Dietary Saccharomyces cerevisiae Ameliorates the Adverse Effects of Aflatoxin B1 on Growth Performance, Haematological and Biochemical Parameters in Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.1.1. AF Production and Diet Preparation
2.1.2. Saccharomyces cerevisiae Culture
2.2. Data Collection and Measurements
2.3. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Haematological Properties
3.3. Blood Biochemistry
3.4. Carcass Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Do, T.H.; Tran, S.C.; Le, C.D.; Nguyen, H.-B.T.; Le, P.-T.T.; Le, H.-H.T.; Le, T.D.; Thai-Nguyen, H.-T. Dietary exposure and health risk characterization of aflatoxin B1, ochratoxin A, fumonisin B1, and zearalenone in food from different provinces in Northern Vietnam. Food Control 2020, 112, 107108. [Google Scholar] [CrossRef]
- Huong, B.T.M.; Tuyen, L.D.; Madsen, H.; Brimer, L.; Friis, H.; Dalsgaard, A. Total Dietary Intake and Health Risks Associated with Exposure to Aflatoxin B(1), Ochratoxin A and Fuminisins of Children in Lao Cai Province, Vietnam. Toxins 2019, 11, 638. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghami, S.S.; Rouhanipour, H.; Sharifi, S.D. Aflatoxin levels in poultry feed: A comparison of mash and pellet forms. Poult. Sci. 2024, 103, 103254. [Google Scholar] [CrossRef]
- Nesic, K.; Jaksic, S.; Popov, N.; Zivkov-Balos, M.; Pajic, M.; Zloh, B.; Polacek, V. In vitro assessment of binding capacity of combined adsorbent (bentonite with yeast cell wall extracts) and aflatoxin B1. Arch. Vet. Med. 2020, 13, 41–52. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Q. Aflatoxin B1 in poultry liver: Toxic mechanism. Toxicon 2023, 233, 107262. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, S.B.; Zhang, Q.; Tan, H.Z. Effects of Aflatoxin B(1) on growth performance, carcass traits, organ index, blood biochemistry and oxidative status in Chinese yellow chickens. J. Vet. Med. Sci. 2023, 85, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Kiarie, E.G.; Yiannikouris, A.; Sun, L.; Karrow, N.A. Nutritional impact of mycotoxins in food animal production and strategies for mitigation. J. Anim. Sci. Biotechnol. 2022, 13, 69. [Google Scholar] [CrossRef]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 Toxicity and Protective Effects of Curcumin: Molecular Mechanisms and Clinical Implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- Gallo, A.; Masoero, F. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital. J. Anim. Sci. 2010, 9, e21. [Google Scholar] [CrossRef]
- Zhao, J.; Shirley, R.B.; Dibner, J.D.; Uraizee, F.; Officer, M.; Kitchell, M.; Vazquez-Anon, M.; Knight, C.D. Comparison of hydrated sodium calcium aluminosilicate and yeast cell wall on counteracting aflatoxicosis in broiler chicks. Poult. Sci. 2010, 89, 2147–2156. [Google Scholar] [CrossRef]
- Aazami, M.H.; Nasri, M.H.F.; Mojtahedi, M.; Mohammadi, S.R. In Vitro Aflatoxin B(1) Binding by the Cell Wall and (1→3)-β-d-Glucan of Baker’s Yeast. J. Food Prot. 2018, 81, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Comi, M.; Vera, P.; Alessandro, A.; Qiu, K.; Wang, J.; Wu, S.-G.; Qi, G.-H.; Zhang, H.-J. Effects of Saccharomyces cerevisiae hydrolysate on growth performance, immunity function, and intestinal health in broilers. Poult. Sci. 2023, 102, 102237. [Google Scholar] [CrossRef] [PubMed]
- Bullerman, L.B. Mycotoxins | Classifications. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4080–4089. [Google Scholar]
- Giambrone, J.J.; Diener, U.L.; Davis, N.D.; Panangala, V.S.; Hoerr, F.J. Effects of Aflatoxin on Young Turkeys and Broiler Chickens1. Poult. Sci. 1985, 64, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Pizzolitto, R.P.; Armando, M.R.; Salvano, M.A.; Dalcero, A.M.; Rosa, C.A. Evaluation of Saccharomyces cerevisiae as an antiaflatoxicogenic agent in broiler feedstuffs. Poult. Sci. 2013, 92, 1655–1663. [Google Scholar] [CrossRef]
- Raz, M.; Bagherzadeh-Kasmani, F.; Karimi-Torshizi, M.A.; Ghazaghi, M.; Mokhtarpour, A.; Mehri, M. Boosting antioxidant defense and enhancing product quality by biochar and probiotics under chronic aflatoxicosis in quails. Poult. Sci. 2025, 104, 105183. [Google Scholar] [CrossRef]
- Sarker, M.T.; Wan, X.L.; Yang, H.M.; Wang, Z.Y. AflatoxinB1 (AFB1) and its toxic effect on the broilers intestine: A review. Vet. Med. Sci. 2023, 9, 1646–1655. [Google Scholar] [CrossRef]
- Insawake, K.; Songserm, T.; Songserm, O.; Rattanakreetakul, C.; Theapparat, Y.; Adeyemi, K.D.; Rassmidatta, K.; Ruangpanit, Y. Influence of phytochemicals on growth performance, gut morphology and ceca microbiome in broilers fed aflatoxin-contaminated diet and raised under high stocking density and heat stress. Poult. Sci. 2025, 104, 105293. [Google Scholar] [CrossRef]
- Guo, J.; Yan, W.-R.; Tang, J.-K.; Jin, X.; Xue, H.-H.; Wang, T.; Zhang, L.-W.; Sun, Q.-Y.; Liang, Z.-X. Dietary phillygenin supplementation ameliorates aflatoxin B1-induced oxidative stress, inflammation, and apoptosis in chicken liver. Ecotoxicol. Environ. Saf. 2022, 236, 113481. [Google Scholar] [CrossRef]
- Lawson, B.; MacDonald, S.; Howard, T.; Macgregor, S.K.; Cunningham, A.A. Exposure of garden birds to aflatoxins in Britain. Sci. Total Environ. 2006, 361, 124–131. [Google Scholar] [CrossRef]
- Cui, Y.; Li, D.; Zhang, M.; Liu, P.; Wang, H.; Li, Y.; Wu, Y. The Effects of Dietary Saccharomyces cerevisiae Supplementation on Gut Microbiota Composition and Gut Health in Aged Labrador Retrievers. Animals. 2024, 14, 1713. [Google Scholar] [CrossRef]
- Maxwell, M.H.; Whitehead, C.C.; Armstrong, J. Haematological and tissue abnormalities in chicks caused by acute and subclinical folate deficiency. Br. J. Nutr. 1988, 59, 73–80. [Google Scholar] [CrossRef]
- Kipkoech, G.; Jepkorir, M.; Kamau, S.; Wanyoko, A.; Kibunja, S.; Amozi Jeremiah, R.; Masese, J.; Ntui-Njock, V.; Mutai, C.; Mwitari, P. Immunomodulatory effects of aflatoxin B1 (AFB1) and the use of natural products to ameliorate its immunotoxic effects: A review [version 2; peer review: 1 approved, 2 approved with reservations]. Open Res. Afr. 2025, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Q.; Wang, J.; Sun, J.; Xiang, Y.; Jin, X. Aflatoxin B1 disrupts the intestinal barrier integrity by reducing junction protein and promoting apoptosis in pigs and mice. Ecotoxicol. Environ. Saf. 2022, 247, 114250. [Google Scholar] [CrossRef] [PubMed]
- He, X.-N.; Zeng, Z.-Z.; Wu, P.; Jiang, W.-D.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Feng, L.; Zhou, X.-Q. Dietary Aflatoxin B1 attenuates immune function of immune organs in grass carp (Ctenopharyngodon idella) by modulating NF-κB and the TOR signaling pathway. Front. Immunol. 2022, 13, 1027064. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.K.; Chang, S.N.; Kang, S.C. The inflammation response and risk associated with aflatoxin B1 contamination was minimized by insect peptide CopA3 treatment and act towards the beneficial health outcomes. Environ. Pollut. 2021, 268, 115713. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Mbajiorgu, C.A. Meta-analysis of Saccharomyces cerevisiae on enhancement of growth performance, rumen fermentation and haemato-biochemical characteristics of growing goats. Heliyon 2023, 9, e14178. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, Y.; Wan, H.; Zhou, X.; Wan, M.; Li, S.; Liu, X. Production of iron-enriched yeast and it’s application in the treatment of iron-deficiency anemia. Biometals 2024, 37, 1023–1035. [Google Scholar] [CrossRef]
- Ghazanfar, S.; Anjum, M.; Azim, A.; Ahmed, I. Effects of dietary supplementation of yeast (Saccharomyces cerevisiae) culture on growth performance, blood parameters, nutrient digestibility and fecal flora of dairy heifers. JAPS J. Anim. Plant Sci. 2015, 25, 53–59. [Google Scholar]
- Mekuria, A.N.; Routledge, M.N.; Gong, Y.Y.; Sisay, M. Aflatoxins as a risk factor for liver cirrhosis: A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2020, 21, 39. [Google Scholar] [CrossRef]
- Moloi, T.P.; Ziqubu, K.; Mazibuko-Mbeje, S.E.; Mabaso, N.H.; Ndlovu, Z. Aflatoxin B1-induced hepatotoxicity through mitochondrial dysfunction, oxidative stress, and inflammation as central pathological mechanisms: A review of experimental evidence. Toxicology 2024, 509, 153983. [Google Scholar] [CrossRef]
- Kalra, A.; Yetiskul, E.; Wehrle, C.J.; Tuma, F. Physiology, Liver; StatPearls: Treasure Island, FL, USA, 2018. [Google Scholar]
- Guo, J.; Xu, L.; Khalouei, H.; Fehr, K.; Senaratne, V.; Ghia, J.E.; Yoon, I.; Khafipour, E.; Plaizier, J.C. Saccharomyces cerevisiae fermentation products reduce bacterial endotoxin concentrations and inflammation during grain-based subacute ruminal acidosis in lactating dairy cows. J. Dairy Sci. 2022, 105, 2354–2368. [Google Scholar] [CrossRef]
- Dávila-Ramírez, J.L.; Carvajal-Nolazco, M.R.; López-Millanes, M.J.; González-Ríos, H.; Celaya-Michel, H.; Sosa-Castañeda, J.; Barrales-Heredia, S.M.; Moreno-Salazar, S.F.; Barrera-Silva, M.A. Effect of yeast culture (Saccharomyces cerevisiae) supplementation on growth performance, blood metabolites, carcass traits, quality, and sensorial traits of meat from pigs under heat stress. Anim. Feed Sci. Technol. 2020, 267, 114573. [Google Scholar] [CrossRef]
- Hieu, H.L.; Khammeng, T. Effect of yeast fermented cassava pulp (FCP) on nutrient digestibility and nitrogen balance of post-weaning pigs. Livest. Res. Rural Dev. 2014, 26, 149. [Google Scholar]
- Muñoz, C.; Wills, D.A.; Yan, T. Effects of dietary active dried yeast (Saccharomyces cerevisiae) supply at two levels of concentrate on energy and nitrogen utilisation and methane emissions of lactating dairy cows. Anim. Prod. Sci. 2017, 57, 656–664. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, T.; Demelash, N.; Zheng, S.; Zhao, W.; Chen, X.; Zhen, Y.; Qin, G. Effect of Yeast Culture (Saccharomyces cerevisiae) on Broilers: A Preliminary Study on the Effective Components of Yeast Culture. Animals 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Rezaeipour, V.; Fononi, H.; Irani, M. Effects of dietary L-threonine and Saccharomyces cerevisiae on performance, intestinal morphology and immune response of broiler chickens. S. Afr. J. Anim. Sci. 2012, 42, 266–273. [Google Scholar] [CrossRef]
- De Chiara, F.; Heebøll, S.; Marrone, G.; Montoliu, C.; Hamilton-Dutoit, S.; Ferrandez, A.; Andreola, F.; Rombouts, K.; Grønbæk, H.; Felipo, V.; et al. Urea cycle dysregulation in non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 905–915. [Google Scholar] [CrossRef]
- Knepper, M.A.; Roch–Ramel, F. Pathways of urea transport in the mammalian kidney. Kidney Int. 1987, 31, 629–633. [Google Scholar] [CrossRef]
| Ingredient, g/kg | Starter | Grower | Finisher |
|---|---|---|---|
| Corn | 495 | 484 | 526 |
| Cassava | 50 | 100 | 100 |
| DDGS corn | 60 | 60 | 60 |
| Palm oil | 21 | 24.7 | 24.3 |
| Limestone | 10.65 | 9.00 | 9.05 |
| Soybean meal | 226 | 221 | 180 |
| Meat and bone meal 45% | 20 | 30 | 30 |
| Extruded soybean | 90 | 50 | 50 |
| Monocalcium phosphate | 5.3 | 1 | 0 |
| Salt | 2.3 | 2.2 | 2.2 |
| Methionine 98% | 2.9 | 2.9 | 3.2 |
| Threonine 98% | 0.9 | 1 | 1.6 |
| Lysine HCL 98.5% | 3.1 | 3,3 | 4.6 |
| Tryptophan | 0.1 | 0.15 | 0.4 |
| Choline chloride 60% | 1 | 1 | 1 |
| Premix * | 11.75 | 9.75 | 7.65 |
| Total Batch | 1000 | 1000 | 1000 |
| Calculated nutrient levels | |||
| Crude protein (min, %) | 21.0 | 19.0 | 18.0 |
| Moisture (max, %) | 13.0 | 13.0 | 13.0 |
| Lysine (min, %) | 1.1 | 1.0 | 0.9 |
| Met + Cys (min, %) | 0.8 | 0.7 | 0.6 |
| ME (Kcal/kg) | 3000 | 3000 | 3100 |
| Phosphorous (min-max, %) | 0.6–1.0 | 0.6–1.0 | 0.6–1.0 |
| Calcium (min-max, %) | 0.5–2.5 | 0.5–2.5 | 0.5–2.5 |
| Crude fibre (max, %) | 4.0 | 4.0 | 4.5 |
| Parameter | CON 1 | SAC 1 | SED | p-Value | AF 2 | SAC 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF0 | AF20 | AF60 | AF0 | AF20 | AF60 | AF | SAC | AF × SAC | AF0 | AF20 | AF60 | CON | SAC | ||
| 0–21 days | |||||||||||||||
| ADFI, g/day | 53.6 bc | 53.1 ab | 52.4 a | 53.5 bc | 54.1 c | 53.9 bc | 0.46 | 0.33 | 0.009 | 0.085 | 53.6 | 53.6 | 53.1 | 53.0 | 53.8 |
| ADG, g/day | 34.1 d | 26.4 abc | 25.6 a | 27.4 c | 26.7 bc | 25.7 ab | 0.72 | <0.001 | <0.001 | <0.001 | 30.7 A | 26.6 B | 25.5 C | 28.6 | 26.6 |
| FCR | 1.57 a | 2.01 b | 2.08 b | 1.96 b | 2.03 b | 2.10 b | 0.088 | <0.001 | 0.016 | 0.020 | 1.77 A | 2.02 B | 2.09 B | 1.89 | 2.03 |
| 21–42 days | |||||||||||||||
| ADFI, g/day | 122 b | 125 e | 123 c | 120 a | 124 d | 132 f | 0.12 | <0.001 | <0.001 | <0.001 | 121 A | 125 B | 127 C | 123 | 125 |
| ADG, g/day | 70.4 | 73.6 | 69.1 | 69.4 | 73.7 | 71.1 | 2.17 | 0.053 | 0.76 | 0.63 | 70.0 A | 73.7 B | 70.1 A | 71.0 | 71.4 |
| FCR | 1.74 | 1.70 | 1.78 | 1.73 | 1.69 | 1.85 | 0.054 | 0.027 | 0.54 | 0.47 | 1.74 AB | 1.70 A | 1.82 B | 1.74 | 1.76 |
| 1–42 days | |||||||||||||||
| ADFI, g/day | 94.1 b | 96.0 c | 94.2 b | 92.9 a | 95.5 c | 100 d | 0.25 | <0.001 | <0.001 | <0.001 | 93.5 A | 95.8 B | 97.1 C | 94.8 | 96.2 |
| ADG, g/day | 53.5 c | 51.3 bc | 48.4 a | 49.6 ab | 51.4 bc | 49.6 ab | 1.07 | 0.009 | 0.21 | 0.014 | 51.5 A | 51.3 A | 49.0 B | 51.0 | 50.2 |
| FCR | 1.76 | 1.87 | 1.95 | 1.87 | 1.86 | 2.02 | 0.041 | <0.001 | 0.035 | 0.13 | 1.82 A | 1.87 A | 1.99 B | 1.86 | 1.92 |
| Parameter | CON 1 | SAC 1 | SED | p-Value | AF 2 | SAC 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF0 | AF20 | AF60 | AF0 | AF20 | AF60 | AF | SAC | AF × SAC | AF0 | AF20 | AF60 | CON | SAC | ||
| Hwt (%) | 27.5 b | 25.5 ab | 26.4 b | 21.7 a | 25.7 ab | 27.8 b | 2.21 | 0.30 | 0.30 | 0.069 | 24.6 | 25.6 | 27.1 | 26.4 | 25.1 |
| WBC (109/L) | 23.6 b | 16.5 a | 13.3 a | 13.3 a | 24.2 b | 15.7 a | 2.90 | 0.030 | 0.98 | 0.001 | 18.5 A | 20.4 B | 14.5 AB | 17.8 | 17.8 |
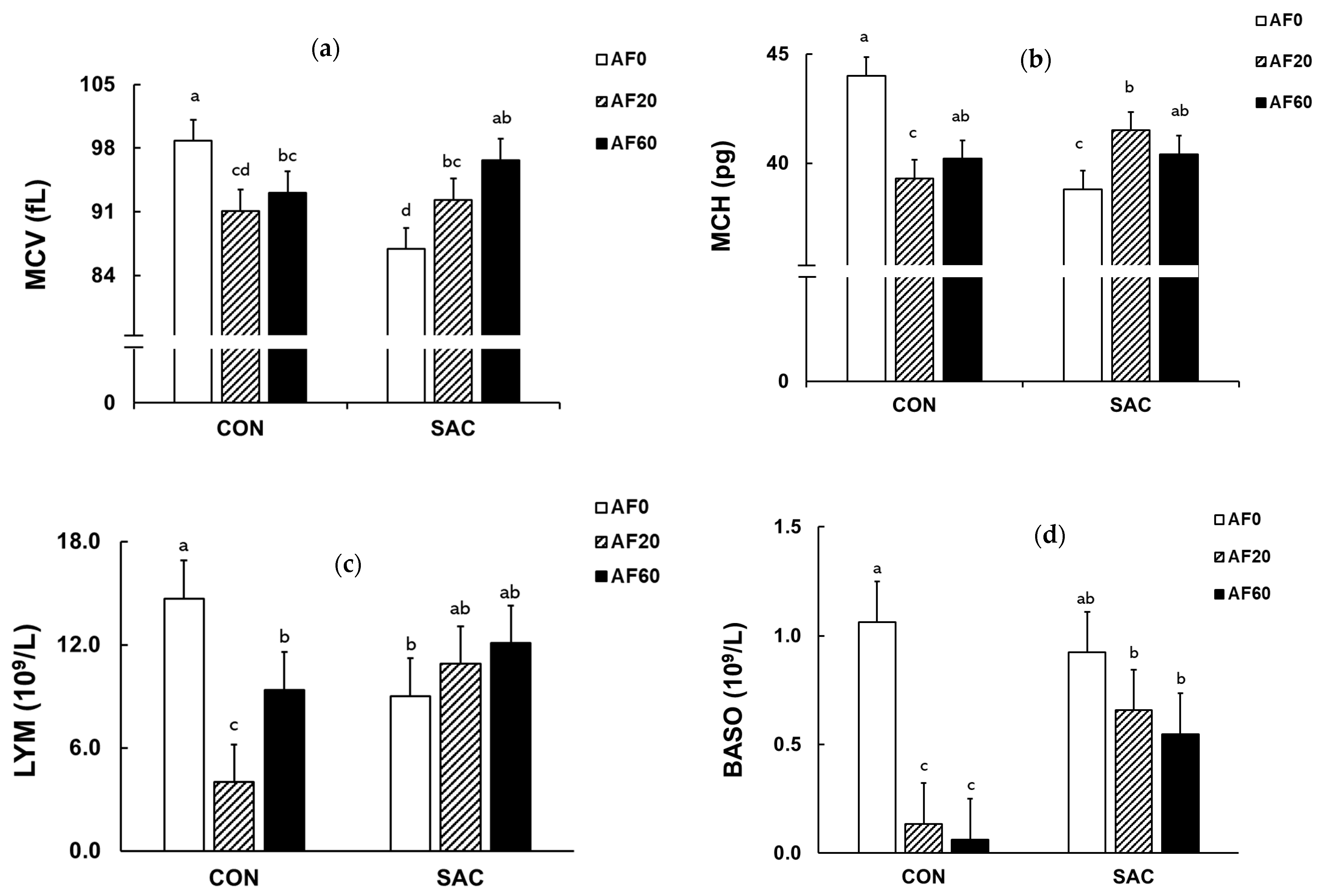
| MCV (fL) | 98.8 d | 91.1 ab | 93.1 bc | 86.9 a | 92.3 bc | 96.7 cd | 2.33 | 0.17 | 0.095 | <0.001 | 92.8 | 91.7 | 94.9 | 94.3 | 92.0 |
| MCH (pg) | 44.0 c | 39.3 a | 40.2 ab | 38.8 a | 41.5 b | 40.4 ab | 0.85 | 0.17 | 0.058 | <0.001 | 41.4 | 40.4 | 40.3 | 41.2 A | 40.2 B |
| LYM (109/L) | 14.70 c | 4.00 a | 9.38 b | 9.01 b | 10.93 bc | 12.10 bc | 2.19 | 0.029 | 0.32 | 0.003 | 11.80 A | 7.43 B | 10,73 A | 9.34 A | 10.62 B |
| BASO (109/L) | 1.062 c | 0.135 a | 0.063 a | 0.925 bc | 0.658 b | 0.548 b | 0.187 | <0.001 | 0.015 | 0.039 | 0.994 A | 0.396 B | 0.305 B | 0.420 A | 0.710 B |
| Parameter | CON 1 | SAC 1 | SED | p-Value | AF 2 | SAC 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF0 | AF20 | AF60 | AF0 | AF20 | AF60 | AF | SAC | AF × SAC | AF0 | AF20 | AF60 | CON | SAC | ||
| Hb (g/L) | 118 | 101 | 110 | 103 | 105 | 107 | 10.6 | 0.59 | 0.47 | 0.46 | 111 | 103 | 109 | 110 | 105 |
| RBC (1012/L) | 2.79 | 2.92 | 3.09 | 2.51 | 2.82 | 3.08 | 0.24 | 0.062 | 0.37 | 0.72 | 2.65 A | 2.87 AB | 3.08 B | 2.93 | 2.80 |
| RDW (%) | 12.1 | 12.0 | 12.3 | 12.1 | 12.2 | 12.3 | 0.47 | 0.78 | 0.86 | 0.97 | 12.1 | 12.1 | 12.3 | 12.1 | 12.2 |
| PLT (109/L) | 5.04 | 4.78 | 5.59 | 5.14 | 5.70 | 7.03 | 0.84 | 0.106 | 0.107 | 0.53 | 5.09 | 5.24 | 6.31 | 5.14 | 5.96 |
| NEU (109/L) | 7.80 | 16.2 | 15.1 | 4.90 | 11.1 | 6.16 | 2.96 | 0.009 | 0.004 | 0.37 | 6.4 A | 13.6 B | 10.6 AB | 13.0 | 7.1 |
| MONO (109/L) | 0.400 | 0.461 | 0.445 | 0.338 | 0.506 | 0.395 | 0.1184 | 0.41 | 0.75 | 0.78 | 0.369 | 0.484 | 0.420 | 0.435 | 0.413 |
| MCHC (g/L) | 442 | 435 | 438 | 463 | 420 | 401 | 18.60 | 0.054 | 0.35 | 0.105 | 453 A | 428 AB | 420 B | 439 | 428 |
| EOS (109/L) | 0.048 | 0.053 | 0.034 | 0.034 | 0.038 | 0.036 | 0.014 | 0.57 | 0.28 | 0.67 | 0.041 | 0.046 | 0.035 | 0.045 | 0.036 |
| Parameter | CON 1 | SAC 1 | SED | p-Value | AF 2 | SAC 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF0 | AF20 | AF60 | AF0 | AF20 | AF60 | AF | SAC | AF × SAC | AF0 | AF20 | AF60 | CON | SAC | ||
| Protein (g/L) | 34.8 | 29.5 | 27.8 | 28.1 | 31.3 | 26.5 | 5.15 | 0.48 | 0.49 | 0.51 | 31.5 | 30.4 | 27.1 | 30.7 | 28.6 |
| Albumin (g/L) | 8.43 | 7.67 | 6.84 | 7.09 | 8.17 | 6.94 | 1.344 | 0.52 | 0.75 | 0.61 | 7.76 | 7.92 | 6.89 | 7.65 | 7.40 |
| Creatine (µmol/L) | 1946 | 11,875 | 12,314 | 6803 | 7500 | 8243 | 5128 | 0.23 | 0.69 | 0.37 | 4375 | 9687 | 10,278 | 8711 | 7515 |
| Urea (mmol/L) | 0.675 | 0.678 | 0.620 | 0.568 | 0.370 | 0.553 | 0.1443 | 0.63 | 0.069 | 0.47 | 0.621 | 0.524 | 0.586 | 0.658 | 0.497 |
| Glucose (mmol/L) | 13.5 | 10.7 | 11.7 | 12.3 | 11.6 | 10.8 | 2.01 | 0.40 | 0.71 | 0.72 | 12.9 | 11.2 | 11.3 | 12.0 | 11.6 |
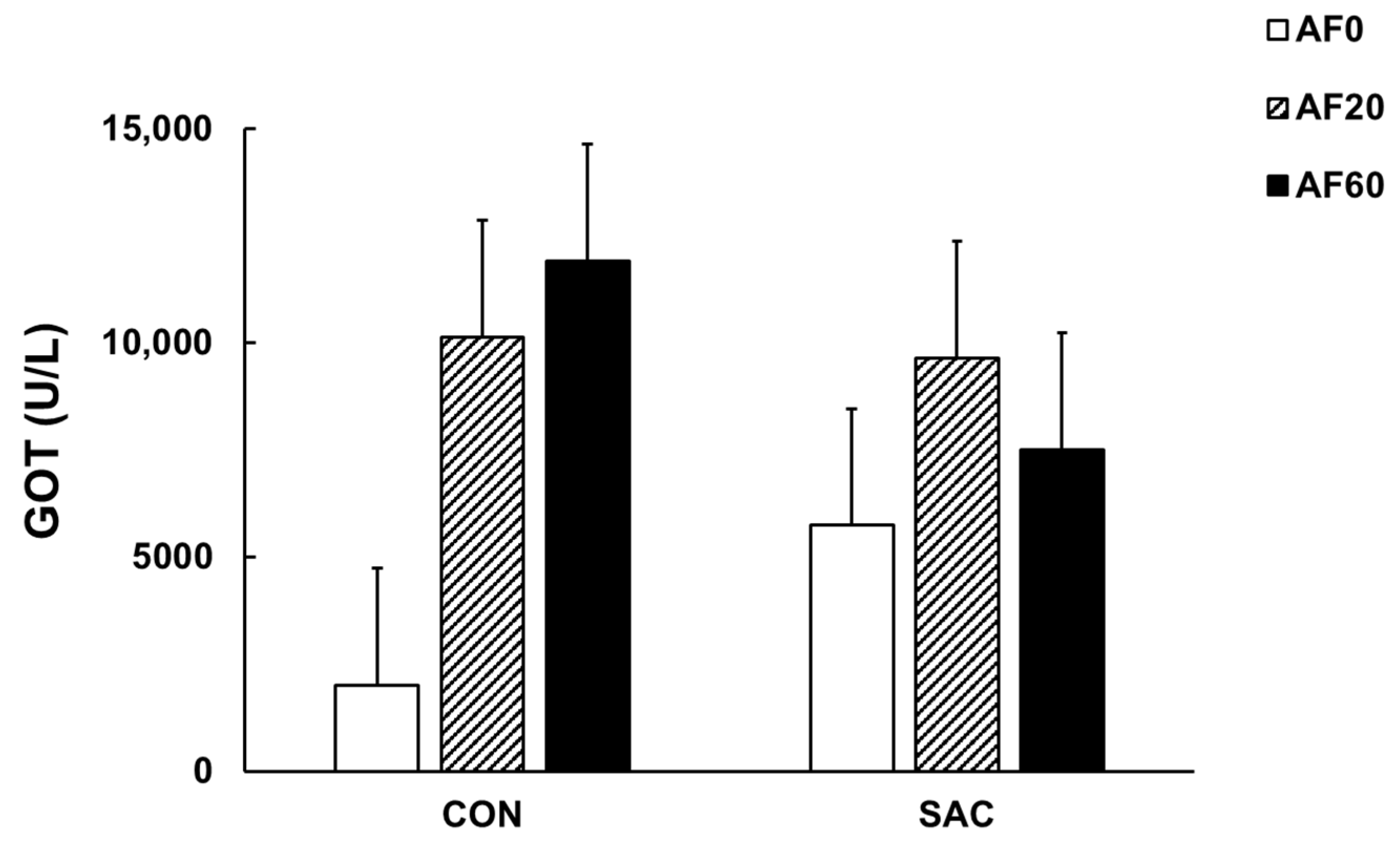
| GOT (U/L) | 2016 | 10,143 | 11,918 | 5733 | 9654 | 7494 | 2732 | 0.009 | 0.80 | 0.14 | 3875 A | 9899 B | 9706 B | 8026 | 7627 |
| GPT (U/L) | 4.45 | 2.98 | 3.51 | 3.97 | 3.26 | 3.54 | 0.72 | 0.13 | 0.89 | 0.75 | 4.21 | 3.12 | 3.52 | 3.65 | 3.59 |
| Parameter | CON 1 | SAC 1 | SED | p-Value | AF 2 | SAC 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF0 | AF20 | AF60 | AF0 | AF20 | AF60 | AF | SAC | AF × SAC | AF0 | AF20 | AF60 | CON | SAC | ||
| Live weight (g) | 2633 | 2378 | 2290 | 2575 | 2455 | 2320 | 148 | 0.033 | 0.85 | 0.81 | 2604 A | 2416 AB | 2305 B | 2433 | 2540 |
| Carcass (g) | 2029 | 1767 | 1600 | 1859 | 1777 | 1722 | 123 | 0.028 | 0.65 | 0.39 | 1944 A | 1772 AB | 1691 B | 1819 | 1786 |
| Carcass (%) | 77.1 b | 74.5 ab | 72.4 a | 72.1 a | 72.3 a | 74.2 ab | 1.65 | 0.47 | 0.083 | 0.028 | 74.6 | 73.4 | 73.3 | 74.6 | 72.9 |
| Thigh (g) | 228 | 204 | 183 | 201 | 175 | 181 | 19.5 | 0.078 | 0.11 | 0.58 | 214 A | 190 AB | 182 B | 205 | 186 |
| Thigh (%) | 11.2 | 11.5 | 11.0 | 10.8 | 9.92 | 10.5 | 0.777 | 0.85 | 0.080 | 0.47 | 11.0 | 10.7 | 10.8 | 11.3 | 10.4 |
| Breast (g) | 266 | 247 | 204 | 253 | 217 | 216 | 28.8 | 0.074 | 0.55 | 0.58 | 260 A | 232 AB | 210 B | 238 | 229 |
| Breast (%) | 13.1 | 14.0 | 12.3 | 13.5 | 12.2 | 12.6 | 1.30 | 0.63 | 0.62 | 0.40 | 13.3 | 13.1 | 12.5 | 13.1 | 12.8 |
| Fat (g) | 15.1 | 14.6 | 16.6 | 16.6 | 18.3 | 20.7 | 4.65 | 0.68 | 0.26 | 0.92 | 15.8 | 16.5 | 18.6 | 15.4 | 18.5 |
| Fat (%) | 0.734 | 0.841 | 1.002 | 0.868 | 1.021 | 1.180 | 0.2203 | 0.20 | 0.21 | 0.99 | 0.801 | 0.931 | 1.091 | 0.859 | 1.023 |
| Gizzard (g) | 37.8 | 42.1 | 38.1 | 36.2 | 41.6 | 38.4 | 4.54 | 0.31 | 0.83 | 0.96 | 37.0 | 41.8 | 38.2 | 39.3 | 38.7 |
| Gizzard (%) | 1.86 | 2.36 | 2.31 | 1.97 | 2.37 | 2.23 | 0.24 | 0.038 | 0.92 | 0.86 | 1.91 A | 2.36 B | 2.27 AB | 2.17 | 2.19 |
| Heart (g) | 10.60 | 11.91 | 8.85 | 12.03 | 9.37 | 9.05 | 1.18 | 0.031 | 0.65 | 0.083 | 11.31 A | 10.60 AB | 8.95 B | 10.5 | 10.1 |
| Heart (%) | 0.522 a | 0.670 c | 0.536 ab | 0.654 bc | 0.528 ab | 0.526 ab | 0.0618 | 0.28 | 0.84 | 0.020 | 0.588 | 0.599 | 0.531 | 0.576 | 0.569 |
| Liver (g) | 59.6 | 63.7 | 62.2 | 59.6 | 72.1 | 55.9 | 6.83 | 0.15 | 0.87 | 0.33 | 59.6 | 67.9 | 59.0 | 61.9 | 62.5 |
| Liver (%) | 2.94 | 3.59 | 3.80 | 3.21 | 4.06 | 3.20 | 0.307 | 0.010 | 0.79 | 0.055 | 3.08 A | 3.83 B | 3.50 AB | 3.44 | 3.49 |
| Spleen (g) | 3.15 | 2.90 | 30.5 | 2.88 | 2.18 | 2.83 | 0.559 | 0.45 | 0.22 | 0.79 | 3.01 | 2.54 | 2.94 | 3.03 | 2.63 |
| Spleen (%) | 0.155 | 0.165 | 0.185 | 0.154 | 0.125 | 0.165 | 0.0312 | 0.39 | 0.278 | 0.68 | 0.155 | 0.145 | 0.175 | 0.168 | 0.148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, D.H.; Nguyen, V.T.; Nguyen, G.T.P.; Nguyen, L.T.T.; Dinh, Y.T.; Dang, H.T.; Nguyen, T.B.; Nguyen, T.H.; Shakeri, M.; Le, H.H. Dietary Saccharomyces cerevisiae Ameliorates the Adverse Effects of Aflatoxin B1 on Growth Performance, Haematological and Biochemical Parameters in Broiler Chickens. Appl. Microbiol. 2025, 5, 99. https://doi.org/10.3390/applmicrobiol5030099
Bui DH, Nguyen VT, Nguyen GTP, Nguyen LTT, Dinh YT, Dang HT, Nguyen TB, Nguyen TH, Shakeri M, Le HH. Dietary Saccharomyces cerevisiae Ameliorates the Adverse Effects of Aflatoxin B1 on Growth Performance, Haematological and Biochemical Parameters in Broiler Chickens. Applied Microbiology. 2025; 5(3):99. https://doi.org/10.3390/applmicrobiol5030099
Chicago/Turabian StyleBui, Doanh Huy, Vinh Thi Nguyen, Giang Thi Phuong Nguyen, Le Thị Tuyet Nguyen, Yen Thi Dinh, Hai Thai Dang, Tiep Ba Nguyen, Thinh Hoang Nguyen, Majid Shakeri, and Hieu Huu Le. 2025. "Dietary Saccharomyces cerevisiae Ameliorates the Adverse Effects of Aflatoxin B1 on Growth Performance, Haematological and Biochemical Parameters in Broiler Chickens" Applied Microbiology 5, no. 3: 99. https://doi.org/10.3390/applmicrobiol5030099
APA StyleBui, D. H., Nguyen, V. T., Nguyen, G. T. P., Nguyen, L. T. T., Dinh, Y. T., Dang, H. T., Nguyen, T. B., Nguyen, T. H., Shakeri, M., & Le, H. H. (2025). Dietary Saccharomyces cerevisiae Ameliorates the Adverse Effects of Aflatoxin B1 on Growth Performance, Haematological and Biochemical Parameters in Broiler Chickens. Applied Microbiology, 5(3), 99. https://doi.org/10.3390/applmicrobiol5030099








