Identification of Filamentous Fungi Present in Prolonged Fermentations of Coffea arabica L. var. Castillo
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation Process Used in This Study
2.2. Sampling and Isolation in Pure Culture
2.3. Macroscopic and Microscopic Characterization
2.4. Taxonomic Classification Based on Metabolic Fingerprinting
2.5. Metataxonomic Classification of Filamentous Fungi
2.6. Determination of Ochratoxin A (OTA)
3. Results
3.1. Macroscopic and Microscopic Filamentous Fungi Characterized
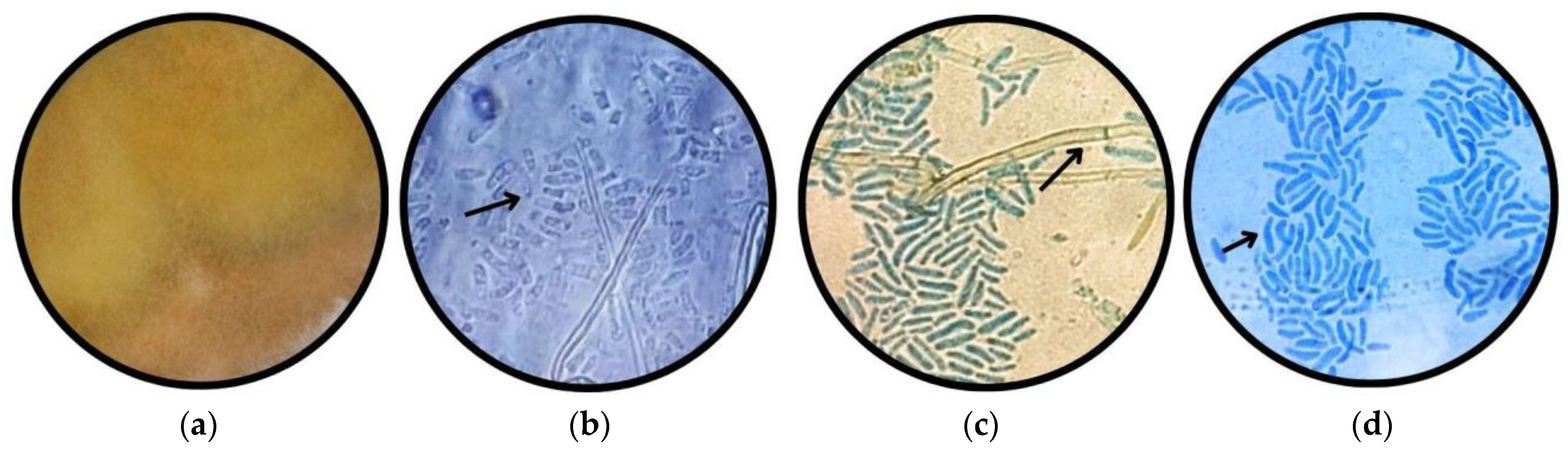
3.1.1. Morphotype 1
3.1.2. Morphotype 2
3.1.3. Morphotype 3
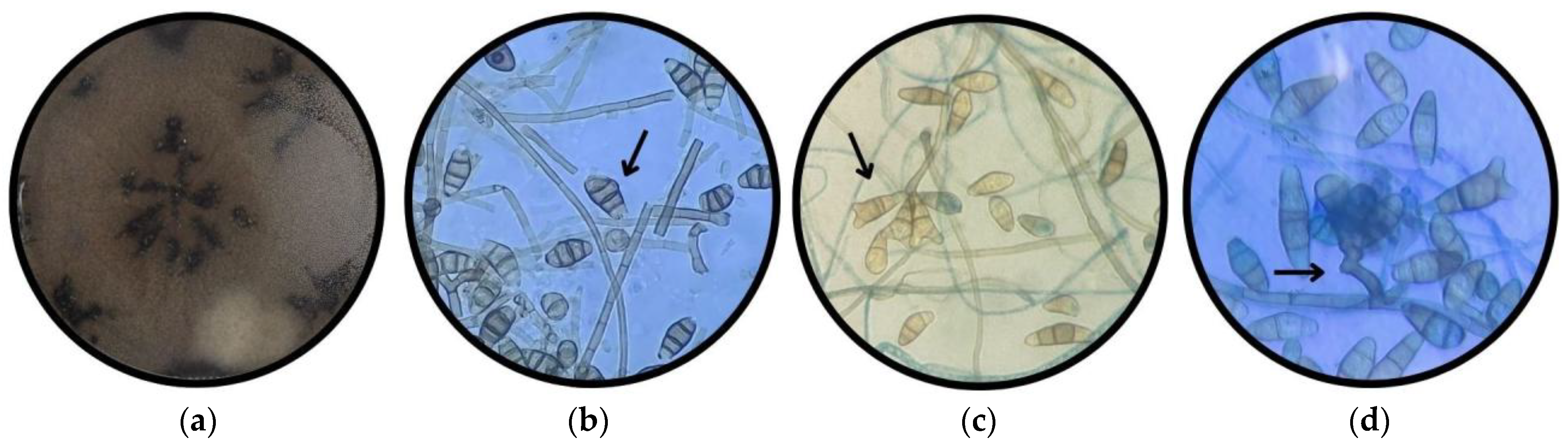
3.1.4. Morphotype 4
3.1.5. Morphotype 5
3.1.6. Morphotype 6
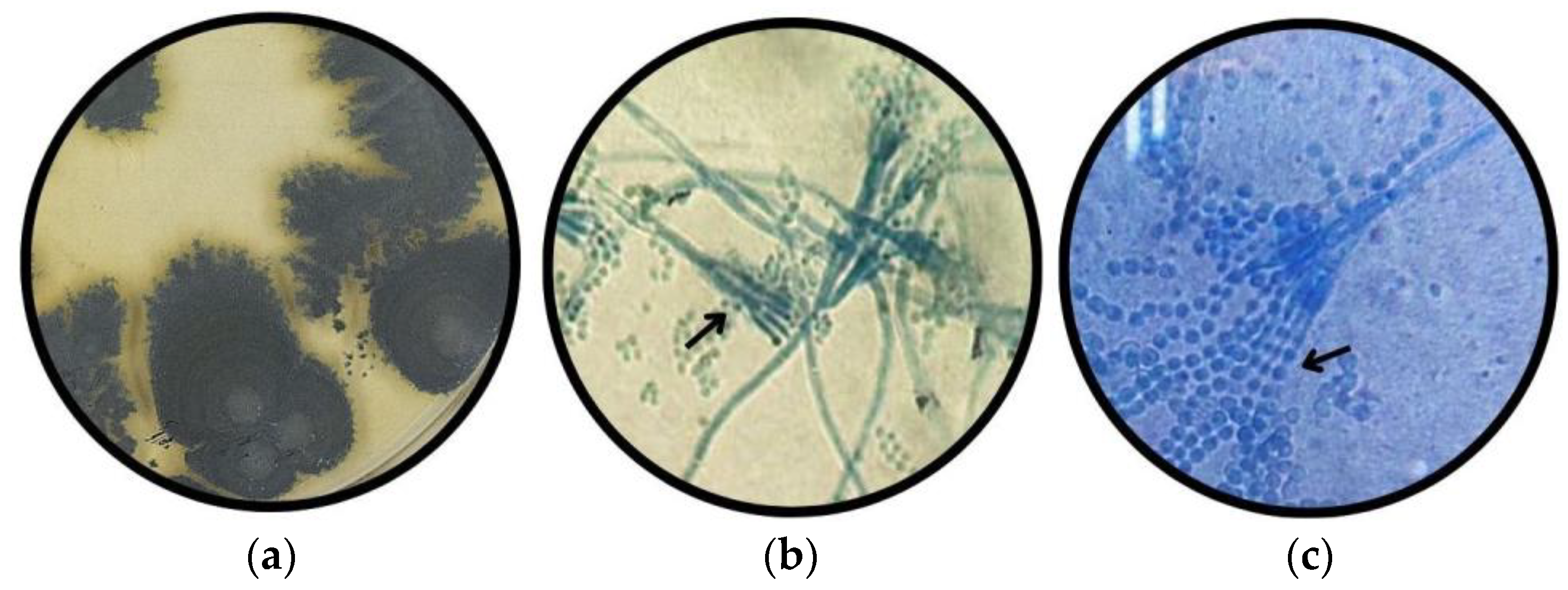
3.1.7. Morphotype 7
3.2. Taxonomic Classification According to Metabolic Fingerprint
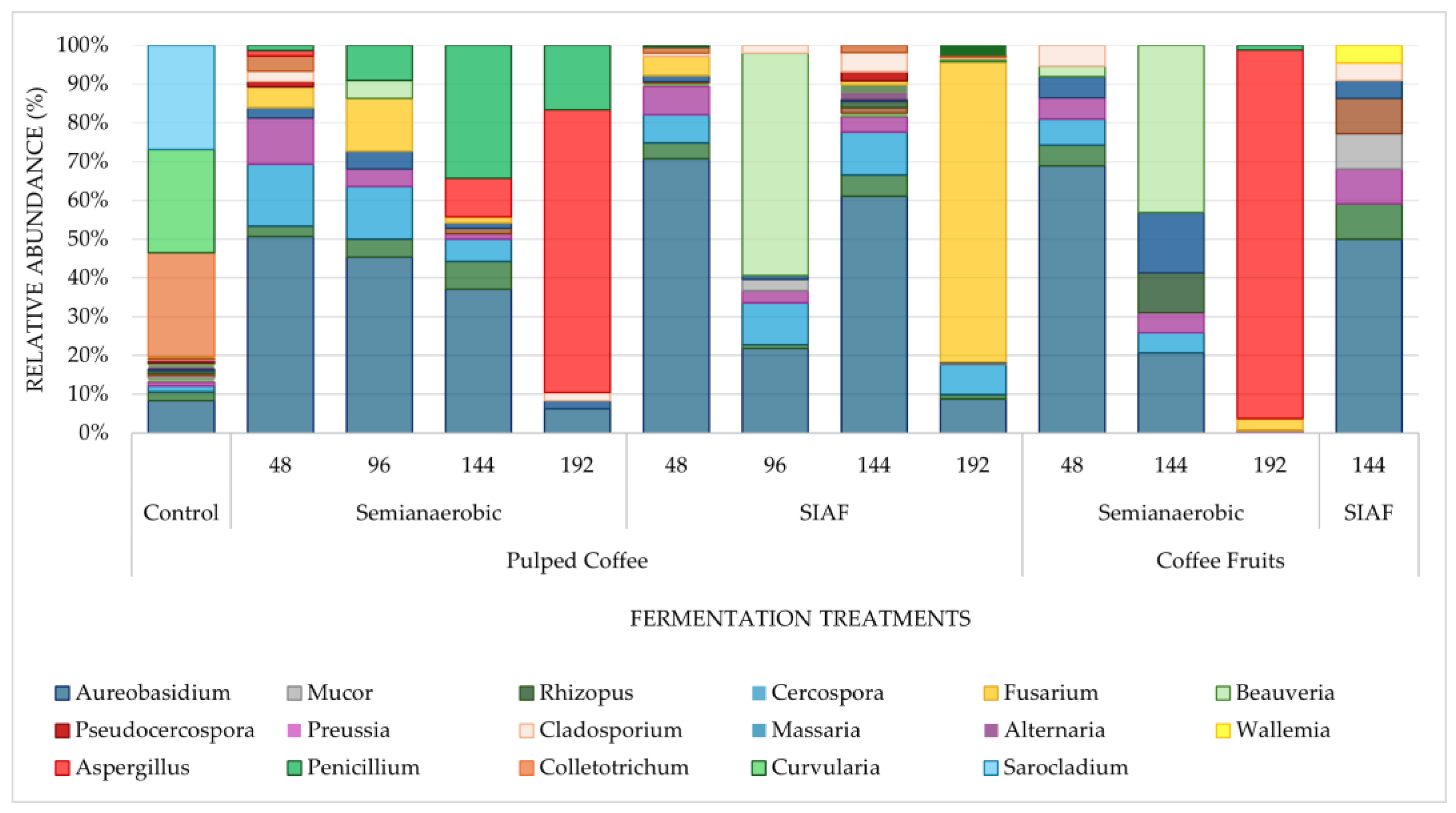
3.3. Taxonomic Classification According to ITS Sequencing
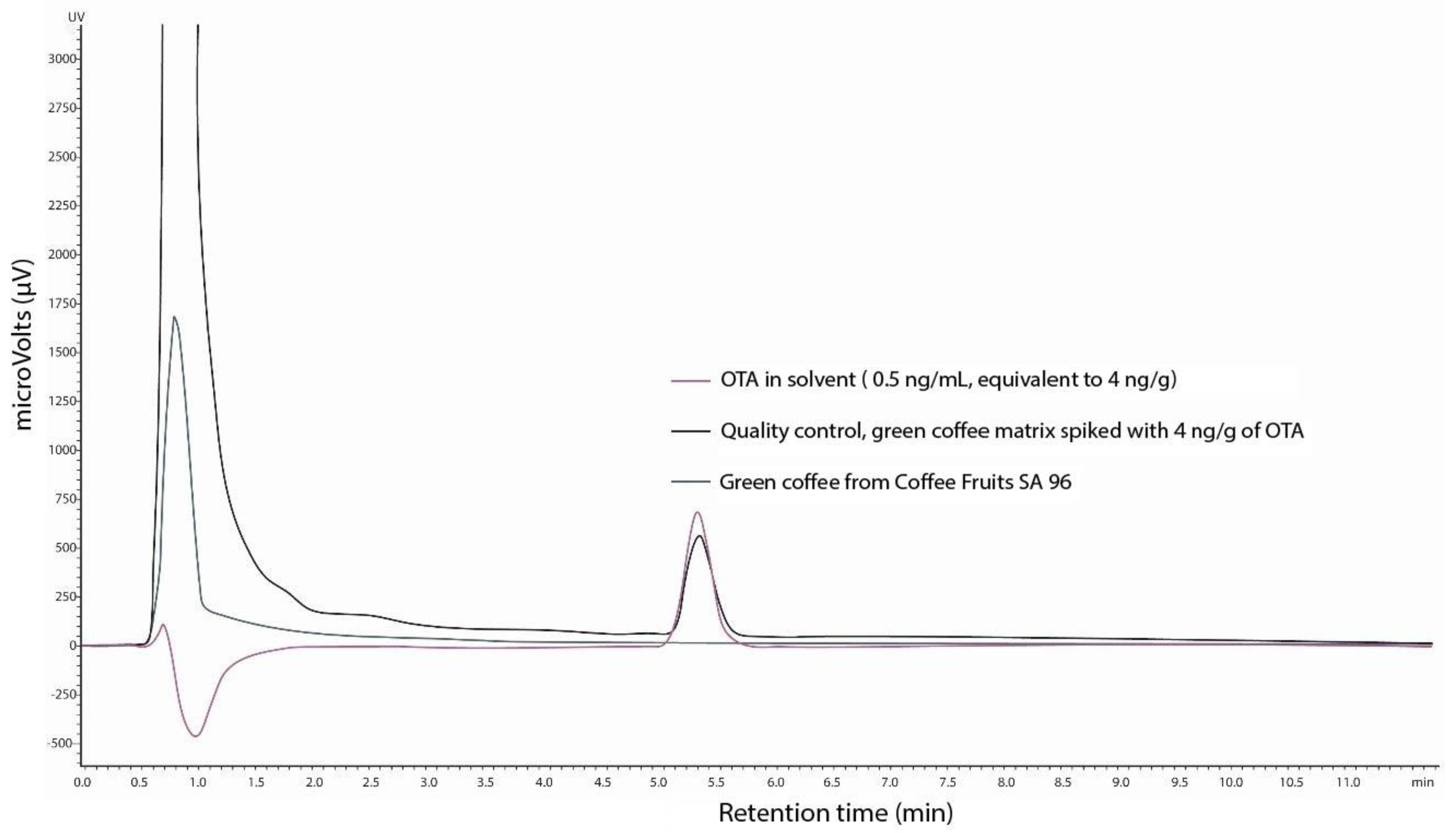
3.4. Quantification of Ochratoxin A (OTA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira Junqueira, A.C.; Melo Pereira, G.V.; Coral Medina, J.D.; Alvear, M.C.R.; Rosero, R.; Carvalho Neto, D.P.; Enríquez, H.G.; Soccol, C.R. First Description of Bacterial and Fungal Communities in Colombian Coffee Beans Fermentation Analysed Using Illumina-Based Amplicon Sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Zhao, J. Coffee Fermentation: Expedition from Traditional to Controlled Process and Perspectives for Industrialization. Appl. Food Res. 2023, 3, 100253. [Google Scholar] [CrossRef]
- Peñuela-Martínez, A.E.; García-Duque, J.F.; Sanz-Uribe, J.R. Characterization of Fermentations with Controlled Temperature with Three Varieties of Coffee (Coffea arabica L.). Fermentation 2023, 9, 976. [Google Scholar] [CrossRef]
- Ferreira, L.J.C.; Souza Gomes, M.; Maciel Oliveira, L.; Diniz Santos, L. Coffee Fermentation Process: A Review. Food Res. Int. 2023, 169, 112793. [Google Scholar] [CrossRef]
- Pereira, L.L.; Junior, D.B.; Souza, L.H.; Gomes, W.S. Relationship Between Coffee Processing and Fermentation|Request PDF. In Quality Determinants in Coffee Production; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Cardoso, W.S.; Agnoletti, B.Z.; Freitas, R.; Abreu Pinheiro, F.; Pereira, L.L. Biochemical Aspects of Coffee Fermentation. In Quality Determinants in Coffee Production; Louzada Pereira, L., Rizzo Moreira, T., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 149–208. [Google Scholar] [CrossRef]
- Mota, M.C.B.; Batista, N.N.; Rabelo, M.H.S.; Ribeiro, D.E.; Borém, F.M.; Schwan, R.F. Influence of Fermentation Conditions on the Sensorial Quality of Coffee Inoculated with Yeast. Food Res. Int. 2020, 136, 109482. [Google Scholar] [CrossRef]
- Pereira, T.S.; Batista, N.N.; Santos Pimenta, L.P.; Martinez, S.J.; Ribeiro, L.S.; Oliveira Naves, J.A.; Schwan, R.F. Self-Induced Anaerobiosis Coffee Fermentation: Impact on Microbial Communities, Chemical Composition and Sensory Quality of Coffee. Food Microbiol. 2022, 103, 103962. [Google Scholar] [CrossRef]
- Silva Vale, A.; Balla, G.; Rodrigues, L.R.S.; Carvalho Neto, D.P.; Soccol, C.R.; Melo Pereira, G.V. Understanding the Effects of Self-Induced Anaerobic Fermentation on Coffee Beans Quality: Microbiological, Metabolic, and Sensory Studies. Foods 2023, 12, 37. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A. Fungi and Food Spoilage, 4th ed.; Springer: New York, NY, USA, 2022. [Google Scholar]
- López-Rodríguez, C.; Verheecke-Vaessen, C.; Strub, C.; Fontana, A.; Schorr-Galindo, S.; Medina, A. Reduction in Ochratoxin A Occurrence in Coffee: From Good Practices to Biocontrol Agents. J. Fungi 2024, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Neto, D.P.; Melo Pereira, G.V.; Finco, A.M.O.; Letti, L.A.J.; Silva, B.J.G.; Vandenberghe, L.P.S.; Soccol, C.R. Efficient Coffee Beans Mucilage Layer Removal Using Lactic Acid Fermentation in a Stirred-Tank Bioreactor: Kinetic, Metabolic and Sensorial Studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- Muñoz, K.; Vega, M.; Rios, G.; Geisen, R.; Degen, G.H. Mycotoxin Production by Different Ochratoxigenic Aspergillus and Penicillium Species on Coffee- and Wheat-Based Media. Mycotoxin Res. 2011, 27, 239–247. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Q.; Wang, H.; Fang, G.; Li, Y.; Zhang, J.; Liu, K. Microbial Characteristics and Functions in Coffee Fermentation: A Review. Fermentation 2024, 11, 5. [Google Scholar] [CrossRef]
- Adhikari, M.; Isaac, E.L.; Paterson, R.R.M.; Maslin, M.A. A Review of Potential Impacts of Climate Change on Coffee Cultivation and Mycotoxigenic Fungi. Microorganisms 2020, 8, 1625. [Google Scholar] [CrossRef]
- Kusumaningrum, H.D.; Rasyidah, M.M. Prevalence of Spoilage Mold in Coffee before and after Brewing. Food Res. 2019, 720–726. [Google Scholar] [CrossRef]
- Neves, T.T.; Brandão, R.M.; Barbosa, R.B.; Cardoso, M.G.; Batista, L.R.; Silva, C.F. Simulation of Coffee Beans Contamination by Aspergillus Species under Different Environmental Conditions and the Biocontrol Effect by Saccharomyces cerevisiae. LWT 2021, 148, 111610. [Google Scholar] [CrossRef]
- Neves, T.T.; Cassimiro, D.M.J.; Souza, J.G.L.; Castro, C.R.S.; Schwan, R.F.; Batista, L.R.; Silva, C.F. Inhibition of Aspergillus spp. Growth and Ochratoxin A Production in Conilon and Arabica Coffees Based-Medium by Saccharomyces cerevisiae. Int. J. Food Microbiol. 2024, 425, 110875. [Google Scholar] [CrossRef]
- Rojas-Pablo, M.; Toledo-Hernández, E.; Rodríguez-Barrera, M.A.; Toribio-Jiménez, J.; Torreblanca-Ramírez, C.; Rosas-Guerrero, V.M.; Salgado-Souto, S.A.; Álvarez-Fitz, P.; Bolaños-Dircio, A.; Romero-Ramírez, Y. Bacillus licheniformis M2-7 Decreases Ochratoxin A Concentrations in Coffee Beans During Storage. Curr. Microbiol. 2024, 81, 62. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Abdi, L.; Coppa, C.F.S.C.; Franco, L.T.; Oliveira, C.A.F. The Concentration and Prevalence of Ochratoxin A in Coffee and Coffee-Based Products: A Global Systematic Review, Meta-Analysis and Meta-Regression. Fungal Biol. 2019, 123, 611–617. [Google Scholar] [CrossRef]
- Humans, I.W.G. on the E. of C. R. to. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Human Immunodeficiency Viruses and Human T-Cell Lymphotropic Viruses; International Agency for Research on Cancer: Lyon, France, 1996. [Google Scholar]
- European Commission. Commission Regulation (EU) 2022/1370 of 5 August 2022 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ochratoxin A in Certain Foodstuffs (Text with EEA Relevance); European Commission: Brussels, Belgium, 2022; Available online: https://eur-lex.europa.eu/eli/reg/2022/1370/oj (accessed on 5 June 2025).
- Lindgren, S.E.; Dobrogosz, W.J. Antagonistic Activities of Lactic Acid Bacteria in Food and Feed Fermentations. FEMS Microbiol. Rev. 1990, 7, 149–163. [Google Scholar] [CrossRef]
- Beugre, G.C.; Kadjo, A.C.; Yao, K.M.; Kone, K.M.; Piro-Metayer, I.; Poss, C.; Durand, N.; Fontana, A.; Guehi, T.S. Sensory Quality of Coffee Beverrage Produced Thereof Linked to the Inhibition of Molds Growth and Ochratoxin a Removal from Coffee Cherries Using Lactobacillus Plantarum Strains. Curr. J. Appl. Sci. Technol. 2023, 42, 10–20. [Google Scholar] [CrossRef]
- Velmourougane, K. Impact of Natural Fermentation on Physicochemical, Microbiological and Cup Quality Characteristics of Arabica and Robusta Coffee. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 233–239. [Google Scholar] [CrossRef]
- Abdel-Hadi, A.; Alshehri, B.; Waly, M.; Aboamer, M.; Banawas, S.; Alaidarous, M.; Palanisamy, M.; Awad, M.; Baazeem, A. Predictive Modeling and Validation on Growth, Production of Asexual Spores and Ochratoxin A of Aspergillus ochraceus Group under Abiotic Climatic Variables. Microorganisms 2021, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Frank, J.; Houbraken, J.; Kujipers, A.; Samson, R. New Ochratoxin A Producing Species of Aspergillus Section Circumdati. Stud. Mycol. 2004, 50, 23–43. Available online: https://orbit.dtu.dk/en/publications/new-ochratoxin-a-producing-species-of-aspergillus-section-circumd (accessed on 9 June 2025).
- Zakaria, L. An Overview of Aspergillus Species Associated with Plant Diseases. Pathogens 2024, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Pacífico, C.; Faria, T.; Oliveira, A.C.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Fungal Contamination in Green Coffee Beans Samples: A Public Health Concern. J. Toxicol. Environ. Health Part A 2017, 80, 719–728. [Google Scholar] [CrossRef]
- Peñuela-Martínez, A.E.; Velasquez-Emiliani, A.V.; Angel, C.A. Microbial Diversity Using a Metataxonomic Approach, Associated with Coffee Fermentation Processes in the Department of Quindío, Colombia. Fermentation 2023, 9, 343. [Google Scholar] [CrossRef]
- Góngora, C.E.; Holguín-Sterling, L.; Pedraza-Claros, B.; Pérez-Salinas, R.; Ortiz, A.; Navarro-Escalante, L. Metataxonomic Identification of Microorganisms during the Coffee Fermentation Process in Colombian Farms (Cesar Department). Foods 2024, 13, 839. [Google Scholar] [CrossRef]
- Peñuela-Martínez, A.E.; Osorio-Giraldo, C.V.; Buitrago-Zuluaga, C.; Medina-Rivera, R.D. Development of Fermentation Strategies for Quality Mild Coffee Production (Coffea arabica L.) Based on Oxygen Availability and Processing Time. Foods 2025, 14, 3001. [Google Scholar] [CrossRef]
- Federación Nacional de Cafeteros. Anuario 2023|Anuario Meteorológico Cafetero; Cenicafé: Chinchiná, Caldas, Colombia, 2023. [Google Scholar]
- Peñuela-Martínez, A.E.; Pabón, J.; Sanz-Uribe, J.R. Método fermaestro: Para determinar la finalización de la fermentación del mucílago de café. Av. Técnic. Cenicafé 2013, 431, 1–8. [Google Scholar] [CrossRef]
- Arias, E.L.; Piñeros, P.A. Aislamiento e Identificación de Hongos Filamentosos de Muestra de suelo de los Páramos de Guasca y Cruz Verde; Pontificia Universidad Javeriana: Bogotá, Colombia, 2008. [Google Scholar]
- Patiño Moscoso, M.A.; Osorio Guerrero, K.V.; Flórez Gómez, D.L.; Sarmiento Moreno, L.F.; Vargas Ramírez, D.N.; Mérida, M.J. Manual Ilustrado de Hongos Presentes en Semillas de Cultivos Semestrales: Arroz, Maíz, Soya y Sorgo; AGROSAVIA: Bogotá, Colombia, 2023. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; APS Press: St. Paul, MN, USA, 1986. [Google Scholar]
- Frąc, M.; Kaczmarek, J.; Jędryczka, M. Metabolic Capacity Differentiates Plenodomus lingam from P. biglobosus Subclade ‘Brassicae’, the Causal Agents of Phoma Leaf Spotting and Stem Canker of Oilseed Rape (Brassica napus) in Agricultural Ecosystems. Pathogens 2022, 11, 50. [Google Scholar] [CrossRef]
- Mycobank. Available online: https://www.mycobank.org/ (accessed on 25 August 2025).
- Index Fungorum-Search Page. Available online: https://www.indexfungorum.org/names/names.asp (accessed on 25 August 2025).
- AOAC 2004.10-2008; Ochratoxin A in Green Coffee. AOAC International: Rockville, MD, USA, 2004.
- Alster, C.J.; Allison, S.D.; Johnson, N.G.; Glassman, S.I.; Treseder, K.K. Phenotypic Plasticity of Fungal Traits in Response to Moisture and Temperature. ISME Commun. 2021, 1, 43. [Google Scholar] [CrossRef]
- Hollingsworth, R.G.; Aristizábal, L.F.; Shriner, S.; Mascarin, G.M.; Moral, R.A.; Arthurs, S.P. Incorporating Beauveria bassiana Into an Integrated Pest Management Plan for Coffee Berry Borer in Hawaii. Front. Sustain. Food Syst. 2020, 4, 22. [Google Scholar] [CrossRef]
- Ferrucho, R.L.; Marín-Ramírez, G.A.; Gaitan, A. Integrated Disease Management for the Sustainable Production of Colombian Coffee. Agronomy 2024, 14, 1286. [Google Scholar] [CrossRef]
- Cruz-O’Byrne, R.; Piraneque-Gambasica, N.; Aguirre-Forero, S. Microbial Diversity Associated with Spontaneous Coffee Bean Fermentation Process and Specialty Coffee Production in Northern Colombia. Int. J. Food Microbiol. 2021, 354, 109282. [Google Scholar] [CrossRef]
- Lu, L.; Tibpromma, S.; Karunarathna, S.C.; Jayawardena, R.S.; Lumyong, S.; Xu, J.; Hyde, K.D. Comprehensive Review of Fungi on Coffee. Pathogens 2022, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Manathunga, K.K.; Gunasekara, N.W.; Meegahakumbura, M.K.; Ratnaweera, P.B.; Faraj, T.K.; Wanasinghe, D.N. Exploring Endophytic Fungi as Natural Antagonists against Fungal Pathogens of Food Crops. J. Fungi 2024, 10, 606. [Google Scholar] [CrossRef]
- Priwiratama, H.; Wiyono, S.; Hidayat, S.H.; Wening, S.; Tondok, E.T. Identification and Characterization of Curvularia, the Causal Agent of Leaf Spot Disease of Oil Palm Seedlings in Indonesia. J. Saudi Soc. Agric. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Akbar, A.; Medina, A.; Magan, N. Resilience of Aspergillus westerdijkiae Strains to Interacting Climate-Related Abiotic Factors: Effects on Growth and Ochratoxin A Production on Coffee-Based Medium and in Stored Coffee. Microorganisms 2020, 8, 1268. [Google Scholar] [CrossRef]
- Manamgoda, D.S.; Cai, L.; McKenzie, E.H.C.; Crous, P.W.; Madrid, H.; Chukeatirote, E.; Shivas, R.G.; Tan, Y.P.; Hyde, K.D. A Phylogenetic and Taxonomic Re-Evaluation of the Bipolaris-Cochliobolus-Curvularia Complex. Fungal Divers. 2012, 56, 131–144. [Google Scholar] [CrossRef]
- Iturrieta-González, I.; Gené, J.; Wiederhold, N.; García, D. Three New Curvularia Species from Clinical and Environmental Sources. Mycokeys 2020, 68, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.M.; Rodríguez-Couto, S.; El-Ghany, M.N.A. Characterization of Penicillium Crustosum L-Asparaginase and Its Acrylamide Alleviation Efficiency in Roasted Coffee Beans at Non-Cytotoxic Levels. Arch. Microbiol. 2021, 203, 2625–2637. [Google Scholar] [CrossRef]
- Melo Pereira, G.V.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting Starter Culture-Controlled Fermentations of Coffee Beans during on-Farm Wet Processing: Growth, Metabolic Analyses and Sensorial Effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef]
- Schoch, C.L.; Shoemaker, R.A.; Hambleton, S.; Spatafora, J.W.; Pedro, W. A Multigene Phylogeny of the Dothideomycetes Using Four Nuclear Loci. Mycologia 2006, 98, 1041–1052. [Google Scholar] [CrossRef]
- Di Francesco, A.; Zajc, J.; Stenberg, J.A. Aureobasidium spp.: Diversity, Versatility, and Agricultural Utility. Horticulturae 2023, 9, 59. [Google Scholar] [CrossRef]
- Silva, C.F.; Batista, L.R.; Schwan, R.F. Incidence and Distribution of Filamentous Fungi during Fermentation, Drying and Storage of Coffee (Coffea arabica L.) Beans. Braz. J. Microbiol. 2008, 39, 521–526. [Google Scholar] [CrossRef]
- Lorenzoni, T.L.; Luz, J.M.R.; Veloso, T.G.R.; Pereira, L.L.; Menezes, K.M.S.; Brioschi Júnior, D.; Kasuya, M.C.M.; Silva, M.D.C.S. Genetic Diversity of the Fungal Community That Contributes to the Sensory Quality of Coffee Beverage after Carbonic Maceration and Fermentation. 3 Biotech 2024, 14, 272. [Google Scholar] [CrossRef]
- Lee, B.-H.; Huang, C.-H.; Liu, T.-Y.; Liou, J.-S.; Hou, C.-Y.; Hsu, W.-H. Microbial Diversity of Anaerobic-Fermented Coffee and Potential for Inhibiting Ochratoxin-Produced Aspergillus Niger. Foods 2023, 12, 2967. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Influence of Temperature and Water Activity on Deleterious Fungi and Mycotoxin Production during Grain Storage. Mycobiology 2017, 45, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Silva Vale, A.; Pereira, C.M.T.; Dea Lindner, J.; Rodrigues, L.R.S.; Kadri, N.K.E.; Pagnoncelli, M.G.B.; Kaur Brar, S.; Soccol, C.R.; Pereira, G.V.M. Exploring Microbial Influence on Flavor Development during Coffee Processing in Humid Subtropical Climate through Metagenetic–Metabolomics Analysis. Foods 2024, 13, 1871. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological Diversity, Evolution and Metabolism of Microbial Communities in the Wet Fermentation of Australian Coffee Beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef]
- Katati, B.; van Diepeningen, A.D.; Njapau, H.; Kachapulula, P.W.; Zwaan, B.J.; Schoustra, S.E. Niche Partitioning Association of Fungal Genera Correlated with Lower Fusarium and Fumonisin-B1 Levels in Maize. BioControl 2024, 69, 185–197. [Google Scholar] [CrossRef]
- Pierzgalski, A.; Bryła, M.; Kanabus, J.; Modrzewska, M.; Podolska, G. Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms. Toxins 2021, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Melo Pereira, G.V.; Mello Sampaio, V.; Wiele, N.; Silva Vale, A.; Carvalho Neto, D.P.; Souza, A.d.F.D.d.; Nogueira dos Santos, D.V.; Ruiz, I.R.; Rogez, H.; Soccol, C.R. How Yeast Has Transformed the Coffee Market by Creating New Flavors and Aromas through Modern Post-Harvest Fermentation Systems. Trends Food Sci. Technol. 2024, 151, 104641. [Google Scholar] [CrossRef]
- Silva, C.F.; Batista, L.R.; Abreu, L.M.; Dias, E.S.; Schwan, R.F. Succession of Bacterial and Fungal Communities during Natural Coffee (Coffea arabica) Fermentation. Food Microbiol. 2008, 25, 951–957. [Google Scholar] [CrossRef] [PubMed]




| Morphotype | Database | Incubation Time (h) | PROB | SIM | DIS | SPECIES ID |
|---|---|---|---|---|---|---|
| 1 | Filamentous Fungi | 24 | --- | 0.16 | 13.82 | Hemicarpenteles paradoux Sarbhoy & Elphick |
| Food or Air | 24 | --- | 0.16 | 13.82 | Hemicarpenteles paradoux Sarbhoy & Elphick | |
| 2 | Filamentous Fungi | 72 | --- | 0.43 | 8.96 | Alternaria alternata (Fries) Keissl, BGA |
| Food or Air | 72 | --- | 0.43 | 8.96 | Alternaria alternata (Fries) Keissl, BGA | |
| 168 | --- | 0.41 | 9.50 | Curvularia lunata var lunata (Wakker) Boedijn BGA | ||
| 3 | Filamentous Fungi | 72 | 0.99 | 0.73 | 4.03 | Cladosporium tenuissimum Cooke BGA |
| Food or Air | 72 | 1.00 | 0.65 | 5.36 | Cladosporium tenuissimum Cooke BGA | |
| 4 | Filamentous Fungi | 96 | --- | 0.57 | 5.90 | Cladosporium sphaerospermum Penzing BGA |
| Food or Air | 96 | --- | 0.57 | 5.90 | Cladosporium sphaerospermum Penzing BGA | |
| 5 | Filamentous Fungi | 96 | 0.98 | 0.62 | 5.78 | Penicillium crustosum Thom BGD |
| Food or Air | 96 | 0.98 | 0.62 | 5.78 | Penicillium crustosum Thom BGD | |
| Penicillium | 96 | 0.98 | 0.62 | 5.78 | Penicillium crustosum Thom BGD | |
| 6 | Filamentous Fungi | 96 | --- | 0.24 | 13.91 | Penicillium roqueforti Thom BGE |
| Food or Air | 48 | --- | 0.38 | 10.21 | Penicillium vulpinum (Cooke & Massee) Seifert & Samson BGA | |
| Penicillium | 48 | --- | 0.31 | 11.36 | Penicillium vulpinum (Cooke & Massee) Seifert & Samson BGA | |
| 7 | Filamentous Fungi | 48 | --- | 0.351 | 9,76 | Penicillium janczewskii Zaleski BGB |
| Food or Air | 96 | --- | 0.39 | 8.03 | Penicillium crustosum Thom BGD | |
| Penicillium | 48 | --- | 0.35 | 11.09 | Penicillium janczewskii Zaleski BGB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buitrago-Zuluaga, C.; Osorio-Giraldo, C.V.; Peñuela-Martínez, A.E. Identification of Filamentous Fungi Present in Prolonged Fermentations of Coffea arabica L. var. Castillo. Appl. Microbiol. 2025, 5, 114. https://doi.org/10.3390/applmicrobiol5040114
Buitrago-Zuluaga C, Osorio-Giraldo CV, Peñuela-Martínez AE. Identification of Filamentous Fungi Present in Prolonged Fermentations of Coffea arabica L. var. Castillo. Applied Microbiology. 2025; 5(4):114. https://doi.org/10.3390/applmicrobiol5040114
Chicago/Turabian StyleBuitrago-Zuluaga, Camila, Carol Vanessa Osorio-Giraldo, and Aida Esther Peñuela-Martínez. 2025. "Identification of Filamentous Fungi Present in Prolonged Fermentations of Coffea arabica L. var. Castillo" Applied Microbiology 5, no. 4: 114. https://doi.org/10.3390/applmicrobiol5040114
APA StyleBuitrago-Zuluaga, C., Osorio-Giraldo, C. V., & Peñuela-Martínez, A. E. (2025). Identification of Filamentous Fungi Present in Prolonged Fermentations of Coffea arabica L. var. Castillo. Applied Microbiology, 5(4), 114. https://doi.org/10.3390/applmicrobiol5040114






