Conversion of Komagataella phaffii Biomass Waste to Yeast Extract Supplement
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of K. phaffii Pellets for Recycled Yeast Extract
2.2. Determination of Optimal Composition of Recycled Yeast Extract in YPD Media
2.3. Culturing of K. phaffii β-Glucosidase-Expressing Clones
2.4. Determination of β-Glucosidase Secretion in IRA1 K. phaffii Clones in Varying Compositions of YPD Media
2.5. Analysis of Reducing Sugars and Total Protein Concentrations
2.6. Statistical Analysis
3. Results
3.1. Selection of Media Types for Expression of β-Glucosidase
3.2. Growth of K. phaffii in Different YPD Media Compositions
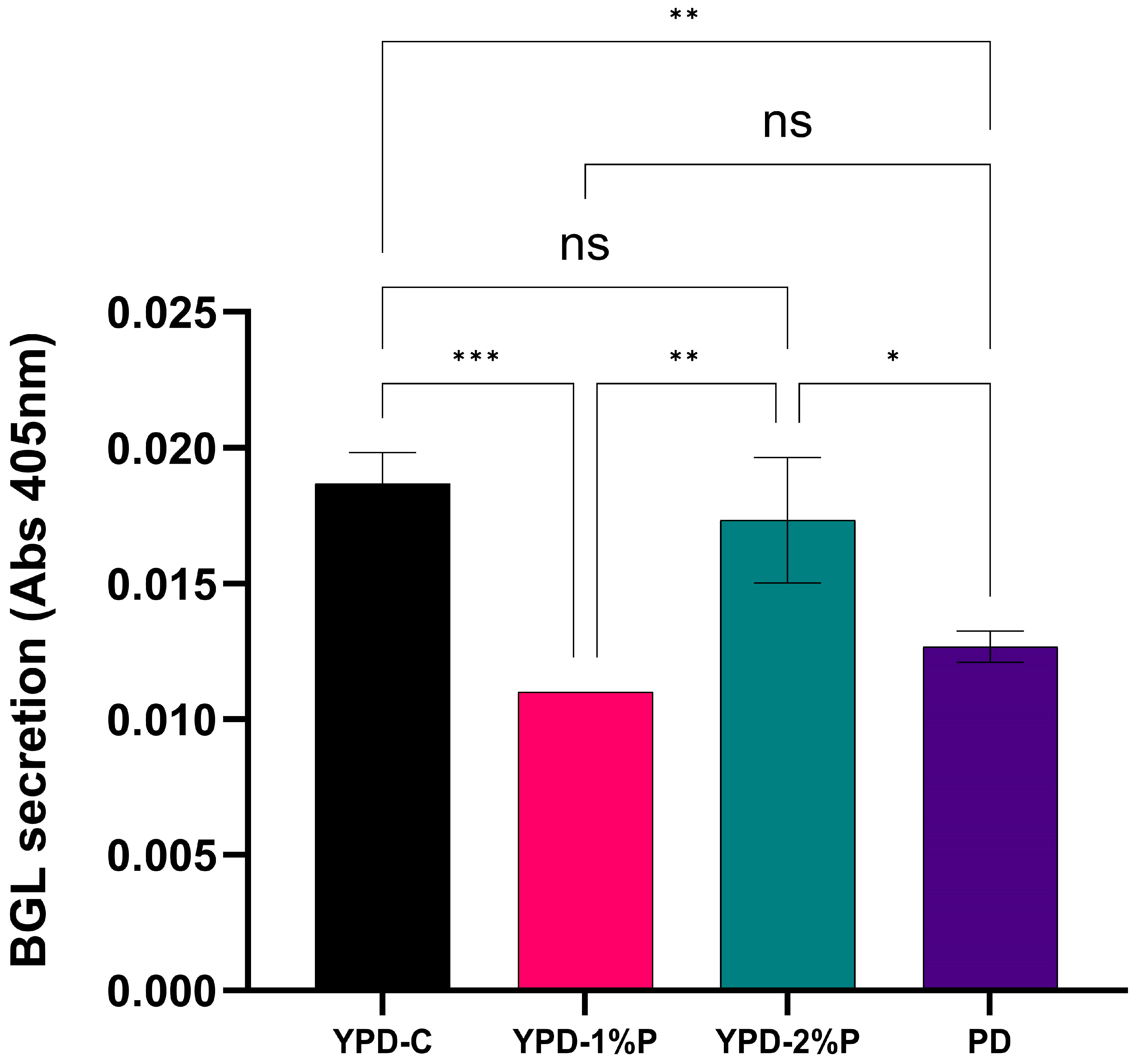
3.3. Expression of β-Glucosidase in Various Media Compositions
3.4. Nutrient Uptake and Carbon Use Efficiency
4. Discussion
4.1. Recycled Yeast Extract Is Soluble at Low Concentrations
4.2. YPD Media Comprised of Recycled Yeast Extract and 2% Peptone Can Effectively Support the Growth of K. phaffii and the Secretion of β-Glucosidase
4.3. YPD Media Comprised of Recycled Yeast Extract Has a Comparable Nutrient Profile to Commercial Yeast Extract Media
4.4. Method Limitations
- Organism-Specific Validation:
- Protein-Specific Results:
- Dependence on Spent Fermentation Conditions:
- Undetermined Quantitative Composition:
- Sterility and Consistency Risks:
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Mattanovich, D.; Sauer, M.; Gasser, B. Yeast biotechnology: Teaching the old dog new tricks. Microb. Cell Factories 2014, 13, 34. [Google Scholar] [CrossRef]
- Demain, A.L.; Phaff, H.J.; Kurtzman, C.P. The industrial and agricultural significance of yeasts. In The Yeasts, 4th ed.; Kurtzman, C.P., Fell, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Chapter 4, pp. 13–19. [Google Scholar]
- Murphy, L.; O’Connell, D.J. The Role of Yeast in the Valorisation of Food Waste. Fermentation 2024, 10, 583. [Google Scholar] [CrossRef]
- Łukaszewicz, M.; Leszczyński, P.; Jabłoński, S.J.; Kawa-Rygielska, J. Potential Applications of Yeast Biomass Derived from Small-Scale Breweries. Appl. Sci. 2024, 14, 2529. [Google Scholar] [CrossRef]
- Nielsen, J. Production of biopharmaceutical proteins by yeast: Advances through metabolic engineering. Bioengineered 2013, 4, 207–211. [Google Scholar] [CrossRef]
- Barone, G.D.; Emmerstorfer-Augustin, A.; Biundo, A.; Pisano, I.; Coccetti, P.; Mapelli, V.; Camattari, A. Industrial Production of Proteins with Pichia pastoris-Komagataella phaffii. Biomolecules 2023, 13, 441. [Google Scholar] [CrossRef]
- Pichia.com. Pichia Produced Products on the Market. Available online: https://pichia.com/science-center/commercialized-products/ (accessed on 30 May 2024).
- Akermann, A.; Weiermüller, J.; Chodorski, J.N.; Nestriepke, M.J.; Baclig, M.T.; Ulber, R. Optimization of bioprocesses with Brewers’ spent grain and Cellulomonas uda. Eng. Life Sci. 2022, 22, 132–151. [Google Scholar] [CrossRef]
- Granget, C.; Manikandan, N.A.; Amulya, K.; Dabros, M.; Fahy, S.; Kelleher, S.M.; Rochfort, K.D.; Gaughran, J.; Freeland, B. Brewer’s spent grain as a self-sufficient feedstock for homofermentative production of optically pure L-lactic acid using Lactobacillus rhamnosus. Environ. Technol. Innov. 2024, 34, 103582. [Google Scholar] [CrossRef]
- Cooray, S.T.; Lee, J.J.L.; Chen, W.N. Evaluation of brewers’ spent grain as a novel media for yeast growth. AMB Express 2017, 7, 117. [Google Scholar] [CrossRef]
- Flores-Copa, V.; Romero-Soto, L.; Romero-Calle, D.; Alvarez-Aliaga, M.T.; Orozco-Gutierrez, F.; Vega-Baudrit, J.; Martín, C.; Carrasco, C. Residual Brewing Yeast as Substrate for Co-Production of Cell Biomass and Biofilm Using Candida maltosa SM4. Fermentation 2021, 7, 84. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Buhler, S.; Matsakas, L. Valorization of Brewers' Spent Grain for the Production of Lipids by Oleaginous Yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef]
- Le, R.K.; Das, P.; Mahan, K.M.; Anderson, S.A.; Wells, T.; Yuan, J.S.; Ragauskas, A.J. Utilization of simultaneous saccharification and fermentation residues as feedstock for lipid accumulation in Rhodococcus opacus. AMB Express 2017, 7, 185. [Google Scholar] [CrossRef]
- Jacob, F.F.; Hutzler, M.; Methner, F.-J. Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: Influence on amino acid and protein content. Eur. Food Res. Technol. 2019, 245, 95–109. [Google Scholar] [CrossRef]
- Sakarika, M.; Kerckhof, F.-M.; Van Peteghem, L.; Pereira, A.; Van Den Bossche, T.; Bouwmeester, R.; Gabriels, R.; Van Haver, D.; Ulčar, B.; Martens, L.; et al. The nutritional composition and cell size of microbial biomass for food applications are defined by the growth conditions. Microb. Cell Factories 2023, 22, 254. [Google Scholar] [CrossRef]
- Ahuja, V.; Arora, A.; Chauhan, S.; Thakur, S.; Jeyaseelan, C.; Paul, D. Yeast-Mediated Biomass Valorization for Biofuel Production: A Literature Review. Fermentation 2023, 9, 784. [Google Scholar] [CrossRef]
- Offei, B.; Braun-Galleani, S.; Venkatesh, A.; Casey, W.T.; O’Connor, K.E.; Byrne, K.P.; Wolfe, K.H. Identification of genetic variants of the industrial yeast Komagataella phaffii (Pichia pastoris) that contribute to increased yields of secreted heterologous proteins. PLoS Biol. 2022, 20, e3001877. [Google Scholar] [CrossRef]
- Iyer, A.; Bestwick, C.S.; Duncan, S.H.; Russell, W.R. Invasive Plants Are a Valuable Alternate Protein Source and Can Contribute to Meeting Climate Change Targets. Front. Sustain. Food Syst. 2021, 5, 575056. [Google Scholar] [CrossRef]
- Zarei, O.; Dastmalchi, S.; Hamzeh-Mivehroud, M. A Simple and Rapid Protocol for Producing Yeast Extract from Saccharomyces cerevisiae Suitable for Preparing Bacterial Culture Media. Iran. J. Pharm. Res. 2016, 15, 907–913. [Google Scholar]
- Inokuma, K.; Hasunuma, T.; Kondo, A. Efficient yeast cell-surface display of exo- and endo-cellulase using the SED1 anchoring region and its original promoter. Biotechnol Biofuels. 2014, 7, 8. [Google Scholar] [CrossRef]
- Vajdič, T.; Erjavec, M.S. Harnessing Environmental Yeasts—Pichia kudriavzevii Strain ZMUM_K002: The Quest for Isolates with Properties for Efficient Biotechnological Applications. Appl. Microbiol. 2025, 5, 30. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Bayarjargal, M.; Munkhbat, E.; Ariunsaikhan, T.; Odonchimeg, M.; Uurzaikh, T.; Gan-Erdene, T.; Regdel, D. Utilization of spent brewer’s yeast Saccharomyces cerevisiae for the production of yeast enzymatic hydrolysate. Mong. J. Chem. 2014, 12, 88–91. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Miao, H.; Tang, X.; Xu, B.; Wu, Q.; Mu, Y.; Huang, Z. Transcriptomic Analysis of Pichia pastoris (Komagataella phaffii) GS115 During Heterologous Protein Production Using a High-Cell-Density Fed-Batch Cultivation Strategy. Front. Microbiol. 2020, 11, 463. [Google Scholar] [CrossRef]
- Murphy, L.; Lynch, C.D.; O’Connell, D.J. Valorisation of Spent Yeast Fermentation Media through Compositional-Analysis-Directed Supplementation. Appl. Microbiol. 2024, 4, 959–971. [Google Scholar] [CrossRef]
- Davami, F.; Eghbalpour, F.; Nematollahi, L.; Barkhordari, F.; Mahboudi, F. Effects of Peptone Supplementation in Different Culture Media on Growth, Metabolic Pathway and Productivity of CHO DG44 Cells; a New Insight into Amino Acid Profiles. Iran. Biomed. J. 2015, 19, 194. [Google Scholar]
- Krahulec, J.; Šafránek, M. Impact of media components from different suppliers on enterokinase productivity in Pichia pastoris. BMC Biotechnol. 2021, 21, 19. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151–166. [Google Scholar] [CrossRef]
- Tachibana, S.; Watanabe, K.; Konishi, M. Estimating effects of yeast extract compositions on Escherichia coli growth by a metabolomics approach. J. Biosci. Bioeng. 2019, 128, 468–474. [Google Scholar] [CrossRef]
- Broach, J.R. Nutritional control of growth and development in yeast. Genetics 2012, 192, 73–105. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Sonderegger, M.; Sauer, U. Evolutionary engineering of Saccharomyces cerevisiae for anaerobic growth on xylose. Appl. Env. Microbiol. 2003, 69, 1990–1998. [Google Scholar] [CrossRef]
- Vu, V.H.; Kim, K. High-cell-density fed-batch culture of Saccharomyces cerevisiae KV-25 using molasses and corn steep liquor. J. Microbiol. Biotechnol. 2009, 19, 1603–1611. [Google Scholar] [CrossRef]
- Ferndahl, C.; Bonander, N.; Logez, C.; Wagner, R.; Gustafsson, L.; Larsson, C.; Hedfalk, K.; Darby, R.A.; Bill, R.M. Increasing cell biomass in Saccharomyces cerevisiae increases recombinant protein yield: The use of a respiratory strain as a microbial cell factory. Microb. Cell Fact. 2010, 9, 47. [Google Scholar] [CrossRef]
- Sherman, F. Getting started with yeast. Methods Enzym. 2002, 350, 3–41. [Google Scholar] [CrossRef]
- Perli, T.; Wronska, A.K.; Ortiz-Merino, R.A.; Pronk, J.T.; Daran, J.M. Vitamin requirements and biosynthesis in Saccharomyces cerevisiae. Yeast 2020, 37, 283–304. [Google Scholar] [CrossRef]
- Wynne, E.; Yoon, J.; Park, D.; Cui, M.; Morris, C.; Lee, J.; Wang, Z.; Yoon, S.; Han, J. Regeneration of Spent Culture Media for Sustainable and Continuous mAb Production via Ion Concentration Polarization. Biotechnol. Bioeng. 2025, 122, 373–381. [Google Scholar] [CrossRef]



| Media Type | Commercial Yeast Extract (g) | Recycled Yeast Extract (mg) | Peptone (%) | Dextrose (%) |
|---|---|---|---|---|
| YPD commercial yeast extract | 10 | 0 | 2% | 2% |
| YPD 10 g/L recycled yeast extract | 0 | 10,000 | 2% | 2% |
| YPD 0.1 g/L recycled yeast extract | 0 | 100 | 2% | 2% |
| YPD 0.05 g/L recycled yeast extract | 0 | 50 | 2% | 2% |
| YPD 15 mg/L recycled yeast extract; 1% peptone | 0 | 15 | 1% | 2% |
| YPD 15 mg/L recycled yeast extract; 2% peptone | 0 | 15 | 2% | 2% |
| PD media (2% peptone; 2% dextrose) | 0 | 0 | 2% | 2% |
| Medium | Average Optical Density at 600 nm | Standard Deviation | Coefficient of Variation |
|---|---|---|---|
| YPD commercial yeast extract | 0.1593 | 0.006 | 3.9% |
| YPD 10 g/L recycled yeast extract | 2.821 | 0.351 | 12.46% |
| YPD 0.1 g/L recycled yeast extract | 1.122 | 0.138 | 12.31% |
| YPD 0.05 g/L recycled yeast extract | 0.7335 | 0.068 | 9.29% |
| YPD 15 mg/L recycled yeast extract; 1% peptone | 0.1482 | 0.005 | 3.58% |
| YPD 15 mg/L recycled yeast extract; 2% peptone | 0.1609 | 0.007 | 4.49% |
| PD media (2% peptone; 2% dextrose) | 0.1344 | 0.007 | 5.15% |
| Medium | Initial Prot. (g/L) | Residual Prot. (g/L) | Consumed ΔProt. (g/L) | % Consumed |
|---|---|---|---|---|
| YPD-C | 26.84 ± 0.25 | 14.31 ± 0.15 | 12.53 ± 0.29 | 46.7 ± 2.6 |
| YPD + 1%P | 13.22 ± 0.18 | 1.96 ± 0.12 | 11.26 ± 0.22 | 85.2 ± 1.9 |
| YPD + 2%P | 17.28 ± 0.16 | 6.86 ± 0.10 | 10.42 ± 0.19 | 60.4 ± 2.1 |
| PD | 15.44 ± 0.15 | 6.26 ± 0.08 | 9.18 ± 0.17 | 59.5 ± 2.5 |
| Medium | Initial Sugar T0 (g/L−1) | Residual Sugar T144 (g/L−1) | Sugar Consumed (g/L−1) |
|---|---|---|---|
| YPD-C | 23.64 ± 0.48 | 3.19 ± 0.27 | 20.45 ± 0.55 |
| YPD + 1%P | 23.80 ± 0.67 | 2.14 ± 0.64 | 21.66 ± 0.92 |
| YPD + 2%P | 23.07 ± 0.35 | 3.51 ± 0.89 | 19.56 ± 0.96 |
| PD | 23.73 ± 0.67 | 3.52 ± 0.76 | 20.20 ± 1.02 |
| Medium | Wet Biomass (g/L−1) | ∆Sugar (g/L−1) | Wet YX/S (g Biomass/g Glucose) |
|---|---|---|---|
| YPD-C | 44.3 ± 1.0 | 20.45 ± 0.55 | 2.16 ± 0.06 |
| YPD + 1% P | 31.9 ± 0.9 | 21.66 ± 0.92 | 1.47 ± 0.07 |
| YPD + 2% P | 42.6 ± 1.0 | 19.56 ± 0.96 | 2.18 ± 0.11 |
| PD | 35.1 ± 1.2 | 20.20 ± 1.02 | 1.74 ± 0.10 |
| PD | 35.1 ± 1.2 | 20.20 ± 1.02 | 1.74 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, L.; O’Connell, D.J. Conversion of Komagataella phaffii Biomass Waste to Yeast Extract Supplement. Appl. Microbiol. 2025, 5, 95. https://doi.org/10.3390/applmicrobiol5030095
Murphy L, O’Connell DJ. Conversion of Komagataella phaffii Biomass Waste to Yeast Extract Supplement. Applied Microbiology. 2025; 5(3):95. https://doi.org/10.3390/applmicrobiol5030095
Chicago/Turabian StyleMurphy, Laura, and David J. O’Connell. 2025. "Conversion of Komagataella phaffii Biomass Waste to Yeast Extract Supplement" Applied Microbiology 5, no. 3: 95. https://doi.org/10.3390/applmicrobiol5030095
APA StyleMurphy, L., & O’Connell, D. J. (2025). Conversion of Komagataella phaffii Biomass Waste to Yeast Extract Supplement. Applied Microbiology, 5(3), 95. https://doi.org/10.3390/applmicrobiol5030095






