Evaluation of the Microalga Graesiella emersonii Growth on Concentrated Cheese Whey Permeate
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Strain
2.2. Cultivation Media
2.3. Experimental Design
2.4. Biomass Productivity
2.5. β-Galactosidase Activity
2.6. Organic C Uptake
2.7. Total Lipid Content
2.8. Fatty Acid Content Analysis with GAS Chromatography—Mass Spectrometry (GC-MS)
2.8.1. Sample Extraction from Biomass
2.8.2. Microalgal Extract Analysis Using GC-MS
2.9. Data Statistical Analysis
3. Results
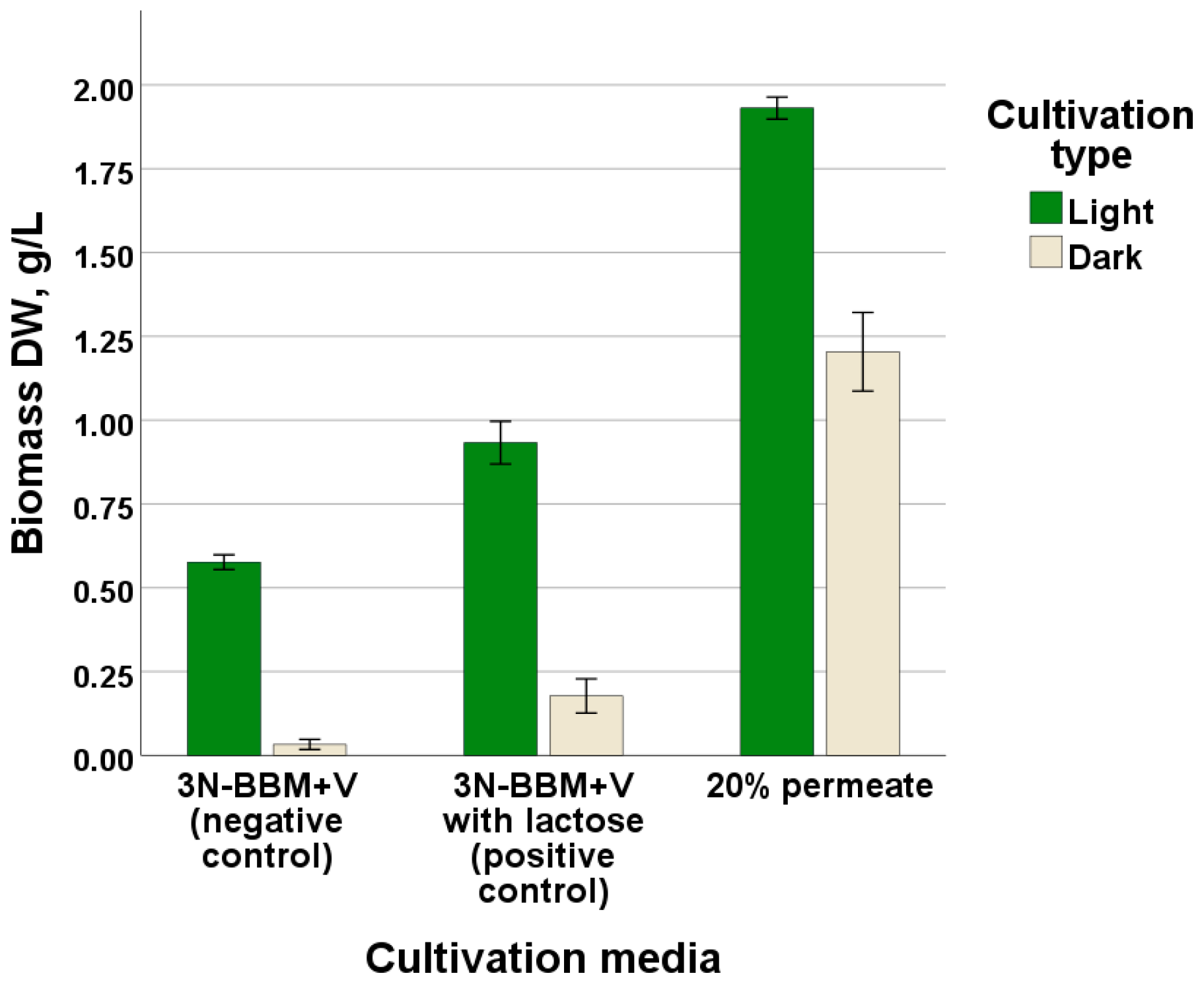
3.1. Optimization of G. emersonii Cultivation Conditions
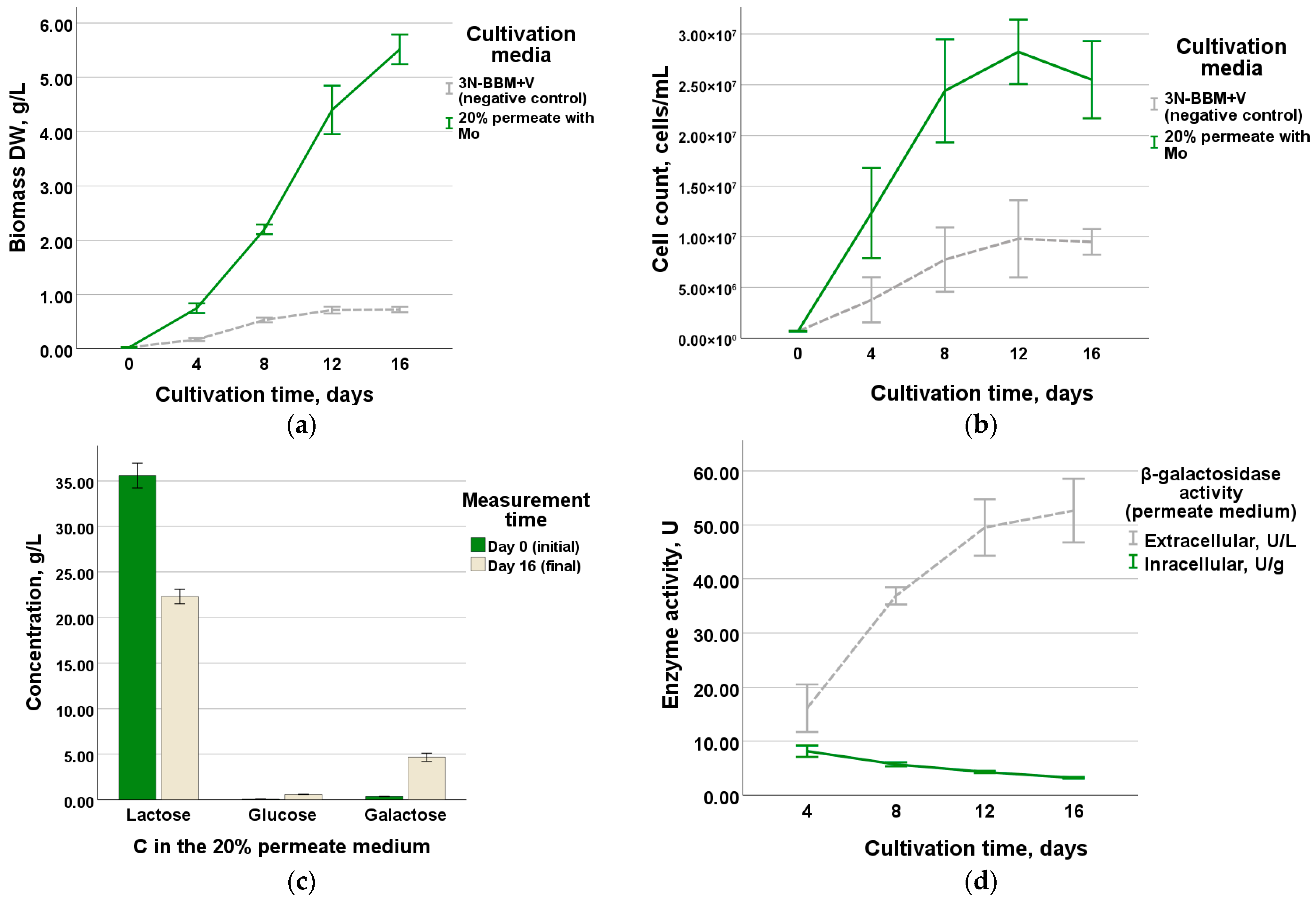
3.2. Evaluation of G. emersonii Growth in Optimized Medium
3.3. Changes in Fatty Acid Compostion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3N-BBM+V | Bold’s basal medium with triple nitrogen and vitamins |
| ANOVA | Analysis of variance |
| DW | Dry weight, g/L |
| GC-MS | Gas chromatography—mass spectrometry |
| MUFA | Monosaturated fatty acids |
| NEA | Normalized enzyme activity, U/g |
| ONPG | Ortho-nitrophenyl-β-galactoside |
| PUFA | Polyunsaturated fatty acids |
| SFA | Saturated fatty acids |
| VEA | Volumetric enzyme activity, U/L |
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new bio technology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and challenges of large-scale cultivation of photosynthetic microalgae and cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Alavianghavanini, A.; Shayesteh, H.; Bahri, P.A.; Vadiveloo, A.; Moheimani, N.R. Microalgae cultivation for treating agricultural effluent and producing value-added products. Sci. Total Environ. 2024, 912, 169369. [Google Scholar] [CrossRef]
- Ozcelik, D.; Suwal, S.; Ray, C.; Tiwari, B.K.; Jensen, P.E.; Poojary, M.M. Valorization of dairy side-streams for the cultivation of microalgae for value added food products. Trends Food Sci. Technol. 2024, 106, 104386. [Google Scholar] [CrossRef]
- Britz, J.T.; van Schalwyk, C.; Hung, Y.T. Treatment of dairy processing wastewaters. In Waste Treatment in the Food Processing Industry; Wang, L.K., Hung, Y.T., Lo, H.H., Yapijakis, C., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–25. [Google Scholar]
- Udourioh, G.A.; Solomon, M.M.; Okolie, J.A. A review of the valorization of dairy industry wastes through thermochemical, biological, and integrated processes for value-added products. Food Sci. Anim. Resour. 2025, 45, 375–408. [Google Scholar] [CrossRef]
- OECD-FAO agricultural Outlook 2022–2031. In World-Wide Electronic Publication; OECD Publishing: Paris, France, 2022; Available online: https://www.oecd.org/en/publications/2022/06/oecd-fao-agricultural-outlook-2022-2031_e00c413c.html (accessed on 6 June 2024).
- Mehner, E.; Fantin, V.; Pizzichini, D.; Vaccari, M. Decentralised by-product valorisation in the dairy value chain: An opportunity for sustainable intensification. J. Clean. Prod. 2024, 478, 143958. [Google Scholar] [CrossRef]
- Kolesovs, S.; Semjonovs, P. Microalgal conversion of whey and lactose containing substrates: Current state and challenges. Biodegradation 2023, 34, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Hidasi, N.; Badary, A.; Jenkins, H.D.; Fields, F.J.; Mayfield, S.P.; Ferrari, S. Selection and characterization of a Parachlorella kessleri microalgal strain able to assimilate lactose, and grow on dairy waste. Biomass Bioenergy 2024, 188, 107344. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.L.; Berthon, J.Y.; Filaire, E. Microalgae n-3 PUFAs production and use in food and feed industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 18, 61–66. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Fan, K.W.; Jiang, Y.; Zhong, Y.; Sun, Z.; Chen, F. Production potential of Chlorella zofingiensis as a feedstock for biodiesel. Bioresour. Technol. 2010, 101, 8658–8663. [Google Scholar] [CrossRef] [PubMed]
- Ki, H.; Kim, E.S.; An, S.M.; Kang, N.S.; Bae, S.S.; Choi, G.; Pan, C.H.; Kim, K.Y.; Patil, J.G.; Cho, K. Enhanced carotenoid production, biodiesel quality, and harvesting efficiency in microalga Graesiella emersonii via heterotrophic cultivation strategy. Algal Res. 2024, 78, 103437. [Google Scholar] [CrossRef]
- Kolesovs, S.; Shvirksts, K.; Grube, M.; Vigants, A. Studies on lactose utilization by the microalga Graesiella emersonii in the 3N-BBM-V model medium. Eur. J. Phycol. 2025, 60, 175–184. [Google Scholar] [CrossRef]
- Kolesovs, S.; Strazdina, I.; Vigants, A. Mixotrophic cultivation of green microalga Graesiella emersonii on cheese whey permeate. Rural Sustain. Res. 2025, 53, 1–7. [Google Scholar] [CrossRef]
- Zanette, C.M.; Mariano, A.B.; Yukawa, Y.S.; Mendes, I.; Spier, M.R. Microalgae mixotrophic cultivation for β-galactosidase production. J. Appl. Phycol. 2019, 31, 1597–1606. [Google Scholar] [CrossRef]
- Bentahar, J.; Deschênes, J.S. Influence of sweet whey permeate utilization on Tetradesmus obliquus growth and β-galactosidase production. Can. J. Chem. Eng. 2021, 100, 1479–1488. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Screening microalgae for producing biofuel precursors from industrial off-gases. Sustainability 2025, 17, 2964. [Google Scholar] [CrossRef]
- Mandal, M.K.; Saikia, P.; Chanu, N.; Chaurasia, N. Modulation of lipid content and lipid profile by supplementation of iron, zinc, and molybdenum in indigenous microalgae. Environ. Sci. Pollut. Res. 2019, 26, 20815–20828. [Google Scholar] [CrossRef] [PubMed]
- Bentahar, J.; Doyen, A.; Beaulieu, L.; Deschênes, J.S. Investigation of β-galactosidase production by microalga Tetradesmus obliquus in determined growth conditions. J. Appl. Phycol. 2019, 31, 301–308. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, P.S.; Del Signore, F.; Canziani, S.; Caggia, C.; Mezzanotte, V.; Ferrer-Ledo, N. Mixotrophic and heterotrophic growth of Galdieria sulphuraria using buttermilk as a carbon source. J. Appl. Phycol. 2023, 35, 2631–2643. [Google Scholar] [CrossRef]
- Tsotsouli, K.; Didos, S.; Koukaras, K.; Argiriou, A. Mixotrophic cultivation of Dunaliella tertiolecta in cheese whey effluents to enhance biomass and exopolysaccharides (EPS) production: Biochemical and functional insights. Mar. Drugs 2025, 23, 120. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef]

| Parameter | Contents | Method |
|---|---|---|
| pH | 6.3 ± 0.1 | pH electrode measurement |
| Dry mater | ~18% (w/v) | Gravimetric method |
| Total carbohydrates | ~17% (w/v) | Mid-infrared spectroscopy |
| - Lactose | 160 g/L | Lactose/galactose enzymatic assay kit |
| - Galactose | 2.5 g/L | Lactose/galactose enzymatic assay kit |
| - Glucose | 0.3 g/L | Glucose enzymatic kit |
| Total proteins | ~1% (w/v) | Mid-infrared spectroscopy |
| Total lipids | 0.02% (w/v) | Mid-infrared spectroscopy |
| Detected Fatty Acids | 3N-BBM+V Photoautotrophic, % | 20% Permeate Mixotrophic, % | 20% Permeate with Mo Mixotrophic, % |
|---|---|---|---|
| Lauric acid | - 1 | 0.62 | 0.51 |
| Myristic acid | 0.30 | 2.82 | 2.53 |
| Pentadecanoic acid | 0.69 | 0.41 | 0.37 |
| 7-Hexadecenoic acid | 1.77 | 1.51 | 1.62 |
| Palmitic acid | 22.57 | 24.07 | 24.30 |
| γ-Linolenic acid | 0.99 | 0.36 | 0.40 |
| Linoleic acid | 15.98 | 4.49 | 4.14 |
| α-Linolenic acid | 15.98 | 8.66 | 5.51 |
| Oleic acid | 28.81 | 21.89 | 24.87 |
| Stearic acid | 1.46 | 11.64 | 11.72 |
| 11-Eicosenoic acid | 1.51 | 1.33 | 1.31 |
| Arachidic acid | 0.22 | 1.28 | 1.25 |
| Heneicosanoic acid | 0.09 | - 1 | - 1 |
| Behenic acid | 0.43 | 1.00 | 0.82 |
| Lignoceric acid | 0.99 | 2.00 | 1.70 |
| Cerotic acid | 2.37 | 11.02 | 11.78 |
| Carboceric acid | 0.30 | 0.23 | 0.23 |
| Montanic acid | 4.69 | 6.38 | 6.67 |
| Melissic acid | 1.03 | 0.28 | 0.28 |
| SFA | 35.14 | 61.75 | 62.16 |
| MUFA | 32.08 | 24.74 | 27.79 |
| PUFA | 32.77 | 13.51 | 10.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesovs, S.; Strazdina, I.; Klavins, L.; Vigants, A. Evaluation of the Microalga Graesiella emersonii Growth on Concentrated Cheese Whey Permeate. Appl. Microbiol. 2025, 5, 124. https://doi.org/10.3390/applmicrobiol5040124
Kolesovs S, Strazdina I, Klavins L, Vigants A. Evaluation of the Microalga Graesiella emersonii Growth on Concentrated Cheese Whey Permeate. Applied Microbiology. 2025; 5(4):124. https://doi.org/10.3390/applmicrobiol5040124
Chicago/Turabian StyleKolesovs, Sergejs, Inese Strazdina, Linards Klavins, and Armands Vigants. 2025. "Evaluation of the Microalga Graesiella emersonii Growth on Concentrated Cheese Whey Permeate" Applied Microbiology 5, no. 4: 124. https://doi.org/10.3390/applmicrobiol5040124
APA StyleKolesovs, S., Strazdina, I., Klavins, L., & Vigants, A. (2025). Evaluation of the Microalga Graesiella emersonii Growth on Concentrated Cheese Whey Permeate. Applied Microbiology, 5(4), 124. https://doi.org/10.3390/applmicrobiol5040124







