Synergies in Green Bioprocessing: A Comprehensive Review of Heterologous Protein Expression and the Transformative Potential of Natural Deep Eutectic Solvents
Abstract
1. Introduction: The Enduring Challenge of Recombinant Protein Production
1.1. The Recombinant Revolution
1.2. Persistent Bottlenecks in Biomanufacturing
1.3. The Emergence of NADESs as a Green and Functional Solvent Platform
1.4. Scope and Thesis of the Review
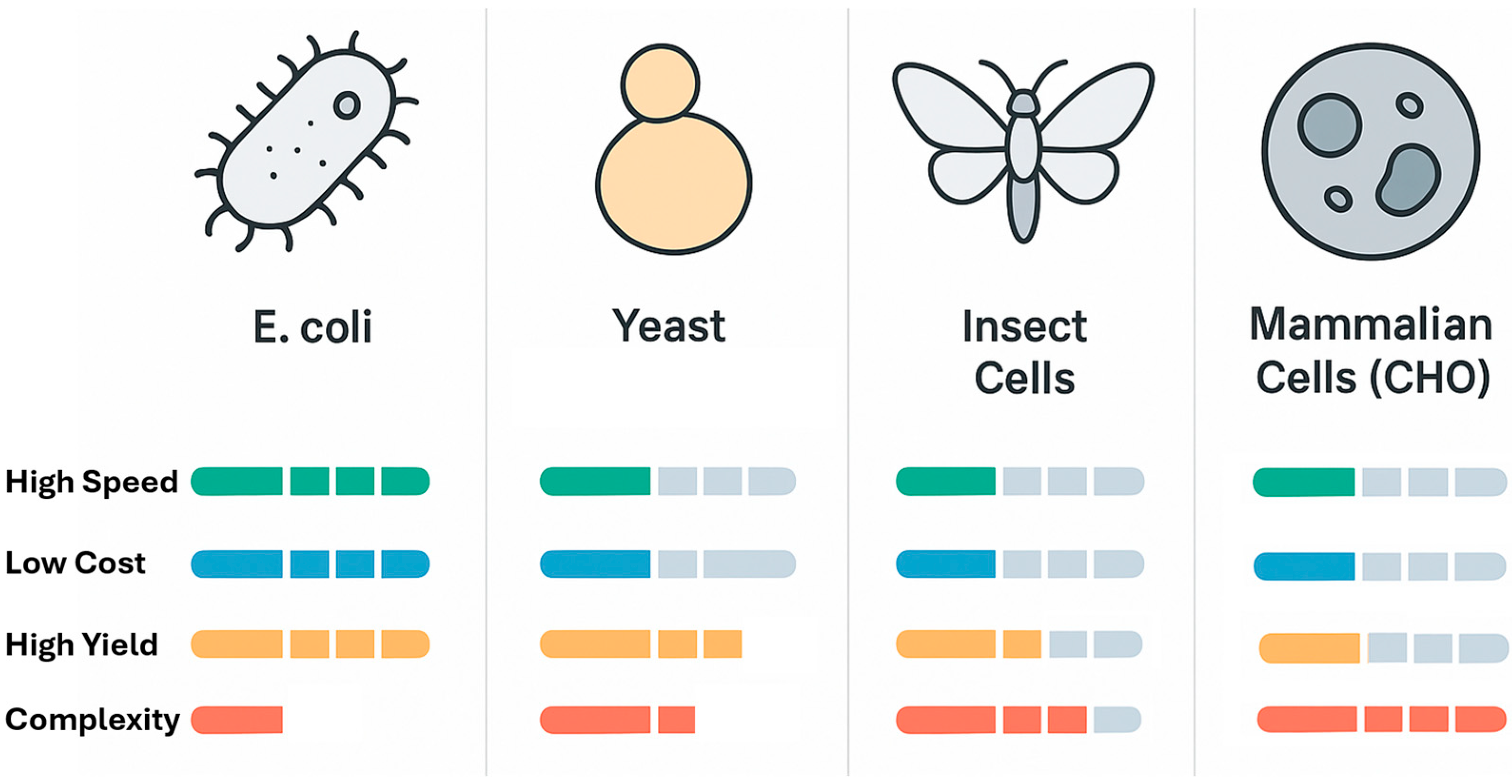
2. The Landscape of Heterologous Protein Expression Platforms
2.1. The Prokaryotic Workhorse: Escherichia coli
2.2. Eukaryotic Microbial Factories: Yeast Systems
2.3. Platforms for Complexity: Insect and Mammalian Cell Systems
2.4. A Comparative Outlook and the Rise of Cell-Free Systems
| Feature | Escherichia coli | Saccharomyces cerevisiae | Komagataella phaffii (Pichia pastoris) | Insect Cells (BEVS) | Mammalian Cells (CHO) | Cell-Free Systems |
|---|---|---|---|---|---|---|
| Speed (Time to Protein) | Very Fast (1–3 days) [7] | Fast (3–7 days) [26] | Fast (3–10 days) [29] | Moderate (7–14 days) [33] | Slow (Weeks to Months for stable line) [35] | Extremely Fast (Hours) [38] |
| Cost (Media & Setup) | Very Low [21] | Low [27] | Low [30] | Moderate [33] | Very High [35] | High (per mg protein) |
| Typical Yield | High (1–10 g/L) [22] | Moderate (0.1–1 g/L) [26] | Very High (1–15+ g/L) [29] | High (0.1–1 g/L) [33] | Moderate to High (0.5–10+ g/L) [35] | Low to Moderate (mg/mL scale) [38] |
| Post-Translational Mods. | None (Limited engineered options) [7] | Yes (Hyperglycosylation) [27] | Yes (Less hyperglycosylation) [28] | Yes (Complex, near-mammalian) [32] | Yes (Authentic, human-like) [35] | Limited (Can be supplemented) [38] |
| Disulfide Bond Formation | Challenging (Requires engineered strains) [23] | Efficient (Secretory pathway) [26] | Efficient (Secretory pathway) [30] | Efficient (Secretory pathway) [33] | Efficient (Secretory pathway) [40] | Possible (Requires redox control) [38] |
| Scalability | Excellent [21] | Excellent [26] | Excellent [30] | Good [34] | Excellent (Industry standard) [36] | Challenging for large scale [39] |
| Key Advantage | Speed, cost, and high yield for simple proteins [7] | GRAS status, well-characterized genetics [26] | Very high cell density and secretion levels [28] | High yield of complex, multi-subunit proteins [32] | Authentic PTMs for therapeutics [35] | Speed, open system for modifications [38] |
| Primary Limitation | Inclusion bodies, lack of PTMs [7] | Hyperglycosylation, lower yields [27] | Methanol use (for AOX1), secretion bottlenecks [29] | More complex workflow than microbes [33] | Slow, expensive, complex media [35] | Cost, scalability, lower yields [38] |
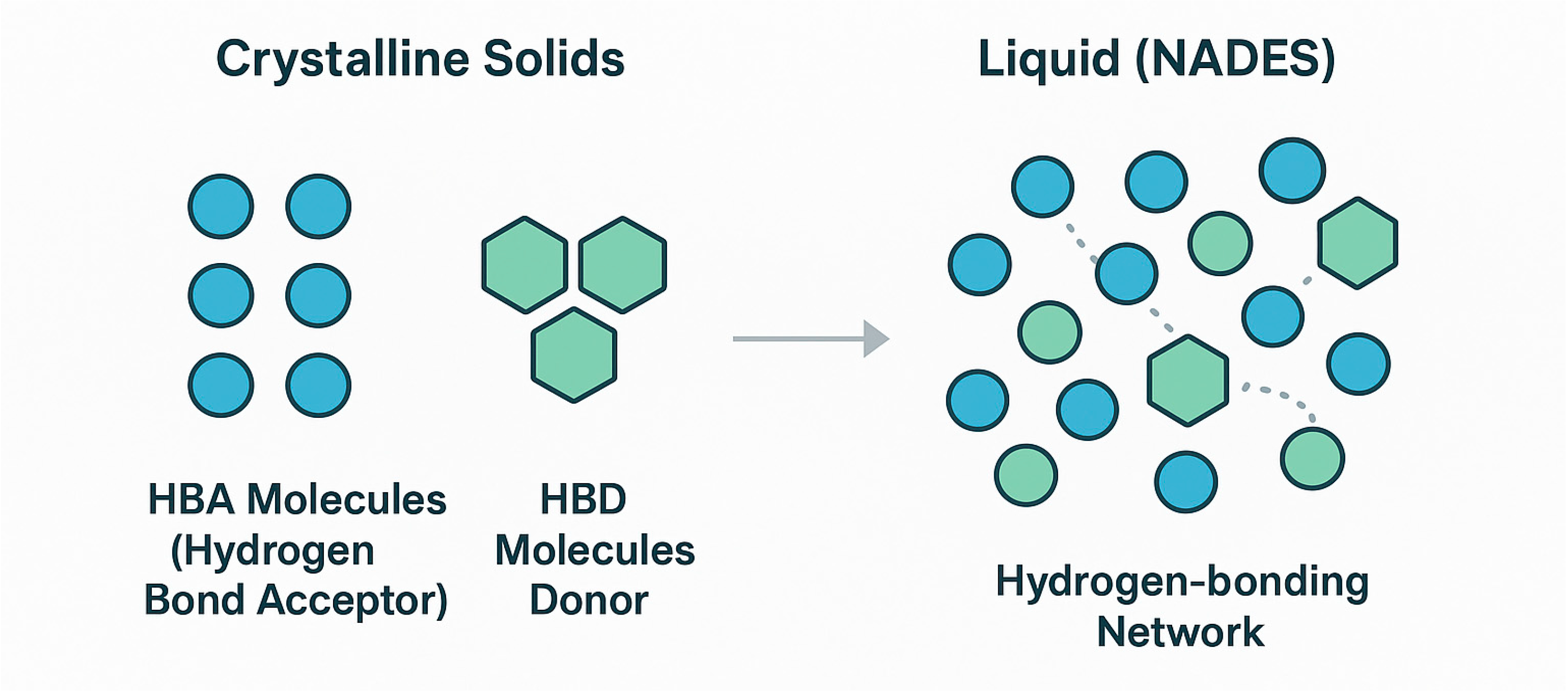
3. Natural Deep Eutectic Solvents (NADESs): Properties and Biotechnological Promise
3.1. Fundamentals: From Eutectic Mixtures to Designer Solvents
3.2. Key Physicochemical Properties for Bioprocessing
3.3. Established Applications in Biotechnology
3.4. The Supramolecular Structure of NADESs in Aqueous Solution
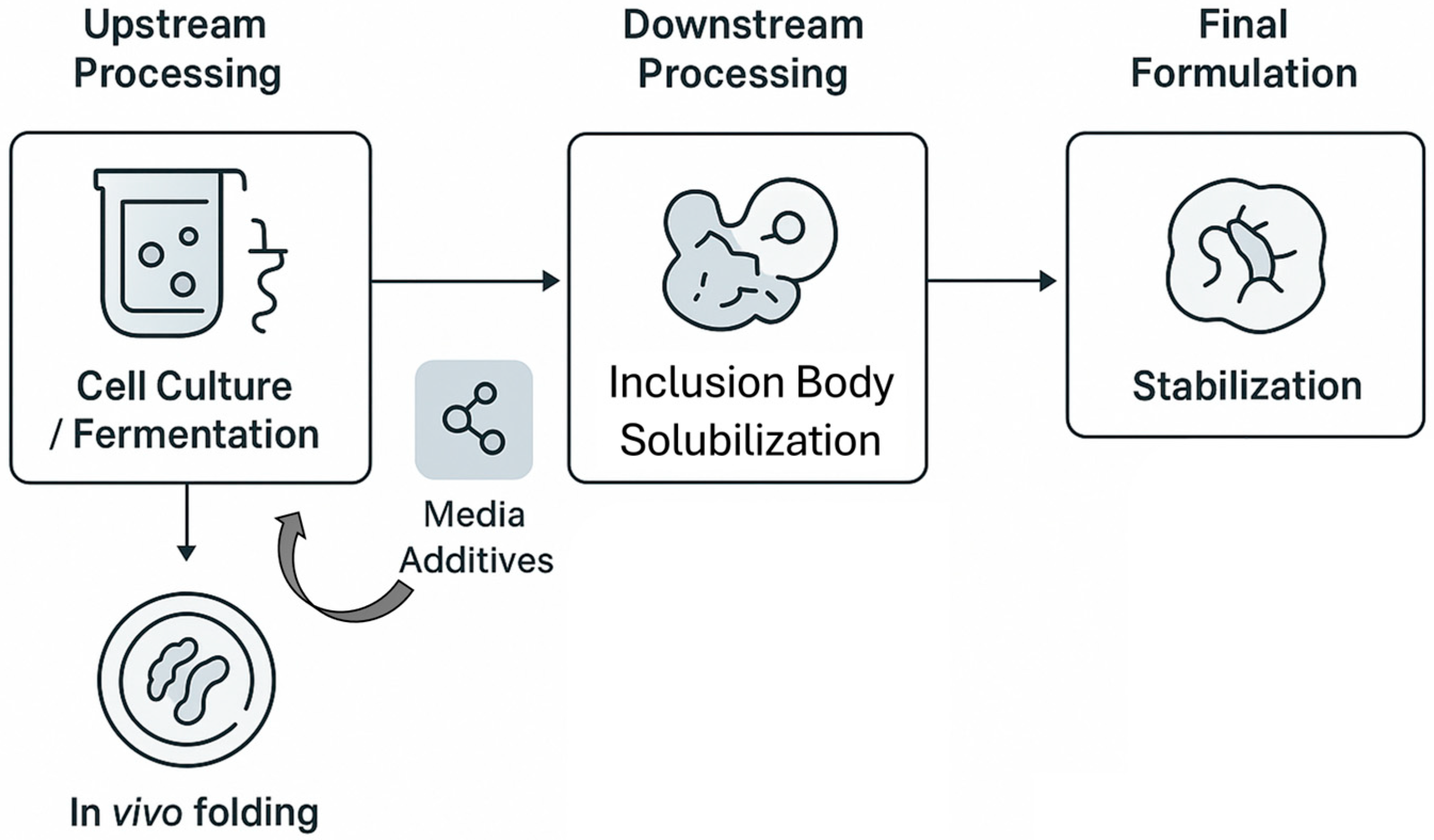
4. Integrating NADESs into the Recombinant Protein Workflow: A Critical Analysis
4.1. Upstream Applications: Enhancing In Vivo Protein Expression and Strain Preservation
4.1.1. Biocompatibility with Production Hosts
4.1.2. Potential of NADESs as Media Additives to Improve Soluble Protein Yield
4.1.3. NADESs as Advanced Cryoprotectants for Engineered Microbial Strains
4.2. Downstream Processing I: A Green Paradigm for Inclusion Body Solubilization and Refolding
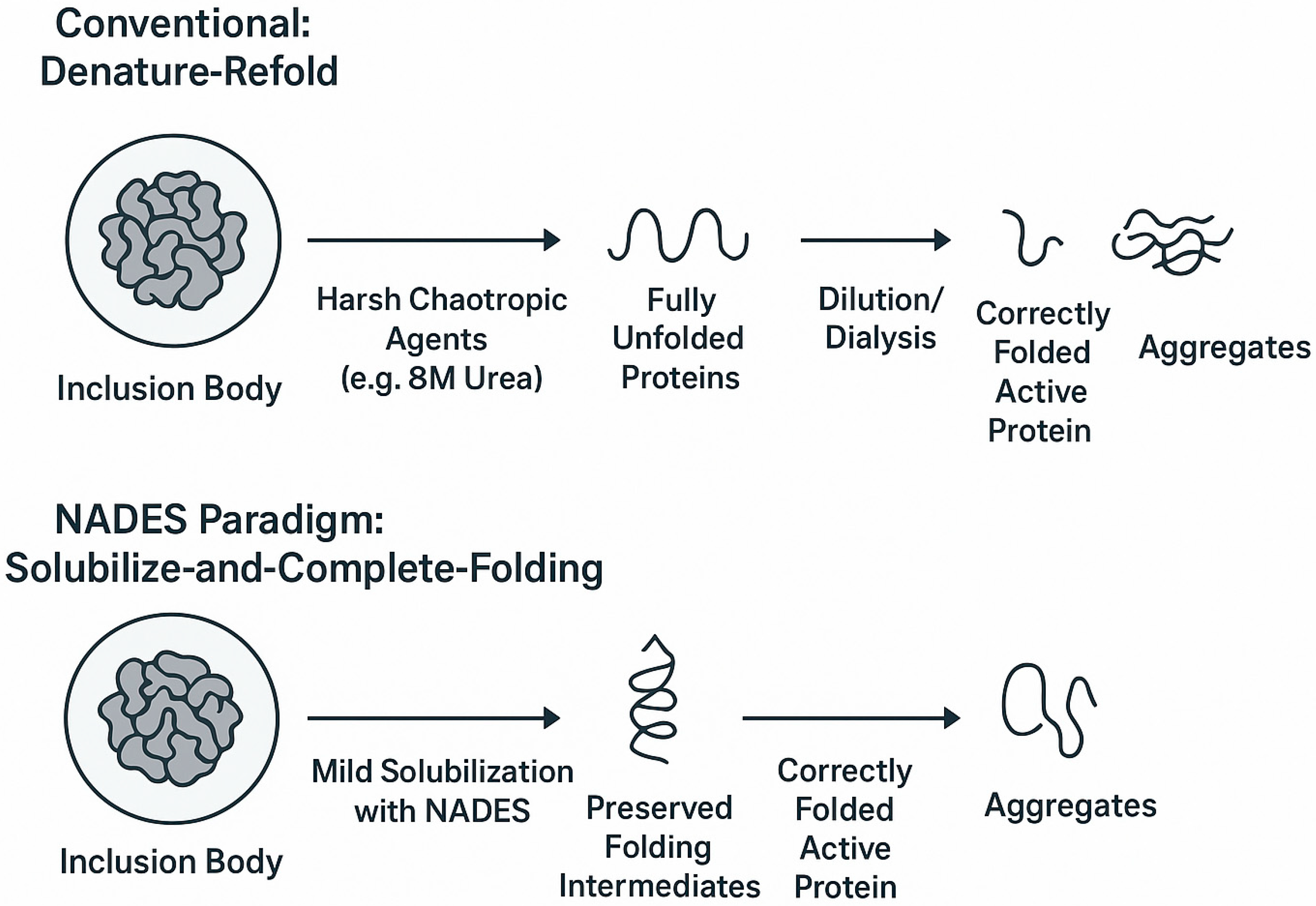
4.2.1. The Conventional Challenge of “Denature-Refold”
4.2.2. A New Strategy: “Solubilize-And-Complete-Folding” with NADESs
4.2.3. Functional Roles of Specific NADESs in Refolding
4.2.4. Mechanistic Synergies: NADESs vs. Individual Stabilizing Agents
4.3. Downstream Processing II: NADESs in Advanced Purification Strategies
4.3.1. Principles of Aqueous Two-Phase Systems (ATPS)
4.3.2. NADES-Based ATPS for Green and Efficient Purification
4.4. Final Formulation: NADESs for Long-Term Protein Stabilization
4.4.1. Mechanisms of Protein Stabilization in NADESs
4.4.2. Prospects for Integrated and Stable Formulations
5. Future Perspectives and Concluding Remarks
5.1. Synthesis and Outlook: Towards a Fully Integrated Bioprocess
5.2. Addressing the Critical Knowledge Gaps
5.3. Charting Future Research Directions
5.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, P.; Mertz, J.E. Personal Reflections on the Origins and Emergence of Recombinant DNA Technology. Genetics 2010, 184, 9–17. [Google Scholar] [CrossRef]
- Cohen, S.N.; Chang, A.C.Y.; Boyer, H.W.; Helling, R.B. Construction of Biologically Functional Bacterial Plasmids In Vitro. Proc. Natl. Acad. Sci. USA 1973, 70, 3240–3244. [Google Scholar] [CrossRef]
- Quianzon, C.C.; Cheikh, I. History of insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 18701. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Wan Rosli, W.R.; Tahir, A.R.M.; Razak, F.S.A.; Kee, P.E.; Ng, H.S.; Chew, Y.-L.; Lee, S.-K.; Ramasamy, M.; Tan, C.S.; et al. Evolving Paradigms of Recombinant Protein Production in Pharmaceutical Industry: A Rigorous Review. Sci 2024, 6, 9. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Bhatwa, A.; Wang, W.; Hassan, Y.I.; Abraham, N.; Li, X.-Z.; Zhou, T. Challenges Associated with the Formation of Recombinant Protein Inclusion Bodies in Escherichia coli and Strategies to Address Them for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 9, 630551. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.M.; Page, R. Strategies to Optimize Protein Expression in E. coli. Curr. Protoc. Protein Sci. 2010, 61, 5.24.1–5.24.29. [Google Scholar] [CrossRef]
- Snoeck, S.; Guidi, C.; De Mey, M. “Metabolic burden” explained: Stress symptoms and its related responses induced by (over)expression of (heterologous) proteins in Escherichia coli. Microb. Cell Fact. 2024, 23, 96. [Google Scholar] [CrossRef]
- Raschmanová, H.; Zamora, I.; Borčinová, M.; Meier, P.; Weninger, A.; Mächler, D.; Glieder, A.; Melzoch, K.; Knejzlík, Z.; Kovar, K. Single-Cell Approach to Monitor the Unfolded Protein Response During Biotechnological Processes with Pichia pastoris. Front. Microbiol. 2019, 10, 335. [Google Scholar] [CrossRef]
- Schramm, F.D.; Schroeder, K.; Jonas, K. Protein aggregation in bacteria. FEMS Microbiol. Rev. 2020, 44, 54–72. [Google Scholar] [CrossRef]
- García-Fruitós, E. Inclusion bodies: A new concept. Microb. Cell Fact. 2010, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Spalding, B.J. Downstream Processing: Key To Slashing Production Costs 100 Fold. Nat. Biotechnol. 1991, 9, 229–232. [Google Scholar] [CrossRef]
- Singh, A.; Upadhyay, V.; Panda, A.K. Solubilization and Refolding of Inclusion Body Proteins. In Insoluble Proteins; Humana Press: New York, NY, USA, 2015; pp. 283–291. [Google Scholar]
- Zhao, D.; Liu, Y.; Zhang, G.; Zhang, C.; Li, X.; Wang, Q.; Shi, H.; Su, Z. Interaction of arginine with protein during refolding process probed by amide H/D exchange mass spectrometry and isothermal titration calorimetry. Biochim. Biophys. Acta–Proteins Proteomics 2015, 1854, 39–45. [Google Scholar] [CrossRef]
- Burnett, M.J.B.; Burnett, A.C. Therapeutic recombinant protein production in plants: Challenges and opportunities. Plants People Planet 2020, 2, 121–132. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef]
- Li, D. Natural deep eutectic solvents in phytonutrient extraction and other applications. Front. Plant Sci. 2022, 13, 1004332. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Karadendrou, M.-A.; Kostopoulou, I.; Kakokefalou, V.; Tzani, A.; Detsi, A. L-Proline-Based Natural Deep Eutectic Solvents as Efficient Solvents and Catalysts for the Ultrasound-Assisted Synthesis of Aurones via Knoevenagel Condensation. Catalysts 2022, 12, 249. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Chen, G.; Cook, J.D.; Ye, W.; Lee, J.E.; Sidhu, S.S. Optimization of peptidic HIV-1 fusion inhibitor T20 by phage display. Protein Sci. 2019, 28, 1501–1512. [Google Scholar] [CrossRef]
- İncir, İ.; Kaplan, Ö. Escherichia coli as a versatile cell factory: Advances and challenges in recombinant protein production. Protein Expr. Purif. 2024, 219, 106463. [Google Scholar] [CrossRef]
- Yu, M.; Tsang, J.S.H. Use of ribosomal promoters from Burkholderia cenocepacia and Burkholderia cepacia for improved expression of transporter protein in Escherichia coli. Protein Expr. Purif. 2006, 49, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb. Cell Fact. 2012, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, S.; Löfblom, J.; Lee, C.; Hjelm, A.; Klepsch, M.; Strous, M.; Drew, D.; Slotboom, D.J.; de Gier, J.-W. Optimizing Membrane Protein Overexpression in the Escherichia coli strain Lemo21(DE3). J. Mol. Biol. 2012, 423, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, F.; Nicastro, R.; Reghellin, V.; Coccetti, P. Post-translational modifications on yeast carbon metabolism: Regulatory mechanisms beyond transcriptional control. Biochim. Biophys. Acta–Gen. Subj. 2015, 1850, 620–627. [Google Scholar] [CrossRef]
- Bonander, N.; Bill, R.M. Optimising Yeast as a Host for Recombinant Protein Production (Review). In Recombinant Protein Production in Yeast; Humana Press: New York, NY, USA, 2012; pp. 1–9. [Google Scholar]
- Kastberg, L.L.B.; Jacobsen, I.H.; Özdemir, E.; Workman, C.T.; Jensen, M.K.; Förster, J. Characterizing heterologous protein burden in Komagataella phaffii. FEMS Yeast Res. 2025, 25, foaf007. [Google Scholar] [CrossRef]
- Türkanoğlu Özçelik, A.; Yılmaz, S.; Inan, M. Pichia pastoris Promoters. In Recombinant Protein Production in Yeast; Humana Press: New York, NY, USA, 2019; pp. 97–112. [Google Scholar]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Sharker, S.M.; Rahman, A. A Review on the Current Methods of Chinese Hamster Ovary (CHO) Cells Cultivation for the Production of Therapeutic Protein. Curr. Drug Discov. Technol. 2021, 18, 354–364. [Google Scholar] [CrossRef]
- Sari-Ak, D.; Alomari, O.; Shomali, R.; Lim, J.; Thimiri Govinda Raj, D. Advances in CRISPR-Cas9 for the Baculovirus Vector System: A Systematic Review. Viruses 2022, 15, 54. [Google Scholar] [CrossRef]
- Possee, R.D.; Chambers, A.C.; Graves, L.P.; Aksular, M.; King, L.A. Recent Developments in the Use of Baculovirus Expression Vectors. Curr. Issues Mol. Biol. 2020, 34, 215–230. [Google Scholar] [CrossRef]
- Hong, Q.; Liu, J.; Wei, Y.; Wei, X. Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development. Vaccines 2023, 11, 1218. [Google Scholar] [CrossRef]
- Li, Z.-M.; Fan, Z.-L.; Wang, X.-Y.; Wang, T.-Y. Factors Affecting the Expression of Recombinant Protein and Improvement Strategies in Chinese Hamster Ovary Cells. Front. Bioeng. Biotechnol. 2022, 10, 880155. [Google Scholar] [CrossRef]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Gecchele, E.; Merlin, M.; Brozzetti, A.; Falorni, A.; Pezzotti, M.; Avesani, L. A Comparative Analysis of Recombinant Protein Expression in Different Biofactories: Bacteria, Insect Cells and Plant Systems. J. Vis. Exp. 2015, 97, e52459. [Google Scholar] [CrossRef]
- Khambhati, K.; Bhattacharjee, G.; Gohil, N.; Braddick, D.; Kulkarni, V.; Singh, V. Exploring the Potential of Cell-Free Protein Synthesis for Extending the Abilities of Biological Systems. Front. Bioeng. Biotechnol. 2019, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, A.; Park, J. Cell-free protein synthesis system: A new frontier for sustainable biotechnology-based products. Biotechnol. Appl. Biochem. 2023, 70, 2136–2149. [Google Scholar] [CrossRef]
- Jenkins, N.; Murphy, L.; Tyther, R. Post-translational Modifications of Recombinant Proteins: Significance for Biopharmaceuticals. Mol. Biotechnol. 2008, 39, 113–118. [Google Scholar] [CrossRef] [PubMed]
- VanderWeide, J.; Tombesi, S.; Castellarin, S.D.; Sabbatini, P. Canopy architecture and fruit microclimate, not ripening-related phytohormones, control phenylpropanoid accumulation in response to early leaf removal in ‘Merlot’ (Vitis vinifera L.) grapevines. Plant Physiol. Biochem. 2020, 157, 291–302. [Google Scholar] [CrossRef]
- Cajnko, M.M.; Vicente, F.A.; Novak, U.; Likozar, B. Natural deep eutectic solvents (NaDES): Translating cell biology to processing. Green Chem. 2023, 25, 9045–9062. [Google Scholar] [CrossRef]
- Mohd Fuad, F.; Mohd Nadzir, M. The formulation and physicochemical properties of betaine-based natural deep eutectic solvent. J. Mol. Liq. 2022, 360, 119392. [Google Scholar] [CrossRef]
- Che Zain, M.S.; Yeoh, J.X.; Lee, S.Y.; Shaari, K. Physicochemical Properties of Choline Chloride-Based Natural Deep Eutectic Solvents (NaDES) and Their Applicability for Extracting Oil Palm Flavonoids. Sustainability 2021, 13, 12981. [Google Scholar] [CrossRef]
- di Lorenzo, R.; Bernardi, A.; Grumetto, L.; Sacchi, A.; Avagliano, C.; Coppola, S.; de Giovanni di Santa Severina, A.F.; Bruno, C.; Paparo, L.; Laneri, S.; et al. Phenylalanine Butyramide Is a New Cosmetic Ingredient with Soothing and Anti-Reddening Potential. Molecules 2021, 26, 6611. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, M.; Hu, T.; Liu, C. Natural deep eutectic solvent—A novel green solvent for protein stabilization. Int. J. Biol. Macromol. 2023, 247, 125477. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Lou, C. Thermodynamic Irreversibility Analysis of Thermal Radiation in Coal-Fired Furnace: Effect of Coal Ash Deposits. Materials 2023, 16, 799. [Google Scholar] [CrossRef]
- Singhvi, P.; Verma, J.; Panwar, N.; Wani, T.Q.; Singh, A.; Qudratullah, M.; Chakraborty, A.; Saneja, A.; Sarkar, D.P.; Panda, A.K. Molecular Attributes Associated with Refolding of Inclusion Body Proteins Using the Freeze–Thaw Method. Front. Microbiol. 2021, 12, 618559. [Google Scholar] [CrossRef]
- Klausser, R.; Kopp, J.; Prada Brichtova, E.; Gisperg, F.; Elshazly, M.; Spadiut, O. State-of-the-art and novel approaches to mild solubilization of inclusion bodies. Front. Bioeng. Biotechnol. 2023, 11, 1249196. [Google Scholar] [CrossRef]
- Handling Inclusion Bodies in Recombinant Protein Expression. Available online: https://www.sigmaaldrich.com/ES/es/technical-documents/protocol/protein-biology/protein-lysis-and-extraction/handling-inclusion-bodies (accessed on 28 September 2025).
- Zeng, Q.; Wang, Y.; Huang, Y.; Ding, X.; Chen, J.; Xu, K. Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 2014, 139, 2565–2573. [Google Scholar] [CrossRef]
- Baynes, B.M.; Wang, D.I.C.; Trout, B.L. Role of Arginine in the Stabilization of Proteins against Aggregation. Biochemistry 2005, 44, 4919–4925. [Google Scholar] [CrossRef]
- Ren, S. Effects of arginine in therapeutic protein formulations: A decade review and perspectives. Antib. Ther. 2023, 6, 265–276. [Google Scholar] [CrossRef]
- Garbe, M.; Lehmann, L.T.; Berger, R.G.; Ersoy, F. Improvement in the Stability and Enzymatic Activity of Pleurotus sapidus Lipoxygenase Dissolved in Natural Deep Eutectic Solvents (NADESs). Life 2024, 14, 271. [Google Scholar] [CrossRef]
- Cayley, S.; Lewis, B.A.; Record, M.T. Origins of the osmoprotective properties of betaine and proline in Escherichia coli K-12. J. Bacteriol. 1992, 174, 1586–1595. [Google Scholar] [CrossRef]
- Hailu, T.T.; Foit, L.; Bardwell, J.C.A. In vivo detection and quantification of chemicals that enhance protein stability. Anal. Biochem. 2013, 434, 181–186. [Google Scholar] [CrossRef]
- Murdock, L.; Burke, T.; Coumoundouros, C.; Culham, D.E.; Deutch, C.E.; Ellinger, J.; Kerr, C.H.; Plater, S.M.; To, E.; Wright, G.; et al. Analysis of Strains Lacking Known Osmolyte Accumulation Mechanisms Reveals Contributions of Osmolytes and Transporters to Protection against Abiotic Stress. Appl. Environ. Microbiol. 2014, 80, 5366–5378. [Google Scholar] [CrossRef]
- Smink, D.; Juan, A.; Schuur, B.; Kersten, S.R.A. Understanding the Role of Choline Chloride in Deep Eutectic Solvents Used for Biomass Delignification. Ind. Eng. Chem. Res. 2019, 58, 16348–16357. [Google Scholar] [CrossRef]
- Jauregi, P.; Esnal-Yeregi, L.; Labidi, J. Natural deep eutectic solvents (NADES) for the extraction of bioactives: Emerging opportunities in biorefinery applications. PeerJ Anal. Chem. 2024, 6, e32. [Google Scholar] [CrossRef]
- James, G.C.; Euston, S.R. Molecular dynamics simulation allows mechanistic understanding of natural deep eutectic solvents action on rapeseed proteins. Food Hydrocoll. 2025, 166, 111328. [Google Scholar] [CrossRef]
- Bittner, J.P.; Smirnova, I.; Jakobtorweihen, S. Investigating Biomolecules in Deep Eutectic Solvents with Molecular Dynamics Simulations: Current State, Challenges and Future Perspectives. Molecules 2024, 29, 703. [Google Scholar] [CrossRef]
- Celebi, A.T.; Vlugt, T.J.H.; Moultos, O.A. Structural, Thermodynamic, and Transport Properties of Aqueous Reline and Ethaline Solutions from Molecular Dynamics Simulations. J. Phys. Chem. B 2019, 123, 11014–11025. [Google Scholar] [CrossRef]
- Sakurai, Y.C.N.; Pires, I.V.; Ferreira, N.R.; Moreira, S.G.C.; da Silva, L.H.M.; da Cruz Rodrigues, A.M. Preparation and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from Araza Pulp (Eugenia stipitata). Foods 2024, 13, 1983. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Shobhna; Kaur, S.; Kashyap, H.K. Influence of Hydration on the Structure of Reline Deep Eutectic Solvent: A Molecular Dynamics Study. ACS Omega 2018, 3, 15246–15255. [Google Scholar] [CrossRef] [PubMed]
- L Nascimento, L.; dos Santos, P.N.A.; Albuquerque, L.; de M. Pita, B.L.; de Almeida, L.S.; de Oliveira, M.L.; de A. Moreira, C.; Teixeira, F.R.P.; Caramão, E.B.; de S. Dias, F.; et al. Ultrasound-Assisted Extraction of Methylxanthines from Cocoa Bean Shells Using Glycerol–Urea Natural Deep Eutectic Solvent (NADES): Optimization and Greenness Assessment. ACS Omega 2025, 10, 44064–44076. [Google Scholar] [CrossRef]
- Gera, R.; Moll, C.J.; Bhattacherjee, A.; Bakker, H.J. Water-Induced Restructuring of the Surface of a Deep Eutectic Solvent. J. Phys. Chem. Lett. 2022, 13, 634–641. [Google Scholar] [CrossRef]
- Al Fuhaid, L.; Khattak, S.; Alghuneim, A.; Gallouzi, I.; Choi, Y.H.; Verpoorte, R.; Witkamp, G.-J.; Farinha, A. NADES as Biocompatible Media for Thermally Stable RNA Molecules. bioRxiv 2025. [Google Scholar] [CrossRef]
- Nystedt, H.L.; Grønlien, K.G.; Tønnesen, H.H. Interactions of natural deep eutectic solvents (NADES) with artificial and natural membranes. J. Mol. Liq. 2021, 328, 115452. [Google Scholar] [CrossRef]
- Yang, T.; Nian, Y.; Lin, H.; Li, J.; Lin, X.; Li, T.; Wang, R.; Wang, L.; Beattie, G.A.; Zhang, J.; et al. Structure and mechanism of the osmoregulated choline transporter BetT. Sci. Adv. 2024, 10, eado6229. [Google Scholar] [CrossRef]
- May, G.; Faatz, E.; Villarejo, M.; Bremer, E. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol. Gen. Genet. MGG 1986, 205, 225–233. [Google Scholar] [CrossRef]
- Perroud, B.; Le Rudulier, D. Glycine betaine transport in Escherichia coli: Osmotic modulation. J. Bacteriol. 1985, 161, 393–401. [Google Scholar] [CrossRef]
- Rumyantsev, A.; Sidorin, A.; Volkov, A.; Al Shanaa, O.; Sambuk, E.; Padkina, M. Transcriptome Analysis Unveils the Effects of Proline on Gene Expression in the Yeast Komagataella phaffii. Microorganisms 2021, 10, 67. [Google Scholar] [CrossRef]
- Cayley, S.; Record, M.T. Roles of Cytoplasmic Osmolytes, Water, and Crowding in the Response of Escherichia coli to Osmotic Stress: Biophysical Basis of Osmoprotection by Glycine Betaine. Biochemistry 2003, 42, 12596–12609. [Google Scholar] [CrossRef]
- Jesus, A.R.; Meneses, L.; Duarte, A.R.C.; Paiva, A. Natural deep eutectic systems, an emerging class of cryoprotectant agents. Cryobiology 2021, 101, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Li, X.; Leong, S.S.J. Refolding of proteins from inclusion bodies: Rational design and recipes. Appl. Microbiol. Biotechnol. 2011, 92, 241–251. [Google Scholar] [CrossRef]
- Singh, A.; Upadhyay, V.; Upadhyay, A.K.; Singh, S.M.; Panda, A.K. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb. Cell Fact. 2015, 14, 41. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T. A ribonuclease from Chinese ginseng (Panax ginseng) flowers. Protein Expr. Purif. 2004, 33, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Ebner, J.; Humer, D.; Klausser, R.; Rubus, V.; Pell, R.; Spadiut, O.; Kopp, J. At-Line Reversed Phase Liquid Chromatography for In-Process Monitoring of Inclusion Body Solubilization. Bioengineering 2021, 8, 78. [Google Scholar] [CrossRef]
- Singh, S.M.; Upadhyay, A.K.; Panda, A.K. Solubilization at high pH results in improved recovery of proteins from inclusion bodies of E. coli. J. Chem. Technol. Biotechnol. 2008, 83, 1126–1134. [Google Scholar] [CrossRef]
- Qi, X.; Sun, Y.; Xiong, S. A single freeze-thawing cycle for highly efficient solubilization of inclusion body proteins and its refolding into bioactive form. Microb. Cell Fact. 2015, 14, 24. [Google Scholar] [CrossRef]
- Shukla, D.; Trout, B.L. Interaction of Arginine with Proteins and the Mechanism by Which It Inhibits Aggregation. J. Phys. Chem. B 2010, 114, 13426–13438. [Google Scholar] [CrossRef]
- Zajac, J.W.P.; Muralikrishnan, P.; Tohidian, I.; Zeng, X.; Heldt, C.L.; Perry, S.L.; Sarupria, S. Flipping Out: Role of Arginine in Hydrophobic Interactions and Biological Formulation Design. Chem. Sci. 2025, 16, 6780–6792. [Google Scholar] [CrossRef]
- Natalello, A.; Liu, J.; Ami, D.; Doglia, S.M.; de Marco, A. The osmolyte betaine promotes protein misfolding and disruption of protein aggregates. Proteins Struct. Funct. Bioinforma. 2009, 75, 509–517. [Google Scholar] [CrossRef]
- Street, T.O.; Bolen, D.W.; Rose, G.D. A molecular mechanism for osmolyte-induced protein stability. Proc. Natl. Acad. Sci. USA 2006, 103, 13997–14002. [Google Scholar] [CrossRef]
- Gomes, I.; Martins, G.F.; Galamba, N. Essential dynamics of ubiquitin in water and in a natural deep eutectic solvent. Phys. Chem. Chem. Phys. 2024, 26, 18244–18255. [Google Scholar] [CrossRef] [PubMed]
- Buarque, F.; Gautério, G.; Coelho, M.; Lemes, A.; Ribeiro, B. Aqueous Two-Phase Systems Based on Ionic Liquids and Deep Eutectic Solvents as a Tool for the Recovery of Non-Protein Bioactive Compounds—A Review. Processes 2022, 11, 31. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Z.; Duan, P.; Li, W.; Zhang, Y.; Wu, J.; Hu, Z.; Roque, R.S.; Liu, J. Cloning, expression, and purification of a highly immunogenic recombinant gonadotropin-releasing hormone (GnRH) chimeric peptide. Protein Expr. Purif. 2006, 50, 163–170. [Google Scholar] [CrossRef]
- De Brabander, P.; Uitterhaegen, E.; Delmulle, T.; De Winter, K.; Soetaert, W. Challenges and progress towards industrial recombinant protein production in yeasts: A review. Biotechnol. Adv. 2023, 64, 108121. [Google Scholar] [CrossRef] [PubMed]
| HBA | HBD | Molar Ratio (HBA:HBD) | Key Property/ Characteristic | Potential Application in Protein Production | Typical Working Concentrations/ Conditions |
|---|---|---|---|---|---|
| Choline Chloride | Urea | 1:2 | Strong protein denaturing and solubilizing capacity; high polarity [16] | Inclusion Body (IB) solubilization; component in refolding buffers [47] | Mild Solubilization: 2–4 M urea equivalent in buffer [48,49] Harsh Solubilization: Used in aqueous solutions equivalent to 6–8 M urea [50] |
| Choline Chloride | Glycerol | 1:2 | High biocompatibility; cryoprotective properties; forms stable ATPS [44] | In vivo media additive; cryopreservation of engineered strains; ATPS for purification [44] | ATPS: 15–40% (w/w) NADESs as the phase-forming component with a salt solution (e.g., K3PO4) [51] |
| Choline Chloride | L-Arginine | Varies | Known aggregation suppressor; enhances protein solubility [14] | Additive in refolding buffers to prevent protein aggregation [14] | Refolding Additive: 0.1–1.0 M arginine in refolding buffer [52,53] |
| Betaine | Sorbitol | 1:1 | Osmoprotectant; excellent protein stabilizer; high biocompatibility [54] | In vivo media additive to enhance soluble expression; excipient for final product formulation [43] | In vivo Media Additive: 1–10 mM added to culture medium for osmoprotection in E. coli [55,56,57] |
| L-Proline | Glycerol | 1:2 | Protein solubilizing agent; osmoprotectant; organocatalytic properties [19] | In vivo media additive; component in mild solubilization/refolding buffers [19] | In vivo Media Additive: ~1 mM added to culture medium for osmoprotection in E. coli [55] |
| Choline Chloride | Lactic Acid | 1:2 | Acidic pH; high solubilizing power for biomass [44] | Pre-treatment of biomass for protein extraction (e.g., plant-based systems) [58] | Biomass Pre-treatment: Used in concentrated form (e.g., >80% w/w) to maximize delignification [58] |
| Menthol | Fatty Acid (e.g., Decanoic Acid) | Varies | Hydrophobic; low water miscibility; can solubilize apolar molecules [18] | Extraction and stabilization of membrane proteins; biphasic reaction systems [18] | Biphasic Systems: Used as a neat, water-immiscible phase for extraction or reaction [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Olivares, J.A.; Coca-Ruiz, V. Synergies in Green Bioprocessing: A Comprehensive Review of Heterologous Protein Expression and the Transformative Potential of Natural Deep Eutectic Solvents. Appl. Microbiol. 2025, 5, 113. https://doi.org/10.3390/applmicrobiol5040113
Martínez-Olivares JA, Coca-Ruiz V. Synergies in Green Bioprocessing: A Comprehensive Review of Heterologous Protein Expression and the Transformative Potential of Natural Deep Eutectic Solvents. Applied Microbiology. 2025; 5(4):113. https://doi.org/10.3390/applmicrobiol5040113
Chicago/Turabian StyleMartínez-Olivares, José Agustín, and Victor Coca-Ruiz. 2025. "Synergies in Green Bioprocessing: A Comprehensive Review of Heterologous Protein Expression and the Transformative Potential of Natural Deep Eutectic Solvents" Applied Microbiology 5, no. 4: 113. https://doi.org/10.3390/applmicrobiol5040113
APA StyleMartínez-Olivares, J. A., & Coca-Ruiz, V. (2025). Synergies in Green Bioprocessing: A Comprehensive Review of Heterologous Protein Expression and the Transformative Potential of Natural Deep Eutectic Solvents. Applied Microbiology, 5(4), 113. https://doi.org/10.3390/applmicrobiol5040113






