Abstract
Coffee pulp, the primary residue generated during the wet processing of Coffea arabica L., is frequently applied directly to fields as a crude soil amendment. However, this practice often lacks proper microbial stabilization, limiting its agronomic potential and posing risks due to the presence of phytotoxic compounds. In Colombia, disease-resistant varieties such as Coffea arabica L. var. Castillo and var. Cenicafé 1, developed by the National Coffee Research Center (Cenicafé), are the amongst the most widely cultivated varieties in the country; however, despite their widespread adoption, the microbial ecology of postharvest residues from these varieties remains poorly characterized. This study aimed to isolate and functionally characterize native microbial communities from the pulp of Coffea arabica var. Castillo and var. Cenicafé 1, and to evaluate their role in postharvest processing and organic waste management. Fresh pulp samples were collected from a wet-processing facility located in tropical mid-elevation zones. A total of 53 microbial isolates were recovered using culture-dependent techniques on selective media targeting yeasts, lactic acid bacteria (LAB), and filamentous fungi. Amplicon sequencing of the 16S rRNA gene (V3–V4 region) and ITS1 region was conducted to profile bacterial and fungal communities, revealing diverse microbial consortia dominated by Aspergillus, Lactobacillus, Leuconostoc, Pichia, and Saccharomyces species. Enzymatic screening indicated high pectinolytic and cellulolytic activity. Composting trials using inoculated pulp showed a ~40% reduction in composting time and improved nutrient content. These findings support the use of native microbiota to enhance composting efficiency and postharvest valorization, contributing to more sustainable and circular coffee systems.
1. Introduction
Coffee (Coffea spp.) is a globally significant crop, both economically and socially, with millions of livelihoods dependent on its cultivation, processing, and export [1,2,3,4]. As one of the most traded agricultural commodities worldwide, coffee drives rural development and foreign exchange earnings in many tropical countries [3,5]. However, the postharvest transformation of coffee cherries into green beans, particularly through the wet-processing method widely applied to Coffea arabica L., involves a sequence of mechanized and water-intensive operations that generate substantial quantities of agro-industrial residues [6,7,8,9]. These include pulp, mucilage, wastewater, and, to a lesser extent, husk and silverskin during later processing stages [7,10,11]. Among these byproducts, coffee pulp, which consists of the outer skin (exocarp) and fleshy layer (mesocarp) removed during the pulping stage, is the most abundant solid residue, often accounting for more than 40–50% of the fresh fruit mass [4,12]. Its generation is therefore directly proportional to global coffee production, which surpasses 10 million tons annually [13,14]. While the economic importance of coffee is well acknowledged, its processing footprint introduces a range of environmental challenges, particularly due to the inadequate valorization of lignocellulosic biomass [4,15]. If not properly managed, these residues contribute to water pollution, greenhouse gas emissions, and local soil degradation, highlighting the need for sustainable and circular approaches to postharvest waste management [15,16,17].
In Colombia, the National Coffee Research Center (Cenicafé) has developed multiple high-performing Coffea arabica L. varieties, including var. Castillo and var. Cenicafé 1, through its plant breeding program [18,19,20]. These varieties combine high yield potential and desirable cup quality with resistance to coffee leaf rust (Hemileia vastatrix) and coffee berry disease (CBD) [20], and have played a central role in national efforts to promote coffee crop renovation [21,22]. As a result, approximately 90% of Colombia’s coffee-growing area is now planted with disease-resistant varieties developed by Cenicafé [21]. While the agronomic and economic benefits of these varieties are well documented, the microbial ecology associated with their postharvest residues, particularly the composition and functional role of native microorganisms inhabiting the coffee pulp, remains poorly understood [7,23,24,25,26]. Investigating the pulp-associated microbiota of highly adopted varieties such as Castillo and Cenicafé 1 offers valuable insights for developing microbial-based innovations in composting, fermentation, and circular waste management strategies [7,15,25,26,27].
Estimates suggest that, for every metric ton of fresh coffee cherries processed through the wet method, approximately 400 to 500 kg of wet pulp are generated as a direct byproduct [4,28,29]. This pulp is a lignocellulosic biomass composed primarily of water, structural polysaccharides including pectin, cellulose, and hemicellulose, and a variety of soluble compounds such as sugars, organic acids, caffeine, and phenolic substances [9,30]. While these compounds confer nutritional and biochemical value, they also pose ecological risks when released into the environment without appropriate treatment [4,9,15]. The high biochemical oxygen demand (BOD) and phytotoxic potential of untreated pulp leachate can negatively impact soil microbial communities, contaminate freshwater systems, and inhibit plant growth in surrounding ecosystems [31,32,33]. Although certain processing residues, such as coffee husks and trunks, have received attention for energy recovery through thermochemical conversion [34,35,36], or as low-cost feedstock for biochar production [37], the valorization of coffee pulp remains limited in both scope and application [4]. In most coffee-producing regions, pulp is either discarded in unregulated open-air systems or subjected to traditional composting practices with minimal control over key physicochemical parameters [38,39]. These methods are often inefficient, and alternative strategies such as black soldier fly (BSF) or vermicomposting have shown promise [40,41]. However, both approaches face limitations, including incomplete stabilization of the substrate in BSF systems and earthworm mortality during the thermophilic phase of composting [38]. As a result, there is a need for optimized microbial-based solutions to ensure efficient, safe, and agronomically valuable pulp management (Figure 1).
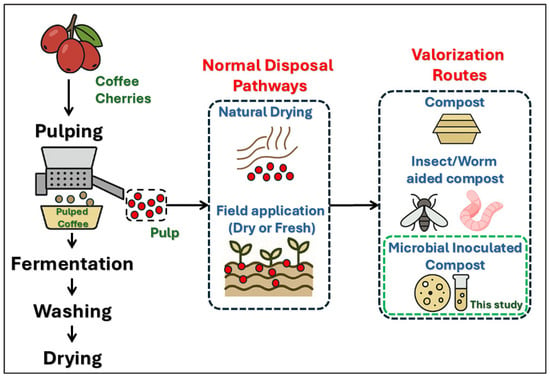
Figure 1.
Conceptual illustration of common disposal practices for coffee pulp and emerging valorization routes, including the microbial inoculation strategy explored in this study. The diagram provides an overview of process pathways; detailed descriptions of microbial functions and enzymatic activities are provided in Section 2.3 and Section 3.2.
Despite these challenges, coffee pulp remains an underexplored biological residue that harbors diverse and functionally active microbial consortia naturally adapted to its physicochemical environment, characterized by high moisture content, acidic pH, and abundant fermentable substrates [42]. Studies have shown that this substrate supports metabolically versatile microorganisms, including non-Saccharomyces yeasts such as Pichia and Hanseniaspora, lactic acid bacteria (LAB) such as Lactobacillus and Leuconostoc species, and filamentous fungi, notably Aspergillus and Penicillium [24]. These native communities possess enzymatic activity capable of degrading complex organic polymers such as pectin, cellulose, and hemicellulose, key constituents of the pulp matrix, thus accelerating decomposition and facilitating nutrient mineralization [24,42,43]. Harnessing this microbiota through selective isolation, enrichment, and functional screening offers promising opportunities for developing bioinoculants to improve composting efficiency and compost quality [44]. Beyond enhancing nitrogen, phosphorus, and potassium retention, microbial bioaugmentation may mitigate phytotoxicity and suppress the growth of spoilage or pathogenic organisms [45]. In coffee postharvest fermentation, these microbial populations may also contribute to mucilage removal, pH stabilization, and the development of flavor precursors [46,47]. Integrating native microbial resources into controlled bioprocesses aligns with broader goals in agroecology and the circular bioeconomy, offering scalable, low-cost strategies to valorize waste streams and enhance sustainability at the farm level [27,31].
In this context, the present study aimed to isolate and functionally characterize native microbial communities from fresh coffee pulp collected at wet-processing facilities in tropical mid-elevation regions. Culture-based isolation and amplicon sequencing of the 16S rRNA (V3–V4) and ITS1 regions were used to identify key bacterial and fungal taxa. Target groups, i.e., yeasts, lactic acid bacteria, and filamentous fungi, were screened for pectinolytic and cellulolytic activities relevant to lignocellulosic degradation. Pilot-scale composting trials were then conducted to assess the capacity of native consortia to accelerate organic matter breakdown and enhance nutrient retention. These combined approaches provide new insights into the functional ecology of coffee pulp microbiota and their potential in postharvest innovation and circular bioeconomy strategies.
2. Materials and Methods
2.1. Study Area and Sample Collection
2.1.1. Geographical Location and Coffee Varieties
The coffee pulp used in this study was obtained from cherries harvested at the “Naranjal” Experimental Station of Cenicafé, located in Chinchiná, Caldas (4.972228, −75.652563), in Colombia’s central coffee-growing region. The station is situated at an altitude of 1381 m above sea level (m.a.s.l.) and is characterized by a humid tropical climate, with average annual temperatures ranging from 19 to 22 °C and total annual precipitation exceeding 2000 mm [48].
The cherries belonged to two Coffea arabica L. varieties of agronomic and commercial importance in Colombia: var. Castillo and var. Cenicafé 1. To ensure uniform physiological maturity, only cherries at ripeness stages 4, 5, and 6 were harvested [49,50]. For each variety, 80 kg of ripe cherries were collected during the main harvest season (October–November 2024), resulting in approximately 35 kg of fresh pulp per batch (Figure 2) [51].

Figure 2.
Fresh coffee pulp from ripe Coffea arabica L. var. Cenicafé 1 cherries at maturity stages 4, 5, and 6 [49,50].
2.1.2. Description of Wet-Processing Facilities
Postharvest processing was conducted at the wet processing facility of the Postharvest Discipline at Cenicafé (4.992275, −75.596716), following a standardized protocol representative of smallholder coffee systems in Colombia [37]. The workflow included hydraulic classification to remove floaters and defective fruits, mechanical pulping, sieve-based separation, fermentation, and washing using the Ecomill® LH300 system (Cenicafé, Manizales, Colombia) [37], followed by mechanical drying [52].
Although the full processing line was applied to the coffee beans, this study focused exclusively on the pulp generated immediately after the mechanical pulping step. The pulp was collected fresh to ensure its integrity as an unaltered lignocellulosic biomass suitable for subsequent physicochemical characterization and pilot-scale composting trials.
2.1.3. Pulp Sampling Procedure
From each 80 kg processing batch, approximately 35 kg of fresh pulp were obtained. Three independent replicates (A, B, and C) per variety were processed under identical conditions, resulting in a total of six pulp samples: three from var. Castillo and three from var. Cenicafé 1.
Each sample was homogenized and packed in sealed, food-grade plastic containers; to maintain sample quality during transport, all containers were placed in controlled-temperature coolers. The samples were transported over a ~4-h drive to the Plant Biotechnology Laboratory, Biological Sciences Department, School of Applied Sciences and Engineering, EAFIT University, Medellín, Colombia.
Upon arrival, samples were stored at 4 °C. Subsamples were immediately used for physicochemical characterization (Section 2.2), while the remaining material was allocated to pilot-scale composting trials (Section 2.5).
2.2. Physicochemical Characterization of Coffee Pulp
Physicochemical analyses were conducted on fresh coffee pulp samples upon arrival at the Plant Biotechnology Laboratory, EAFIT University. Each analysis was performed in triplicate per sample to ensure analytical reliability. The characterization included moisture content, pH, and temperature, as well as the determination of lignocellulosic fractions, macronutrient content, and soluble compounds relevant to composting performance and microbial activity.
2.2.1. Moisture Content, pH, and Temperature
Moisture content was determined by gravimetric analysis. Approximately 10 g of fresh pulp were weighed and dried at 105 °C in a forced-air convection oven (Memmert UN110, Schwabach, Germany) until constant weight was achieved, as specified in the international standard ISO6673:2003 [53]. The initial pH was measured by suspending 10 g of pulp in 100 mL of distilled water (1:10 w/v), homogenizing the mixture for 30 min, and measuring with a benchtop pH meter (Mettler Toledo SevenCompact S220, Greifensee, Switzerland), previously calibrated with standard buffers (pH 4.00 and 7.00). Sample temperature upon arrival was recorded by inserting a digital probe thermometer (Testo 108, Titisee-Neustadt, Germany) into the center of the container.
2.2.2. Lignocellulosic Composition
The lignocellulosic composition was analyzed to determine the structural carbon content of the coffee pulp. The contents of cellulose, hemicellulose, and lignin were quantified using the Van Soest sequential fiber analysis method, involving neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) extractions. The procedures were conducted using an Ankom 200 Fiber Analyzer (ANKOM Technology, Macedon, NY, USA), with appropriate filter bags and reagents. These measurements enabled an assessment of the material’s recalcitrance and its potential degradation pathway during composting.
2.2.3. Nutrient and Soluble Compound Analysis
Total nitrogen (N) content was determined using the Kjeldahl digestion and distillation method, employing a semi-automatic digestion unit (Kjeltec™ 8200, FOSS, Hilleroed, Denmark) and a distillation unit (Kjeltec™ 8400, FOSS, Denmark). Available phosphorus (P) was quantified by the molybdenum blue method using a UV-Vis spectrophotometer (Thermo Scientific Genesys 150, Waltham, MA, USA), and potassium (K) was measured by flame photometry (Jenway PFP7, Dunmow, UK).
While total soluble solids were measured as °Brix using a digital refractometer (Atago PAL-1, Tokyo, Japan), reducing sugars were determined spectrophotometrically using the 3,5-dinitrosalicylic acid (DNS) method, with absorbance readings at 540 nm. These analyses provided critical information on the nutrient availability and microbial substrate potential of the pulp prior to composting.
2.3. Microbial Isolation and Functional Screening
2.3.1. Culture-Dependent Isolation Techniques
Fresh coffee pulp samples from Coffea arabica var. Castillo and var. Cenicafé 1 were homogenized in sterile phosphate-buffered saline (PBS, pH 7.2; Gibco™, Thermo Fisher Scientific, Waltham, MA, USA) at a 1:10 (w/v) ratio under aseptic conditions. The homogenates were serially diluted (10−1 to 10−6) in sterile 0.85% saline solution (Oxoid™, Thermo Fisher, Waltham, MA, USA), and 100 µL aliquots from appropriate dilutions were spread in triplicate onto selective agar media using sterile disposable spreaders (VWR International, Radnor, PA, USA). Plates were incubated under optimized conditions depending on the target microbial group: aerobic incubation at 28 ± 2 °C for yeasts and filamentous fungi (48–120 h), and anaerobic incubation using AnaeroGen™ sachets (Oxoid™) in Oxoid™ anaerobic jars for LAB (72 h). Distinct colony morphotypes were purified by repeated streaking on the same media and stored in 25% sterile glycerol (Sigma-Aldrich®, St. Gallen, Switzerland) at −80 °C in cryovials (Nalgene®, Thermo Fisher Scientific, Waltham, MA, USA) for further characterization.
2.3.2. Selective Media and Functional Group Targeting
Targeted microbial groups were isolated using the following selective media: Yeasts were cultured on Yeast Peptone Dextrose Agar (YPDA; Sigma-Aldrich®) supplemented with 100 mg L−1 chloramphenicol (Sigma-Aldrich®) to inhibit bacterial growth. Lactic acid bacteria (LAB) were isolated on de Man, Rogosa, and Sharpe (MRS) agar (Oxoid™, Thermo Fisher) supplemented with 0.05% (w/v) cysteine-HCl (Merck®, Rahway, NJ, USA) and incubated under anaerobic conditions. Filamentous fungi were recovered on Potato Dextrose Agar (PDA; Merck®) supplemented with 50 mg L−1 streptomycin (Sigma-Aldrich®) to suppress bacterial overgrowth. Representative isolates of the dominant morphotypes were subsequently selected for downstream functional screening.
2.3.3. Enzymatic (Pectinolytic and Cellulolytic) Activity Assays
Pectinolytic potential was evaluated by spot-inoculating isolates on Pectin Agar plates (1% citrus pectin, 0.5% yeast extract, 1.5% agar, pH 6.5). Plates were incubated at 30 °C for 72 h. After incubation, plates were flooded with 1% cetyltrimethylammonium bromide (CTAB), and zones of clearance around colonies were recorded as indicative of pectinase production.
Cellulase activity was assessed on Carboxymethyl Cellulose (CMC) agar (1% CMC, 0.2% NaNO3, 0.1% KH2PO4, 0.05% MgSO47H2O, 0.05% KCl, 0.02% yeast extract, 1.5% agar). Following incubation at 30 °C for 5 days, plates were stained with 0.1% Congo red solution for 15 min and destained with 1 M NaCl for 10 min. Clear zones surrounding colonies indicated cellulase activity.
The Pectinolytic Index and Cellulolytic Index were calculated as the ratio between halo diameter (mm) and colony diameter (mm) at the time of reading (Index = halo/colony). Halo diameters were measured along two perpendicular axes with a digital caliper and averaged. Colony diameters were measured on the same plates immediately before halo reading. Higher index values indicate proportionally greater extracellular enzyme diffusion relative to colony size.
2.3.4. Pathogenicity and Mycotoxigenicity Screening
Hemolytic activity was tested on 5% sheep blood agar to assess pathogenic potential; isolates exhibiting β-hemolysis were flagged for exclusion. Mycotoxigenic potential of fungal isolates was evaluated using thin-layer chromatography (TLC). Cultures were grown in YES (Yeast Extract Sucrose) broth for 10 days at 28 °C and extracted with chloroform. Extracts were spotted on silica gel TLC plates alongside aflatoxin standards and developed in a chloroform:methanol (98:2 v/v) solvent system. Detection was performed under UV light at 365 nm.
2.4. Amplicon Sequencing and Bioinformatic Analysis
Total genomic DNA was extracted from 0.25 g of homogenized coffee pulp using the DNeasy PowerSoil Pro Kit (QIAGEN, Hilden, Germany), following the manufacturer’s protocol. DNA concentration and purity were verified with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific), and integrity was confirmed by 1% agarose gel electrophoresis; three biological replicates per sample type were pooled equimolarly for sequencing.
2.4.1. DNA Extraction, Amplicon Library Preparation, and Sequencing
For bacterial profiling, the V3–V4 region of the 16S rRNA gene was amplified using primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GACTACHVGGGTATCTAATCC-3′). For fungal communities, the ITS1 region was amplified using primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2 (5′-GCTGCGTTCTTCATCGATGC-3′). Amplicon libraries were prepared using Illumina’s dual-index strategy and sequenced on a MiSeq platform (Illumina, San Diego, CA, USA) using a 2 × 300 bp paired-end configuration.
DNA extraction blanks (negative controls) containing only extraction reagents were processed in parallel with the coffee pulp samples to monitor potential contamination. These controls were subjected to PCR amplification and included in the sequencing run. No contaminant sequences of relevance were detected after quality filtering.
2.4.2. Taxonomic Assignment and Diversity Metrics
Raw sequencing data were processed using the QIIME2 pipeline (version 2023.2). After demultiplexing, reads were quality-filtered, trimmed, and denoised using DADA2 to generate amplicon sequence variants (ASVs). Taxonomic assignment was performed against the SILVA database (release 138.1) for 16S rRNA and UNITE database (version 9.0) for ITS1 sequences using a Naive Bayes classifier. Alpha diversity metrics (Chao1 richness, Shannon index) and beta diversity (Bray–Curtis dissimilarity) were calculated. Principal Coordinates Analysis (PCoA) was used to visualize differences in microbial community structure across sample types.
2.5. Pilot-Scale Composting Trials
2.5.1. Experimental Setup and Treatment Design
Pilot-scale composting experiments were conducted under controlled conditions at the Plant Biotechnology Laboratory, EAFIT University. Coffee pulp from two Coffea arabica varieties, var. Castillo and var. Cenicafé 1, was composted in both inoculated and uninoculated conditions to evaluate the effect of microbial inoculation on composting dynamics. For each variety, three replicates were processed with inoculation and three without, resulting in a total of 12 composting units (2 varieties × 2 treatments × 3 replicates).
Each treatment was carried out in 20 L perforated plastic bins, which served as micro-composting reactors. Bins were passively aerated through lateral and basal perforations and placed on raised metal grids to ensure proper drainage and airflow. To standardize initial composting conditions, all treatments received a blend of sugarcane bagasse (as bulking agent) and fresh cow manure (as nitrogen source) to adjust the initial C/N ratio and moisture content. Moisture was brought to approximately 75% (wet basis) using distilled water when required, and the C/N ratio was adjusted to ~25:1 based on pulp composition and amendment ratios. No chemical or thermal pretreatments were applied prior to composting.
In the inoculated treatments, a microbial inoculum prepared from mature, previously stabilized coffee pulp compost was added at 10% w/w (fresh basis) [54,55]. The uninoculated treatments followed the same protocol but without the addition of the inoculum.
2.5.2. Inoculum Preparation and Application
The microbial inoculum was prepared from mature coffee pulp compost previously stabilized for a minimum of 90 days under controlled conditions. The compost was sieved through a 2 mm mesh to remove coarse particles and homogenized to ensure uniformity. To reactivate the microbial community, the compost was hydrated to 60% (wb) moisture content and incubated at ambient temperature (25 ± 2 °C) for 48 h before application. A 10% w/w inoculum (based on fresh pulp weight) was thoroughly mixed into the pulp matrix of the inoculated treatments prior to composting [55]. The uninoculated treatments followed an identical protocol, excluding the addition of the inoculum.
2.5.3. Monitoring of Composting Parameters (Temperature, Moisture, and pH)
Composting temperature was monitored daily using a digital compost thermometer (REOTEMP® Heavy Duty, San Diego, CA, USA) inserted into the core of each pile. Probes were positioned at the vertical center of the composting mass, approximately 15 cm from the surface, to capture representative core temperatures. Aeration was passive, provided by lateral and basal perforations in the bins, with no forced air supply. Ambient temperature at the composting site was recorded daily using a digital thermohygrometer to account for environmental influences on pile temperature dynamics. Temperature data were used to identify the mesophilic and thermophilic phases and assess microbial activity. Moisture content was determined weekly using the gravimetric method, and samples were adjusted with distilled water to maintain levels between 55% and 65% (wb). pH was measured weekly from a 1:10 pulp-to-water suspension using a benchtop pH meter (Mettler Toledo SevenCompact S220, Switzerland).
2.5.4. Organic Matter and Lignocellulose Degradation Evaluation
To evaluate organic matter degradation over time, subsamples were collected each 15 days. Organic matter content was calculated as volatile solids (VS) loss after ignition at 550 °C in a muffle furnace (Nabertherm LT 5/11, Lilienthal, Germany). The degradation of cellulose, hemicellulose, and lignin was assessed using the Van Soest method with an Ankom 200 Fiber Analyzer (ANKOM Technology, USA), following the same procedure described in Section 2.2.2.
2.5.5. Final Compost Characterization
Nutrient Content (NPK)
At the end of the composting period, the stabilized compost from each treatment was analyzed for total nitrogen (N) using the Kjeldahl method, and for available phosphorus (P) and potassium (K) by spectrophotometry and flame photometry, respectively (as described in Section 2.2.3). These parameters were used to assess the agronomic quality of the final compost product.
Maturity and Phytotoxicity Tests
Compost maturity was evaluated by measuring C/N ratio, color and odor changes, and temperature stability over the final week. Additionally, a phytotoxicity test was conducted using Lactuca sativa L. (lettuce) seed germination assays in a compost–water extract (1:10 v/v). The germination index (GI) was calculated by comparing seed germination and root elongation in compost extract versus distilled water. A GI above 80% was considered indicative of mature, non-phytotoxic compost [56].
2.6. Statistical Analysis
All statistical analyses were performed using RStudio v.4.3.1. Prior to inferential testing, the Shapiro–Wilk test was used to assess normality, and Levene’s test (car package) was applied to evaluate homogeneity of variances.
For parametric datasets (e.g., temperature, VS reduction, nutrient concentrations), one-way analysis of variance (ANOVA) followed by Tukey’s Honest Significant Difference (HSD) test was used to determine differences among treatments and cultivars. For non-normally distributed or heteroscedastic data, the Kruskal–Wallis test with Dunn’s post hoc test was employed (FSA package). A significance threshold of p < 0.05 was adopted for all analyses.
To evaluate microbial diversity and community structure, amplicon sequencing data were processed using QIIME2 v2023.2 [57]. Alpha diversity metrics, including Shannon index, Observed ASVs, and Faith’s Phylogenetic Diversity, were calculated and compared using Kruskal–Wallis tests.
Beta diversity was assessed using Bray–Curtis dissimilarity, followed by a Principal Coordinates Analysis (PCoA) performed in the vegan package. To test for significant differences in community composition among cultivars and treatments, a PERMANOVA (Permutational Multivariate Analysis of Variance; adonis() function in vegan) was performed with 999 permutations. All data visualization was conducted using ggplot2 [58].
3. Results and Discussion
3.1. Microbial Diversity Associated with Coffee Pulp
3.1.1. Culture-Dependent Isolation and Functional Groups
A total of 53 microbial isolates were recovered from fresh coffee pulp obtained from Coffea arabica L. var. Castillo and var. Cenicafé 1 across three replicates. Culture-dependent isolation on selective media yielded 20 yeast isolates (YPDA), 18 lactic acid bacteria (LAB) isolates (MRS), and 15 filamentous fungi isolates (PDA). Colony morphology and pigmentation varied markedly among isolates, highlighting the ecological diversity of microbial niches within the coffee pulp matrix.
Preliminary Gram staining and microscopic examination revealed that the yeast colonies predominantly belonged to the genera Pichia and Saccharomyces, consistent with their established presence in coffee fermentation ecosystems [59]. LAB isolates exhibited rod- and coccus-shaped morphologies, were catalase-negative, and showed acid tolerance (Table 1), suggesting likely affiliation with the genera Lactobacillus and Leuconostoc, as previously reported in coffee mucilage fermentations [60,61,62]. Filamentous fungal isolates displayed dense sporulation and pigmentation characteristic of Aspergillus and Penicillium spp., which are recognized for their lignocellulolytic capabilities [63,64,65].

Table 1.
Microbial isolates recovered from coffee pulp by culture-dependent techniques.
These functional groups are crucial during early composting stages for acidification, enzymatic hydrolysis of mucilage, and initial substrate breakdown. Morphological variation and pigmentation patterns, including spore color and colony texture, served as preliminary indicators of functional specialization [66,67]. Although no phylogenetic tree was generated for the culture-based isolates, taxonomic assignment was supported by colony morphology, microscopic features, Gram staining (for bacteria), and genus- or species-level identification through functional and biochemical traits, as presented in Table 1.
3.1.2. Amplicon-Based Community Profiling
Amplicon sequencing of the V3–V4 region of the 16S rRNA gene and ITS1 region of fungal rDNA provided a comprehensive profile of the microbial communities present in the coffee pulp samples. Across all samples, a total of 4112 bacterial and 2678 fungal ASVs were identified after quality filtering and chimera removal.
Bacterial communities were dominated by members of the Firmicutes and Actinobacteria phyla, particularly Lactobacillus, Leuconostoc, Bacillus, and Streptomyces species. The presence of Bacillus and Streptomyces aligns with their known role in protein hydrolysis, fiber degradation, and secondary metabolite detoxification during composting [68,69,70]. In contrast, Lactobacillus and Leuconostoc were more abundant in freshly collected samples, reflecting early colonization and fermentative activity.
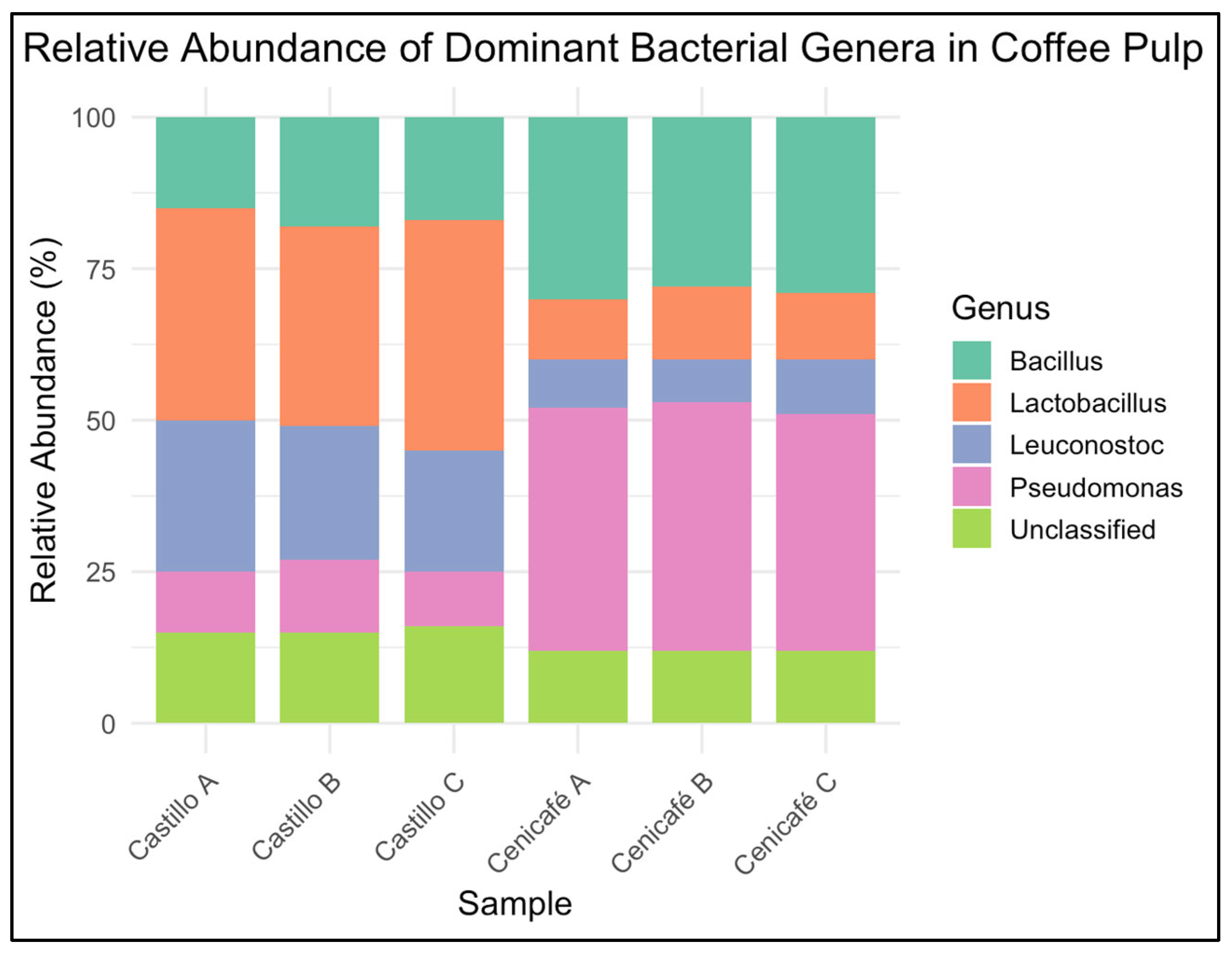
Fungal communities were primarily composed of Aspergillus, Pichia, Saccharomyces, and Trichoderma spp., with relative abundances varying by cultivar and site. To evaluate the taxonomic structure of bacterial communities associated with coffee pulp from the two Coffea arabica L. varieties, we performed high-throughput 16S rRNA (V3–V4) amplicon sequencing and analyzed the relative abundance of dominant genera. The results reveal clear differences in community composition between varieties. Figure 3 shows the relative abundance of dominant bacterial genera (n = 3 biological replicates per variety) in coffee pulp from Coffea arabica L. var. Castillo and Cenicafé 1.
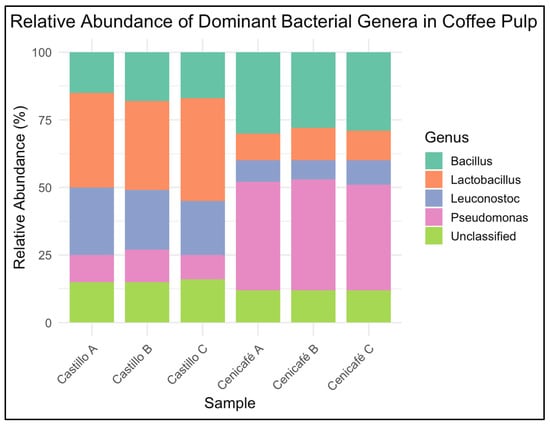
Figure 3.
Relative abundance of dominant bacterial genera in coffee pulp from Coffea arabica L. var. Castillo and var. Cenicafé 1.
Castillo samples were enriched in fermentative genera such as Lactobacillus, Leuconostoc, and Bacillus, whereas Cenicafé 1 samples exhibited a higher relative abundance of Pseudomonas and Streptomyces, genera commonly associated with aerobic environments and organic matter turnover. These cultivar-specific profiles suggest that intrinsic pulp chemistry and microenvironmental factors may shape microbial assembly during early postharvest stages [71,72]. In the Castillo pulp, Lactobacillus (24.6%), Leuconostoc (18.3%), and Bacillus (15.1%) were the most abundant bacterial genera, while in the Cenicafé 1 pulp, Pseudomonas (21.8%), Streptomyces (17.5%), and Lactobacillus (14.2%) dominated. For fungal communities, the Castillo pulp was enriched in Pichia (26.4%), Saccharomyces (19.8%), and Aspergillus (16.5%), whereas the Cenicafé 1 pulp showed higher relative abundance of Aspergillus (23.7%), Trichoderma (18.9%), and Pichia (15.4%). No novel genera were detected; however, the specific cultivar-linked patterns in abundance and community structure have not been previously described for Colombian coffee pulp.
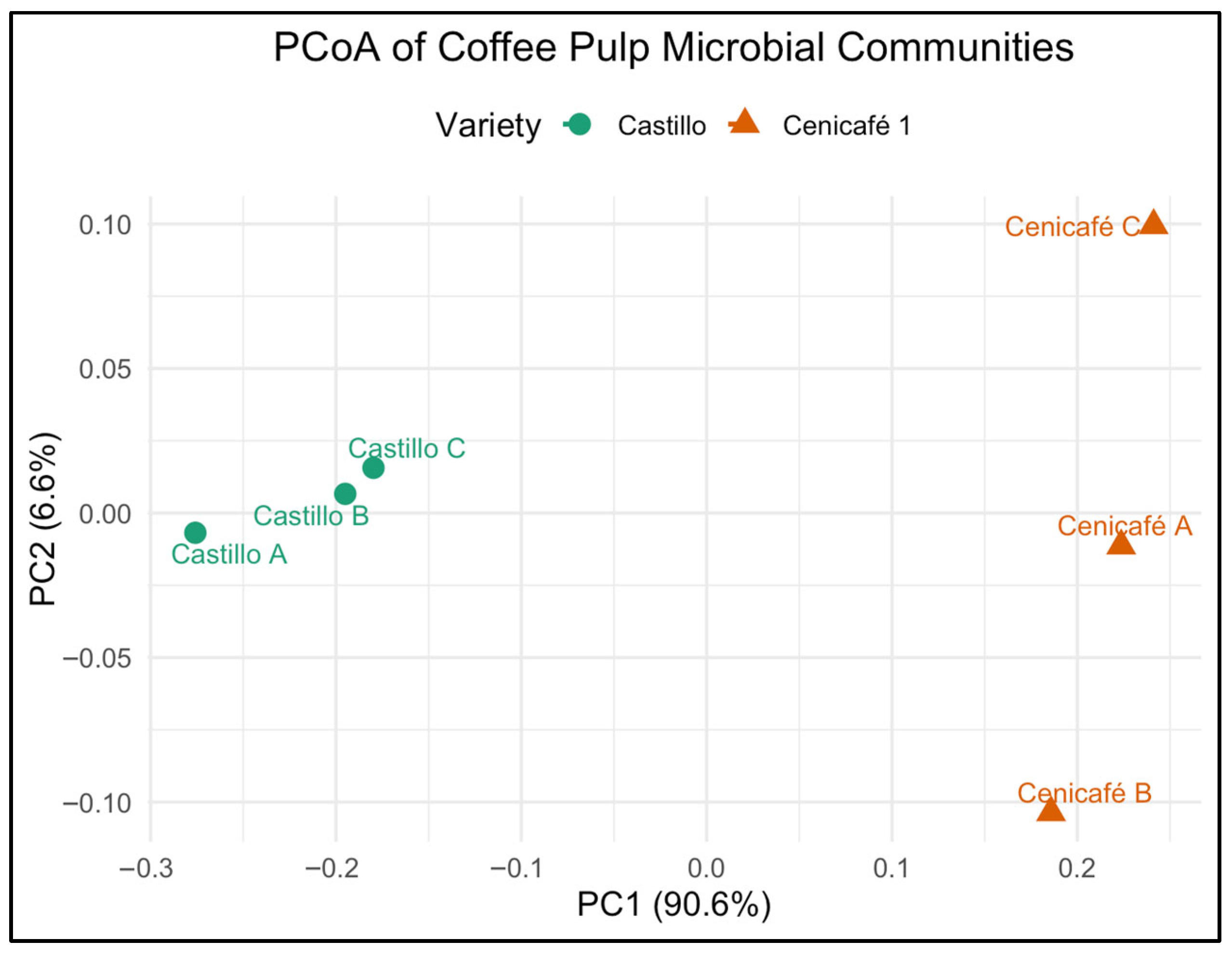
PCoA revealed a clear separation between microbial communities from the pulp of Coffea arabica L. var. Castillo and var. Cenicafé 1 (Figure 4). The first principal coordinate (PC1), accounting for 90.6% of the variation, distinctly discriminated between the two varieties, indicating that genotype-specific pulp chemistry or fermentation microenvironments significantly shaped microbial assembly.
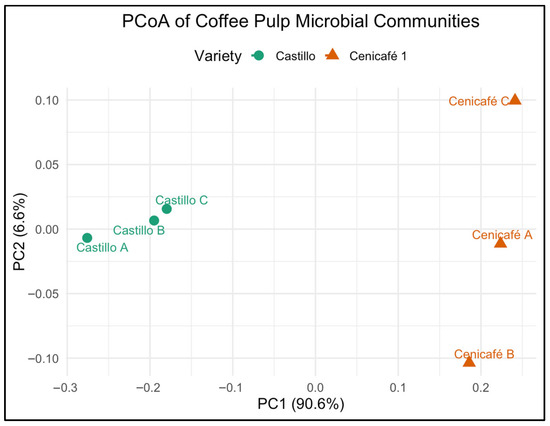
Figure 4.
Principal coordinates analysis (PCoA) of bacterial community composition in coffee pulp from Coffea arabica L. var. Castillo and var. Cenicafé 1.
The analysis of the dataset showed that PC1 (90.6% of variation) was positively correlated with the relative abundances of Lactobacillus, Leuconostoc, and Bacillus, and negatively correlated with Pseudomonas and Streptomyces. PC2 (4.3% of variation) was primarily influenced by variation in Aspergillus and Trichoderma abundances. The separation along PC1 reflects that the pulp from var. Castillo tends to harbor higher proportions of fermentative bacteria (Lactobacillus, Leuconostoc) and spore-forming decomposers (Bacillus), whereas the pulp from var. Cenicafé 1 is enriched in aerobic degraders (Pseudomonas, Streptomyces). Variation along PC2 indicates differential colonization by fungal taxa such as Aspergillus and Trichoderma. These correlations support that the clustering observed in the PCoA plot (Figure 4) is driven by both bacterial and fungal community shifts linked to cultivar-specific pulp composition and potential functional roles during postharvest transformation.
To evaluate within-sample microbial diversity, alpha diversity metrics were calculated for both bacterial and fungal communities [73]. As shown in Table 2, bacterial richness (Chao1) and diversity (Shannon Index) were slightly higher in the Cenicafé 1 pulp compared to Castillo. A similar trend was observed for fungal communities, with Cenicafé 1 exhibiting greater diversity and richness. These findings suggest that the pulp from Cenicafé 1 may harbor a more complex microbial ecosystem, potentially influenced by genotype-specific physicochemical traits.

Table 2.
Alpha diversity metrics for bacterial (16S rRNA) and fungal (ITS1) communities associated with coffee pulp from Coffea arabica var. Castillo and var. Cenicafé 1. Values represent Shannon diversity and Chao1 richness estimates per variety.
These results confirm that coffee pulp harbors a rich and functionally relevant microbiome, with both core fermentative taxa (e.g., Lactobacillus, Pichia) and specialized decomposers (e.g., Bacillus, Aspergillus) contributing to its postharvest transformation. Sequencing depth was sufficient to represent the dominant bacterial and fungal taxa, as reflected in the consistently high post-filter read counts and stable ASV richness across biological replicates. Given that the study’s primary objective was to compare alpha diversity between cultivars, Shannon index and Chao1 richness were selected as the most relevant indicators. Rarefaction curves and Faith’s Phylogenetic Diversity, although valuable in other contexts, were not included in this paper to maintain focus on the comparative diversity patterns most pertinent to our objectives.
3.2. Enzymatic Potential of Native Isolates
3.2.1. Pectinolytic and Cellulolytic Activities
Representative isolates from each functional group were screened for hydrolytic enzyme production using agar plate assays. Pectinolytic activity was the highest in Aspergillus niger and Pichia kudriavzevii isolates, with clear halo formation on pectin agar indicating active pectin degradation [74,75]. These enzymes are crucial for mucilage breakdown during postharvest fermentation, facilitating the removal of the sticky layer surrounding coffee beans [76].
Cellulolytic activity was most pronounced in Trichoderma harzianum, Bacillus subtilis, and Streptomyces spp., which formed clear zones on CMC agar. The enzymatic index (halo diameter/colony diameter) exceeded 2.0 in the most active isolates, indicating strong potential for lignocellulosic biomass deconstruction. These microbes are also implicated in the early thermophilic stages of composting, where rapid breakdown of fibrous material is essential for microbial succession and pile aeration [77].
The enzymatic screening of representative microbial isolates revealed diverse functional profiles relevant to organic matter degradation. As summarized in Table 3, Aspergillus niger (F1) and Pichia kudriavzevii (Y2) displayed high pectinolytic activity, with pectinolytic index values of 2.8 and 2.1, respectively. These isolates were recovered from the Cenicafé 1 and Castillo pulps, underscoring the presence of functionally specialized yeasts and filamentous fungi in both varieties.

Table 3.
Summary of enzymatic activity of representative microbial isolates. Values represent mean ± standard error (SE) of three independent replicates for each isolate. Indices were calculated as halo diameter/colony diameter (mm/mm).
In contrast, strong cellulolytic activity was observed in Trichoderma harzianum (F5, cellulolytic index = 3.2) and Bacillus subtilis (B3, cellulolytic index = 2.8), suggesting a complementary role in lignocellulose degradation. These taxa are commonly associated with thermotolerant composting phases and may contribute to enhanced fiber breakdown during aerobic decomposition [77]. The functional classification of isolates supports their targeted application in composting and postharvest processing systems.
3.2.2. Comparison Across Strains and Genera
Quantitative differences in enzymatic activity were observed not only between functional groups but also between strains within the same genus. For instance, Aspergillus isolates from Cenicafé 1 exhibited significantly higher pectinase indices compared to those from Castillo, suggesting strain-level adaptation or substrate-specific expression profiles.
This enzymatic heterogeneity highlights the potential of microbial consortia over monocultures for functional inoculation, where complementary enzyme systems can act synergistically to accelerate decomposition. The high cellulase production by Trichoderma and Streptomyces also indicates potential secondary benefits for soil organic matter dynamics, particularly under acidic tropical conditions [78].
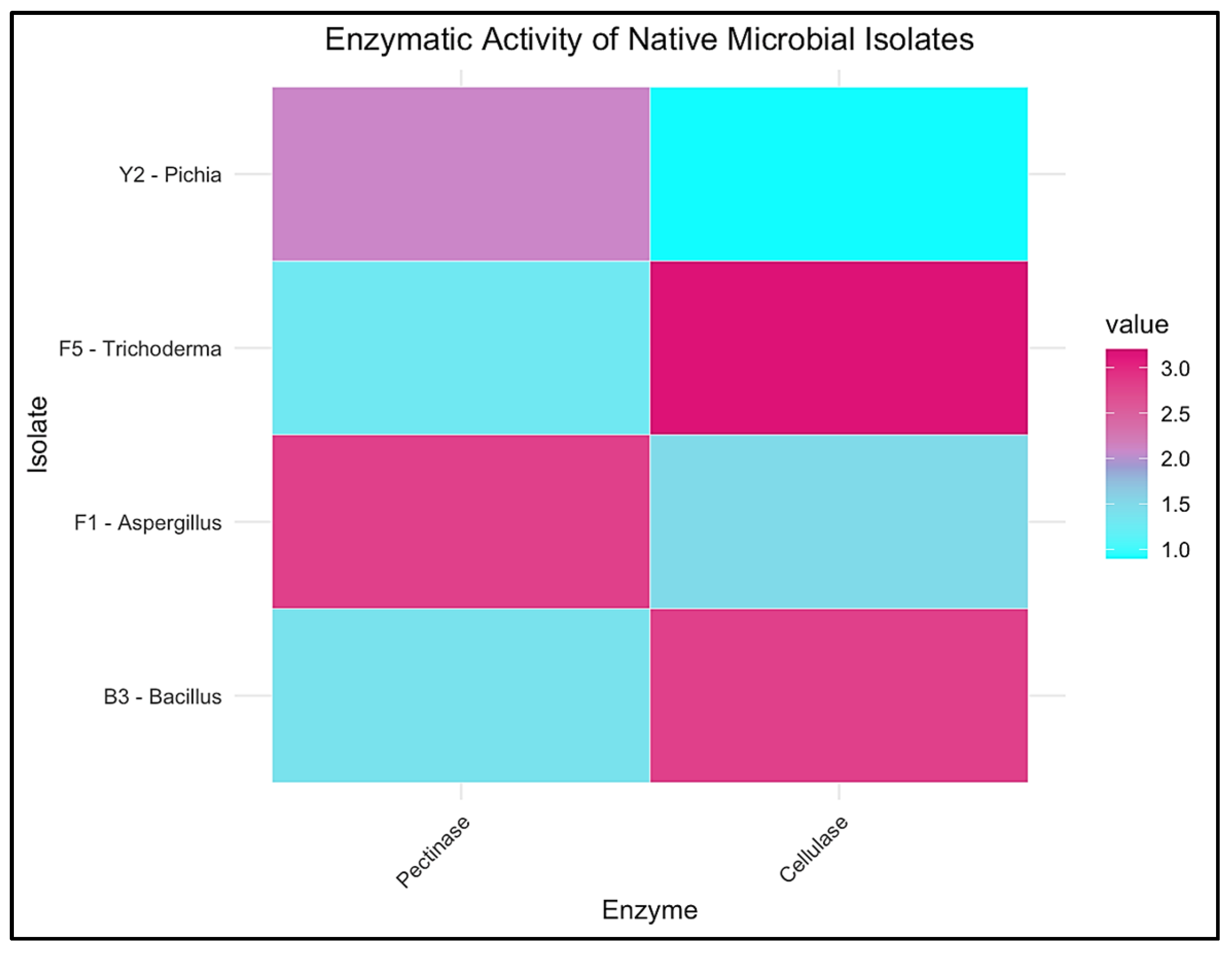
To visualize and compare the functional potential of key microbial isolates, a heatmap of enzymatic activity was constructed based on pectinolytic and cellulolytic index values, as seen in Figure 5 (n = 3 independent replicates per isolate), which clearly distinguishes the isolates according to their dominant enzymatic traits. Aspergillus niger (F1) and Pichia kudriavzevii (Y2) were identified as strong pectinase producers, which is relevant for mucilage removal during coffee fermentation. In contrast, Trichoderma harzianum (F5) and Bacillus subtilis (B3) displayed enhanced cellulase activity, underscoring their potential role in fiber degradation during composting [78].
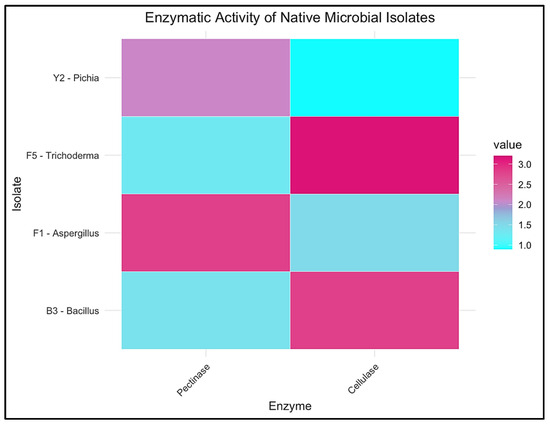
Figure 5.
Heatmap of pectinolytic and cellulolytic activities of native microbial isolates from Coffea arabica pulp. Color intensity corresponds to enzymatic index values based on halo-to-colony diameter ratios.
These findings support the hypothesis that native pulp-associated microbes offer functional advantages for postharvest valorization of coffee residues, and they could be selectively used to enhance the degradation of pectic and lignocellulosic compounds in both fermentation and composting systems.
3.2.3. Implications for Lignocellulose Breakdown and Mucilage Removal
The combined enzymatic capabilities of the isolates reflect a robust potential to support both postharvest coffee fermentation (via mucilage degradation) and composting efficiency (via lignocellulose breakdown). These traits are particularly relevant for on-farm applications where rapid pulp stabilization and transformation into nutrient-rich compost are desired [79].
Moreover, the absence of mycotoxigenic or phytopathogenic strains, confirmed via ITS and 16S annotations, supports the biosafety of these native isolates for biotechnological applications [80,81]. This aligns with previous reports demonstrating the functional and ecological safety of native microbial inoculants sourced from agro-industrial residues [82,83].
Together, these results validate the selection of native microbial consortia for integrated use in sustainable coffee postharvest systems, reinforcing the circular bioeconomy model for coffee-producing regions [27,84].
3.3. Composting Performance and Substrate Transformation
3.3.1. Temperature Profiles, Decomposition Rates, and Composting Time
All composting treatments exhibited the expected temperature evolution, characterized by rapid microbial activation during the first 72 h. In the inoculated treatments, core temperatures rose above 55 °C and reached peak values of 56.3 °C (Castillo) and 57.1 °C (Cenicafé 1). In contrast, the uninoculated treatments peaked at only 46.1 °C and 47.4 °C (Table 4), falling below the optimal thermophilic threshold required for effective sanitization and substrate degradation.

Table 4.
Composting performance indicators for coffee pulp from Coffea arabica cultivars Castillo and Cenicafé 1. Values represent the mean results per treatment over a 45-day composting period under controlled pilot-scale conditions.
The thermophilic phase was substantially longer in the inoculated treatments, 8.7 days (Castillo) and 10.3 days (Cenicafé 1), compared to just 4.3 and 4.9 days in the uninoculated composts. This prolonged exposure to elevated temperatures likely promoted enhanced microbial enzymatic activity and facilitated a more extensive breakdown of organic matter, as confirmed by differences in mass loss, volatile solids (VS) reduction, and fiber degradation [85].
The moisture content across all treatments was initially adjusted to approximately 75%, then maintained within the optimal range of 55–65% through periodic manual mixing and water supplementation [86]. Although moisture loss followed similar trends across treatments, inoculated piles exhibited slightly faster drying toward the end of the process due to more intense microbial heat generation [87].
pH dynamics followed a typical composting trajectory. Initial values ranged from 4.5 to 5.1, reflecting the inherent acidity of fresh coffee pulp [88]. In the first two weeks, microbial metabolism and ammonification led to a steady rise in pH [89]. By day 30, pH values had stabilized between 7.5 and 8.2 in inoculated treatments and 6.8 to 7.3 in uninoculated ones. The higher final pH observed in inoculated composts is favorable for microbial succession and enzymatic degradation and may also contribute to the lower phytotoxicity observed at the end of the process [90].
By day 45, the inoculated compost piles had returned to ambient temperature, indicating the end of the active degradation phase. In this study, biological stability was defined as the point at which compost temperatures remained at ambient levels for at least three consecutive days, pH showed no further change, and germination index values exceeded 80%, indicating the absence of phytotoxic effects. CO2 evolution testing was not performed. These piles reached full biological stability by day 53, as evidenced by neutral pH, germination indices above 85%, and stabilized C/N ratios. This demonstrates that microbial inoculation, when combined with adequate substrate conditioning and passive aeration, can reduce the composting period to approximately 7–8 weeks, compared to the 90–120 days typically reported for passive, uninoculated coffee pulp composting [28]. In contrast, the uninoculated composts showed slower thermal decline and remained biologically immature by day 53, as evidenced by persistent acidic odors, lower temperatures, and germination indices below 70% [44,91].
Decomposition metrics further underscored the benefit of inoculation. Inoculated composts achieved mass losses of 41.2% (Castillo) and 43.9% (Cenicafé 1), with corresponding volatile solids (VS) reductions of 39.8% and 40.6%, respectively. Uninoculated treatments, by contrast, achieved only 27.6% and 29.0% mass loss, and 25.1% and 26.4% VS reduction. Similarly, hemicellulose and cellulose degradation were substantially higher in inoculated treatments. Lignin reduction, though modest across all treatments (9.3–15.1%), followed the same trend, consistent with expectations for short-cycle aerobic composting systems [92].
3.3.2. Nutrient Enhancement in Final Product
Final compost analyses revealed a clear enrichment in nutrient content in all treatments, with inoculated composts consistently outperforming their uninoculated counterparts [93]. This pattern reflects both the concentration effect of organic matter mineralization and the enhanced nutrient retention promoted by microbial activity [94,95].
Total nitrogen (N) increased from initial pulp values (1.2–1.4%) to 1.92% in inoculated Castillo and 2.08% in inoculated Cenicafé 1, compared to only 1.36% and 1.41%, respectively, in the uninoculated treatments (Table 5). These differences suggest that inoculation contributed to more efficient microbial assimilation and stabilization of nitrogen, likely due to sustained thermophilic activity and improved substrate utilization [96]. The slightly higher N in Cenicafé 1 treatments may reflect cultivar-specific pulp chemistry or higher microbial activity, although the differences were not statistically significant (p > 0.05).

Table 5.
Average nutrient composition of composted Coffea arabica pulp (Castillo and Cenicafé 1) under inoculated and uninoculated conditions. Values are % dry weight; Fe and Zn converted from mg/kg. C/N ratio and organic matter included as maturity indicators.
Similarly, phosphorus (P) and potassium (K) levels were significantly higher in inoculated composts, reaching 0.54% and 2.6% (Castillo) and 0.59% and 2.8% (Cenicafé 1), compared to 0.32% and 1.5% and 0.35% and 1.7%, respectively, in their uninoculated equivalents. These results underscore the potential of inoculation not only to accelerate decomposition but also to enhance the agronomic value of the final product [97].
Among secondary macronutrients, calcium (Ca), magnesium (Mg), and sulfur (S) also followed the same trend, with higher concentrations consistently observed in inoculated composts [97,98]. For instance, Ca content increased from 0.87% to 1.15% in Castillo and from 0.91% to 1.22% in Cenicafé 1.
Micronutrients including iron (Fe) and zinc (Zn) were present at beneficial levels across all treatments, but again, higher values were recorded in inoculated composts. For Fe, levels increased from 0.037% to 0.046% (Castillo) and 0.039% to 0.0488% (Cenicafé 1); for Zn, from 0.008% to 0.011% and 0.0091% to 0.0124%, respectively. These concentrations, while modest, may contribute to improving micronutrient status in deficient soils, especially in places with degraded fertility [99,100].
Compost maturity indicators further supported the effectiveness of inoculation. The C/N ratio dropped to 12.5 (Castillo) and 11.8 (Cenicafé 1) in inoculated treatments, compared to 18.4 and 17.2 in the uninoculated ones, values that exceed the commonly accepted threshold for mature compost (C/N ≤ 15) [101,102]. Additionally, the organic matter content remained higher in inoculated composts (48.7% and 50.3%) than in their uninoculated counterparts (43.2% and 44.5%), suggesting a more effective preservation of humified carbon fractions [103].
Taken together, these results confirm that microbial inoculation not only accelerates composting and organic matter degradation, but also leads to greater nutrient recovery and improved compost maturity. While minor differences were observed between the two cultivars, the effect of inoculation was the dominant factor, confirming the value of this strategy in optimizing compost quality from coffee pulp. These outcomes align with prior findings on composted agro-industrial residues and reinforce the potential of inoculated composting systems for sustainable soil fertility management in coffee-producing regions [104,105].
3.3.3. Evaluation of Phytotoxicity, Safety, and Potential Scalability
Phytotoxicity was evaluated using Lactuca sativa seed germination assays, which are commonly used to assess compost maturity and the presence of inhibitory compounds [106]. Only the inoculated treatments exceeded the critical germination index (GI) threshold of 80%, with Castillo (inoculated) reaching 88.7% and Cenicafé 1 (inoculated) 92.4% (Table 1). In contrast, uninoculated treatments showed significantly lower GIs, 64.2% (Castillo) and 66.8% (Cenicafé 1), indicating residual phytotoxicity and incomplete stabilization [107].
These results confirm that microbial inoculation significantly improves compost maturity and biological safety, likely due to the more complete degradation of phytotoxic compounds such as caffeine, phenolics, and organic acids, which are naturally present in raw coffee pulp [43,88]. The longer thermophilic phase in inoculated compost, especially in Cenicafé 1, likely facilitated the transformation or volatilization of these inhibitory metabolites, contributing to the higher GI observed [101].
From a safety perspective, the absence of unpleasant odors, the stabilization of pH, and the elevated GI values in inoculated treatments suggest that the resulting composts are suitable for agronomic use, including in seedling production and organic substrates [108]. Meanwhile, the lower GI values in uninoculated composts highlight the limitations of passive composting systems, which may fail to detoxify pulp residues within 53 days.
From a practical standpoint, the combination of native microbial inoculation, low-cost bulking materials (e.g., sugarcane bagasse and cow manure), and passively aerated bins offers a scalable composting model for smallholder coffee producers [17,28]. The protocol requires minimal infrastructure, is adaptable to local conditions, and significantly improves compost quality and process efficiency. Furthermore, the consistency of performance across both varieties suggests that the inoculated composting approach is robust and broadly transferable across genotypes and growing environments. The dominant bacterial and fungal taxa identified in the inoculated composts, Bacillus, Streptomyces, Lactobacillus, Aspergillus, and Trichoderma, are predominantly facultative or obligate aerobic species, consistent with the oxygen availability provided by passive aeration. Facultative aerobes such as Lactobacillus can maintain metabolic activity under microaerophilic conditions during localized oxygen depletion, whereas obligate aerobes (Bacillus, Streptomyces, Aspergillus, Trichoderma) are responsible for the majority of lignocellulose degradation during the thermophilic and maturation phases.
4. Conclusions
This study demonstrates that fresh pulp from Coffea arabica L. var. Castillo and var. Cenicafé 1 harbors a diverse microbiome shaped by cultivar-specific traits and postharvest conditions. Culture-dependent isolation and amplicon sequencing identified fermentative taxa (Lactobacillus, Pichia, Saccharomyces) and lignocellulolytic decomposers (Bacillus, Streptomyces, Aspergillus, Trichoderma), with slightly greater richness in Cenicafé 1.
Functional screening revealed complementary enzymatic capacities, with pectinase-producing yeasts and fungi aiding mucilage degradation and cellulase-producing bacteria and filamentous fungi driving fiber decomposition. The absence of mycotoxigenic or phytopathogenic species supports their safe application as bioinoculants in postharvest and composting systems.
Pilot-scale composting showed that inoculation with native consortia accelerated stabilization by ~40%, producing nutrient-enriched compost in 53 days with higher N, P, K, Ca, Mg, S, Fe, and Zn, lower C/N ratios, and germination indices above 85%. These results highlight the potential of pulp-associated microbiota to enhance coffee postharvest processing, improve compost quality, and support circular bioeconomy strategies in coffee-producing regions. Future work should focus on validating these results under field-scale conditions and developing optimized inoculant formulations to facilitate adoption by smallholder coffee producers.
Author Contributions
Conceptualization, P.A.F.-V. and E.D.-D.; methodology, P.A.F.-V.; validation, P.A.F.-V. and E.D.-D.; formal analysis, P.A.F.-V.; investigation, P.A.F.-V.; resources, E.D.-D.; data curation, P.A.F.-V.; writing—original draft preparation, P.A.F.-V.; writing—review and editing, E.D.-D.; visualization, P.A.F.-V.; supervision, E.D.-D.; project administration, P.A.F.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union’s Horizon EUSPA-2022-SPACE research and innovation actions under grant agreement No. 101131859 (COMUNIDAD). The APC was supported by an editorial discount provided by the publisher.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the laboratory team at the Plant Biotechnology Laboratory, Biological Sciences Department, School of Applied Sciences and Engineering, EAFIT University, for their technical support in sample processing, microbial isolation, and composting analyses. The authors also acknowledge the administrative and logistical assistance provided by the Postharvest Discipline at Cenicafé during sample collection and experimental setup.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Acquaticci, L.; Angeloni, S.; Cela, N.; Galgano, F.; Vittori, S.; Caprioli, G.; Condelli, N. Impact of Coffee Species, Post-Harvesting Treatments and Roasting Conditions on Coffee Quality and Safety Related Compounds. Food Control 2023, 149, 109714. [Google Scholar] [CrossRef]
- Ahmed, H.; Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Toward Circular Economy: Potentials of Spent Coffee Grounds in Bioproducts and Chemical Production. Biomass 2024, 4, 286–312. [Google Scholar] [CrossRef]
- Amrouk, E.M.; Palmeri, F.; Magrini, E. Global Coffee Market and Recent Price Developments; FAO: Rome, Italy, 2025; p. 10. [Google Scholar]
- Rahmah, D.M.; Mardawati, E.; Kastaman, R.; Pujianto, T.; Pramulya, R. Coffee Pulp Biomass Utilization on Coffee Production and Its Impact on Energy Saving, CO2 Emission Reduction, and Economic Value Added to Promote Green Lean Practice in Agriculture Production. Agronomy 2023, 13, 904. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef] [PubMed]
- de Bomfim, A.S.C.; de Oliveira, D.M.; Walling, E.; Babin, A.; Hersant, G.; Vaneeckhaute, C.; Dumont, M.-J.; Rodrigue, D. Spent Coffee Grounds Characterization and Reuse in Composting and Soil Amendment. Waste 2023, 1, 2–20. [Google Scholar] [CrossRef]
- Dadi, D.; Daba, G.; Beyene, A.; Luis, P.; Van der Bruggen, B. Composting and Co-Composting of Coffee Husk and Pulp with Source-Separated Municipal Solid Waste: A Breakthrough in Valorization of Coffee Waste. Int. J. Recycl. Org. Waste Agric. 2019, 8, 263–277. [Google Scholar] [CrossRef]
- Lemma, D.B.; Debebe, W.A. Wet Coffee Processing Wastewater Treatment by Using an Integrated Constructed Wetland. Desalination Water Treat. 2023, 304, 97–111. [Google Scholar] [CrossRef]
- Král, E.; Rukov, J.L.; Mendes, A.C. Coffee Cherry on the Top: Disserting Valorization of Coffee Pulp and Husk. Food Eng. Rev. 2024, 16, 146–162. [Google Scholar] [CrossRef]
- Ijanu, E.M.; Kamaruddin, M.A.; Norashiddin, F.A. Coffee Processing Wastewater Treatment: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Appl. Water Sci. 2019, 10, 11. [Google Scholar] [CrossRef]
- Staš, J.; Houdkova, M.; Banout, J.; Duque-Dussán, E.; Roubík, H.; Kokoska, L. Adaptation and Validation of a Modified Broth Microdilution Method for Screening the Anti-Yeast Activity of Plant Phenolics in Apple and Orange Juice Models. Life 2024, 14, 938. [Google Scholar] [CrossRef]
- Sánchez-Reinoso, A.D.; Ávila-Pedraza, E.Á.; Lombardini, L.; Restrepo-Díaz, H. The Application of Coffee Pulp Biochar Improves the Physical, Chemical, and Biological Characteristics of Soil for Coffee Cultivation. J. Soil Sci. Plant Nutr. 2023, 23, 2512–2524. [Google Scholar] [CrossRef]
- Pascucci, F. The State of the Global Coffee Sector. In Sustainability in the Coffee Supply Chain: Tensions and Paradoxes; Pascucci, F., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 57–75. ISBN 978-3-031-72502-9. [Google Scholar]
- Poyilil, S.; Palatel, A.; Chandrasekharan, M. Physico-Chemical Characterization Study of Coffee Husk for Feasibility Assessment in Fluidized Bed Gasification Process. Environ. Sci. Pollut. Res. 2022, 29, 51041–51053. [Google Scholar] [CrossRef] [PubMed]
- Serna-Jiménez, J.A.; Siles, J.A.; de los Ángeles Martín, M.; Chica, A.F. A Review on the Applications of Coffee Waste Derived from Primary Processing: Strategies for Revalorization. Processes 2022, 10, 2436. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Barthel, M.; Velthof, G.; Westerik, D.; Rahn, E.; Pulleman, M.; Six, J.; Giller, K.E. Assessing Greenhouse Gas Emissions from Post-Harvest Residue Management in Coffee and Cocoa Production Systems; Wageningen University & Research: Wageningen, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Flores-Solórzano, S.B.; Huerta-Lwanga, E.; Cuevas-González, R.; Guillén-Navarro, K. Optimal Conditions to Produce Extracts of Compost and Vermicompost from Oil Palm and Coffee Pulp Wastes. J. Mater. Cycles Waste Manag. 2022, 24, 801–810. [Google Scholar] [CrossRef]
- Duque-Orrego, H.; Salazar, H.M.; Rojas-Sepúlveda, L.A.; Gaitán, Á. Análisis Económico de Tecnologías Para La Producción de Café En Colombia. Cenicafé 2021. [Google Scholar] [CrossRef]
- Flórez, C.; Arias, J.; Maldonado, C. Variedades Castillo Zonales Resistencia a La Roya Con Mayor Productividad. Cenicafé 2018, 489, 1–8. [Google Scholar] [CrossRef]
- Flórez, C.; Maldonado, C.; Cortina, H.; Moncada, M.; Montoya-Restrepo, E.C.; Ruales, L.; Muñoz-Unigarro, C.; Rendón, J.; Duque-Orrego, H. Cenicafé 1: Nueva Variedad de Porte Bajo, Altamente Productiva, Resistente a La Roya y al CBD, Con Mayor Calidad Física Del Grano. Av. Téc. Cenicafé 2016, 469, 1–8. [Google Scholar] [CrossRef]
- Centro Nacional de Investigaciones de Café. Guía Más Agronomía, Más Productividad, Más Calidad, 3rd ed.; Cenicafé: Manizales, Colombia, 2021; ISBN 978-958-8490-49-6. [Google Scholar]
- Rendón, J.R. Producción de Café Variedad Castillo® En Altas Densidades de Siembra Con Uno y Dos Tallos Por Sitio. Rev. Cenicafé 2021, 72, e72106. [Google Scholar] [CrossRef]
- Duong, B.; Marraccini, P.; Maeght, J.-L.; Vaast, P.; Lebrun, M.; Duponnois, R. Coffee Microbiota and Its Potential Use in Sustainable Crop Management. A Review. Front. Sustain. Food Syst. 2020, 4, 607935. [Google Scholar] [CrossRef]
- Vinícius de Melo Pereira, G.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial Ecology and Starter Culture Technology in Coffee Processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef]
- Fu, X.; Li, G.; Li, Y.; Li, Y.; Bi, X.; Huang, J.; Yang, Y.; Yu, H.; Liu, D.; Hu, F.; et al. Fermentation with Coffee Berry Peels Induces Spatiotemporal Changes in Microbial Communities Leading to Unique Aroma of Coffee Berries. Int. J. Food Prop. 2024, 27, 657–673. [Google Scholar] [CrossRef]
- Tenea, G.N.; Cifuentes, V.; Reyes, P.; Cevallos-Vallejos, M. Unveiling the Microbial Signatures of Arabica Coffee Cherries: Insights into Ripeness Specific Diversity, Functional Traits, and Implications for Quality and Safety. Foods 2025, 14, 614. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Venkatkarthick, R.; SenthilKannan, K.; Kuppam, C.; Mary Stephy, G.; Kamyab, H.; Chen, W.-H.; Thomas, J.; Ngamcharussrivichai, C. Advanced Technologies on the Sustainable Approaches for Conversion of Organic Waste to Valuable Bioproducts: Emerging Circular Bioeconomy Perspective. Fuel 2022, 324, 124313. [Google Scholar] [CrossRef]
- Mebrate, A.; and Kippie, T. Effect of Coffee Pulp Compost and P Fertilizer on Yield and Yield Components of Maize (Zea Mays L.) in Gedeo Zone, Southern Ethiopia. Compost. Sci. Util. 2021, 29, 21–36. [Google Scholar] [CrossRef]
- Montilla-Pérez, J.; Arcila-Pulgarín, J.; Aristizábal-Loaiza, M.; Montoya-Restrepo, E.C.; Puerta-Quintero, G.I.; Oliveros-Tascón, C.E.; Cadena-Gómez, G. Caracterización de Algunas Propiedades Físicas y Factores de Conversión Del Café Durante El Proceso de Beneficio Húmedo Tradicional. Rev. Cenicafé 2008, 59, 120–142. [Google Scholar]
- Pérez-Sariñana, B.Y.; Saldaña-Trinidad, S. Chemistry and Biotransformation of Coffee By-Products to Biofuels. In The Question of Caffeine; Books on Demand: Hamburg, Germany, 2017. [Google Scholar]
- Pagliarini, E.; Totaro, G.; Saccani, A.; Gaggìa, F.; Lancellotti, I.; Di Gioia, D.; Sisti, L. Valorization of Coffee Wastes as Plant Growth Promoter in Mulching Film Production: A Contribution to a Circular Economy. Sci. Total. Environ. 2023, 871, 162093. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Physicochemical Properties and Valorization of the Mucilage Byproduct from Colombian Coffea arabica L. as a Powdered Food Hydrocolloid. ACS Food Sci. Technol. 2024, 4, 2895–2906. [Google Scholar] [CrossRef]
- Sierra-López, L.D.; Hernandez-Tenorio, F.; Marín-Palacio, L.D.; Giraldo-Estrada, C. Coffee Mucilage Clarification: A Promising Raw Material for the Food Industry. Food Humanit. 2023, 1, 689–695. [Google Scholar] [CrossRef]
- Duque Dussán, E.; Bappah, M.; Sanz-Uribe, J.; Nainggolan, E.A. Thermo-Chemical Characterization of Coffee Husk from a New Variety (Coffea arabica L. Var. Cenicafé 1) for Biofuel Production. Sci. Agric. Bohem. 2025, 56, 1–22. [Google Scholar] [CrossRef]
- Manrique, R.; Vásquez, D.; Ceballos, C.; Chejne, F.; Amell, A. Evaluation of the Energy Density for Burning Disaggregated and Pelletized Coffee Husks. ACS Omega 2019, 4, 2957–2963. [Google Scholar] [CrossRef]
- Manrique, R.; Vásquez, D.; Chejne, F.; Pinzón, A. Energy Analysis of a Proposed Hybrid Solar–Biomass Coffee Bean Drying System. Energy 2020, 202, 1–8. [Google Scholar] [CrossRef]
- Duque-Dussán, E.; Sanz-Uribe, J.R.; Banout, J. Design and Evaluation of a Hybrid Solar Dryer for Postharvesting Processing of Parchment Coffee. Renew. Energy 2023, 215, 118961. [Google Scholar] [CrossRef]
- Thurston, R.W.; Morris, J.; Steiman, S. Coffee: A Comprehensive Guide to the Bean, the Beverage, and the Industry; Bloomsbury Publishing PLC: London, UK, 2013; ISBN 978-1-4422-1442-2. [Google Scholar]
- la Rosa, J.M.D.; Pérez-Dalí, S.M.; Campos, P.; Sánchez-Martín, Á.; González-Pérez, J.A.; Miller, A.Z. Suitability of Volcanic Ash, Rice Husk Ash, Green Compost and Biochar as Amendments for a Mediterranean Alkaline Soil. Agronomy 2023, 13, 1097. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N.; Sinha, A.K. Conversion of Spent Coffee and Donuts by Black Soldier Fly (Hermetia Illucens) Larvae into Potential Resources for Animal and Plant Farming. Insects 2021, 12, 332. [Google Scholar] [CrossRef]
- Hutabarat, D.J.C.; Mangindaan, D. Cultivation of Black Soldier Fly (Hermetia Illucens) Larvae for the Valorization of Spent Coffee Ground: A Systematic Review and Bibliometric Study. Agriculture 2024, 14, 205. [Google Scholar] [CrossRef]
- Kandasamy, S.; Muthusamy, G.; Balakrishnan, S.; Duraisamy, S.; Thangasamy, S.; Seralathan, K.-K.; Chinnappan, S. Optimization of Protease Production from Surface-Modified Coffee Pulp Waste and Corncobs Using Bacillus Sp. by SSF. 3 Biotech 2016, 6, 167. [Google Scholar] [CrossRef]
- Ameca, G.M.; Cerrilla, M.E.O.; Córdoba, P.Z.; Cruz, A.D.; Hernández, M.S.; Haro, J.H. Chemical Composition and Antioxidant Capacity of Coffee Pulp. Ciênc. E Agrotecnologia 2018, 42, 307–313. [Google Scholar] [CrossRef]
- San Martin Ruiz, M.; Reiser, M.; Kranert, M. Enhanced Composting as a Way to a Climate-Friendly Management of Coffee by-Products. Environ. Sci. Pollut. Res. 2020, 27, 24312–24319. [Google Scholar] [CrossRef]
- Jain, R.; Pattanaik, L.; Padhi, S.K.; Naik, S.N. Role of Microbes and Microbial Consortium in Solid Waste Management. In Environmental and Agricultural Microbiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 383–422. ISBN 978-1-119-52589-9. [Google Scholar]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The Role of Wet Fermentation in Enhancing Coffee Flavor, Aroma and Sensory Quality. Eur. Food Res. Technol. 2021, 247, 485–498. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. The Role of Microbes in Coffee Fermentation and Their Impact on Coffee Quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and Agronomic Performance of the Coffee Crop in the Context of Climate Change and Global Warming: A Review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef]
- Peñuela-Martínez, A.E.; Guerrero, Á.; Sanz-Uribe, J.R. Cromacafé® Herramienta Para Identificar Los Estados de Madurez de Las Variedades de Café de Fruto Rojo. Av. Téc. Cenicafé 2022, 535, 1–8. [Google Scholar] [CrossRef]
- Pineda, M.F.; Tinoco, H.A.; Lopez-Guzman, J.; Perdomo-Hurtado, L.; Cardona, C.I.; Rincon-Jimenez, A.; Betancur-Herrera, N. Ripening Stage Classification of Coffea arabica L. Var. Castillo Using a Machine Learning Approach with the Electromechanical Impedance Measurements of a Contact Device. Mater. Today Proc. 2022, 1675, 1–26. [Google Scholar] [CrossRef]
- García, L.J.C.; Posada-Suárez, H.; Läderach, P. Recommendations for the Regionalizing of Coffee Cultivation in Colombia: A Methodological Proposal Based on Agro-Climatic Indices. PLoS ONE 2014, 9, e113510. [Google Scholar] [CrossRef] [PubMed]
- Duque-Dussán, E.; Banout, J. Improving the Drying Performance of Parchment Coffee Due to the Newly Redesigned Drying Chamber. J. Food Process Eng. 2022, 45, e14161. [Google Scholar] [CrossRef]
- ISO 6673:2003; Green Coffee—Determination of Loss in Mass at 105 Degrees C. ISO: Geneva, Switzerland, 2003.
- Dimitrijević, S.; Milić, M.; Buntić, A.; Dimitrijević-Branković, S.; Filipović, V.; Popović, V.; Salamon, I. Spent Coffee Grounds, Plant Growth Promoting Bacteria, and Medicinal Plant Waste: The Biofertilizing Effect of High-Value Compost. Sustainability 2024, 16, 1632. [Google Scholar] [CrossRef]
- Duque-Buitrago, L.-F.; Calderón-Gaviria, K.-D.; Torres-Valenzuela, L.-S.; Sánchez-Tamayo, M.-I.; Plaza-Dorado, J.-L. Modulating Coffee Fermentation Quality Using Microbial Inoculums from Coffee By-Products for Sustainable Practices in Smallholder Coffee Production. Sustainability 2025, 17, 1781. [Google Scholar] [CrossRef]
- Lončarić, Z.; Galić, V.; Nemet, F.; Perić, K.; Galić, L.; Ragályi, P.; Uzinger, N.; Rékási, M. The Evaluation of Compost Maturity and Ammonium Toxicity Using Different Plant Species in a Germination Test. Agronomy 2024, 14, 2636. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Wickham, H. Data Analysis. In Use R! Springer International Publishing: Cham, Switzerland, 2016; pp. 189–201. ISBN 978-3-319-24275-0. [Google Scholar]
- Shen, X.; Wang, Q.; Wang, H.; Fang, G.; Li, Y.; Zhang, J.; Liu, K. Microbial Characteristics and Functions in Coffee Fermentation: A Review. Fermentation 2024, 11, 5. [Google Scholar] [CrossRef]
- Polanía Rivera, A.M.; López Silva, J.; Torres-Valenzuela, L.S.; Plaza Dorado, J.L. Development of Starter Inoculum for Controlled Arabica Coffee Fermentation Using Coffee By-Products (Pulp and Mucilage Broth), Yeast, and Lactic Acid Bacteria. Fermentation 2024, 10, 516. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Yeasts Are Essential for Mucilage Degradation of Coffee Beans during Wet Fermentation. Yeast 2023, 40, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.L.; Pereira, P.V.; Bertoli, L.D.; Silveira, D.L.; Batista, N.N.; Pinheiro, P.F.; De Souza Carneiro, J.; Schwan, R.F.; De Assis Silva, S.; Coelho, J.M.; et al. Fermentation of Coffea Canephora Inoculated with Yeasts: Microbiological, Chemical, and Sensory Characteristics. Food Microbiol. 2021, 98, 103786. [Google Scholar] [CrossRef] [PubMed]
- Núñez Pérez, J.; Chávez Arias, B.S.; De La Vega Quintero, J.C.; Zárate Baca, S.; Pais-Chanfrau, J.M. Multi-Objective Statistical Optimization of Pectinolytic Enzymes Production by an Aspergillus Sp. on Dehydrated Coffee Residues in Solid-State Fermentation. Fermentation 2022, 8, 170. [Google Scholar] [CrossRef]
- Carota, E.; Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Lignocellulolytic Potential of the Recently Described Species Aspergillus Olivimuriae on Different Solid Wastes. Appl. Sci. 2021, 11, 5349. [Google Scholar] [CrossRef]
- Alabdalall, A.H.; Almutari, A.A.; Aldakeel, S.A.; Albarrag, A.M.; Aldakheel, L.A.; Alsoufi, M.H.; Alfuraih, L.Y.; Elkomy, H.M. Bioethanol Production from Lignocellulosic Biomass Using Aspergillus Niger and Aspergillus Flavus Hydrolysis Enzymes through Immobilized S. Cerevisiae. Energies 2023, 16, 823. [Google Scholar] [CrossRef]
- Krull, R.; Wucherpfennig, T.; Esfandabadi, M.E.; Walisko, R.; Melzer, G.; Hempel, D.C.; Kampen, I.; Kwade, A.; Wittmann, C. Characterization and Control of Fungal Morphology for Improved Production Performance in Biotechnology. J. Biotechnol. 2013, 163, 112–123. [Google Scholar] [CrossRef]
- Cárdenas, D.E.; Aguilar, C.; Bhatta, U.; Bugingo, C.; Cochran-Murray, S.; Gazis, R.; Miles, T.D.; Jurick, W.; Naegele, R.P.; Quesada-Ocampo, L.; et al. Rotten to the Core: Challenges with Postharvest Disease Management of Fruit Crops. Plant Dis. 2025, 1–70. [Google Scholar] [CrossRef]
- Applications of Streptomyces spp. Enhanced Compost in Sustainable Agriculture. In Soil Biology; Springer International Publishing: Cham, Switzerland, 2020; pp. 257–291. ISBN 978-3-030-39172-0.
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. A Review on Bacterial Contribution to Lignocellulose Breakdown into Useful Bio-Products. Int. J. Environ. Res. Public. Health 2021, 18, 6001. [Google Scholar] [CrossRef]
- Bhimani, A.A.; Bhimani, H.D.; Vaghela, N.R.; Gohel, S.D. Cultivation Methods, Characterization, and Biocatalytic Potential of Organic Solid Waste Degrading Bacteria Isolated from Sugarcane Rhizospheric Soil and Compost. Biologia 2024, 79, 953–974. [Google Scholar] [CrossRef]
- Velásquez, S.; Banchón, C. Influence of Pre-and Post-Harvest Factors on the Organoleptic and Physicochemical Quality of Coffee: A Short Review. J. Food Sci. Technol. 2023, 60, 2526–2538. [Google Scholar] [CrossRef]
- Vale, A.d.S.; Pereira, C.M.T.; De Dea Lindner, J.; Rodrigues, L.R.S.; Kadri, N.K.E.; Pagnoncelli, M.G.B.; Kaur Brar, S.; Soccol, C.R.; Pereira, G.V. de M. Exploring Microbial Influence on Flavor Development during Coffee Processing in Humid Subtropical Climate through Metagenetic–Metabolomics Analysis. Foods 2024, 13, 1871. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J. Alpha Diversity. In Bioinformatic and Statistical Analysis of Microbiome Data; Springer International Publishing: Cham, Switzerland, 2023; pp. 289–333. ISBN 978-3-031-21390-8. [Google Scholar]
- De Souza, T.S.P.; Kawaguti, H.Y. Cellulases, Hemicellulases, and Pectinases: Applications in the Food and Beverage Industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Sohail, M.; Barzkar, N.; Michaud, P.; Tamadoni Jahromi, S.; Babich, O.; Sukhikh, S.; Das, R.; Nahavandi, R. Cellulolytic and Xylanolytic Enzymes from Yeasts: Properties and Industrial Applications. Molecules 2022, 27, 3783. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed]
- Finore, I.; Feola, A.; Russo, L.; Cattaneo, A.; Di Donato, P.; Nicolaus, B.; Poli, A.; Romano, I. Thermophilic Bacteria and Their Thermozymes in Composting Processes: A Review. Chem. Biol. Technol. Agric. 2023, 10, 7. [Google Scholar] [CrossRef]
- Chen, M.; Li, Q.; Liu, C.; Meng, E.; Zhang, B. Microbial Degradation of Lignocellulose for Sustainable Biomass Utilization and Future Research Perspectives. Sustainability 2025, 17, 4223. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Qiu, H.; Anning, D.K.; Li, M.; Wang, Y.; Zhang, C. Effects of C/N Ratio on Lignocellulose Degradation and Enzyme Activities in Aerobic Composting. Horticulturae 2021, 7, 482. [Google Scholar] [CrossRef]
- Maral-Gül, D.; Eltem, R. Evaluation of Bacillus Isolates as a Biological Control Agents against Soilborne Phytopathogenic Fungi. Int. Microbiol. 2024, 28, 75–89. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Oufensou, S.; Zeidan, R.; Migheli, Q.; Jaoua, S. Microbial Volatilome in Food Safety. Current Status and Perspectives in the Biocontrol of Mycotoxigenic Fungi and Their Metabolites. Biocontrol Sci. Technol. 2023, 33, 499–538. [Google Scholar] [CrossRef]
- Clara Ivette, R.-M.; Luis Alberto, M.-G.; Luis Galdino, G.-P.; Francisco Alexander, R.-M.; Reiner, R.-R. Advances and Challenges in the Production and Use of Native Bacteria as Plant Probiotics in Agronomic Applications: A Mexican Review. J. Agric. Food Res. 2025, 21, 101917. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.M.; Parra Cota, F.I.; Cira Chávez, L.A.; García Ortega, L.F.; Estrada Alvarado, M.I.; Santoyo, G.; de los Santos-Villalobos, S. Microbial Inoculants in Sustainable Agriculture: Advancements, Challenges, and Future Directions. Plants 2025, 14, 191. [Google Scholar] [CrossRef]
- Gil-Gómez, J.A.; Florez-Pardo, L.M.; Leguizamón-Vargas, Y.C. Valorization of Coffee By-Products in the Industry, a Vision towards Circular Economy. Discov. Appl. Sci. 2024, 6, 480. [Google Scholar] [CrossRef]
- Ravindran, B.; Awasthi, M.K.; Karmegam, N.; Chang, S.W.; Chaudhary, D.K.; Selvam, A.; Nguyen, D.D.; Rahman Milon, A.; Munuswamy-Ramanujam, G. Co-Composting of Food Waste and Swine Manure Augmenting Biochar and Salts: Nutrient Dynamics, Gaseous Emissions and Microbial Activity. Bioresour. Technol. 2022, 344, 126300. [Google Scholar] [CrossRef]
- Wang, X.; Sale, P.; Hunt, J.; Clark, G.; Wood, J.L.; Franks, A.E.; Reddy, P.; Jin, J.; Joseph, S.; Tang, C. Enhancing Growth and Transpiration Efficiency of Corn Plants with Compost Addition and Potential Beneficial Microbes under Well-Watered and Water-Stressed Conditions. Plant Soil 2025, 1–19. [Google Scholar] [CrossRef]
- Sheng, C.; Yao, C. Review on Self-Heating of Biomass Materials: Understanding and Description. Energy Fuels 2022, 36, 731–761. [Google Scholar] [CrossRef]
- Rohaya, S.; Anwar, S.H.; Amhar, A.B.; Sutriana, A.; Muzaifa, M. Antioxidant Activity and Physicochemical Composition of Coffee Pulp Obtained from Three Coffee Varieties in Aceh, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2023, 1182, 012063. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Zhang, X.; Liu, J.; Xu, X.; Wang, S.; Lan, T.; Zhang, K.; Gao, F.; He, Q.; et al. Mechanisms and Effects of Novel Ammonifying Microorganisms on Nitrogen Ammonification in Cow Manure Waste Composting. Waste Manag. 2023, 169, 167–178. [Google Scholar] [CrossRef]
- Ellafi, A.; Dali, A.; Mnif, S.; Ben Younes, S. Microbial Enzymatic Degradation, Spectral Analysis and Phytotoxicity Assessment of Congo Red Removal By Bacillus spp. Catal. Lett. 2023, 153, 3620–3633. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Landeka Dragičević, T. The Bacterial Degradation of Lignin—A Review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Zainudin, M.H.M.; Zulkarnain, A.; Azmi, A.S.; Muniandy, S.; Sakai, K.; Shirai, Y.; Hassan, M.A. Enhancement of Agro-Industrial Waste Composting Process via the Microbial Inoculation: A Brief Review. Agronomy 2022, 12, 198. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, K.; Yang, Y.; Gao, B.; Zheng, H. Effects of Biochar on the Accumulation of Necromass-Derived Carbon, the Physical Protection and Microbial Mineralization of Soil Organic Carbon. Crit. Rev. Environ. Sci. Technol. 2024, 54, 39–67. [Google Scholar] [CrossRef]
- Li, J.-Y.; Chen, P.; Li, Z.-G.; Li, L.-Y.; Zhang, R.-Q.; Hu, W.; Liu, Y. Soil Aggregate-Associated Organic Carbon Mineralization and Its Driving Factors in Rhizosphere Soil. Soil Biol. Biochem. 2023, 186, 109182. [Google Scholar] [CrossRef]
- Chen, X.; Du, G.; Wu, C.; Li, Q.; Zhou, P.; Shi, J.; Zhao, Z. Effect of Thermophilic Microbial Agents on Nitrogen Transformation, Nitrogen Functional Genes, and Bacterial Communities during Bean Dregs Composting. Environ. Sci. Pollut. Res. 2022, 29, 31846–31860. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Zafar, U.; Khan, A.; Haq, T.; Mujahid, T.; Wali, M. Effectiveness of Compost Inoculated with Phosphate Solubilizing Bacteria. J. Appl. Microbiol. 2022, 133, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.A.; Alharby, H.F.; Abdelfattah, M.A.; Mohamed, I.A.A.; Hakeem, K.R.; Rady, M.M.; Shaaban, A. Spirulina Platensis-Inoculated Humified Compost Boosts Rhizosphere Soil Hydro-Physico-Chemical Properties and Atriplex Nummularia Forage Yield and Quality in an Arid Saline Calcareous Soil. J. Soil Sci. Plant Nutr. 2023, 23, 2215–2236. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Metwaly, M.M.; Afifi, A.A.; D’Antonio, P.; Scopa, A. Assessment of Soil Fertility Status under Soil Degradation Rate Using Geomatics in West Nile Delta. Land 2022, 11, 1256. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Fawzy, Z.F.; Elsakhawy, T.; Omara, A.E.-D.; Amer, M.; Faizy, S.E.-D.; Abowaly, M.; El-Henawy, A.; Kiss, A.; et al. Nano-Restoration for Sustaining Soil Fertility: A Pictorial and Diagrammatic Review Article. Plants 2022, 11, 2392. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, J.; Yang, Y.; Liu, Y.; Zhang, L.; Wang, G.; Liu, G.; Dang, R.; Li, G.; Yuan, J. Determining the Extraction Conditions and Phytotoxicity Threshold for Compost Maturity Evaluation Using the Seed Germination Index Method. Waste Manag. 2023, 171, 502–511. [Google Scholar] [CrossRef]
- Dume, B.; Hanc, A.; Svehla, P.; Michal, P.; Chane, A.D.; Nigussie, A. Composting and Vermicomposting of Sewage Sludge at Various C/N Ratios: Technological Feasibility and End-Product Quality. Ecotoxicol. Environ. Saf. 2023, 263, 115255. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Choudhury, M.; Sharma, P.C.; Priyanka; Jat, H.S.; Jat, M.L.; Kar, S. Stability of Humic Acid Carbon under Conservation Agriculture Practices. Soil Tillage Res. 2022, 216, 105240. [Google Scholar] [CrossRef]
- Nadaf, S.A.; Shivaprasad, P.; Babou, C.; Hariyappa, N.; Chandrashekar, N.; Kumari, P.; Sowmya, P.R.; Hareesh, S.B.; Pati, N.R.; Nagaraja, J.S.; et al. Coffee (Coffea spp.). In Soil Health Management for Plantation Crops: Recent Advances and New Paradigms; Thomas, G.V., Krishnakumar, V., Eds.; Springer Nature: Singapore, 2024; pp. 337–389. ISBN 978-981-97-0092-9. [Google Scholar]
- Kiup, E.; Swan, T.; Field, D. Soil Management Practices in Coffee Farming Systems in the Asia-Pacific Region and Their Relevance to Papua New Guinea: A Systematic Review. Soil Use Manag. 2025, 41, e70068. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Q.; Zhang, Y.; Yang, L.; Zeng, Z.; Zhou, Y.; Chen, B. Advance in the Thermoinhibition of Lettuce (Lactuca sativa L.) Seed Germination. Plants 2024, 13, 2051. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, G.; Chen, W.; Yang, Y.; Ma, R.; Li, D.; Shen, Y.; Li, G.; Yuan, J. Phytotoxicity of Farm Livestock Manures in Facultative Heap Composting Using the Seed Germination Index as Indicator. Ecotoxicol. Environ. Saf. 2022, 247, 114251. [Google Scholar] [CrossRef]
- Antunes, L.F.D.S.; Vaz, A.F.D.S.; Martelleto, L.A.P.; Leal, M.A.D.A.; Alves, R.D.S.; Ferreira, T.D.S.; Rumjanek, N.G.; Correia, M.E.F.; Rosa, R.C.C.; Guerra, J.G.M. Sustainable Organic Substrate Production Using Millicompost in Combination with Different Plant Residues for the Cultivation of Passiflora Edulis Seedlings. Environ. Technol. Innov. 2022, 28, 102612. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).