An Ab Initio Investigation of the Hydration of Tin(II)
Abstract
1. Introduction
2. Materials and Methods
3. Results
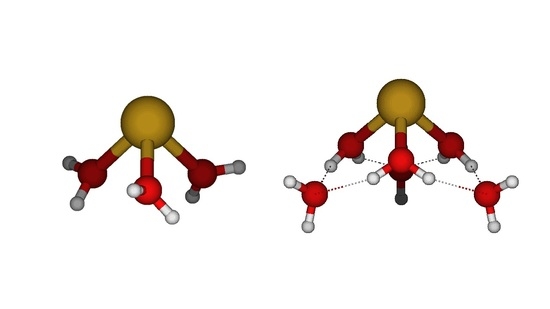
3.1. A Survey of Structures
- The monoaquatin(II) remained as C2v at all levels;
- The most stable diaquatin(II) remained as the bent C2 at all levels except HF/LANL2MB (C2v planar). The linear holodirected D2d structure was approximately 50 kJ/mol higher in energy, but the unstable bent Cs structure was only slightly higher in energy (<1 kJ/mol for nonminimal basis sets). All attempts to generate a [1+1] structure instead resulted in proton transfer to give a SnOH+ + H3O+ complex, which was 25–40 kJ/mol higher in energy;
- The most stable triaquatin(II) remained as the pyramidal C3 at all levels. The two pyramidal C3v structures were 12–25 kJ/mol higher in energy, whereas the planar holodirected D3h and D3 structures were 60–90 kJ/mol higher in energy. The stable [2+1] C2v structure was 25–50 kJ/mol higher in energy;
- The most stable tetraaquatin(II) was usually the see-saw C2, but it could be the C2v #3 (HF/LANL2DZ, HF/B+) or Cs (HF/LANL2MB). The C2v #3 was slightly higher in energy (<2 kJ/mol), with the other C2v structures being higher (15–25 kJ/mol). The holodirected D2d #1, #2, S4, and D2 structures were much higher in energy (50–80 kJ/mol). The Cs #2 [3+1] structure was always competitive in energy, and usually lower, than the tetracoordinate structure;
- The most stable pentaaquatin(II) was the square pyramidal Cs (if it exists), which is closely related to the C2v #1 structure (<3 kJ/mol). The other three C2v structures were ~25 kJ/mol higher in energy. The stable [4+1] and [3+2] structures were competitive in energy, and sometimes lower, depending on level of theory;
- The most stable hexaaquatin(II) was the distorted octahedral C3 (if it exists). The octahedral Th structure was ~30 kJ/mol higher in energy;
- Of the 16 different C2v heptaaquatin(II) structures tried, none were stable, and either possessed imaginary modes or dissociated to a [6+1], [5+2], or [4+3] structure. Structures #1–#4, and #11, always dissociated. Structures #5–#8, and #16, usually remained as 7-coordinates. The remaining structures usually dissociated at most levels. Of the 7-coordinate C2v structures, #8 and #16 were the lowest in energy. All of the 7-coordinate structures dissociated at HF/LANL2MB. Upon desymmetrization of the remaining 7-coordinate C2v structures to C2, nearly all dissociated to [6+1], [5+2], or [4+3] structures. The only exceptions, C2 #10 and #15 at HF/CEP-31G*, possessed imaginary B modes, of which one corresponds to a water molecule moving to the second hydration shell;
- Of the octaaquatin(II) structures, two D4h and two D4d structures (point group order h = 16) were first examined. Multiple imaginary modes were present;
- ○
- For the D4d #1 and #2 structures, desymmetrization along the A2 imaginary mode gave the same S8 structure; along the B1 imaginary mode, they gave the same D4 #2 structure; along the B2 imaginary mode, they gave the C4v #1 and #2 structures; and along the imaginary E1 mode, they gave the same C2v #1 structure (via Cs);
- ○
- For the D4h #1 and #2 structures, desymmetrization along the A1u imaginary mode gave the D4 #2 structure found before; along the A2g imaginary mode, they gave the same C4h #1 structure; along the A2u imaginary mode, they gave the C4v #3 [4+4] and #4 structures; along the B2g imaginary mode (D4h #1), the D2h #1 structure ascended in symmetry to D4h #2; along the B1g (D4h #2) imaginary mode, they gave the D2h #2 structure; along the B1u imaginary mode, they gave the same D2d #1 structure; along the B2u mode, they gave the D2d #2 and #4 structures, respectively; and along the Eg and Eu modes, they gave D2h #3 and #4 (via C2h and C2v);
- Examination of lower symmetry structures (h = 8) gave the following results:
- ○
- For the S8 #1 structure, all E1 and B imaginary modes corresponded to the expulsion of water molecules to the second hydration shell. Desymmetrization along the B mode gave the [4+4] C4;
- ○
- For the D4 #2 structure, the E mode corresponded to the expulsion of water molecules to the second hydration shell. Desymmetrization along the A2 mode gave either a [4+4] C4 structure or a C4 #2 structure, whereas along the B1 mode, it gave the D2 #1 structure;
- ○
- For the C4h #1 structure, the imaginary Eu mode corresponded to the expulsion of two water molecules. Desymmetrization along the imaginary Au mode for the most part gave either a [4+4] C4 structure or ascended in symmetry to S8; along the imaginary Bu mode at HF/CEP-4G and HF/LANL2MB, it gave a D2d #5 and S4 structure, respectively. This D2d #5 structure was then rerun at all levels;
- ○
- For the D2d structures, desymmetrization along an A2 imaginary mode would give an S4 structure; along a B1 imaginary mode, they gave a D2 structure; along a B2 imaginary mode, they gave a C2v structure; along an E mode, they gave either a C2 or Cs structure. Along the A2 mode, an S4 #2 or #4 structure typically resulted, or ascension in symmetry to the D2d #5; along the B1 mode, there was usually ascension to D4 #2; along the B2 mode, there was dissociation to a [6+2] or [4+4] structure; and along an E mode, dissociation would occur;
- ○
- For the D2h structures, desymmetrization along the imaginary Au mode would give a D2 structure, and along the imaginary Bng modes, a C2h structure was given. In all cases, these desymmetrized, and most ascended in symmetry to structures already found (D2 #5, D4 #2, C4h #1). The Bnu modes corresponded to the expulsion of water molecules from the first hydration sphere;
- Examination of lower symmetry structures (h = 4) gave the following results:
- ○
- For the C2v structures, desymmetrization along the A2 mode would give a C2 structure, and along the B1 or B2 mode, different Cs structures were given. For the C2v structures, at least one of the imaginary B modes in each structure corresponded to dissociation to a [6+2] structure, whereas desymmetrization along the A2 mode led to a [4+4] or [4+2+2] structure;
- ○
- For the C4 and S4 structures, the imaginary E mode corresponded to dissociation to a [6+2] structure, whereas desymmetrization along the B mode to give a C2 structure resulted in dissociation;
- ○
- For the D2 structures, at least one of the imaginary B2 or B3 modes corresponded to dissociation to a [6+2] structure, whereas desymmetrization along the B1 mode to give a C2 structure resulted in dissociation to a [6+2] or [4+4];
- Based on these results, we must conclude that a stable 8-coordinate octaaquatin(II) ion cannot exist.
- Of the enneaaquatin(II) structures, four D3h structures (point group order h = 12) were first examined. Multiple imaginary modes were present. Desymmetrization along the A1” mode would yield D3 structures; along A2′, C3h structures were given; and along A2”, C3v structures were given. A common D3 #1 structure was found for most, and in some cases gave an additional [6+3] structure. Two possible C3h structures were found, and in some cases gave an additional [6+3] structure. Four possible C3v structures were found, and in some cases gave additional [6+3] structures. At least one of the degenerate modes corresponded to the expulsion of water molecule(s) from the inner coordination shell;
- ○
- For the D3 structure, desymmetrization along the A2 mode gave a C3 #1 [6+3] structure. At least one of the E modes corresponded to the loss of water molecules from the first hydration shell.
- ○
- For the C3h structures, desymmetrization along the A” mode gave either the C3 #1 [6+3] structure above or a new C3 #3 [6+3] structure (or occasionally [3+3+3]). At least one of the E modes corresponded to the loss of water molecules from the first hydration shell.
- ○
- For the C3v structures, desymmetrization along the A2 mode gave one of the C3 [6+3] structures (or occasionally [3+3+3]) found above. At least one of the E modes corresponded to the loss of water molecules from the first hydration shell.
- Based on these results, we must conclude that a stable 9-coordinate enneaaquatin(II) ion cannot exist.
3.2. The Sn–O Distance
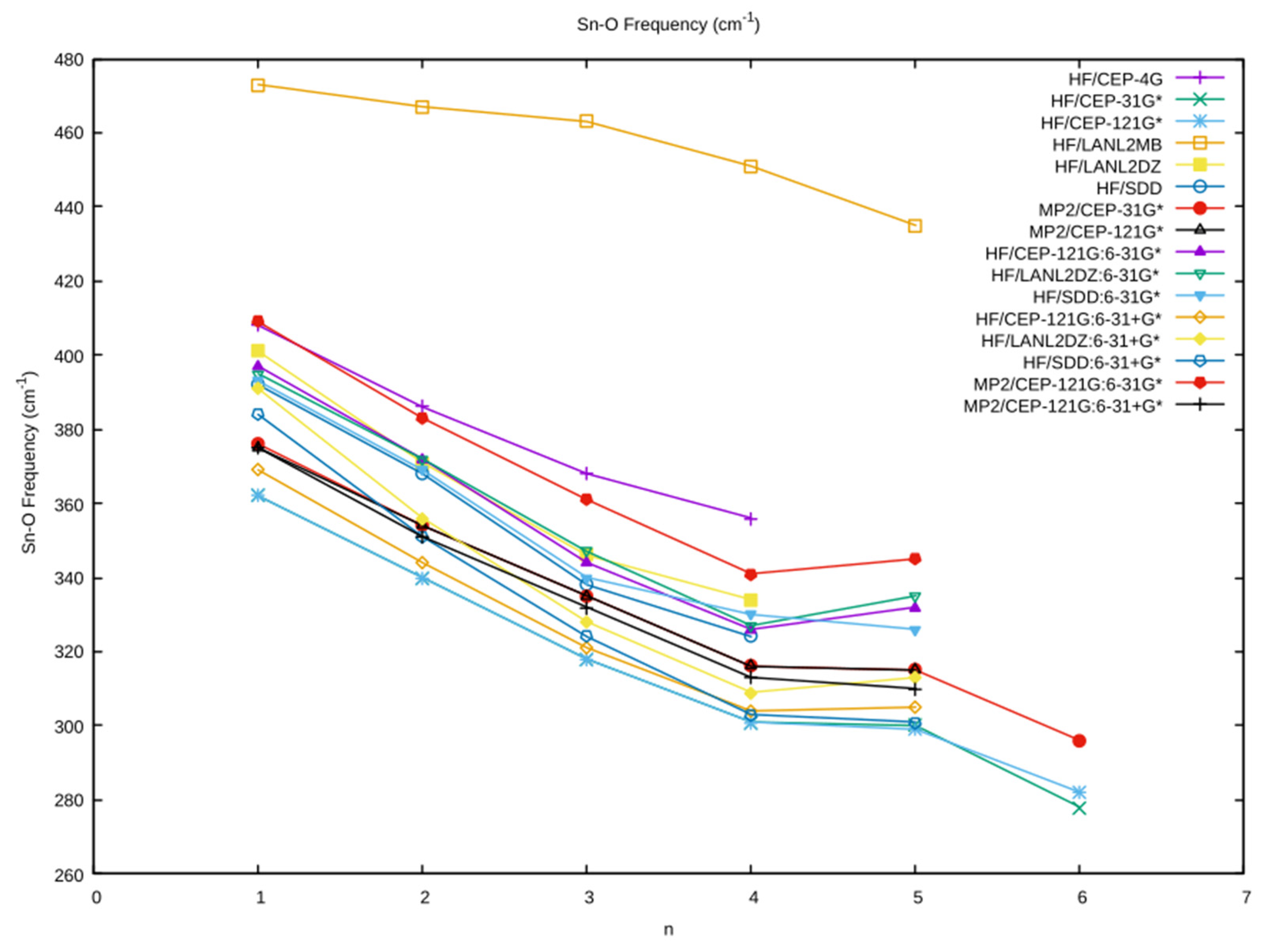
3.3. The Sn–O Vibrational Frequency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richens, D.T. The Chemistry of Aqua Ions; Wiley: Chichester, UK, 1997. [Google Scholar]
- Baes, C.F., Jr.; Mesmer, R.E. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Thallium(III) and Mercury(II). J. Solution Chem. 2020, 49, 1419–1429. [Google Scholar] [CrossRef]
- Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Lead(II). Liquids 2022, 2, 39–49. [Google Scholar] [CrossRef]
- Cox, H.; Stace, A.J. Molecular View of the Anomalous Acidities of Sn2+, Pb2+, and Hg2+. J. Am. Chem. Soc. 2004, 126, 3939–3947. [Google Scholar] [CrossRef]
- Johansson, G.; Ohtaki, H. An X-Ray Investigation of the Hydrolysis Products of Tin(II) in Solution. Acta Chem. Scand. 1973, 27, 643–660. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, T.; Lindqvist, O.; Claeson, T.; Boyce, J.B. EXAFS and X-ray diffraction studies of the hydration structure of stereochemically active Sn(II) ions in aqueous solution. Chem. Phys. Lett. 1982, 93, 528–532. [Google Scholar] [CrossRef]
- Tobias, R.S. Studies on the Hydrolysis of Metal Ions. 21. The Hydrolysis of the Tin(II) Ion, Sn2+. Acta Chem. Scand. 1958, 12, 198–223. [Google Scholar] [CrossRef]
- Grimvall, S. On the Crystal Structure of Sn3O(OH)2SO4. Acta Chem. Scand. 1973, 27, 1447. [Google Scholar] [CrossRef]
- Davies, C.G.; Donaldson, J.D.; Laughlin, D.R.; Howie, R.A.; Beddoes, R. Crystal structure of tritin(II) dihydroxide oxide sulfate. J. Chem. Soc. Dalton Trans. 1975, 2241–2244. [Google Scholar] [CrossRef]
- Hofer, T.S.; Pribil, A.B.; Randolf, B.R.; Rode, B.M. Structure and Dynamics of Solvated Sn(II) in Aqueous Solution: An ab Initio QM/MM MD Approach. J. Am. Chem. Soc. 2005, 127, 14231–14238. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 98, Revision A.9; Gaussian, Inc.: Pittsburgh, PA, USA, 1998. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.02; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Pye, C.C.; Gunasekara, C.M.; Rudolph, W.W. An ab initio investigation of bismuth hydration. Can. J. Chem. 2007, 85, 945–950. [Google Scholar] [CrossRef]
- Pye, C.C.; Whynot, D.C.M.; Corbeil, C.R.; Mercer, D.J. Desymmetrization in geometry optimization: Application to an ab initio study of copper(I) hydration. Pure Appl. Chem. 2020, 92, 1643–1654. [Google Scholar] [CrossRef]
- Pye, C.C.; Gilbert, C.R. An ab initio investigation of the second hydration shell of metal cations. Comput. Appl. Chem. 2020, 208, 395–397. [Google Scholar] [CrossRef]
- Persson, I. Structures of Hydrated Metal Ions in Solid State and Aqueous Solution. Liquids 2022, 2, 210–242. [Google Scholar] [CrossRef]
- Hennings, E.; Schmidt, H.; Kohler, M.; Voigt, W. Crystal structure of tin(II) perchlorate trihydrate. Acta Crystallogr. E 2014, 70, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.; D’Angelo, P.; Lundberg, D. Hydrated and Solvated Tin(II) Ions in Solution and the Solid State, and a Coordination Chemistry Overview of the d10s2 Metal Ions. Chem. Eur. J. 2017, 22, 18583–18592. [Google Scholar] [CrossRef] [PubMed]


| Basis Set/Pseudopotential | HF | MP2 |
|---|---|---|
| CEP-4G | 2.2098 | n/c |
| CEP-31G* | 2.3001 | 2.2978 |
| CEP-121G* | 2.2963 | 2.2964 |
| LANL2MB | 2.0616 | n/c |
| LANL2DZ | 2.2202 | n/c |
| SDD | 2.2898 | n/c |
| A | 2.2624 | 2.2606 |
| B | 2.2397 | n/c |
| C | 2.3005 | n/c |
| A+ | 2.2797 | 2.2887 |
| B+ | 2.2610 | n/c |
| C+ | 2.3241 | n/c |
| Expt. XRD [19] | 2.201(7) | |
| Expt. XRD [20] | 2.208(9) | |
| Expt. EXAFS xtal. [20] | 2.209(3) | |
| Expt. EXAFS soln. [20] | 2.219(3) | |
| Expt. LAXS soln. [20] | 2.206(2) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pye, C.C.; Gunasekara, C.M. An Ab Initio Investigation of the Hydration of Tin(II). Liquids 2022, 2, 465-473. https://doi.org/10.3390/liquids2040027
Pye CC, Gunasekara CM. An Ab Initio Investigation of the Hydration of Tin(II). Liquids. 2022; 2(4):465-473. https://doi.org/10.3390/liquids2040027
Chicago/Turabian StylePye, Cory C., and Champika Mahesh Gunasekara. 2022. "An Ab Initio Investigation of the Hydration of Tin(II)" Liquids 2, no. 4: 465-473. https://doi.org/10.3390/liquids2040027
APA StylePye, C. C., & Gunasekara, C. M. (2022). An Ab Initio Investigation of the Hydration of Tin(II). Liquids, 2(4), 465-473. https://doi.org/10.3390/liquids2040027