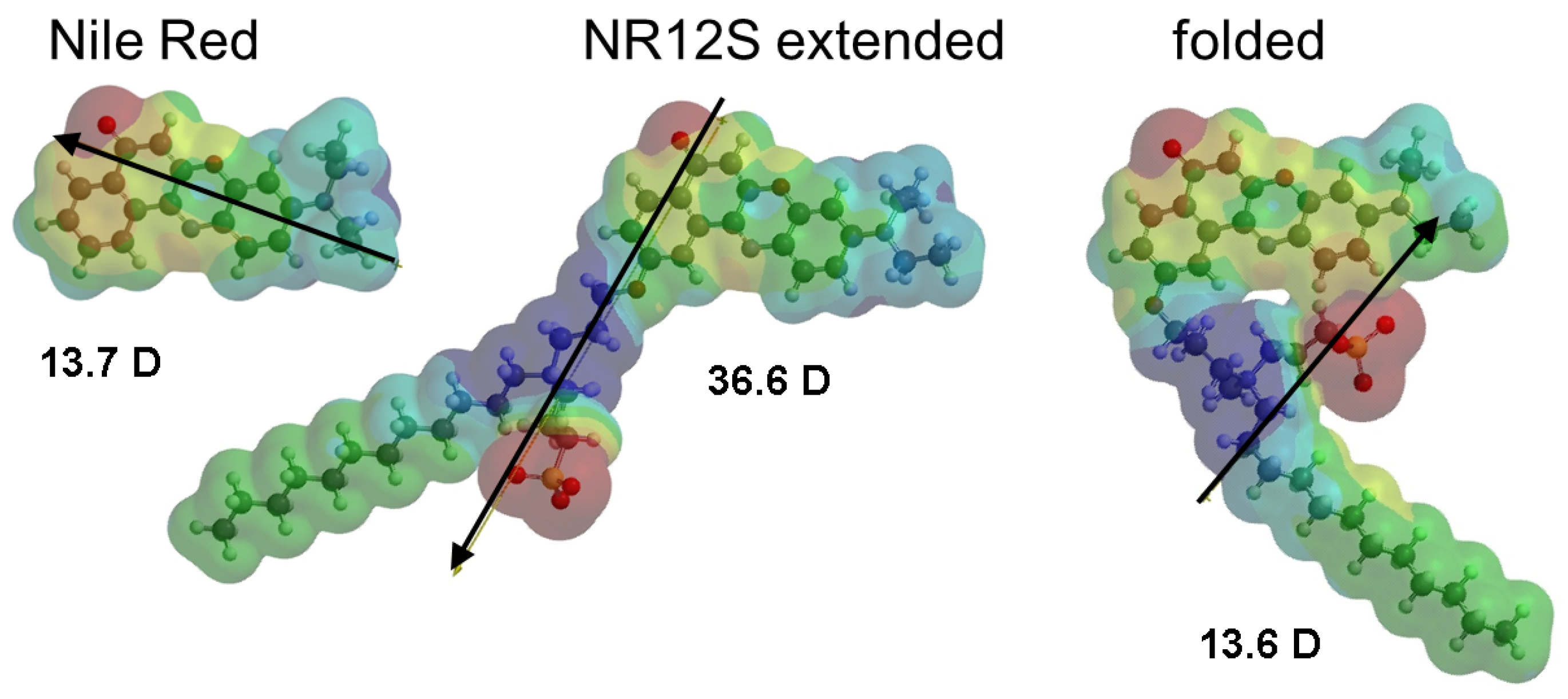
3.4.2. Impact on Conformation: Fluorene and Nile Red Series
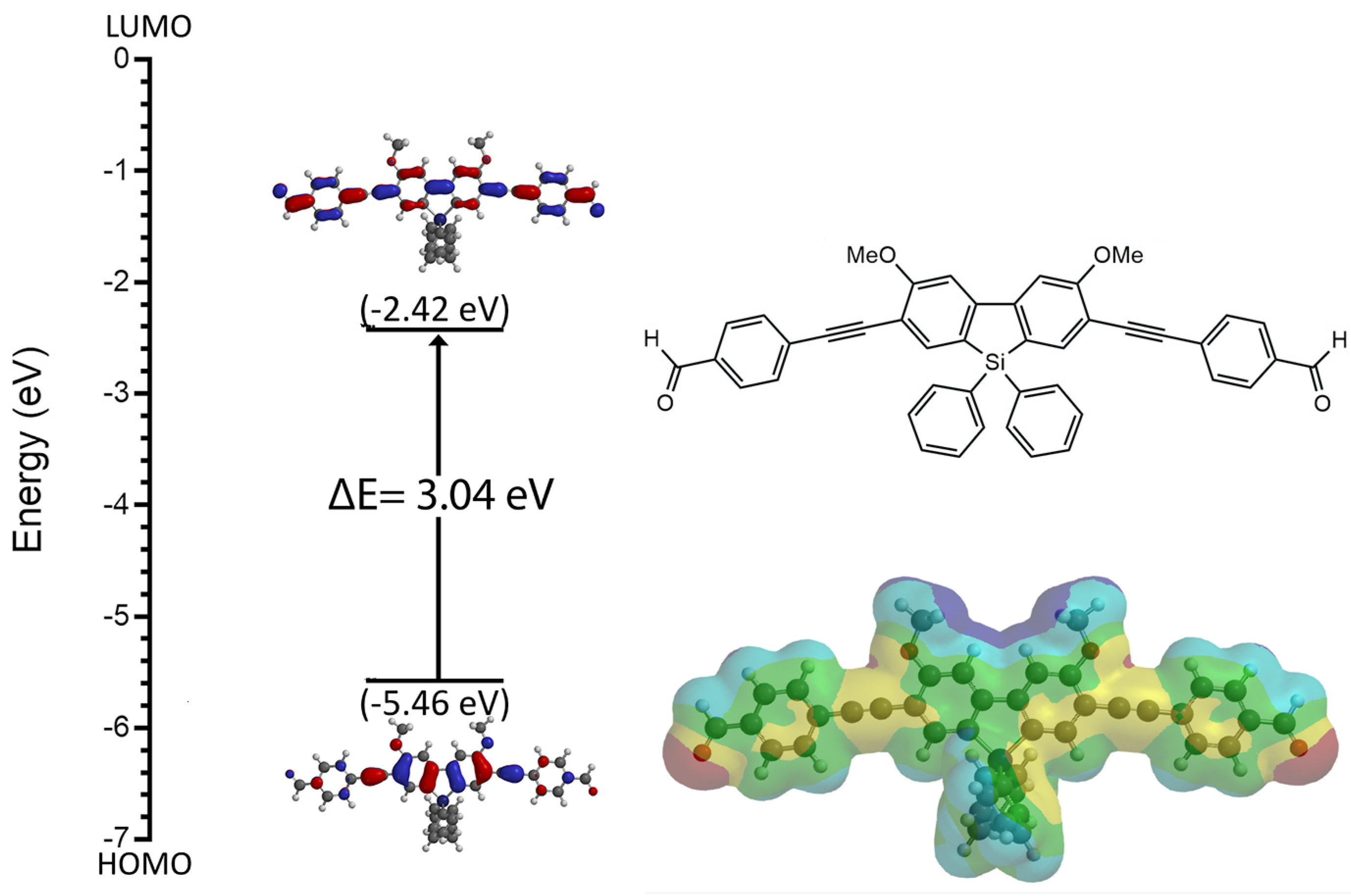
3.4.3. Use of DFT in Probe Design: 2,7-Disubstituted Metallafluorenes
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef]
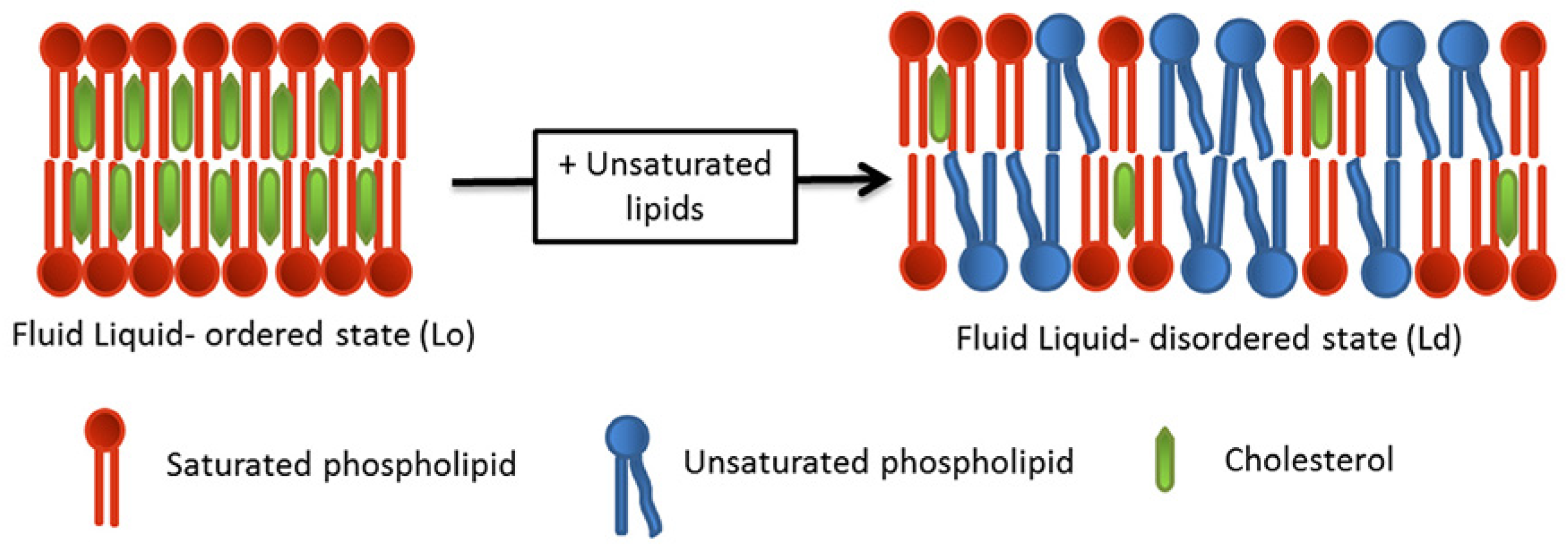
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Levental, I.; Levental, K.R.; Heberle, F.A. Lipid Rafts: Controversies Resolved, Mysteries Remain. Trends Cell Biol. 2020, 30, 341–353. [Google Scholar] [CrossRef]
- Klymchenko, A.S.; Kreder, R. Fluorescent probes for lipid rafts: From model membranes to living cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Ten Hagen, T.L. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ashoka, A.H.; Ashokkumar, P.; Kovtun, Y.P.; Klymchenko, A.S. Solvatochromic Near-Infrared Probe for Polarity Mapping of Biomembranes and Lipid Droplets in Cells under Stress. J. Phys. Chem. Lett. 2019, 10, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Danylchuk, D.I.; Jouard, P.-H.; Klymchenko, A.S. Targeted Solvatochromic Fluorescent Probes for Imaging Lipid Order in Organelles under Oxidative and Mechanical Stress. J. Am. Chem. Soc. 2021, 143, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Darwich, Z.; Klymchenko, A.S.; Kucherak, O.A.; Richert, L.; Mely, Y. Detection of apoptosis through the lipid order of the outer plasma membrane leaflet. Biochim. Biophys. Acta 2012, 1818, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Shynkar, V.V.; Klymchenko, A.S.; Kunzelmann, C.; Duportail, G.; Muller, C.D.; Demchenko, A.P.; Freyssinet, J.M.; Mely, Y. Fluorescent biomembrane probe for ratiometric detection of apoptosis. J. Am. Chem. Soc. 2007, 129, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Ma, Y.; Yang, Y.; Lv, H.; Wang, J.; Wang, T.; Liu, F.; Xu, S.; Jiang, Z.; Lin, W. Lighting up the changes of plasma membranes during apoptosis with fluorescent probes. Coord. Chem. Rev. 2023, 476, 214926. [Google Scholar] [CrossRef]
- Yang, S.T.; Kreutzberger, A.J.B.; Kiessling, V.; Ganser-Pornillos, B.K.; White, J.M.; Tamm, L.K. HIV virions sense plasma membrane heterogeneity for cell entry. Sci. Adv. 2017, 3, e1700338. [Google Scholar] [CrossRef] [PubMed]
- Carravilla, P.; Nieva, J.L.; Eggeling, C. Fluorescence Microscopy of the HIV-1 Envelope. Viruses 2020, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.; Waithe, D.; Carravilla, P.; Huarte, N.; Galiani, S.; Enderlein, J.; Eggeling, C. Envelope glycoprotein mobility on HIV-1 particles depends on the virus maturation state. Nat. Comm. 2017, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Lorizate, M.; Brugger, B.; Akiyama, H.; Glass, B.; Muller, B.; Anderluh, G.; Wieland, F.T.; Krausslich, H.G. Probing HIV-1 membrane liquid order by Laurdan staining reveals producer cell-dependent differences. J. Biol. Chem. 2009, 284, 22238–22247. [Google Scholar] [CrossRef]
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. FRET as a biomolecular research tool—Understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef]
- Shaya, J.; Collot, M.; Benailly, F.; Mahmoud, N.; Mely, Y.; Michel, B.Y.; Klymchenko, A.S.; Burger, A. Turn-on Fluorene Push-Pull Probes with High Brightness and Photostability for Visualizing Lipid Order in Biomembranes. ACS Chem. Biol. 2017, 12, 3022–3030. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chemistry 2016, 22, 13764–13782. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Di, C.; Hu, Y.; Munyemana, J.C.; Shu, Y.; Wang, J.-H.; Qiu, H. A novel colorimetric and red-emitting fluorescent probe based on benzopyrylium derivatives for selective detection and imaging of SO2 derivatives in cells and zebrafish. Dye. Pigment. 2023, 212, 111129. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Wang, W.; Luo, J.-G.; Kong, L. Red-Emitting Fluorescent Probe for Detection of γ-Glutamyltranspeptidase and Its Application of Real-Time Imaging under Oxidative Stress in Cells and In Vivo. Anal. Chem. 2018, 90, 7467–7473. [Google Scholar] [CrossRef]
- Kucherak, O.A.; Oncul, S.; Darwich, Z.; Yushchenko, D.A.; Arntz, Y.; Didier, P.; Mely, Y.; Klymchenko, A.S. Switchable nile red-based probe for cholesterol and lipid order at the outer leaflet of biomembranes. J. Am. Chem. Soc. 2010, 132, 4907–4916. [Google Scholar] [CrossRef]
- Misra, R.M.; Bhattacharyya, S.P. Intramolecular Charge Transfer: Theory and Applications, 1st ed.; Wiley-VCH.: Weinheim, Germany, 2018. [Google Scholar]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Sun, R.; Wan, W.; Jin, W.; Bai, Y.; Xia, Q.; Wang, M.; Huang, Y.; Zeng, L.; Sun, J.; Peng, C.; et al. Derivatizing Nile Red fluorophores to quantify the heterogeneous polarity upon protein aggregation in the cell. Chem. Commun. 2022, 58, 5407–5410. [Google Scholar] [CrossRef]
- Liu, L.; Lei, Y.; Zhang, J.; Li, N.; Zhang, F.; Wang, H.; He, F. Rational Design for Multicolor Flavone-Based Fluorophores with Aggregation-Induced Emission Enhancement Characteristics and Applications in Mitochondria-Imaging. Molecules 2018, 23, 2290. [Google Scholar] [CrossRef] [PubMed]
- Shaya, J.; Fontaine-Vive, F.; Michel, B.Y.; Burger, A. Rational Design of Push-Pull Fluorene Dyes: Synthesis and Structure-Photophysics Relationship. Chemistry 2016, 22, 10627–10637. [Google Scholar] [CrossRef] [PubMed]
- Tarai, A.; Huang, M.; Das, P.; Pan, W.; Zhang, J.; Gu, Z.; Yan, W.; Qu, J.; Yang, Z. ICT and AIE Characteristics Two Cyano-Functionalized Probes and Their Photophysical Properties, DFT Calculations, Cytotoxicity, and Cell Imaging Applications. Molecules 2020, 25, 585. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Y.; Chen, Y.; Zheng, J.; Zhang, J.; Li, R.; Wan, H.; Yin, J.; Yuan, Z.; Chen, H. A Family of Push-Pull Bio-Probes for Tracking Lipid Droplets in Living Cells with the Detection of Heterogeneity and Polarity. Anal. Chim. Acta 2020, 1096, 166–173. [Google Scholar] [CrossRef]
- Jarrett-Noland, S.; McConnell, W.; Braddock-Wilking, J.; Dupureur, C. Solvatochromic Behavior of 2,7-Disubstituted Sila- and Germafluorenes. Chemosensors 2023, 11, 160. [Google Scholar] [CrossRef]
- Mes, G.; de Jong, B.; van Ramesdonk, H.; Verhoeven, J.; Warman, J.; de Haas, M.; Horsman-van den Dool, L. Excited-State Dipole Moment and Solvatochromism of Highly Fluorescent Rod-Shaped Bichromophoric Molecules. J. Am. Chem. Soc. 1984, 106, 6524–6528. [Google Scholar] [CrossRef]
- Kawski, A.; Bojarski, P.; Kuklinski, B. Estimation of ground- and excited-state dipole moments of Nile Red dye from solvatochromic effect on absorption and fluorescence spectra. Chem. Phys. Lett. 2008, 463, 410–412. [Google Scholar] [CrossRef]
- Vequi-Suplicy, C.C.; Coutinho, K.; Lamy, M.T. Electric dipole moments of the fluorescent probes Prodan and Laurdan: Experimental and theoretical evaluations. Biophys. Rev. 2014, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chattopadhyay, A.; Samanta, A.; Soujanya, T. Dipole Moment Change of NBD Group upon Excitation Studied Using Solvatochromic and Quantum Chemical Approaches: Implications in Membrane Research. J. Phys. Chem. 1994, 98, 2809–2812. [Google Scholar] [CrossRef]
- Patil, O.; Ingalgondi, P.; Mathapati, G.; Gounalli, S.; Sankarappa, T.; Hanagodimath, S. Ground and Excited State Dipole Moments of a Dye. J. Appl. Phys. 2016, 8, 55–59. [Google Scholar]
- Gulseven Sidir, Y.; Sidir, I. Solvent effect on the absorption and fluorescence spectra of 7-acetoxy-6-(2,3-dibromopropyl)-4,8-dimethylcoumarin: Determination of ground and excited state dipole moments. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Yablon, D.G.; Schilowitz, A.M. Solvatochromism of Nile Red in nonpolar solvents. Appl. Spectrosc. 2004, 58, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.E.; Blanco, J.B.; Imperiali, B. Photophysics and biological applications of the environment-sensitive fluorophore 6-N,N-dimethylamino-2,3-naphthalimide. J. Am. Chem. Soc. 2005, 127, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Manjuladevi, V.; Gupta, R.; Kumari, S. Solvatochromism of tricycloquinazoline based disk-shaped liquid crystal: A potential molecular probe for fluorescence imaging. RSC Adv. 2015, 5, 84592–84600. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 87th ed.; Lide, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Thipperudrappa, J. Analaysis of solvatochromism of a biologically active ketocyanine dye using different solvent polarity scales and estimation of dipole moments. Int. J. Life Sci. Pharm. Res. 2014, 4, 1–11. [Google Scholar]
- Bakhshiev, N.G. Universal Intermolecular Interactions and Their Effect on the Position the Electronic Spectra of Molecules in Two Component Solutions. Opt. Spektrosk. 1964, 16, 821–832. [Google Scholar]
- Chamma, A.; Viallet, P. Determination du moment dipolaire d'une molecule dans un etat excite singulet. Comptes Rendus Acad. Des. Sci. 1970, 27, 1901–1904. [Google Scholar]
- Kumari, R.; Varghese, A.; George, L. Estimation of Ground-State and Singlet Excited-State Dipole Moments of Substituted Schiff Bases Containing Oxazolidin-2-One Moiety through Solvatochromic Methods. J. Fluor. 2017, 27, 151–165. [Google Scholar] [CrossRef]
- Kucherak, O.A.; Didier, P.; Mély, Y.; Klymchenko, A.S. Fluorene Analogues of Prodan with Superior Fluorescence Brightness and Solvatochromism. J. Phys. Chem. Lett. 2010, 1, 616–620. [Google Scholar] [CrossRef]
- Renuka, C.G.; Shivashankar, K.; Boregowda, P.; Bellad, S.S.; Muregendrappa, M.V.; Nadaf, Y.F. An Experimental and Computational Study of 2-(3-Oxo-3H-benzo[f] chromen-1-ylmethoxy)-Benzoic Acid Methyl Ester. J. Solut. Chem. 2017, 46, 1535–1555. [Google Scholar] [CrossRef][Green Version]
- Aaron, J.-J.; Buna, M.; Parkanyi, C.; Antonious, M.S.; Tine, A.; Cisse, L. Quantitative Treatment of the Effect of Solvent on the Electronic Absorption and Fluorescence Spectra of Substituted Coumarins: Evaluation of the First Excited Singlet-State Dipole Moments. J. Fluor. 1995, 5, 337–347. [Google Scholar] [CrossRef]
- Cha, S.; Choi, M.G.; Jeon, H.R.; Chang, S.K. Negative Solvatochromism of Merocyanine Dyes: Application as Water Content Probes for Organic Solvents. Sens. Actuators B Chem. 2011, 157, 14–18. [Google Scholar] [CrossRef]
- Arathi, A.S.; Mallick, S.; Koner, A.L. Tuning Aggregation-Induced Emission of 2,3-Napthalimide by Employing Cyclodextrin Nanocavities. ChemistrySelect 2016, 1, 3535–3540. [Google Scholar] [CrossRef]
- Pandey, A.; Rai, R.; Pal, M.; Pandey, S. How polar are choline chloride-based deep eutectic solvents? Phys. Chem. Chem. Phys. 2014, 16, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Choo, H.J.; Jung, S.Y.; Ko, Y.G.; Park, W.H.; Jeon, S.J.; Kim, C.H.; Joo, T.; Cho, B.R. A two-photon fluorescent probe for lipid raft imaging: C-laurdan. Chembiochem 2007, 8, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Niko, Y.; Kawauchi, S.; Konishi, G. Solvatochromic pyrene analogues of Prodan exhibiting extremely high fluorescence quantum yields in apolar and polar solvents. Chemistry 2013, 19, 9760–9765. [Google Scholar] [CrossRef]
- Giordano, L.; Shvadchak, V.V.; Fauerbach, J.A.; Jares-Erijman, E.A.; Jovin, T.M. Highly Solvatochromic 7-Aryl-3-hydroxychromones. J. Phys. Chem. Lett. 2012, 3, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, J.; Dong, H.; Zhang, S.; Xu, W.; Wang, J.; Gao, P.; Peng, X. Fluorene-Derived Two-Photon Fluorescent Probes for Specific and Simultaneous Bioimaging of Endoplasmic Reticulum and Lysosomes: Group-Effect and Localization Hua. J. Mater. Chem. B 2013, 1, 5450–5455. [Google Scholar] [CrossRef]
- Chen, R.F.; Fan, Q.L.; Liu, S.J.; Zhu, R.; Pu, K.Y.; Huang, W. Fluorene and Silafluorene Conjugated Copolymer: A New Blue Light-Emitting Polymer. Synth. Met. 2006, 156, 1161–1167. [Google Scholar] [CrossRef]
- Yang, T.; Zuo, Y.; Zhang, Y.; Gou, Z.; Wang, X.; Lin, W. Novel Fluorene-Based Fluorescent Probe with Excellent Stability for Selective Detection of SCN- and Its Applications in Paper-Based Sensing and Bioimaging. J. Mat. Chem. B 2019, 7, 4649–4654. [Google Scholar] [CrossRef]
- Niko, Y.; Klymchenko, A.S. Emerging Solvatochromic Push-Pull Dyes for Monitoring the Lipid Order of Biomembranes in Live Cells. J. Biochem. 2021, 170, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Auvray, M.; Bolze, F.; Clavier, G.; Mahuteau-Betzer, F. Silafluorene as a promising core for cell-permeant, highly bright and two-photon excitable fluorescent probes for live-cell imaging. Dye. Pigment. 2021, 187, 109083–109091. [Google Scholar] [CrossRef]
- Li, L.; Xu, C.; Li, S. Synthesis and photophysical properties of highly emissive compounds containing a dibenzosilole core. Tet. Lett. 2010, 51, 622–624. [Google Scholar] [CrossRef]
- Hammerstroem, D.W.; Braddock-Wilking, J.; Rath, N.P. Synthesis and characterization of luminescent 2,7-disubstituted silafluorenes. J. Organomet. Chem. 2016, 813, 110–118. [Google Scholar] [CrossRef]
- Hammerstroem, D.W.; Braddock-Wilking, J.; Rath, N.P. Luminescent 2,7-disubstituted germafluorenes. J. Organomet. Chem. 2017, 830, 196–202. [Google Scholar] [CrossRef]
- Germann, S.; Jarrett, S.J.; Dupureur, C.M.; Rath, N.P.; Gallaher, E.; Braddock-Wilking, J. Synthesis of Luminescent 2-7 Disubstituted Silafluorenes with alkynyl-carbazole, -phenanthrene, and -benzaldehyde substituents. J. Organomet. Chem. 2020, 927, 121514. [Google Scholar] [CrossRef]
- Braddock-Wilking, J.; Dupureur, C.; Germann, S.; Spikes, H. Luminescent Silafluorene and Germafluorene Compounds. U.S. Patent 20220162235, 26 May 2022. [Google Scholar]
- Spikes, H.J.; Jarrett-Noland, S.J.; Germann, S.M.; Braddock-Wilking, J.; Dupureur, C.M. Group 14 Metallafluorenes as Sensitive Luminescent Probes of Surfactants in Aqueous Solution. J. Fluor. 2021, 31, 961–969. [Google Scholar] [CrossRef]
- Spikes, H.J.; Jarrett-Noland, S.J.; Germann, S.M.; Olivas, W.; Braddock-Wilking, J.; Dupureur, C.M. Group 14 Metallafluorenes for Lipid Structure Detection and 2 Cellular Imaging. Chem. Proc. 2021, 5, 83–88. [Google Scholar] [CrossRef]
- Xiong, J.F.; Li, J.X.; Mo, G.Z.; Huo, J.P.; Liu, J.Y.; Chen, X.Y.; Wang, Z.Y. Benzimidazole derivatives: Selective fluorescent chemosensors for the picogram detection of picric acid. J. Org. Chem. 2014, 79, 11619–11630. [Google Scholar] [CrossRef] [PubMed]
- Reiser, A.; Leyshon, L.; Saunders, D.; Mijovic, M.; Bright, A.; Bogie, J. Fluorescence of Aromatic Benzoxazole Derivatives. J. Am. Chem. Soc. 1972, 94, 2414–2421. [Google Scholar] [CrossRef]
- Barwiolek, M.; Wojtczak, A.; Kozakiewicz, A.; Babinska, M.; Tafelska-Kaczmarek, A.; Larsen, E.; Szlyk, E. The synthesis, characterization and fluorescence properties of new benzimidazole derivatives. J. Lum. 2019, 211, 88–95. [Google Scholar] [CrossRef]
- Dupureur, C.; Jarrett-Noland, S.; McConnell, W.; Gnawali, G.; Germann, S.; Braddock-Wilking, J. Chimeric Environment Sensitive Fluorescent Probes. Provisional Patent Application 63/511,403, 30 June 2023. [Google Scholar]
| Solvent | Dielectric Constant b | Refractive Index b η | ET30 c | f1,η) d |
|---|---|---|---|---|
| Water | 80.1 | 1.3330 | 63.1 | 0.3217 |
| Glycol | 37.0 | 1.4385 | 56.3 | 0.2719 |
| Methanol | 32.7 | 1.3284 | 55.4 | 0.3086 |
| Ethanol | 24.5 | 1.3614 | 51.9 | 0.2911 |
| Dichloromethane | 8.93 | 1.4241 | 40.7 | 0.2172 |
| Tetrahydrofuran | 7.58 | 1.4072 | 37.4 | 0.2096 |
| Ethyl acetate | 6.00 | 1.3724 | 38.1 | 0.1993 |
| Toluene | 2.38 | 1.4969 | 33.9 | 0.01350 |
| Dioxane | 2.22 | 1.4224 | 36.0 | 0.02164 |
| Carbon tetrachloride | 2.24 | 1.4601 | 32.4 | 0.01400 |
| Acetonitrile | 37.5 | 1.3441 | 45.6 | 0.30500 |
| Dimethylformamide | 36.7 | 1.4305 | 43.2 | 0.27440 |
| DMSO | 46.7 | 1.4783 | 45.1 | 0.26340 |
| Chloroform | 4.81 | 1.4458 | 39.1 | 0.14700 |
| Cyclohexane | 2.02 | 1.4262 | 30.9 | -0.001600 |
| n-hexane | 1.88 | 1.3749 | 31.0 | -0.0014 |
| Acetone | 20.7 | 1.3587 | 42.2 | 0.2842 |
| Benzene | 2.27 | 1.5011 | 34.3 | 0.001700 |
| Diethyl ether | 4.33 | 1.3524 | 34.5 | 0.16760 |
| Probe | Structure | Lippert–Mataga Slope | ET30 Slope | Dipole Moment, D a | Ref. |
|---|---|---|---|---|---|
| Nile Red |  | 4083 | c | 8.0 b | [21,36] |
| NR12S | 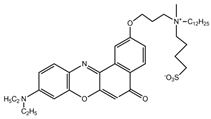 | 12,860 | 200 | 14–37 d | [21] |
| Prodan | 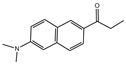 | 3881 b | 127 | 7.7 | [49] |
| C laurdan |  | 6334 | 82 | 6.9 | [50] |
| PA | 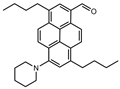 | 7256 b | 154 | 6.0 | [51] |
| PK | 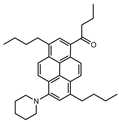 | 9503 | 95 | 4.8 | [51] |
| 7AMC | 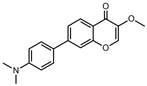 | 11,840 | 307 | 9.2 | [52] |
| FR0 | 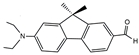 | 18380 | 267 | 9.2 | [44] |
| FR1/PP3 |  | 14,170 | 280 | 13.4 | [26] |
| FR2/PP6 | 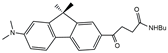 | 14,880 | 212 | 6.4 | [26] |
| FR3 | 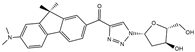 | 12,880 | 200 | 6.4–12.2 d | [16] |
| FR4 | 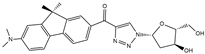 | 16,720 | 265 | 2.2–13.5 d | [16] |
| FR8 | 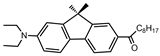 | 13,440 | 320 | 9.56 | [44] |
| 2,5APMC |  | c | 88 | 4.5 b | [40] |
| 2BME |  | 11,640 | c | 2.0–7.8 e | [45] |
| MF1 |  | 293 | 4.6 | 3.0 | [29] |
| MF2 | 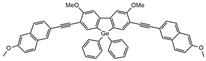 | 157 | 2.5 | 3.2 | [29] |
| MF3 |  | 124 | 2.1 | 2.0 | [29] |
| MF4 | 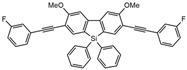 | 253 | 4.3 | 2.2 | [29] |
| MF5 | 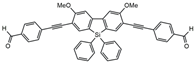 | 6500 | 126 | 8.8 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupureur, C.M. Use of DFT Calculations as a Tool for Designing New Solvatochromic Probes for Biological Applications. Liquids 2024, 4, 148-162. https://doi.org/10.3390/liquids4010007
Dupureur CM. Use of DFT Calculations as a Tool for Designing New Solvatochromic Probes for Biological Applications. Liquids. 2024; 4(1):148-162. https://doi.org/10.3390/liquids4010007
Chicago/Turabian StyleDupureur, Cynthia M. 2024. "Use of DFT Calculations as a Tool for Designing New Solvatochromic Probes for Biological Applications" Liquids 4, no. 1: 148-162. https://doi.org/10.3390/liquids4010007
APA StyleDupureur, C. M. (2024). Use of DFT Calculations as a Tool for Designing New Solvatochromic Probes for Biological Applications. Liquids, 4(1), 148-162. https://doi.org/10.3390/liquids4010007







