Cold-Resistant Lactic Acid Bacteria from Zamorano-Leonesa Donkey Milk: Isolation, Functional Screening, and Genome-Based Insights for Technological Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of Lactic Acid Bacteria (LAB) Strains
2.3. Identification of Bacterial Strains by 16S rRNA Amplification and Sequencing
2.4. Fresh Donkey Milk Fermentation
2.5. Qualitative Screening of Acid and Bile Tolerance
2.6. Genome Analysis, Metabolic Pathways and Secondary Metabolites
2.7. Data/Statistical Analysis
2.8. Antibiotic Susceptibility Testing
3. Results
3.1. Isolation and Identification of Bacterial Strains from Storage Zamorano-Leonese Donkey Milk
3.2. Donkey Milk Fermentation Assay
3.3. Preliminary Screening for Acid and Bile Resistance
3.4. Genome Analysis of L. mesenteroides B8 and L. paracasei subsp. tolerans B19 from Donkey Milk
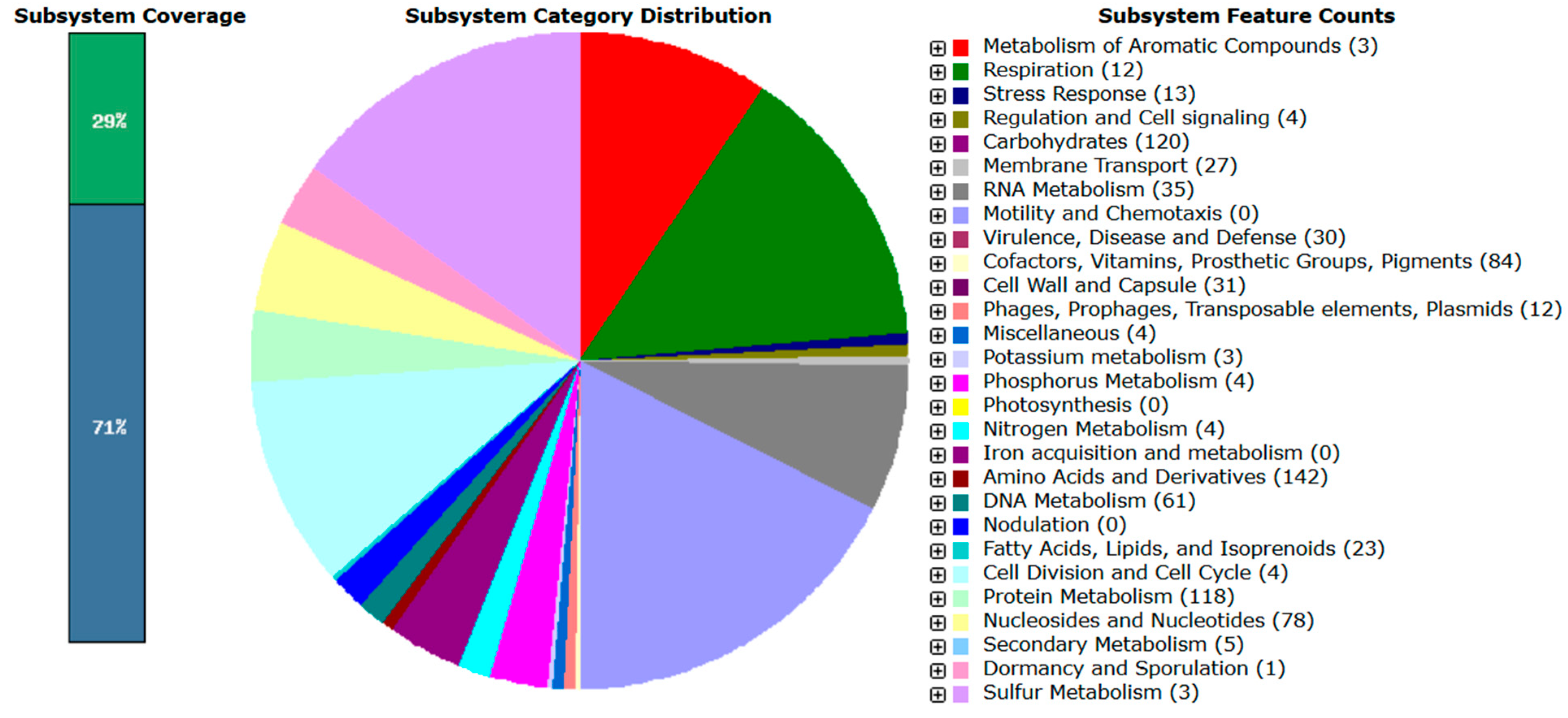
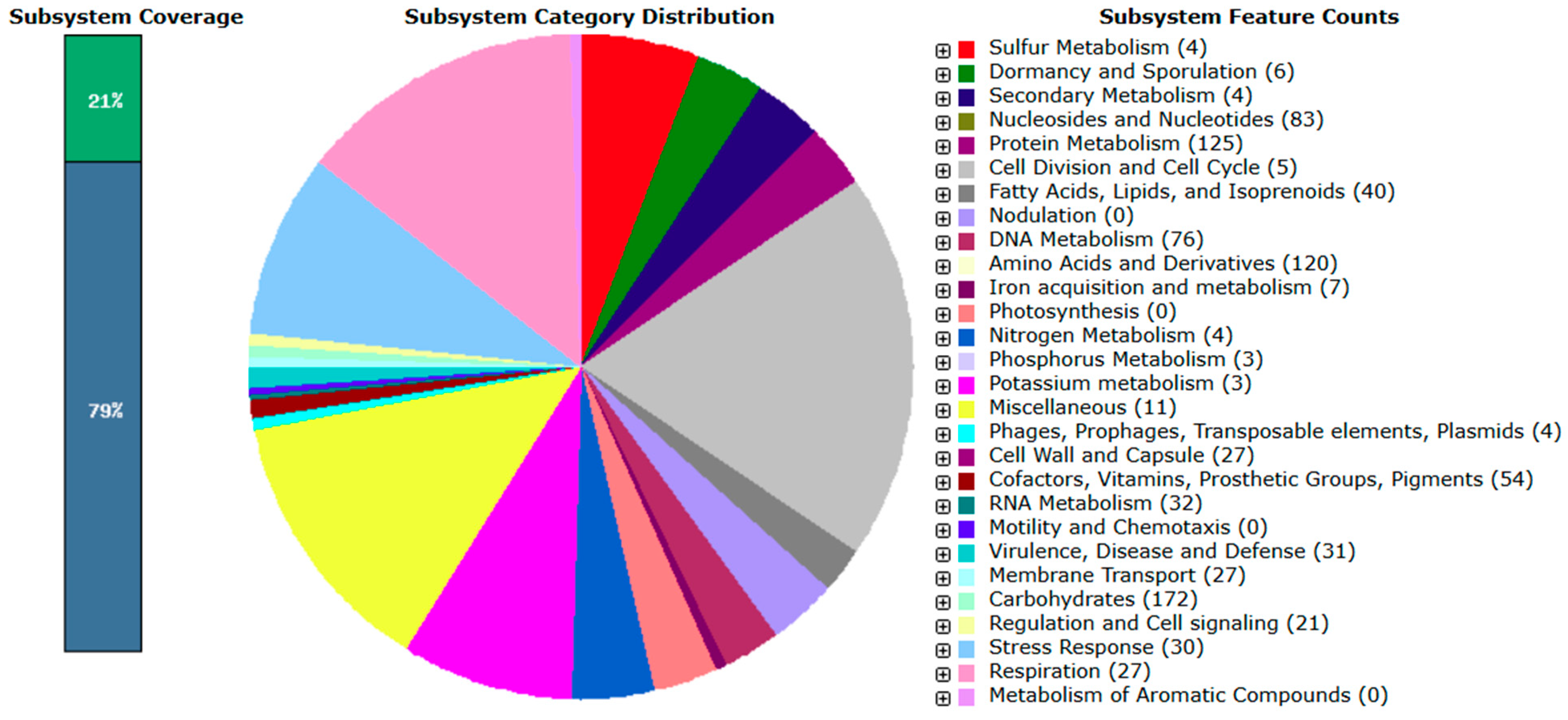
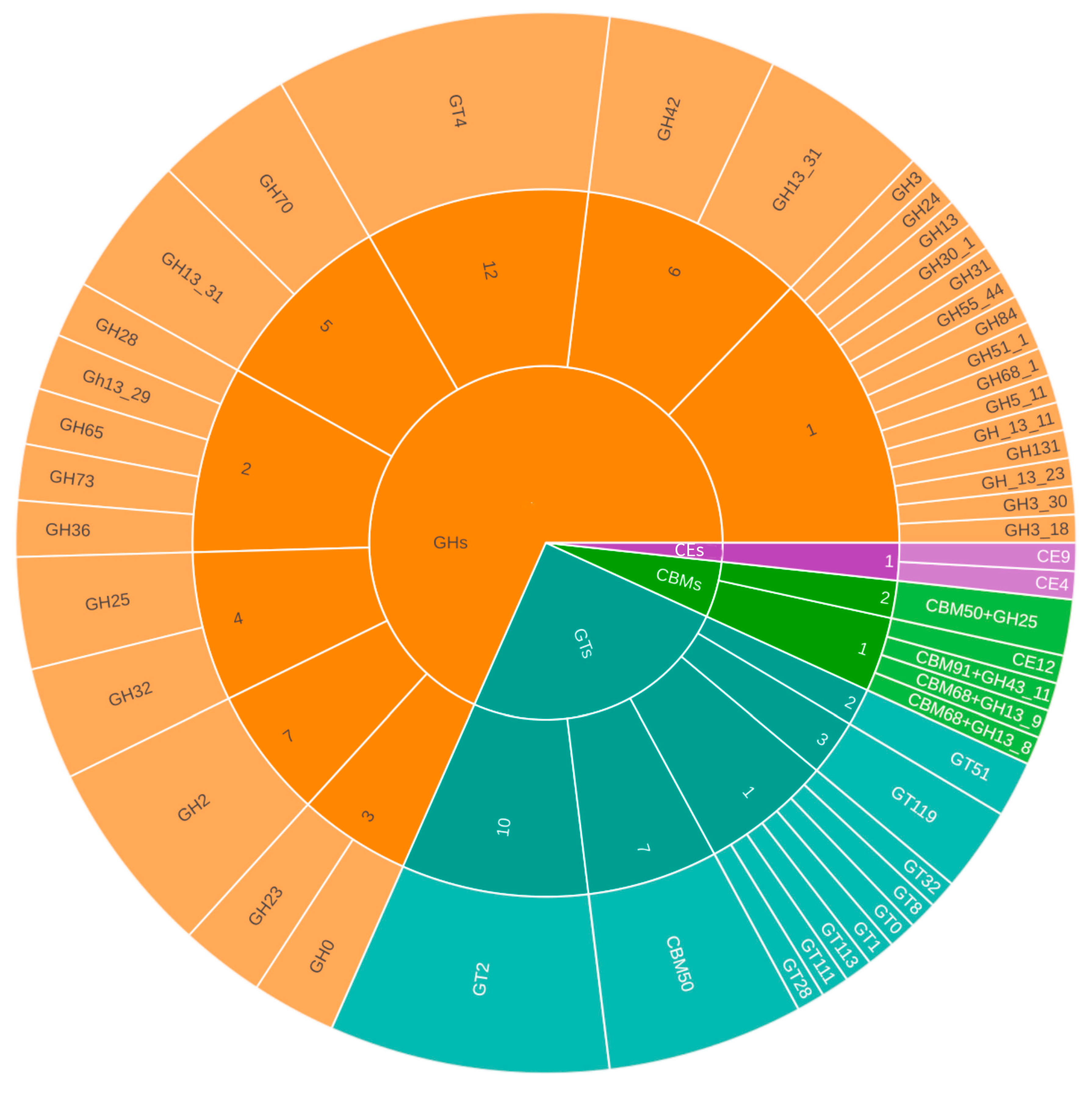
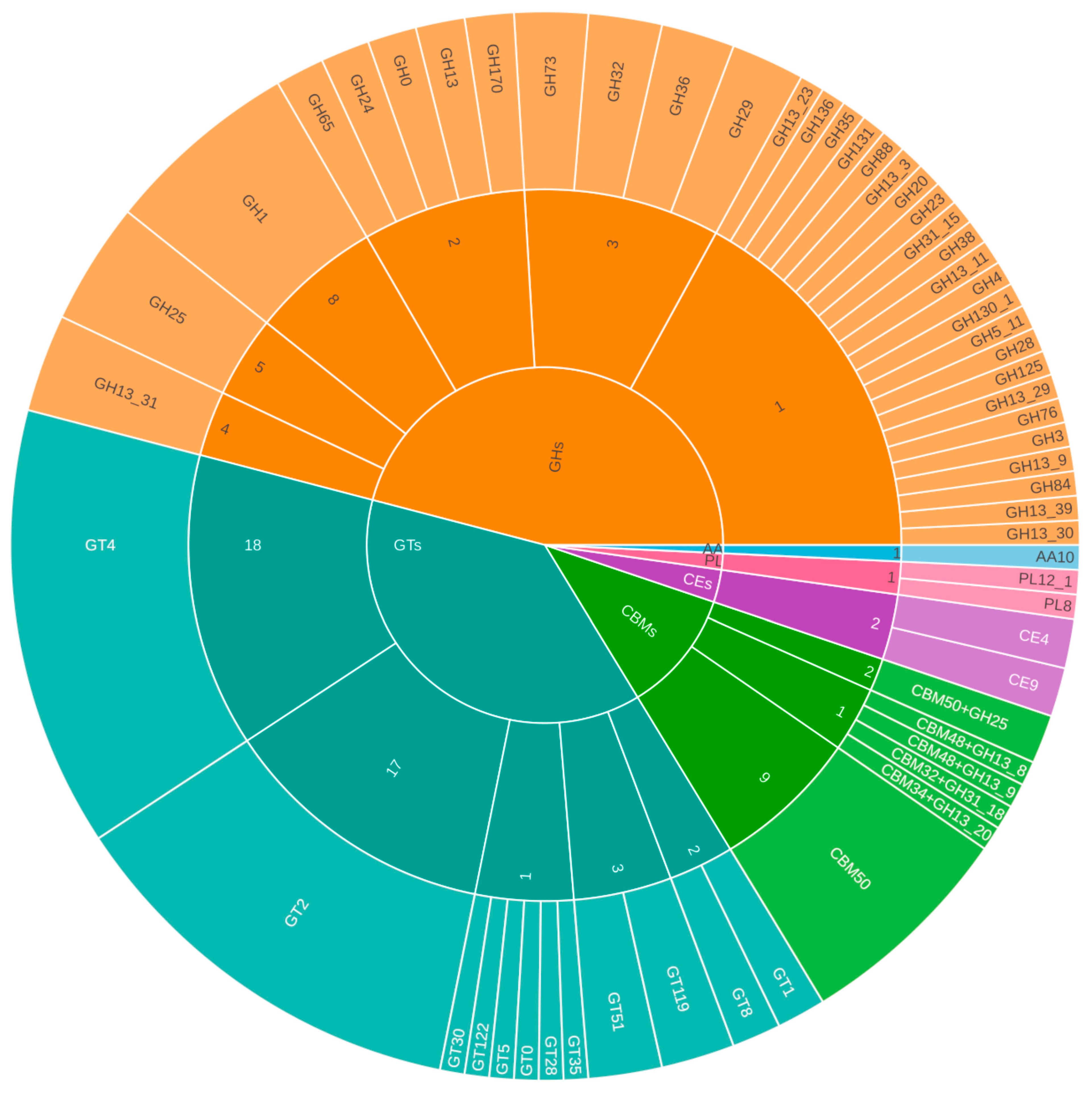
3.4.1. CAZymes
3.4.2. antiSMASH Analysis
3.4.3. Prokka
3.5. Antibiotic Susceptibility and Genome-Based AMR Assessment
4. Discussion
4.1. Ecological and Technological Relevance
4.2. Functional Performance and Stress Tolerance
4.3. Genome-Based Functional Insights
4.4. Biotechnological Implication
4.5. Antibiotic Susceptibility and AMR Genomic Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| CAZy | Carbohydrate active enzyme |
| CARD | Comprehensive Antibiotic Resistance Database |
| CDSs | Coding Sequences |
| EFSA | European Food Safety Authority |
| HPP | High pressure process |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| RiPPs | Ribosomally synthesized and post-translationally modified proteins |
References
- Sarti, L.; Martini, M.; Brajon, G.; Barni, S.; Salari, F.; Altomonte, I.; Ragona, G.; Mori, F.; Pucci, N.; Muscas, G.; et al. Donkey’s Milk in the Management of Children with Cow’s Milk Protein Allergy: Nutritional and Hygienic Aspects. Ital. J. Pediatr. 2019, 45, 102. [Google Scholar] [CrossRef]
- Albertos, I.; López, M.; Jiménez, J.M.; Cao, M.J.; Corell, A.; Castro-Alija, M.J. Characterisation of Zamorano-Leonese donkey milk as an alternative sustainably produced protein food. Front. Nutr. 2022, 9, 872409. [Google Scholar] [CrossRef]
- Martini, M.; Altomonte, I.; Licitra, R.; Salari, F. Nutritional and nutraceutical quality of donkey milk. J. Equine Vet. Sci. 2018, 65, 33–37. [Google Scholar] [CrossRef]
- Singh, M.P.; Vashisht, P.; Singh, L.; Awasti, N.; Sharma, S.; Mohan, C.; Charles, A.P.R. Donkey milk as a non-bovine alternative: A review of its nutri-functional properties, applications, and challenges. J. Food Sci. Technol. 2024, 61, 1652–1661. [Google Scholar] [CrossRef]
- Plotuna, A.M.; Hotea, I.; Ban-Cucerzan, A.; Badea, C.; Mladin, A.; Tîrziu, E. The microbial landscape of donkey milk: A systematic review. Rom. J. Vet. Sci. 2025, 58, 2. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 on the Hygiene of Food of Animal Origin. Off. J. Eur. Union 2004, L 139, 55–205. [Google Scholar]
- Codex Alimentarius. General Principles of Food Hygiene CXC 1-1969; 2020 Revision; FAO: Rome, Italy, 2020. [Google Scholar]
- Papademas, P.; Kamilari, E.; Aspri, M.; Anagnostopoulos, D.A.; Mousikos, P.; Kamilaris, A.; Tsaltas, D. Investigation of donkey milk bacterial diversity by 16S rDNA high-throughput sequencing on a Cyprus donkey farm. J. Dairy Sci. 2021, 104, 167–178. [Google Scholar] [CrossRef]
- Axelsson, L.; Fontana, A.; Morelli, L.; von Wright, A. Lactic Acid Bacteria: An Introduction to Taxonomy, Physiology, and Molecular Biology. In Lactic Acid Bacteria; CRC Press: Boca Raton, FL, USA, 2024; pp. 3–27. [Google Scholar]
- Hernández-Figueroa, R.H.; López-Malo, A.; Mani-López, E. Lactic acid bacteria-derived exopolysaccharides: Dual roles as functional ingredients and fermentation agents in food applications. Fermentation 2025, 11, 538. [Google Scholar] [CrossRef]
- Cirat, R.; Capozzi, V.; Benmechernene, Z.; Spano, G.; Grieco, F.; Fragasso, M. LAB antagonistic activities and their significance in food biotechnology: Molecular mechanisms, food targets, and other related traits of interest. Fermentation 2024, 10, 222. [Google Scholar] [CrossRef]
- Farid, W.; Masud, T.; Sohail, A.; Ahmad, N.; Naqvi, S.S.; Khan, S.; Manzoor, M.F. Gastrointestinal transit tolerance, cell surface hydrophobicity, and functional attributes of Lactobacillus acidophilus strains isolated from Indigenous Dahi. Food Sci. Nutr. 2021, 9, 5092–5102. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Pellegrino, M.S.; Frola, I.D.; Natanael, B.; Gobelli, D.; Nader-Macias, M.E.; Bogni, C.I. In vitro characterization of lactic acid bacteria isolated from bovine milk as potential probiotic strains to prevent bovine mastitis. Probiotics Antimicrob. Proteins 2019, 11, 74–84. [Google Scholar] [CrossRef]
- Islam, M.Z.; Uddin, M.E.; Rahman, M.T.; Islam, M.A.; Harun-ur-Rashid, M. Isolation and characterization of dominant lactic acid bacteria from raw goat milk: Assessment of probiotic potential and technological properties. Small Rumin. Res. 2021, 205, 106532. [Google Scholar] [CrossRef]
- Patil, A.; Disouza, J.; Pawar, S. Shelf life stability of encapsulated lactic acid bacteria isolated from sheep milk thrived in different milk as natural media. Small Rumin. Res. 2019, 170, 19–25. [Google Scholar] [CrossRef]
- Massouras, T.; Bitsi, N.; Paramithiotis, S.; Manolopoulou, E.; Drosinos, E.H.; Triantaphyllopoulos, K.A. Microbial profile, antibacterial properties and chemical composition of raw donkey milk. Animals 2020, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Greifová, G.; Drobná, E.; Olejníková, P.; Greif, G.; Greifová, M. Isolation, characterisation and technological properties of raw donkey’s milk isolate, Lacticaseibacillus paracasei, compared to raw goat’s and cow’s milk isolates. Czech J. Food Sci. 2025, 43, 118–128. [Google Scholar] [CrossRef]
- Negm El-Dein, A.; Noor El-Deen, A.; Tolba, S.; El-Shatoury, E.; Awad, G.; Ibrahim, M.; Farid, M. Probiotic properties and bile salt hydrolase activity of some isolated lactic acid bacteria. Egypt. J. Microbiol. 2017, 52, 87–100. [Google Scholar] [CrossRef]
- Mejía-Caballero, A.; López-Sánchez, R.; Ramos-Cerrillo, B.; Garciarrubio, A.; Segovia, L. Genomic insights into habitat adaptation of Lactobacillus species. World J. Microbiol. Biotechnol. 2025, 41, 61. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, X.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Comparative genomics and specific functional characteristics analysis of Lactobacillus acidophilus. Microorganisms 2021, 9, 1992. [Google Scholar] [CrossRef]
- Wels, M.; Siezen, R.; van Hijum, S.; Kelly, W.J.; Bachmann, H. Comparative genome analysis of Lactococcus lactis indicates niche adaptation and resolves genotype/phenotype disparity. Front. Microbiol. 2019, 10, 4. [Google Scholar] [CrossRef]
- Menéndez-Cañamares, S.; Blázquez, A.; Albertos, I.; Poveda, J.; Díez, A. Probiotic Bacillus subtilis SB8 and edible coatings for sustainable fungal disease management in strawberry. Biol. Control 2024, 196, 105572. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Vinderola, G.; Gueimonde, M.; Gomez-Gallego, C.; Delfederico, L.; Salminen, S. Correlation between in vitro and in vivo assays in selection of probiotics from traditional species of bacteria. Trends Food Sci. Technol. 2017, 68, 83–90. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Medema, M.H.; Weber, T. The antiSMASH database version 4: Additional genomes and BGCs, new sequence-based searches and more. Nucleic Acids Res. 2024, 52, D586–D589. [Google Scholar] [CrossRef]
- Zheng, J.; Ge, Q.; Yan, Y.; Zhang, X.; Huang, L.; Yin, Y. dbCAN3: Automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 2023, 51, W115–W121. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Aarestrup, F.M. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Fiocco, D.; Albenzio, M.; Spano, G.; Capozzi, V. Microbial populations of fresh and cold-stored donkey milk by high-throughput sequencing provide indication for a correct management of this high-value product. Appl. Sci. 2020, 10, 2314. [Google Scholar] [CrossRef]
- Akpinar, A.; Yerlikaya, O. Some potential beneficial properties of Lacticaseibacillus paracasei subsp. paracasei and Leuconostoc mesenteroides strains originating from raw milk and kefir grains. J. Food Process. Preserv. 2021, 45, e15986. [Google Scholar] [CrossRef]
- Hayek, S.A.; Gyawali, R.; Aljaloud, S.O.; Krastanov, A.; Ibrahim, S.A. Cultivation media for lactic acid bacteria used in dairy products. J. Dairy Res. 2019, 86, 490–502. [Google Scholar] [CrossRef]
- Djerrab, L.; Chekroud, Z.; Rouabhia, A.; Dems, M.A.; Attailia, I.; Garcia, L.I.R.; Smadi, M.A. Potential use of Bacillus paramycoides for the production of the biopolymer polyhydroxybutyrate from leftover carob fruit agro-waste. AIMS Microbiol. 2022, 8, 318. [Google Scholar] [CrossRef]
- Singhal, N.; Singh, N.S.; Mohanty, S.; Kumar, M.; Virdi, J.S. Rhizospheric Lactobacillus plantarum (Lactiplantibacillus plantarum) strains exhibit bile salt hydrolysis, hypocholesterolemic and probiotic capabilities in vitro. Sci. Rep. 2021, 11, 15288. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, L.; Tian, F.; Zhao, J.; Zhai, Q. Identification of Novel Bile Salt-Tolerant Genes in Lactobacillus Using Comparative Genomics and Its Application in the Rapid Screening of Tolerant Strains. Microorganisms 2022, 10, 2371. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.J.N.; Costa, I.M.; Rocha, W.C.D.; Silva, J.F.; Sousa, L.F.; Pereira, A.L.F.; Gomes, M.M.; Albuquerque, R.F.; Nascimento, J.S.; Oliveira, M.E.G.; et al. Lactococcus lactis GV103, potentially probiotic, applied in the development of lactose-free fermented milk. Braz. J. Microbiol. 2025, 56, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y.; Li, L.; Li, Y.; Yuan, L.; E, Y.; Qiao, J. Positive Regulation of the DLT Operon by TCSR7 Enhances Acid Tolerance of Lactococcus lactis F44. J. Dairy Sci. 2022, 105, 7940–7950. [Google Scholar] [CrossRef]
- Kandasamy, S.; Lee, K.H.; Yoo, J.; Yun, J.; Kang, H.B.; Kim, J.E.; Ham, J.S. Whole genome sequencing of Lacticaseibacillus casei KACC92338 strain with strong antioxidant activity reveals genes and gene clusters of probiotic and antimicrobial potential. Front. Microbiol. 2024, 15, 1458221. [Google Scholar] [CrossRef]
- Samodra, E.M.A.; Suroto, D.; Utami, T.; Wikandari, R.; Rahayu, E.S. Cold Stress Response Genes of Lactiplantibacillus plantarum subsp. plantarum Mut-3 and Lactiplantibacillus plantarum subsp. plantarum Mut-7 Support the Ability to Survive in Low-Temperature Conditions. HAYATI J. Biosci. 2023, 30, 65–70. [Google Scholar] [CrossRef]
- Bucka-Kolendo, J.; Sokołowska, B. Impact of High Hydrostatic Pressure on the Single Nucleotide Polymorphism of Stress-Related dnaK, hrcA, and ctsR in Lactobacillus Strains. Qual. Assur. Saf. Crops Foods 2022, 14, 54–66. [Google Scholar] [CrossRef]
- Nöldeke, E.R.; Stehle, T. Unraveling the Mechanism of Peptidoglycan Amidation by the Bifunctional Enzyme Complex GatD/MurT: A Comparative Structural Approach. Int. J. Med. Microbiol. 2019, 309, 151334. [Google Scholar] [CrossRef]
- Han, S.; Elnar, A.G.; Lim, C.; Kim, G.B. Complete Genome Sequence of Bacteriocin-Producing Ligilactobacillus salivarius B4311 Isolated from Fecal Samples of Broiler Chicken with Anti-Listeria Activity. J. Anim. Sci. Technol. 2024, 66, 232–236. [Google Scholar] [CrossRef]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and Utility of the Antioxidant Potential of Probiotic Lactobacilli and Bifidobacteria as Representatives of the Human Gut Microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef]
- Goyal, D.; Swaroop, S.; Prakash, O.; Pandey, J. Survival Strategies in Cold-Adapted Microorganisms. In Survival Strategies in Cold-Adapted Microorganisms; Springer: Singapore, 2021; pp. 173–186. [Google Scholar] [CrossRef]
- Martínez-Miranda, J.G.; Chairez, I.; Durán-Páramo, E. Mannitol Production by Heterofermentative Lactic Acid Bacteria: A Review. Appl. Biochem. Biotechnol. 2022, 194, 2762–2795. [Google Scholar] [CrossRef] [PubMed]
- Darsanaki, R.K.; Aliabadi, M.A.; Chakoosari, M.M.D. Antibiotic resistance of lactic acid bacteria. Sci. J. Microbiol. 2013, 2, 201–206. [Google Scholar] [CrossRef]
| Bacterial Isolate | Closest Match (Database) | Nucleotide Identity (%) |
|---|---|---|
| B1.2 | Enterococcus gallinarum 4493 | 99.9% |
| B2 | Enterobacter roggenkampii EN-117 | 100% |
| B3 | Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 | 100% |
| B4 | Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 | 100% |
| B5 | Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 | 100% |
| B.6.1.1 | Enterobacter kobei DSM 13645 | 100% |
| B.6.1.2 | Enterobacter kobei DSM 13645 | 100% |
| B7 | Enterobacter asburiae BY4 | 100% |
| B8 | Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 | 100% |
| B9 | Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 | 100% |
| B12 | Bacillus tequilensis KCTC13622 | 100% |
| B13 | Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 | 100% |
| B15 | Leuconostoc mesenteroides subsp. mesenteroides ATCC 8293 | 100% |
| B19 | Lacticaseibacillus paracasei subsp. tolerans ATCC 25599 | 100% |
| Treatment | Incubation Time | ||
|---|---|---|---|
| Bacterial isolate | 24 h | 48 h | 72 h |
| Control | 4.67 ± 0.153 Ba | 4.43 ± 0.08 Ab | 4.47 ± 0.09 ABc |
| B3 | 5.06 ± 0.055 Bb | 4.96 ± 0.05 Ae | 4.91 ± 0.01 Af |
| B4 | 6.4 ± 0.1 Cf | 4.85 ± 0.09 Bde | 4.61 ± 0.05 Ad |
| B5 | 5.85 ± 0.07 Be | 5.74 ± 0.06 Ae | 5.62 ± 0.03 Ag |
| B8 | 5.03 ± 0.057 Bb | 4.85 ± 0.09 Ade | 4.82 ± 0.02 Ae |
| B9 | 6.65 ± 0.14 Bg | 4.72 ± 0.05 Ac | 4.63 ± 0.03 Ad |
| B12 | 5.85 ± 0.06 Ae | 5.95 ± 0.05 Bf | 5.94 ± 0.03 ABh |
| B13 | 5.62 ± 0.11 Bcd | 4.44 ± 0.07 Ab | 4.34 ± 0.03 Ab |
| B19 | 5.96 ± 0.06 Be | 4.11 ± 0.04 Aa | 4.04 ± 0.04 Aa |
| Bacterial Isolate | Acid Tolerance (pH 2.5) | Bile Tolerance (0.3% ox bile) |
|---|---|---|
| B3 | w | − |
| B4 | w | w |
| B5 | + | + |
| B8 | + | + |
| B9 | + | w |
| B12 | w | + |
| B13 | − | − |
| B19 | + | + |
| Features | L. mesenteroides subsp. mesenteroides B8 | L. paracasei subsp. tolerans B19 |
|---|---|---|
| Genome size (bp) | 2,119,290 | 3,154,893 |
| L50 | 3 | 8 |
| N50 | 278,678 | 109,143 |
| GC content | 37.7 | 46.2 |
| Number of contigs | 27 | 81 |
| Predicted coding sequences | 2169 | 3275 |
| Subsystem | 210 | 229 |
| Number of RNAs | 69 | 68 |
| Cluster | Type | Genome Location |
|---|---|---|
| Cluster 1 | Cytokinins, Terpenoids precursors | 359,483–406,232 |
| Cluster 2 | T3KPS | 64,089–105,246 |
| Cluster 3 | β-lactones | 56,530–88,656 |
| Cluster 4 | Lincosamides | 49,540–114,758 |
| Cluster | Type | Genome Location |
|---|---|---|
| Cluster 1 | Similar RiPPs | 1038–11,555 |
| Cluster 2 | Terpenoids precursors | 93,206–114,057 |
| Cluster 3 | Similar RiPPs | 1–8996 |
| Cluster 4 | Similar RiPPs | 2801–13,798 |
| Gene ID | Gene Name | Function |
|---|---|---|
| Cold stress resistance | ||
| fig|6666666.1141134.peg.248 | cspLA_1 | Cold shock protein CspLA. |
| fig|6666666.1141134.peg.1486 | cspLA_2 | Cold shock protein CspLA. |
| fig|6666666.1141134.peg.1612 | cspG | Cold shock CspLG. |
| Biosynthesis proteins | ||
| fig|6666666.1141134.peg.1663 | coaBC | Bifunctional coenzyme A biosynthesis protein CoaBC |
| fig|6666666.1141134.peg.1912 | gshAB | Bifunctional glutathione biosynthesis protein GshAB |
| fig|6666666.1141134.peg.369 | ribD | Riboflavin biosynthesis protein RibD |
| fig|6666666.1141134.peg.371 | ribBA | Riboflavin biosynthesis protein RibBA |
| Adhesion Genes | ||
| fig|6666666.1141134.peg.1759 | dltA | D-alanine and D-alanyl ligase transport proteins |
| fig|6666666.1141134.peg.1762 | dltD | DltD protein |
| fig|6666666.1141134.peg.1761 | dltC | D-alanyl transport protein |
| Acid stress resistance | ||
| fig|6666666.1141134.peg.1713 | aroE | Kimate dehydrogenase (NADP (+)) |
| fig|6666666.1141134.peg.2121 | npr | NADH peroxidase |
| fig|6666666.1141134.peg.11 | murE | Murein endolytic transglycosylase |
| Carbohydrate metabolism genes | ||
| fig|6666666.1141134.peg.504 | bglB | Thermostable beta-glucosidase B |
| fig|6666666.1141134.peg.771 | lacZ | Beta-galactosidase |
| fig|6666666.1141134.peg.392 | bgaA | Beta-galactosidase BgaA |
| fig|6666666.1141134.peg.668 | GanA | Beta-galactosidase GanA |
| Gene ID | Gene Name | Function |
|---|---|---|
| General stress | ||
| fig|6666666.1141135.peg.2309 | gspA | General stress protein A |
| Gastrointestinal survival | ||
| fig|6666666.1141135.peg.622 | uspA | Universal stress protein UspA |
| fig|6666666.1141135.peg.3160 | msrA | Methionine sulfoxide reductase peptide MsrA |
| fig|6666666.1141135.peg.2333 | msrB | Methionine sulfoxide reductase peptide MsrB |
| Termic shock resistance | ||
| fig|6666666.1141135.peg.1977 | hrcA | Heat-inducible transcription repressor HrcA |
| fig|6666666.1141135.peg.643 | ctsR | Transcriptional regulator CtsR |
| fig|6666666.1141135.peg.1979 | dnaK | DnaK cofactor, |
| fig|6666666.1141135.peg.1980 | dnaJ | DnaJ chaperone protein |
| Adhesion Genes and cell wall synthesis | ||
| fig|6666666.1141135.peg.2181 | lysM | LysM domain-containing protein |
| fig|6666666.1141135.peg.2469 | pilO | Type 4a pilus biogenesis protein PilO |
| fig|6666666.1141135.peg.3111 | murT | MurT subunit of lipid isoglutaminyl synthase II |
| Acid stress resistance | ||
| fig|6666666.1141134.peg.1713 | aroE | Kimate dehydrogenase (NADP (+)) |
| fig|6666666.1141134.peg.2121 | npr | NADH peroxidase |
| fig|6666666.1141134.peg.11 | murE | Murein endolytic transglycosylase |
| Characteristic probiotic genes | ||
| fig|6666666.1141135.peg.400 | lacB | LacB subunit of galactose-6-phosphate isomerase |
| fig|6666666.1141135.peg.2703 | lacT | LacT anti-transcriptional terminator |
| fig|6666666.1141135.peg.618 | gbuC | Glycine betaine/carnitine transporter-binding protein GbuC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulnes, D.; Albertos, I.; Jiménez, J.-M.; Castro-Alija, M.J.; Díez-Méndez, A. Cold-Resistant Lactic Acid Bacteria from Zamorano-Leonesa Donkey Milk: Isolation, Functional Screening, and Genome-Based Insights for Technological Applications. Appl. Microbiol. 2025, 5, 135. https://doi.org/10.3390/applmicrobiol5040135
Bulnes D, Albertos I, Jiménez J-M, Castro-Alija MJ, Díez-Méndez A. Cold-Resistant Lactic Acid Bacteria from Zamorano-Leonesa Donkey Milk: Isolation, Functional Screening, and Genome-Based Insights for Technological Applications. Applied Microbiology. 2025; 5(4):135. https://doi.org/10.3390/applmicrobiol5040135
Chicago/Turabian StyleBulnes, David, Irene Albertos, José-María Jiménez, María José Castro-Alija, and Alexandra Díez-Méndez. 2025. "Cold-Resistant Lactic Acid Bacteria from Zamorano-Leonesa Donkey Milk: Isolation, Functional Screening, and Genome-Based Insights for Technological Applications" Applied Microbiology 5, no. 4: 135. https://doi.org/10.3390/applmicrobiol5040135
APA StyleBulnes, D., Albertos, I., Jiménez, J.-M., Castro-Alija, M. J., & Díez-Méndez, A. (2025). Cold-Resistant Lactic Acid Bacteria from Zamorano-Leonesa Donkey Milk: Isolation, Functional Screening, and Genome-Based Insights for Technological Applications. Applied Microbiology, 5(4), 135. https://doi.org/10.3390/applmicrobiol5040135










