Applied Microbiology for Sustainable Agricultural Development
Abstract
1. Introduction
- -
- -
- -
- No-tillage: Improves water retention, reduces soil erosion, and increases soil organic matter.
- -
- Agroforestry: Boosts biodiversity and positively impacts water, climate, and ecological balance [3].
- -
- Precision farming: Optimizes resource use (water, energy, fertilizers, plant protection products) through accurate monitoring [4].
- -
- -
- -
- -
- -
2. Materials and Methods
3. Review
3.1. Agricultural Microbiome
3.2. Sustainable Green Chemistry
3.3. Biofertilizers
3.4. Development of EM and Biofertilizer Formulation
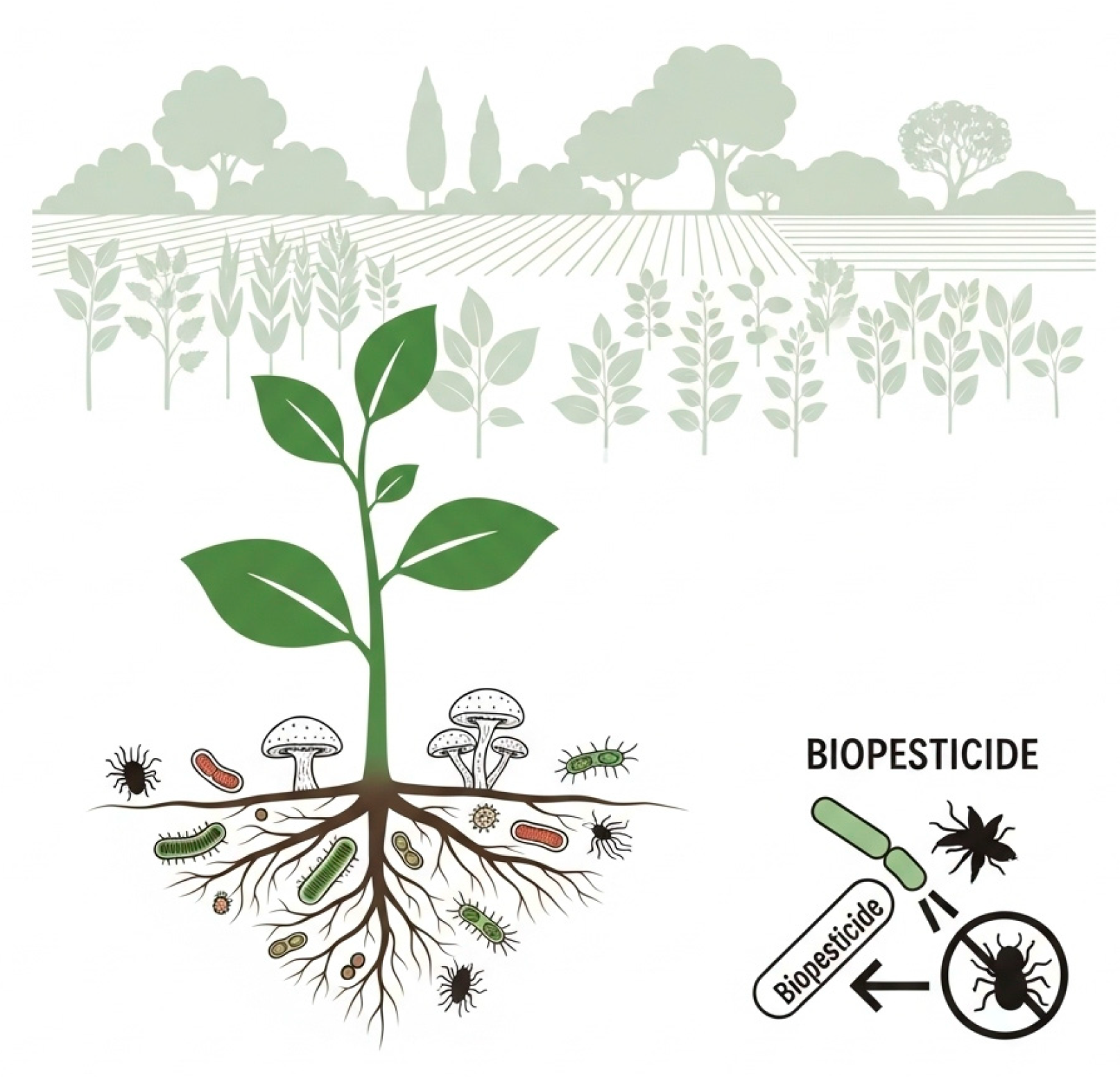
3.5. Biopesticides
3.6. Bioherbicides
- Traditional microorganisms (e.g., Fusarium, Colletotrichum, Phoma, Streptomyces), soil microorganisms and parasitic fungi, natural plant extracts (Solanum habrochaites, pelargonate, essential oils), and even seaweed products (Ascophyllum nodosum). This reflects the global trend away from synthetic herbicides towards natural, biodegradable, and environmentally safer compounds.
- Commercialization status and regionality: Some bioherbicides are available locally (e.g., Di-Bak® in Australia, Bio-Phoma™ in Canada, Kichawi Kill™ in Kenya). Many products are “experimental” or “discontinued,” which shows the difficulties with their sustainable commercialization, including problems with mass production, field efficacy, and registration. There is still a lack of international standardization, and registration is often limited to regions where weed is a problem.
- Practical challenges: Bioherbicides are also accompanied by practical challenges, as they are often less fast and selective than synthetic agents; they can act slower and require specific conditions (humidity, temperature). Some of them only act on selected species (e.g., Colletotrichum only on Aeschynomene). Storage problems and shorter shelf life are often mentioned as barriers.
- Ecological and agrotechnical importance: Bioherbicides as a key tool in sustainable agriculture or in integrated plant protection (IPM) and in organic production systems. They can also reduce the problem of herbicide resistance, e.g., in the case of Conyza canadensis (resistant to glyphosate), Albifimbria verrucaria is the answer to this challenge.
- Promising research directions: Microorganisms such as Trichoderma, Phoma, and Fusarium oxysporum are intensively studied as carriers of new bioactive metabolites. There is also growing interest in multi-component products (e.g., Di-Bak®—a mixture of three fungi) and target application systems (e.g., capsules, implants, nanoformulation).
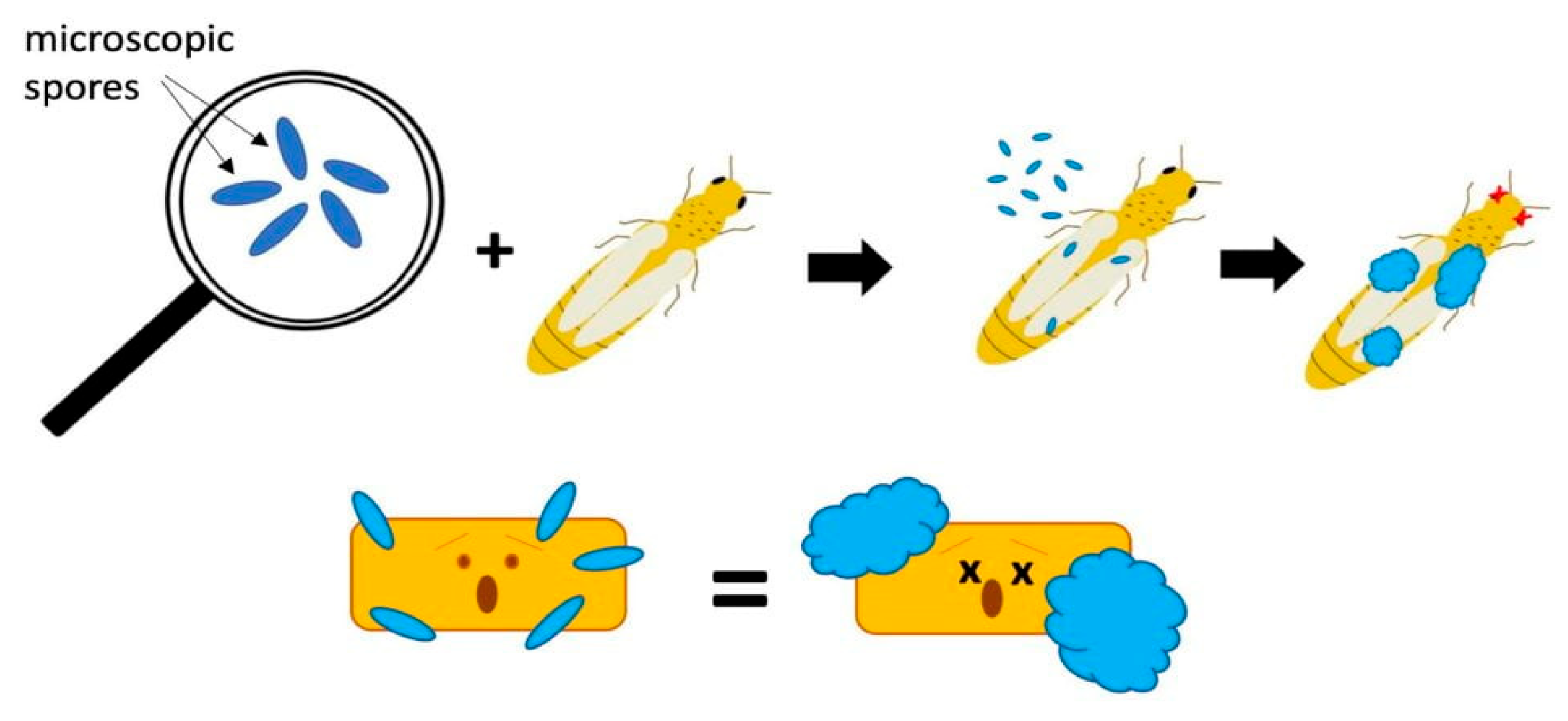
3.7. Bioinsecticides
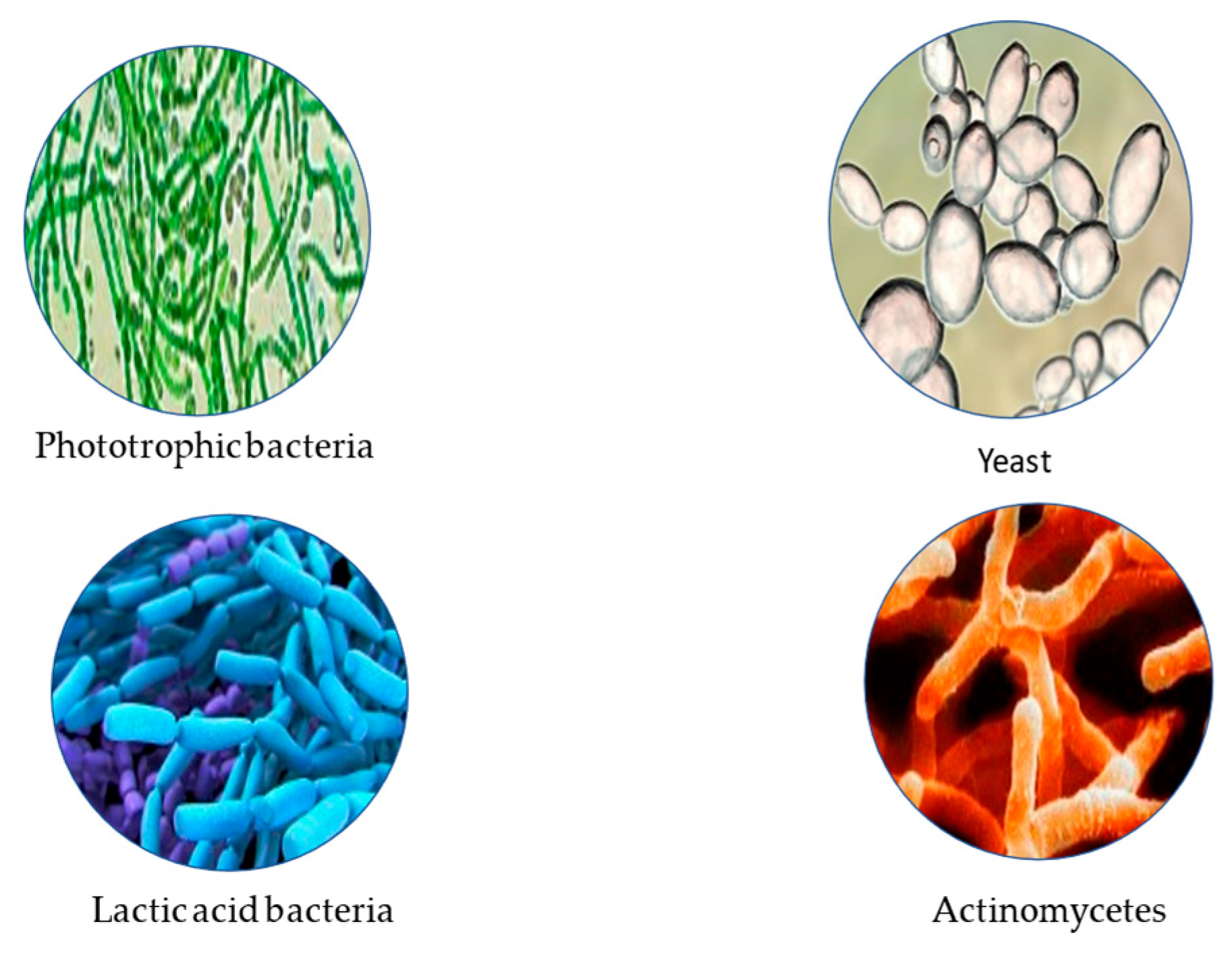
3.8. Effective Microorganisms
3.8.1. Microorganisms in the Influence of Plant Resistance
3.8.2. EMs in Plant Cultivation
3.8.3. Microbiological Preparations in Composting
3.8.4. EMs in Food Processing
3.8.5. Mycorrhizal Preparations
3.8.6. Microorganisms in Aerobic Nitrification and Denitrification
3.8.7. EM and Germanium (Ge)
3.8.8. Inhibition of the Growth of Various Pathogenic Microorganisms Using EMs
3.9. Microbiological Organisms in Sustainable Agriculture
3.10. Soil and Microorganisms: Basics and Impact of EMs
Soil Microorganisms Enhance Plant Hea
4. Discussion
4.1. Criteria for Assessing the Effectiveness of Microbial Technologies
- -
- -
- -
- -
- Economic and Practical Aspects: Cost-effectiveness for the farmer (cost–benefit ratio), ease of use, and stability of preparations and their compliance with existing agricultural practices and organic certification requirements [123].
- -
- Research institutions and universities, such as the Institute of Soil Science and Plant Cultivation—National Research Institute (IUNG-PIB) in Puławy, Poland, are key centers that conduct research on microbiological preparations, evaluate their effectiveness, and create guidelines. Their scientific publications and guides for farmers often include detailed criteria [54,104,112].
- -
- Universities of life sciences (e.g., Poznań University of Life Sciences, Maria Curie-Skłodowska University in Lublin, which is mentioned in the text in the context of research on biopreparations) and agricultural and microbiological faculties around the world. Researchers from these institutions publish articles in peer-reviewed scientific journals that present research results in accordance with these criteria.
- -
- International research centers, including organizations such as the International Center for Tropical Agriculture (CIAT), the International Maize and Wheat Improvement Center (CIMMYT), or the World Agroforestry Center (ICRAF), also conduct extensive research in this area.
- -
- Agricultural and development organizations, such as the Food and Agriculture Organization of the United Nations (FAO), which promotes sustainable agricultural practices and often publishes reports and guidelines on the use of innovative technologies, including microbiological ones, to improve food and environmental security [47].
- -
- As an organization promoting organic agriculture, the International Federation of Organic Agriculture Movements (IFOAM Organics International) sets standards and criteria for products permitted for use in this method of cultivation, which often include detailed guidelines for organic preparations [47].
- -
- In Poland, national ministries of agriculture and regulatory agencies, such as the Ministry of Agriculture and Rural Development, together with subordinate institutions (such as IUNG-PIB), develop and implement regulations on the registration and marketing of microbiological fertilizer products, which require an assessment of their efficacy and safety [50,51,52].
- -
- In order to obtain registration and introduce products to the market, biotechnology companies and producers of biopreparations must comply with and present data confirming the efficacy of their products in accordance with the guidelines of regulatory bodies. They often cite their own studies and studies of independent institutes [51,52].
- -
- World Organization for Animal Health (WOAH) and World Health Organization (WHO) mainly focus on animal and human health, but their interest in antimicrobial resistance (AMR) makes them indirectly raise the effectiveness criteria for alternatives to antibiotics, including microbiological solutions in agriculture, in order to reduce the use of chemical plant protection and veterinary products that may contribute to AMR [47].
4.2. Numerical Estimates of the Possible Impact of Using Technology in Various Technological Processes
4.3. Comparative Assessment of the Impact of Existing Technologies on Product Safety and Production Efficiency
- a.
- Bioherbicides
- Production Efficiency:
- Potential: The texts indicate that bioherbicides, such as those produced from Lasiodiplodia theobromae or L. pseudotheobromae, can be effective in controlling monocotyledonous and dicotyledonous weeds. This offers a promise for effective control [68].
- Challenges:
- ○
- ○
- Limited Commercial Application: Despite promising research, “only a few live microbial bioherbicides have been approved for commercial use and have been placed on the market, but their use has unfortunately been very limited for a number of reasons, mainly economic.” This indicates a barrier to widespread adoption [69,70,77,83].
- ○
- Product Safety:
- Advantages:
- ○
- Natural Origin: Bioherbicides are produced from microorganisms, plant extracts, and minerals (e.g., sulfur, although the text notes sulfur is more of a fungicide than herbicide). This makes them generally “safer and more environmentally friendly” than synthetic pesticides because they are often biodegradable and have less toxicity to non-target organisms [68].
- ○
- ○
- b.
- Bioinsecticides
- Production Efficiency:
- Challenges:
- ○
- ○
- Environmental Conditions: The effectiveness of preparations containing live fungal spores (e.g., Beauveria bassiana) depends on environmental conditions (humidity, temperature) conducive to germination and infection [68].
- Product Safety:
- Advantages:
- ○
- ○
- ○
- ○
- c.
- Effective Microorganisms (EMs)
- Production Efficiency:
- Potential:
- ○
- ○
- ○
- ○
- ○
- Challenges:
- ○
- Controversy and Inconsistent Results: The text frankly admits there are “reports of both benefits from their use and their ineffectiveness.” It notes that “some studies, e.g., Van Vliet et al. [111], did not show a significant effect.”
- ○
- Liberal Registration Procedure: Criticisms of the “liberal registration procedure,” which “indicates the need for further research on the mechanisms of EM action.”
- ○
- Competition with Native Microflora: In the context of composting, the lack of an EM effect could be due to “competition with native microflora or parasitism/antibiosis phenomena.”
- ○
- Not a Panacea: It is emphasized that “their role as a panacea for all problems should not be overestimated.”
- Product Safety:
- Advantages:
- ○
- ○
- ○
- d.
- Mycorrhizal Preparations
- Production Efficiency:
- Potential:
- ○
- Increased Nutrient Uptake: Mycorrhizal fungi enhance the uptake of water and minerals (nitrogen, phosphorus, potassium, micronutrients) by plants [40].
- ○
- Stress Resistance: Increased resistance to frost and salinity.
- ○
- Improved Plant Growth and Appearance: More abundant flowering, better rooting.
- ○
- ○
- Product Safety:
- Advantages:
- ○
- ○
- No Chemical Residues: They do not introduce artificial substances into the environment or products.
- ○
- e.
- Microorganisms in Aerobic Nitrification and Denitrification (Wastewater Treatment Perspective)
- Process Efficiency (not necessarily agricultural production, but technological processes):
- Potential:
- ○
- ○
- ○
- Product Safety (more “Environmental Safety” and “Water Quality”):
- Advantages:
- ○
- Reduced Nitrogen Pollution: Effectively remove nitrogen compounds that cause water eutrophication and air quality deterioration and negatively affect human and animal health. This is crucial for aquatic environmental safety [59].
- ○
- Minimized Emissions: These processes convert harmful forms of nitrogen (e.g., ammonia) into harmless nitrogen gas (N2), reducing pollution [85].
4.4. Microbiome in the Fight Against Abiotic Stresses: Recent Achievements and Perspectives in Sustainable Agriculture
4.4.1. Role of the Rhizosphere in Drought Tolerance
- -
- Increase water availability: By producing exopolysaccharides (EPSs), which improve soil aggregation and water retention. Some strains can also affect root architecture, increasing their ability to explore soil volume.
- -
- Modulate plant hormones: By producing auxins, gibberellins, or cytokinins, which help plants maintain turgor, delay wilting, and stimulate root growth in search of water [76].
- -
4.4.2. Arbuscular Mycorrhizal Fungi (AMF)-Advanced Mechanisms
- -
- Change osmoregulation: Many studies have confirmed that AMF affects the accumulation of osmolytes, such as proline, soluble sugars, and amino acids, which protect plant cells from damage by dehydration.
- -
- Antioxidation: They activate the antioxidant system of plants (increased activity of enzymes such as superoxide dismutase, catalase, peroxidase), reducing the production of reactive oxygen species (ROS) under stress.
- -
4.4.3. Endophytes—Hidden Heroes of Immunity
- -
- Improve salinity tolerance: They can reduce sodium absorption by plants, increase tolerance to osmotic stress, and also modify the expression of genes related to ion transport and defense responses.
- -
- -
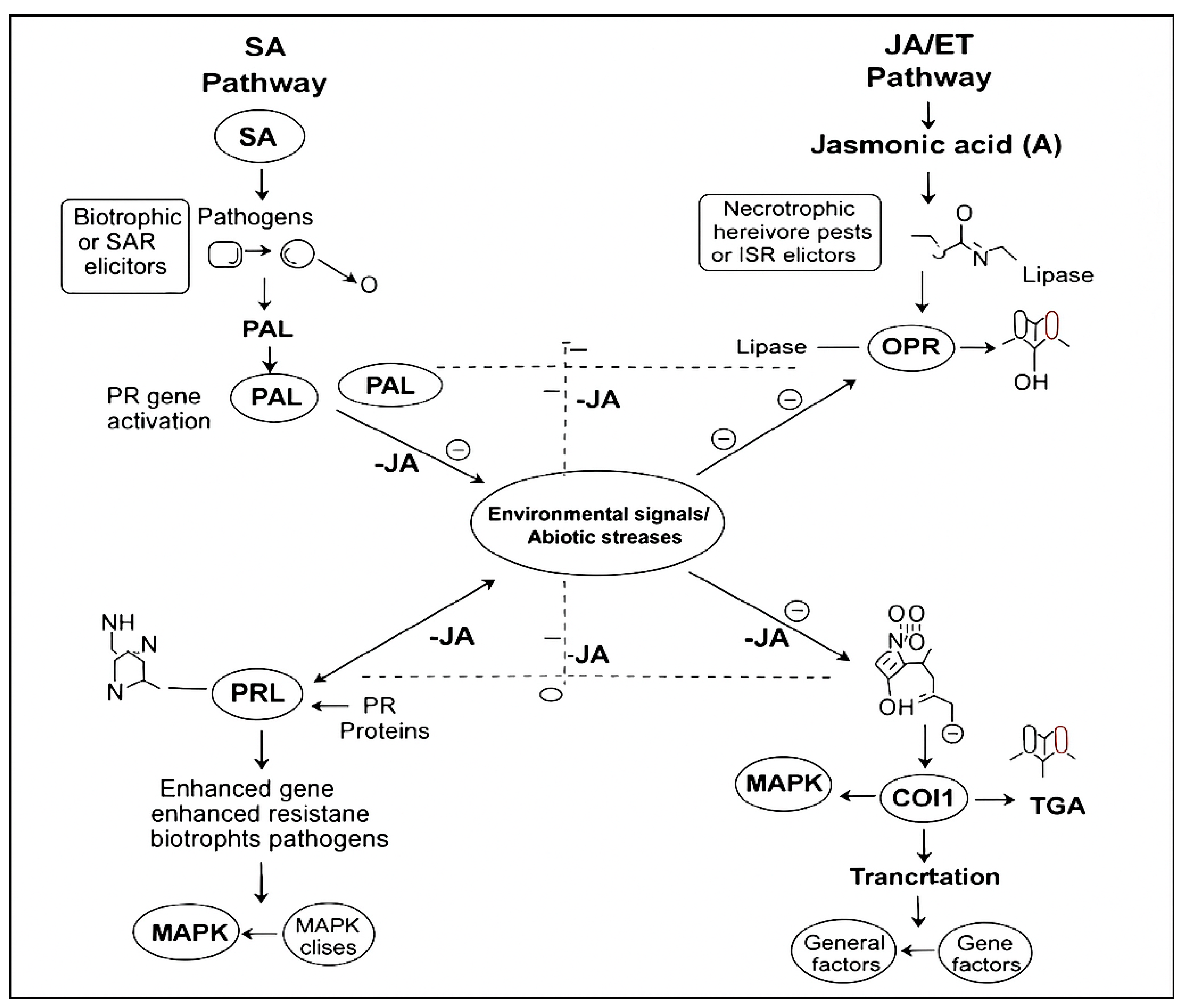
- Induce systemic immunity: By activating signaling pathways such as salicylic acid (SA) and jasmonic acid (JA), similar to PGPR, but acting systemically from within the plant [12].
4.4.4. Latest Achievements and Research Directions
4.4.5. Summary and Outlook
4.5. Challenges, Knowledge Gaps, and Future Research Directions
- ▪
- Soil type: Different soils have different physicochemical properties and native microbial communities, which affect the adaptation and functioning of introduced microorganisms.
- ▪
- Climate: Temperature, humidity, and rainfall dramatically affect microbial activity and survival.
- ▪
- Plant species and variety: Each plant interacts uniquely with the soil microbiome, meaning that solutions often need to be tailored to specific crops.
- ▪
4.6. Regulatory Issues and Farmer Acceptance
5. Conclusions
- -
- Microbial preparations are revolutionizing agricultural efficiency and environmental management, generating specific benefits confirmed by research, such as:
- -
- The use of Nod inoculants accelerates germination and increases the number of root nodules, which translates into a reduced need for synthetic nitrogen-containing fertilizers (up to 90%) and increased biomass production.
- -
- Mycorrhizal preparations have demonstrated the ability to increase nutrient (P, N) uptake by at least 20% and improve water uptake, resulting in reduced yield losses (up to 10%) in drought conditions.
- -
- Natural Plant Protection: Fungal biopreparations and other microbiological solutions effectively protect plants from pathogens. Bioinsecticides can achieve pest control efficacy of ≥70% population reduction, while simultaneously reducing yield losses by at least 10%.
- -
- Bioherbicides contribute to reduced chemical residues in the product (target: ≥90%) and reduced toxicity to non-target organisms (target: ≤5% mortality/damage). Bioherbicides can reduce weed mass by up to 80% and increase crop yields by ≥5%.
- -
- Abiotic Stress Resistance: Soil and plant microflora play a key role in increasing plant resistance to drought and salinity. Microbiological preparations can reduce stress symptoms (e.g., salinity) by at least 15%, improving plant tolerance.
- -
- Soil Health Regeneration: The use of effective microorganisms (EMs) can increase soil organic matter content (by at least 0.1% per year) and nutrient availability (P, K, N) by at least 10%. They also improve microbial balance and soil structure, increasing water retention.
- -
- Circular Economy: Microorganisms are essential for transforming organic waste into valuable resources (compost, biogas). Microorganisms in wastewater nitrification/denitrification processes can achieve total nitrogen (TN) removal efficiency of ≥90%, reducing treatment costs by at least 10% and shortening hydraulic retention time by at least 15%.
- -
- Precision Agriculture and Microbiome Engineering: Omics technologies (e.g., metagenomics, metatranscriptomics) enable a detailed understanding of the microbiome, enabling the design of precise, tailored biopreparations and the modification of microorganisms to enhance their effectiveness.
- -
- Risk of Resistance: Like synthetic alternatives, microbial technologies can lead to the development of resistance in weeds, diseases, and pests. This requires continuous monitoring and product rotation.
- -
- Release of GMOs into the Environment: The potential release of genetically modified microorganisms (GMOs) raises ethical and regulatory concerns that require a robust legal framework and transparent dialogue.
- -
- Variability and Understanding Limitations: Results are sometimes inconsistent due to complex environmental interactions. For example, the effectiveness of EMs can be variable, with yield impacts ranging from −5% to +20%, depending on specific growing conditions. Continued investment in research and development (R&D) is essential to fully understand the possibilities and limitations, standardize applications, and predict outcomes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | arbuscular mycorrhizal fungi |
| AOA | ammonia-oxidizing archaea |
| AOB | ammonia-oxidizing bacteria |
| COD | chemical oxygen demand |
| DO | dissolved oxygen |
| DS | drought stress |
| EM | effective microorganism |
| EPA | Environmental Protection Agency |
| EPS | extracellular polymeric substance |
| FF | French fries |
| GV | granulosis |
| HDL | high-density lipoprotein fraction |
| HN-AD | heterotrophic nitrification and aerobic denitrification |
| HNADM | heterotrophic nitrification with oxygen denitrifying microorganisms |
| HRAC | Herbicide Resistance Action Committee |
| LDL | low-density lipoprotein fraction |
| LEMs | effective local microorganisms |
| MBBR | moving bed biofilm reactor |
| MBR | membrane bioreactor |
| MRLs | maximum residue levels |
| MoA in Pesticides | mode of action |
| NPV | nuclear polyhedrosis virus |
| NOB | nitrite-oxidizing bacteria |
| Nod | biofertilizers containing rhizobia Nod |
| PBS | polybutylene succinate |
| PC | potato chips |
| PGPR | plant-growth-promoting rhizobacteria |
| PP | pomelo peel |
| PSI | photosystem I |
| PSII | photosystem II bs |
| QS | secretion of quorum sensing |
| RSM | response surface methodology |
| SBBR | sequencing batch biofilm reactor |
| SBR | sequencing batch reactor |
| SHARON | single reactor high activity ammonium removal over nitrite |
| SND | simultaneous heterotrophic nitrification and aerobic denitrification |
| SwE | seaweed extract |
| tEM | thermoacids of effective microorganisms |
| tEMA | thermoacids of effective microorganisms with shading |
| tEMB | thermoacids of effective microorganisms without shading |
| TIN | total inorganic nitrogen |
| TN | total nitrogen |
| TP | total phosphorus |
References
- Kucharski, J.; Jastrzębska, E. The role of effective microorganisms in shaping the microbiological properties of soil. Ecol. Eng. 2005, 12, 295–296. (In Polish) [Google Scholar]
- Shah, K.K.; Tripathi, S.; Tiwari, I.; Shrestha, J.; Modi, B.; Paudel, N.; Das, B.D. Role of soil microbes in sustainable crop production and soil health: A review. Agric. Sci. Technol. 2021, 13, 109–118. [Google Scholar] [CrossRef]
- Sarveswaran, S.; Johar, V.; Singh, V.; Ragunanthan, C. Agroforestry: A Way Forward for Sustainable Development. Ecol. Environ. Conserv. 2023, 29, S300–S309. [Google Scholar] [CrossRef]
- Li, X.; Guo, Q.; Wang, Y.; Xu, J.; Wei, Q.; Chen, L.; Liao, L. Enhancing Nitrogen and Phosphorus Removal by Applying Effective Microorganisms to Constructed Wetlands. Water 2020, 12, 2443. [Google Scholar] [CrossRef]
- Coskun, M.; Coskun, M.; Cayir, A. Frequencies of micronuclei (MNi), nucleoplasm bridges (NPBs), and nuclear buds (NBUDs) in farmers exposed to pesticides in Çanakkale, Turkey. Environ. Int. 2011, 1, 93–96. [Google Scholar] [CrossRef]
- Wong, R.H.; Chang, S.Y.; Ho, S.W. Polymorphisms in metabolic GSTP1 and DNA-repair XRCC1 genes with an increased risk of DNA damage in pesticide-exposed fruit growers. Mutat. Res. 2008, 654, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, B.; Vambol, V.; Krochmal-Marczak, B.; Messaoudi, M.; Skiba, D.; Pszczółkowski, P.; Barbaś, P.; Farhan, A.K. Green Technology as a Way of Cleaning the Environment from Petroleum Substances in South-Eastern Poland. Front. Biosci. (Elite Ed.) 2022, 14, 28. [Google Scholar] [CrossRef]
- Wang, C. Green Technology Innovation, Energy Consumption Structure and Sustainable Improvement of Enterprise Performance. Sustainability 2022, 14, 10168. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 Implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards the List of Approved Active Substances Text with EEA Relevance. OJ L 153, 11.6.2011, pp. 1–186 (BG, ES, CS, DA, DE, ET, EL, EN, FR, IT, LV, LT, HU, MT, NL, PL, PT, RO, SK, SL, FI, SV). Available online: http://data.europa.eu/eli/reg_impl/2011/540/oj (accessed on 20 July 2025).
- European Food Safety Authority. Foreword. EFSA J. 2012, 10, sf101. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/sf101 (accessed on 17 December 2021).
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance gibberellins. EFSA J. 2012, 10, 2502–2551. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance Bacillus thuringiensis ssp. tenebrionis strain NB-176. EFSA J. 2013, 11, 3024–3059. [Google Scholar]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance carbon dioxide. EFSA J. 2013, 11, 3053–3153. [Google Scholar] [CrossRef]
- European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance orange oil. EFSA J. 2013, 11, 3090–3144. [Google Scholar] [CrossRef]
- Active Substances, Safeners and Synergists (1465 Matching Records). 2025. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 2 July 2025).
- Ortiz, A.; Sansinenea, E. Chapter 1. Bacillus thuringiensis based biopesticides for integrated crop management. In Advances in Bio-Inoculant Science, Biopesticides; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Singh, A.K., Eds.; Woodhead Publishing: Cambridge, UK, 2022; pp. 1–6. ISBN 9780128233559. [Google Scholar] [CrossRef]
- Kapka-Skrzypczak, L.; Cyranka, M.; Kruszewski, M.; Turski, W.A. Plant protection products and farmers’ health—Biomarkers and the possibility of using them to assess exposure to pesticides. Med. Ogólna I Nauk. O Zdrowiu 2011, 17, 28–32. (In Polish) [Google Scholar]
- Skiba, D.; Sawicka, B.; Pszczółkowski, P.; Barbaś, P.; Krochmal-Marczak, B. Impact of crop management and weed control systems of very early potatoes on weed infestation, biodiversity, and tuber health safety. Life 2021, 11, 826. [Google Scholar] [CrossRef]
- Bolognesi, C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat. Res. 2003, 3, 251–272. [Google Scholar] [CrossRef]
- Barbaś, P.; Sawicka, B. Comparison of the profitability of various methods of weed infestation in edible potato cultivation. Probl. Agric. Eng. 2017, 2, 5–15. (In Polish) [Google Scholar]
- Nowacka, A.; Gnusowski, B.; Dąbrowski, J. Remains of protection measures in agricultural crops. The remains. Prog. Plant Prot. /Postępy W Ochr. Roślin 2006, 47, 79–90. (In Polish) [Google Scholar]
- Martínez-Valenzuela, C.; Gómez-Arroyo, S.; Villalobos-Pietrini, R.; Waliszewski, S.; Calderón-Segura, M.E.; Félix-Gastélum, R.; Álvarez-Torres, A. Genotoxic biomonitoring of agricultural workers exposed to pesticides in the north of Sinaloa State, Mexico. Environ. Int. 2009, 35, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, G.M.; Azevedo, M.B.; Silva, L.B. Cytogenetic biomonitoring of Brazilian workers exposed to pesticides. Micronucleus analysis in buccal epithelial cells of soybean growers. Mutat. Res. 2009, 1–2, 1–4. [Google Scholar] [CrossRef]
- Crestani, M.; Menezes, C.; Glusczak, L.; Miron, D.d.S.; Spanevello, R.; Silveira, A.; Gonçalves, F.F.; Zanella, R.; Loro, V.L. Effects of Clomazone Herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 2007, 67, 2305–2311. [Google Scholar] [CrossRef]
- Santos, M.D.; Crestani, M.; Shettinger, M.R.; Morsch, V.M. Effects of the herbicides clomazone, quinclorac, and metsulfuron methyl on acetylcholinesterase activity in the silver catfish (Rhamadia quelen). Ecotoxicol. Environ. Saf. 2005, 6, 398–403. [Google Scholar] [CrossRef] [PubMed]
- OEPP/EPPO. PP1/213(4) Analiza ryzyka oporności Biuletyn. Bull. OEPP EPPO Bull. 2015, 45, 371–387. [Google Scholar] [CrossRef]
- Alam, A.; Bibi, F.; Deshwal, K.; Sahariya, A.; Bhardwaj, C.; Emmanuel, I. Biofortification of primary crops to eliminate latent hunger: An overview. Nat. Resour. Hum. Health 2022, 2, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, Y.; Qi, X.; Zhang, S.; Zhi, C.; Meng, H.; Cheng, Z. Effects of exogenous Germanium and effective microorganisms on germanium accumulation and nutritional qualities of garlic (Allium sativum L.). Sci. Hortic. 2021, 283, 110114. [Google Scholar] [CrossRef]
- Gupta, K. Profiles of the bio-fertilizer industry on the market. In Biofertilizers: Research and Impact; Inamuddin, Ahamed, M.I., Boddula, R., Rezakazemi, M., Eds.; Wiley-Scrivener: Salem, MA, USA, 2021. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and bioavailability of Zn, Fe and Se in wheat: Present status and future prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef]
- El-Ghwas, D.E.; Elkhateeb, W.A.; Daba, G.M. Fungi: The Next Generation of Biofertilizers. Environ. Sci. Arch. 2022, 2, 34–41. [Google Scholar] [CrossRef]
- Singh, M.; Biswas, S.K.; Nagar, D.; Lal, K.; Singh, J. Effect of biofertilizer growth parameters and potato yield. inside J. Curr. Microbiol. Regret. Sci. 2017, 6, 1717–1724. [Google Scholar]
- De Assis, R.M.A.; Carneiro, J.J.; Medeiros, A.P.R.; de Carvalho, A.A.; da Cunha Honorato, A.; Carneiro, M.A.C.; Bertolucci, S.K.V.; Pinto, J.E.B.P. Arbuscular mycorrhizal fungi and organic manure enhance growth and accumulation of citral, total phenols, and flavonoids in Melissa officinalis L. Ind. Crops Prod. 2020, 158, 112981. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protect photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protect maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2020, 143, 111934. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and Arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [Google Scholar] [CrossRef]
- Paravar, A.; Farahani, S.M.; Rezazadeh, A. Lallemantia response to drought stress and the use of Arbuscular mycorrhizal fungi. Crops Ind. Prod. 2021, 172, 114002. [Google Scholar] [CrossRef]
- Plouznikoff, K.; Asins, M.J.; de Boulois, H.D.; Carbonell, E.A.; Declerck, S. Genetic analysis of tomato root colonization by arbuscular mycorrhizal fungi. Ann. Bot. 2019, 124, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Abdelraouf, E.; Bicego, B.; Joshi, V.; Garcia, A.G. Deficit irrigation: A viable option for sustainable confection sunflower (Helianthus annuus L.) production in the semi-arid US. Irrig. Sci. 2018, 36, 319–328. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhang, Q.; Li, S.; Sun, Y.; Lu, W.; Ma, C. Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Express 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, Z.; Mohsenzadeh, S.; Rowshan, V.; Zarei, M. Mitigation of water deficit stress in Dracocephalum moldavica L. by symbiotic association with soil microorganisms. Sci. Hortic. 2020, 272, 109549. [Google Scholar] [CrossRef]
- Ramos-González, M.I.; Matilla, M.A.; Quesada, J.M.; Ramos, J.L.; Espinosa-Urgel, M. Using genomics to discover bacterial lifestyle determinants in the rhizosphere. In Molecular Microbiological Ecology of the Rhizosphere; de Bruijn, F.J., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; ISBN 9781118296172. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Zhang, B.-X.; Lu, C.-L.; Yang, Z.-Q.; Yuan, L. Novel biofilm-inspired encapsulation technology enhances the viability of probiotics during processing, storage, and delivery. Trends Food Sci. Technol. 2025, 160, 105032. [Google Scholar] [CrossRef]
- Ferrando, L.; Rariz, G.; Martínez-Pereyra, A.; Fernández-Scavino, A. Endophytic diazotrophic communities from rice roots are diverse and weakly associated with soil diazotrophic community composition and soil properties. J. Appl. Microbiol. 2024, 135, 157. [Google Scholar] [CrossRef]
- Kowalska, J.; Niewiadomska, J.; Głuchowska, K.; Kaczmarek, D. Impact of fertilizers and soil properties in the case of Solanum tuberosum L. during conversion to organic farming. Appl. Ecol. Environ. Res. 2017, 15, 369–839. [Google Scholar] [CrossRef]
- Council Directive 91/414/EEC of 15 July 1991 Concerning the Placing of Plant Protection Products on the Market Official Journal L 230, 19/08/1991 pp. 0001. Available online: https://www.fao.org/faolex/results/details/es/c/LEX-FAOC018635/ (accessed on 10 July 2023).
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council on the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC103352 (accessed on 14 December 2022).
- Villaverde, J.J.; Sevilla-Morán, B.; Sandín-España, P.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides in the framework of the European. Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2014, 70, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, Z.; Guo, P.; Wang, R.; Liu, T.; Luo, J.; Hao, B.; Wang, Y.; Guo, W. Synergistic mechanisms of bioorganic fertilizer and AMF driving rhizosphere bacterial community to improve phytoremediation efficiency of multiple HMs-contaminated saline soil. Sci. Total Environ. 2023, 883, 163708. [Google Scholar] [CrossRef]
- Yang, Y. The Application of Genomics in Agriculture. Agric. Sci. Food Process. 2025, 2, 26–46. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Zeng, D.-W.; Zhu, Y.-F.; Zhou, M.-H.; Kondo, A.; Hasunuma, T.; Zhao, X.-Q. Fermentation design and process optimization strategy based on machine learning. BioDesign Res. 2025, 7, 100002. [Google Scholar] [CrossRef]
- Sawicka, B.; Egbuna, C.; Nayak, A.K.; Kala, S. Chapter 2. Plant diseases, pathogens, and diagnosis. PART I. Green approach to pest and disease control. In Natural Remedies for Pest, Disease and Weed Control; Egbuna, C., Sawicka, B., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2020; pp. 1–16. ISBN 978-0-12-819304-4. [Google Scholar] [CrossRef]
- Crandall, L.; Zaman, R.; Duthie-Holt, M.; Jarvis, W.; Erbilgin, N. Navigating the Semiochemicals Landscape: Attraction of Subcortical Beetle Communities to Bark Beetle Pheromones, Fungal and Host Tree Volatiles. Insects 2025, 16, 57. [Google Scholar] [CrossRef]
- Han, X.; Li, S.; Zeng, Q.; Sun, P.; Wu, D.; Wu, J.; Yu, X.; Lai, Z.; Milne, R.J.; Kang, Z.; et al. Genetic engineering, including genome editing, for enhancing broad-spectrum disease resistance in crops. Plant Commun. 2025, 6, 101195. [Google Scholar] [CrossRef]
- Vaitkeviciene, N. The Effect of Biodynamic Preparations on the Accumulation of Biologically Active Compounds in the Tubers of Different Genotypes of Ware Potatoes. Ph.D. Thesis, Agricultural Sciences, Agronomy (01A), ASU, Akademija, Kaunas, Lithuania, 2016; p. 212. [Google Scholar]
- Vaitkeviciene, N.; Jariene, E.; Danilcenko, H.; Sawicka, B. Effect of biodynamic preparations on the content of some mineral elements and starch in tubers of three colored potato cultivars. J. Elem. 2016, 21, 927–935. [Google Scholar]
- Keidan, M. Optimization of Winter Oilseed Rape Technological Parameters in the Organic Farming System. Ph.D. Thesis, Aleksandras Stulginskis University, Kaunas, Lithuania, 2018; p. 221. [Google Scholar]
- Adetunji, C.O.; Oloke, J.K.; Phazang, P.; Sarin, N.B. Influence of eco-friendly phytotoxic metabolites from Lasiodiplodia pseudotheobromae C1136 on physiological, biochemical, and ultrastructural changes on tested weeds. Environ. Sci. Pollut. Res. 2020, 27, 9919–9934. [Google Scholar] [CrossRef]
- Levickienė, D.; Jarienė, E.; Gajewski, M.; Danilčenko, H.; Vaitkevičienė, N.; Przybył, J.L.; Sitarek, M. Influence of harvest time on biologically active compounds and antioxidant activity in mulberry leaves in Lithuania. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 431–436. [Google Scholar] [CrossRef]
- Levickienė, D. The Influence of the Biodynamic Preparations on the Soil Properties and Accumulation of Bioactive Compounds in the Leaves of White Mulberry (Morus alba L.). Ph.D. Thesis, Aleksandras Stulginskis University, Kaunas, Lithuania, 2018; p. 212. [Google Scholar]
- Vlahova, V. Use of BD preparation 500 for organically cultivated pepper (Capsicum annuum L.). New Knowl. J. Sci. 2020, 9, 85–96. [Google Scholar]
- Stanek-Tarkowska, J.; Szostek, M.; Rybak, M. Effect of Different Doses of Ash from Biomass Combustion on the Development of Diatom Assemblages on Podzolic Soil under Oilseed Rape Cultivation. Agronomy 2021, 11, 2422. [Google Scholar] [CrossRef]
- Ngouajio, M.; McGiffen, M.E.; Hembree, K.J. Tolerance of tomato cultivars to velvetleaf interference. Weed Sci. 2009, 49, 91–98. [Google Scholar] [CrossRef]
- Goldwasser, Y.; Lanini, W.; Wrobel, R. Tolerance of tomato varieties to lespedeza dodder. Weed Sci. 2009, 49, 520–523. [Google Scholar] [CrossRef]
- Andrew, I.K.S.; Storkey, J.; Sparke, D.L. A review of the potential for competitive cereal cultivars as a tool in integrated weed management. Weed Res. 2015, 55, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Pan, Z.; Bajsa-Hirschel, J.; Douglas, B.C. The potential future roles of natural compounds and microbial bioherbicides in weed management in crops. Adv. Weed Sci. 2022, 40, e020210054. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejpias, F.J.R.; Molinillo, J.M.G. Recent advances in allopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Farooq, M.; Bajwa, A.A.; Cheema, S.A.; Cheema, Z.A. Application of allelopathy in crop production. Int. J. Agric. Biol. 2013, 15, 1367–1378. [Google Scholar] [CrossRef]
- Portela, V.O.; Moro, A.; Santana, N.A.; Baldoni, D.B.; de Castro, I.A.; Antoniolli, Z.I.; Dalcol, I.I.; Seminoti Jacques, R.J. First report on the production of phytotoxic metabolites by Mycoleptodiscus indicus under optimized conditions of submerged fermentation. Environ. Technol. 2022, 43, 1458–1470. [Google Scholar] [CrossRef]
- Boligłowa, E. Potato protection against diseases and pests using Effective Microorganisms (EM) with herbs. In Selected Ecological Issues in Modern Agriculture; Zbytek, Z., Ed.; PIMR: Poznań, Poland, 2005; pp. 165–170. (In Polish) [Google Scholar]
- Janas, R. Possibilities of using effective microorganisms in ecological systems of crop production. Probl. Agric. Eng. 2009, 3, 111–119. (In Polish) [Google Scholar]
- Ji, B.; Hu, H.; Zhao, Y.; Mu, X.; Liu, K.; Li, C. Effects of deep tillage and straw returning on soil microorganism and enzyme activities. Sci. World J. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, M.; Szmigiel, A.; Ropek, D. Effectiveness of potato protection production using selected insecticides to combat potato beetles (Leptinotarsa decemlineata Say). Acta Sci. Pol. Ser. Agric. 2009, 8, 5–14. [Google Scholar]
- Kumar, A.; Singh, V.K.; Singh, P.; Mishr, K. Purification of Environmental Pollutants via Microbes, 1st ed.; Vipin, A.K., Singh, K., Singh, P., Mishra, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; p. 474. ISBN 978012821199. [Google Scholar]
- Daniel, C.; Wyssa, E. Field applications of Beauveria bassiana to control the Rhizolites cerasi fruit fly. J. Appl. Entomol. 2010, 134, 675–681. [Google Scholar] [CrossRef]
- Rudeen, M.L.; Jaronski, S.T.; Petzold-Maxwell, J.L.; Gassmann, A.J. Entomopathogenic fungi in cornfields and their potential to fight the larvae of western corn rootworm Diabrotica virgifera. J. Invertebr. Pathol. 2013, 114, 329–332. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Tangtrakulwanich, K.; Wau, S.; Miller, J.H.; Ophus, V.L.; Prewett, J.; Jaroski, S.T. Evaluation of the effectiveness of enteropathy in the control of wireworms (Coleoptera: Elateridae) on spring wheat. J. Invertebr. Pathol. 2014, 120, 43–49. [Google Scholar] [CrossRef]
- Trawczyński, C. The influence of irrigation and Effective Microorganisms on quantity and chemical composition of the yields of plants cultivation in organic crop rotation on light soil. J. Res. Appl. Agric. Eng. 2012, 57, 148–154. [Google Scholar]
- Laznik, Ž.; Tóth, T.; Lakatos, T.; Vidrih, M.; Tran, S. Inspection of Colorado potato beetle (L. decemlineata Say) on potato under field conditions: Comparison of the effectiveness of the two strains Steinernema feltier (Filipe) and spraying with thiamethoxam. J. Plant Dis. Prot. 2010, 117, 129–135. [Google Scholar] [CrossRef]
- Lepiarczyk, A.; Kulig, B.; Stępnik, K. The effect of simplified soil cultivation and forecrops on LAI development of selected winter wheat varieties for crop rotation. Fragm. Agron. 2005, 2, 98–105. [Google Scholar]
- Xu, H.L. Effects of a microbial inoculant and organic fertilizer on the growth, photosynthesis, and yield of sweet corn. J. Crop Prod. 2000, 3, 183–214. [Google Scholar] [CrossRef]
- Emitazi, G.; Nader, A.; Etemadifar, Z. Effect of nitrogen fixing bacteria on growth of potato tubers. Adv. Food Sci. 2004, 26, 56–58. [Google Scholar]
- Kalitkiewicz, A.; Kępińska, E. The use of Rhizobacteria to stimulate plant growth. Biotechnology 2008, 2, 102–114. [Google Scholar]
- Górski, R.; Kleiber, T. Effect of Effective Microorganisms (EM) on nutrient contents in substrate and development and yielding of rose (Rosa x hybrida) and gerbera (Gerbera jamesonii). Ecol. Chem. Eng. S 2010, 17, 505–513. [Google Scholar]
- Kaczmarek, Z.; Owczarzak, W.; Mrugalska, L.; Grzelak, M. Effect of effective microorganisms on selected physical and water properties of arable-humus levels of mineral soils. J. Res. Appl. Agric. Eng. 2007, 52, 73–77. (In Polish) [Google Scholar]
- Kaczmarek, Z.; Jakubas, M.; Grzelak, M.; Mrugalska, L. Impact of the addition of various doses of Effective Microorganisms to arable-humus horizons of mineral soils on their physical and water properties. J. Res. Appl. Agric. Eng. 2008, 53, 118–121. [Google Scholar]
- Kaczmarek, Z.; Wolna-Murawska, A.; Jakubas, M. Change in the number of selected groups of soil microorganisms and enzymatic activity in soil inoculated with effective microorganisms (EM). J. Res. Appl. Agric. Eng. 2008, 53, 122–128. [Google Scholar]
- Szembowski, B. Experiences of a Farm in Trankwice with the EM-FarmingTM Biotechnology. In Natural Probiotic Microorganisms; Associate Ecosystem: Lichen, Poland, 2009; pp. 56–58. (In Polish) [Google Scholar]
- Kosicka, D.; Wolna-Murawka, A.; Trzeciak, M. Influence of microbiological preparations on soil and plant growth and development. Kosmos 2015, 64, 327–335. (In Polish) [Google Scholar]
- Kołodziejczyk, M. Effectiveness of nitrogen fertilization and application of microbial preparations in potato cultivation. Turk. J. Agric. For. 2014, 38, 299–310. [Google Scholar] [CrossRef]
- Kołodziejczyk, M. Effect of nitrogen fertilization and microbial populations on potato yielding. Plant Soil Environ. 2014, 60, 379–386. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. The impact of the application of biopreparations and fungicides on the yield and selected parameters of the seed value of seed potatoes. Acta Agroph. 2018, 25, 239–255. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. The impact of the use of fungicides, microbiological preparations, and herbal extracts on the shaping of the potato yield. Fragm. Agron. 2018, 35, 81–93. [Google Scholar]
- Pszczółkowski, P.; Krochmal-Marczak, B.; Sawicka, B.; Pszczółkowski, M. Effect of the use of effective microorganisms on the color of raw potato tuber flesh for food processing. Appl. Sci. 2021, 11, 8959. [Google Scholar] [CrossRef]
- Sawicka, B.; Pszczółkowski, P.; Kiełtyka-Dadasiewicz, A.; Ćwintal, M.; Krochmal-Marczak, B. Effect of effective microorganisms on the quality of potatoes in food processing. Appl. Sci. 2021, 11, 1415. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Awino, R.; Njeru, E.M.; Maingi, J.M. Potential Use of Beneficial Microorganisms for Soil Amelioration, Phytopathogen Biocontrol, and Sustainable Crop Production in Smallholder Agroecosystems. Front. Sustain. Food Syst. 2021, 5, 130. [Google Scholar] [CrossRef]
- Galarreta, J.I.R.; Ezpelata, B.; Pascualena, J.; Ritter, E. Combining ability in early generations of potato breeding. Plant Breed. 2006, 125, 183–186. [Google Scholar] [CrossRef]
- Kolasa-Wiącek, A. Will Effective Microorganisms Revolutionize the World? Post. Technol. Convert Food 2010, 1, 66–69. (In Polish) [Google Scholar]
- Kowalska, J.; Sosnowska, D.; Remlein-Starosta, D.; Drożdżyński, D.; Wojciechowska, R.; Łopatka, W. Effective Microorganisms in Organic Farming; IOR-PIB: Poznań, Poland, 2021; Available online: https://www.ior.poznan.pl/plik,3661,sprawozdanie-mikroorganizmy-w-rol-eko-2011-pdf.pdf (accessed on 20 May 2025).
- Barbaś, P.; Skiba, D.; Pszczółkowski, P.; Sawicka, B. Natural Resistance of Plants. Subject: Biochemistry & Molecular Biology. E Scholary Community Encyclopedia. 2022. Available online: https://encyclopedia.pub/entry/34721 (accessed on 16 November 2022).
- Martyniuk, S. Effective and ineffective microbiological preparations used in the protection and cultivation of plants as well as reliable and unreliable methods of their evaluation. Post. Microbiol. 2011, 50, 321–328. [Google Scholar]
- Okorski, M.; Majchrzak, B. Fungi colonizing pea seeds after applying the EM 1 microbiological preparation. Prog. Plant Prot./Postępy W Ochr. Roślin 2008, 48, 1314–1318. [Google Scholar]
- Higa, T. Effective microorganisms, concepts, and the latest technological achievements. Materials from the Effective Microorganisms Conference for Sustainable Agriculture and Environment. In Proceedings of the 4th Kyusei International Wildlife Agriculture Conference, Bellingham, WA, USA, 19–21 June 1998; pp. 247–248. [Google Scholar]
- Dach, J.; Wolna-Maruwka, B.; Zbytek, Z. Influence of effective microorganisms’ addition (EM) on composting process and gaseous emission intensity. J. Res. Appl. Agric. Eng. 2009, 54, 49–55. [Google Scholar]
- Higa, T. Effective microorganisms—Technology of the 21st century. In Proceedings of the “Effective Microorganisms in the World”, London, UK, 23 July 2005; pp. 20–24. [Google Scholar]
- Zarzecka, K.; Gugała, M. Effect of UGmax soil fertilizer on the potato yield and its structure. Bull. IHAR 2013, 267, 107–112. (In Polish) [Google Scholar] [CrossRef]
- Baranowska, A.; Zarzecka, K.; Gugała, M.; Mystkowska, I. The effect of fertilizer on UGmax soil on the presence of Streptomyces scabies on edible potato tubers. J. Ecol. Eng. 2018, 3, 68–73. [Google Scholar] [CrossRef]
- Van Vliet, P.C.J.; Bloem, J.; de Goede, R.G.M. Microbial diversity, nitrogen loss and grass production after the addition of Effective Microorganisms® (EM) to slurry manure. Appl. Soil Ecol. 2006, 32, 188–198. [Google Scholar] [CrossRef]
- Martyniuk, S. Production of microbiological preparations on the example of symbiotic bacteria in legumes. J. Res. Appl. Agric. Eng. 2010, 55, 20–23. (In Polish) [Google Scholar]
- Martyniuk, S.; Księżak, J. Evaluation of pseudo-microbial biopreparations used in plant production. Pol. Agron. J. 2011, 6, 27–33. [Google Scholar]
- Paśmionka, I.; Kotarba, K. Possibilities of using effective microorganisms in environmental protection. Cosm. Probl. Biol. Sci. 2015, 64, 173–184. [Google Scholar]
- Ding, L.-N.; Li, Y.-T.; Wu, Y.-Z.; Li, T.; Geng, R.; Cao, J.; Zhang, W.; Tan, X.-L. Plant Disease Resistance-Related Signaling Pathways: Recent Progress and Future Prospects. Int. J. Mol. Sci. 2022, 23, 16200. [Google Scholar] [CrossRef]
- Okumoto, S.; Shintani, M.; Higa, T. The use of effective microorganisms and biochar inhibits the transfer of radioactive cesium from soil to plants during continuous Komatsu cultivation. In Proceedings of the International Scientific Conference “Radiobiology: Present”, Institute of Radiobiology of the NAS of Belarus, Gomel, Belarus, 26–27 September 2019; p. 22. [Google Scholar]
- Nigussie, A.; Dume, B.; Ahmed, M.; Mamuye, M.; Ambaw, G.; Berhiun, G.; Biresaw, A.; Aticho, A. Effect of microbial inoculation on nutrient turnover and lignocellulose degradation during composting: A meta-analysis. Waste Manag. 2021, 125, 220–234. [Google Scholar] [CrossRef]
- Allahverdiyev, S.R.; Kırdar, E.; Gunduz, G.; Kadimaliyev, D.; Revin, V.; Filonenko, V.; Rasulova, D.A.; Abbasova, Z.I.; Gani-Zade, S.I.; Zeynalova, E.M. Effective Microorganisms (EM). Technology in Plants. Technology 2011, 14, 103–106. [Google Scholar]
- Pszczółkowski, P.; Sawicka, B.; Danilcenko, H.; Jariene, E. The role of microbiological preparations in improving the quality of potato tubers. In Proceedings of the International Scientific Conference: ‘New trends in food safety and quality’, Aleksandras Stulginskis University, Akademija, Lithuania, 5–7 October 2017; pp. 22–23. [Google Scholar]
- Barbaś, P.; Aslan, H.; Aslan, I.; Skiba, D.; Otekunrin, O.A.; Sawicka, B. Prospects for using pesticides in agriculture. Agron. Sci.–Former. Ann. UMCS Sect. E Agric. 2023, 58, 97–120. [Google Scholar] [CrossRef]
- Sawicka, B. Rate of spread of fungal diseases on potato plants as affected by application of a biostimulator and foliar fertilizer. In Biostimulators in Modern Agriculture. Solanaceous Crops; Dąbrowski, Z., Ed.; Wieś Jutra, Limited: Warsaw, Poland, 2008; pp. 68–76. ISBN 83-89503-55-7. [Google Scholar]
- Boligłowa, E.; Gleń, K. Assessment of effective microorganism activity (EM) in winter wheat protection against fungal diseases. Ecol. Chem. Eng. A 2018, 15, 23–27. [Google Scholar]
- Sawicka, B.; Pszczółkowski, P.; Noaema, A.H.; Krochmal-Marczak, B.; Kiełtyka-Dadasiewicz, A. Effective microorganisms in agriculture and food processing. In Contemporary Research on the State of the Environment/Contemporary Research on the State of the Environment and the Medicinal Use of Plants; Cheil, M., Skoczylas, M.M., Eds.; University of Life Sciences in Lublin: Lublin, Poland, 2019; ISBN 978-83-7259-310-8. Available online: https://up.lublin.pl/wp-content/uploads/2021/02/Wsp%C3%B3%C5%82czesne-badania-nad-stanem-%C5%9Brodowiska-i-leczniczym-wykorzystaniem-ro%C5%9Blin.pdf (accessed on 17 July 2025). (In Polish)
- Tommonaro, G.; Abbamondi, G.R.; Mikołaja, B.; Poli, A.; D’Angelo, C.; Iodice, C.; De Prisco, R. Productivity and Nutritional Trait Improvements with Various Tomatoes Grown Effective Microorganisms Technology. Agriculture 2021, 11, 112. [Google Scholar] [CrossRef]
- Quiroz, M.; Céspedes, C. Bokashi as a fix and nitrogen source for sustainable farming systems: An overview. J. Soil Sci. Plant Nutr. 2019, 19, 237–248. [Google Scholar] [CrossRef]
- Marczakiewicz, J. Another year with EM Biotechnology at RZD SGGW Chylice. Natural Probiotic Microorganisms; Ecosystem Association Publishing House: Licheń, Poland, 2009; p. 65. (In Polish) [Google Scholar]
- Mathews, S.; Gowrilekshmi, R. Solid Waste Management with Effective Microbial (EM) Technology. J. Curr. Microbiol. Appl. Sci. 2016, 5, 804–815. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, H.; Zhang, J.; Shuguang, C.; Yangqing, C.; Lei, W.Y.; Zhang, R.; Song, L. Simultaneous heterotrophic nitrification and aerobic denitrification (SND) for nitrogen removal: Review and future prospects. Environ. Adv. 2022, 9, 100254. [Google Scholar] [CrossRef]
- Al-Taweil, H.I.; Bin Osman, M.; Hamid, A.A.; Yusoff, W.M. Development of microbial inoculants and the impact of soil application on rice seedlings growth. Am. J. Agric. Biol. Sci. 2009, 4, 79–82. [Google Scholar] [CrossRef]
- Szewczuk, C.; Sugier, D.; Baran, S.; Bielińska, E.J.; Gruszczyk, M. The impact of fertilizing agents and different doses of fertilizers on selected soil chemical properties as well as the yield and quality traits of potato tubers. Ann. Univ. Mariae Curie-Skłodowska Sect. E Agric. 2016, LXXI, 65–79. [Google Scholar][Green Version]
- Solarska, E. Development of ecological hop production technology. In Proceedings of the 1st Lublin Scientific and Technical Conference—Microorganisms in Environmental Revitalization—Science and Practice, Lublin, Poland, 23–24 March 2010; pp. 56–64. (In Polish). [Google Scholar]
- Henry, A.B.; Maung, C.E.H.; Kim, K.Y. Metagenomic analysis reveals enhanced biodiversity and composting efficiency of lignocellulosic waste by thermoacidophile effective microorganism (tEM). J. Environ. Manag. 2020, 276, 111252. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S. Impact of inoculation with local effective microorganisms on soil nitrogen cycling and legume productivity using composted broiler litter. Appl. Soil Ecol. 2020, 154, 103567. [Google Scholar] [CrossRef]
- Faturrahman, L.; Meryandini, A.; Junior, M.Z.; Rusmana, I. The Role of Agarolytic Bacteria in Enhancing Physiological Function for Digestive System of Abalone (Haliotis asinine). J. Appl. Environ. Biol. Sci. 2015, 5, 49–56. [Google Scholar]
- Gacka, S.; Kolbusz, S. Biotechnology EM-FarmingTM—Comprehensive, natural, solution in animal production ensuring animal welfare. In Natural Probiotic Microorganisms; Ekosystem Association Publishing House: Licheń, Poland, 2009; pp. 102–103. (In Polish) [Google Scholar]
- Tsatsakis, A.M.; Nawaz, M.A.; Kouretas, D.; Balias, G.; Savolainen, K.; Tutelyan, V.A.; Golokhvast, K.S.; Lee, J.D.; Chung, J.G. Envirtmental impacts of Genetically Modified Plants: A Review. Environ. Res. 2017, 156, 818–833. [Google Scholar] [CrossRef]
- Pniewska, I. Wpływ Efektywnych Mikroorganizmów (EM) i Dokarmiania Pozakorzeniowego Nawozami Typu Alkalin na Plonowanie i Cechy Jakościowe Fasoli Szparagowej. Ph.D. Thesis, UPH, Siedlce, Poland, 2015. (In Polish). [Google Scholar]
- Fatunbi, O.; Ncube, L. Activities of Effective Microorganism (EM) on the Nutrient Dynamics of Different Organic Materials Applied to Soil. Am. Eurasian J. Agron. 2009, 2, 26–35. [Google Scholar]
- Safwat, M.; Safwat Rozaik, E. Growth Inhibition of Various Pathogenic Microorganisms Using Effective Microorganisms (EM). Int. J. Res. Eng. 2017, 4, 283–286. [Google Scholar] [CrossRef]
- Gopalasamy, R. The role of microorganisms in sustainable development Envtl. Int. J. Sci. Res. 2019, 6, 413. [Google Scholar]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 80, 149319. [Google Scholar] [CrossRef] [PubMed]
- Correia, T.S.; Lara, T.S.; Santos, J.A.d.; Sousa, L.D.S.; Santana, M.D.F. Arbuscular Mycorrhizal Fungi Promote Physiological and Biochemical Advantages in Handroanthus serratifolius Seedlings. Plants 2022, 11, 2731. [Google Scholar] [CrossRef]
- Martin, F.M.; van der Heijden, M.A.G. The mycorrhizal symbiosis: Research frontiers in genomics, ecology, and agricultural application. New Phytol. 2024, 242, 1486–1506. [Google Scholar] [CrossRef]
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen cycling during wastewater treatment. Adv. Appl. Microbiol. 2019, 106, 113–192. [Google Scholar]
- Xi, H.; Zhou, X.; Arslan, M.; Luo, Z.; Wei, J.; Wu, Z.; El-Din, M.G. Heterotrophic nitrification and aerobic denitrification process: Promising but a long way to go in the wastewater treatment. Sci. Total Environ. 2022, 805, 150212. [Google Scholar] [CrossRef]
- Türkmen, F.U.; Önalan, F.E.S. The impact of climate change on the sustainability of food security. In Climate Change and Future of Agriculture; Springer: Berlin/Heidelberg, Germany, 2024; pp. 169–189. [Google Scholar]
- Lei, Y.; Wang, Y.; Liu, H.; Xi, C.; Song, L. A novel heterotrophic nitrifying and aerobic denitrifying bacterium, Jobelihle taiwanensis DN-7, can remove high-strength ammonium. Appl. Microbiol. Biotechnol. 2016, 100, 4219–4229. [Google Scholar] [CrossRef]
- Su, J.F.; Shi, J.X.; Huang, T.L.; Ma, F. Kinetic analysis of simultaneous denitrification and biomineralization of novel Acinetobacter sp. CN86. Mar. Pollut. Bull. 2016, 109, 87–94. [Google Scholar] [CrossRef]
- Su, J.F.; Shi, J.X.; Ma, F. Aerobic denitrification and biomineralization by a novel heterotrophic bacterium, Acinetobacter sp. H36. Mar. Pollut. Bull. 2017, 116, 209–215. [Google Scholar] [CrossRef]
- Jetten, M.S. The microbial nitrogen cycle. Environ. Microbiol. 2008, 10, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Q.; Ma, T.; Wang, M.; Ni, J. Genomic insights into metabolic potentials of two simultaneous aerobic denitrification and phosphorus removal bacteria, Achromobacter sp. GAD3 and Agrobacterium sp. LAD9. FEMS Microbiol. Ecol. 2018, 94, 223. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, B.; An, Q.; Wang, X.; Zhang, Y.X. Kinetic characteristics and modelling of growth and substrate removal by Alcaligenes faecalis strain NR. Bioprocess. Biosyst. Eng. 2016, 39, 593–601. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Fan, W.; Wang, J. Heterotrophic nitrification and aerobic denitrification by a novel Acinetobacter sp. ND7 isolated from municipal activated sludg. Bioresour. Technol. 2020, 301, 122749. [Google Scholar] [CrossRef]
- Msambwa, M.M.; Daniel, K.; Lianyu, C. Integration of information and communication technology in secondary education for better learning: A systematic literature review. Soc. Sci. Humanit. Open 2024, 10, 101203. [Google Scholar] [CrossRef]
- Padhi, S.K.; Maiti, N.K. Molecular insight into the dynamic central metabolic pathways of Achromobacter xylosoxidans CF-S36 during heterotrophic nitrogen removal processes. J. Biosci. Bioeng. 2017, 123, 46–55. [Google Scholar] [CrossRef]
- Medhi, K.; Singhal, A.; Chauhan, D.K.; Thakur, I.S. Investigating the nitrification and denitrification kinetics under aerobic and anaerobic conditions by Paracoccus denitrificans ISTOD1. Bioresour. Technol. 2017, 242, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Pan, L.L.; Lev, N.; Tang, X. Characterization of novel Bacillus strain N31 from mariculture water capable of halophilic heterotrophic nitrification-aerobic denitrification. J. Biosci. Bioeng. 2017, 124, 564–571. [Google Scholar] [CrossRef]
- He, T.; Xie, D.; Li, Z.; Ni, J.; Sun, Q. Ammonium stimulates nitrate reduction during simultaneous nitrification and denitrification process by Arthrobacter arilaitensis Y-10. Bioresour. Technol. 2017, 239, 66–73. [Google Scholar] [CrossRef]
- Zhao, B.; He, Y.L.; Hughes, J.; Zhang, X.F. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR. Bioresour. Technol. 2010, 101, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Y.X.; Liang, X.; Zhao, S.Q.; Wang, J.P.; Xia, Z.H. Nitrogen removal characteristics of a heterotrophic nitrifier Acinetobacter junii YB and its potential application for the treatment of high-strength nitrogenous wastewater. Bioresour. Technol. 2015, 193, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Ye, Y.; He, Q.; Zhang, S. Isolation and characterization of Pseudoxanthomonas sp. Strain YP1 capable of denitrifying phosphorus removal (DPR). Geomicrobiol. J. 2018, 35, 537–543. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, X.; Li, W.; Qin, W.; Wang, P. Characteristics of heterotrophic nitrifying bacterium strain SFA13 isolated from the Songhua River. Ann. Microbiol. 2015, 66, 271–278. [Google Scholar] [CrossRef]
- Xu, X.; Yang, K.; Dou, Y. High-end equipment development task decomposition and scheme selection method. J. Syst. Eng. Electron. 2021, 32, 118–135. [Google Scholar] [CrossRef]
- Luyckx, M.; Reins, L. The Future of Farming: The (Non)-Sense of Big Data Predictive Tools for Sustainable EU Agriculture. Sustainability 2022, 14, 12968. [Google Scholar] [CrossRef]
- Bremmer, J.; Riemens, M.; Reinders, M. The Future of Crop Protection in Europe. Panel for the Future of Science and Technology. European Parliamentary Research Service Scientific Foresight Unit (STOA) PE 656.330—February 2021 EN. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2021/656330/EPRS_STU(2021)656330_EN.pdf (accessed on 5 November 2021).
- Mann, R.S.; Kaufman, P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012, 9, 185–202. [Google Scholar] [CrossRef]


| Microbe/References | Weed Target(s) and Status | Trade Name | Year of Introduction or Registration/State |
|---|---|---|---|
| Albifimbria verrucaria (formerly Myrothecium verrucaria) | Conyza canadensis (glyphosate-resistant)—Experimental | - | 2023 |
| Alternaria cassia Bannon, 1988 | Cassia obtusifolia C. coccidentalis Crotalaria spectabilis—Never commercialized | Casst™ | Never |
| Alternaria destruens Bewick et al., 1984 | Cusucta spp.—Discontinued | Smolder™ | 2005 |
| Ascophyllum nodosum (seaweed) | Various weeds—Available | BioWeed USA | USA |
| Chondrostereum purpureum Hintz 2007 | Populus and Alnus spp.—Unknown | Chontrol™ | 2004 |
| Citronella oil (22.9%) | Jacobaea vulgaris | Barrier H | 2015 (EU, Japan, USA) |
| Colletotrichum acutatum Morris 1989 | Hakea sericea—Discontinued | Hakatak | 1990 |
| Colletotrichum echinochloae | Echinochloa crus-galli—Experimental | - | 2023 |
| Colletotrichum gloeosporioides f. sp. aeschynomene Cartwright et al., 2010 | Aeshynomene vigrinica—Available on demand | Collego® | 1982 |
| Colletotrichum gloeosporioides f. sp. aeschynomene | Aeschynomene virginica—Available | LockDownTM | 2006 (USA) |
| Colletotrichum gloeosporioides f. sp. Malvae Boyetchko et al. 2007 | Acacia mearnsii and A. pycnantha—Discontinued | Stumpout™ | 1997 |
| Cylindrobasidium laeve. Morris et al., 1999 | Acacia mearnsii and A. pycnantha—Discontinued | Stumpout™ | 1997 |
| Fusarium oxysporum f. sp. strigae | Striga hermonthica—Under development | Kichawi Kill™ | 2023 (Kenya) |
| Lasiodiplodia pseudotheobromae, Macrophomina phaseolina, Parkinsonia aculeata—Available | Neoscytalidium novaehollandiae (Di-Bak® Parkinsonia) | Di Bak® | 2019 (Australia) |
| Pelargonic acid (natural fatty acid) | Grassy and broadleaf weeds | Katana® | 2016 (USA) |
| Phoma macrostoma | Broadleaf weeds—Available | Bio-Phoma™ | 2016 (Canada) |
| Phoma macrostoma Bailey et al., 2011 | Many broadleaves weed species—Available | Bio-Phoma™ | 2016 |
| Phytophthora palmivora Ridings 1986 | Morrenia odorata—Discontinued | DeVine® | 1982 |
| Pine oil + sugar formula | Herbaceous and grassy weeds—Available | Bioweed™ | Australia/West Asia |
| Pseudomonas fluorescens Kennedy et al., 2001 | Bromus tectorum—Discontinued | D7® | 2014 |
| Puccinia canaliculata Phatak et al., 1983 | Cyperus esculentus—Discontinued | Dr. Biosedge™ | 1987 |
| Puccinia thlaspeos Knopp et al., 2002 | Isatis tinctorial—Discontinued | Woad Warrior® | 2002 |
| Sclerotinia minor Watson 2018 | Taraxacum officinale—Discontinued | Sarritor® | 2009 |
| Several fungi Gale and Goutler 2013 | Parkinsonia aculeate—Available | Di-Bak® | 2019 |
| Solanum habrochaites—plant extract | Various weeds | Available WeedLock | 2017 (Malasya) |
| Streptomyces hygroscopicus (Bialaphos) | Broad-spectrum post-emergence herbicide | Bialaphos® | 2016 (East Asia) |
| Streptomyces scabies O’Sullivan et al. 2015 | Several grass and broadleaf weeds—Never commercialized | Opportune™ | 2012 |
| Tobacco mild green mosaic vírus Charudattan and Hiebert 2007 | Solanum viarum—Available | SolviNix™ | 2014 |
| Trichoderma koningiopsis and others | Euphorbia heterophylla, Bidens pilosa, Conyza bonariensis—Experimental | - | 2023 |
| Xanthomonas campestris pv. poae Imaizaumi et al., 1999 | Poa annua—Discontinued | Camperico™ | 1997 |
| Advantages | Disadvantages |
|---|---|
| Increasing food production: Some microorganisms are beneficial to plants and help in their growth and production. They can also help produce food by fermenting and processing food. | Uncontrolled growth of microorganisms: In some cases, microorganisms can grow uncontrollably and become harmful to plants, soil, and human health. |
| Plant protection: Some microorganisms are able to fight plant diseases and pests, reducing the use of harmful pesticides and other chemicals. | Environmental pollution: Some microorganisms, such as E. coli bacteria, can cause soil and water pollution, which is a public health risk. |
| Improving soil quality: Microorganisms can help enrich the soil with nutrients and improve its structure, which positively affects the health of plants. | GMO risks: Some microbial technologies, such as genetic engineering, can lead to genetically modified organisms (GMOs), which raise social and ethical concerns. |
| Sustainable agriculture: The use of microorganisms can aid sustainable agriculture by reducing the use of harmful chemicals and improving soil quality. | Costs: Some systems and technologies using microorganisms can be expensive, which is a barrier to their widespread use. |
| Origin of Species | Specification of Species | References |
|---|---|---|
| Activated sludge | Acinetobacter sp. ND7 | [153] |
| Ochrobactrum anthropic LJ81 | [147] | |
| Alcaligenes faecalis NR | [152] | |
| Achromobacter sp. GAD3 | [151] | |
| Agrobacterium sp. LAD9 | [151] | |
| Acinetobacter sp. SZ28 | [127] | |
| Acinetobacter sp. WB-1 | [127] | |
| Ochrobactrum sp. KSS10 | [28] | |
| Pseudomonas stutzeri CFY1 | [152] | |
| Thauera sp. FDN-01 | [154] | |
| Diaphorobacter sp. PD-7 | [127] | |
| Artificial lake | Acinetobacter sp. H36 | [148] |
| Acinetobacter sp. CN86 | [148] | |
| Domestic wastewater | Achromobacter xylosoxidans CF-S36 | [155] |
| Paracoccus denitrificans ISTOD1 | [156] | |
| Klebsiella pneumoniae CF-S9 | [155] | |
| Drinking water reservoir | Zoogloea sp. N299 | [157] |
| Flooded paddy soil | Arthrobacter arilaitensis Y-10 | [158] |
| Pseudomonas tolaasii strain Y-11 | [158] | |
| Laboratory-scale MBR | Acinetobacter calcoaceticus HNR | [159] |
| Bacillus methylotrophicus L7 | [127] | |
| Serratia sp. LJ-1 | [129] | |
| Laboratory-scale SBR | Acinetobacter junii YB | [160] |
| Pseudoxanthomonas sp. YP1 | [161] | |
| Landfill leachate | Zobellella taiwanensis DN- | [147] |
| Seabed sludge | Paracoccus versutus LYM | [162] |
| Songhua River | Microbacterium esteraromaticum SFA13 | [163] |
| Wastewater system | Acinetobacter sp. YS2 | [144] |
| Cupriavidus sp. S1 | [161] | |
| Pseudomonas sp. yy7 | [152] | |
| Rhodococcus sp. CPZ24 | [152] |
| Source | Target Weeds | Ecosystem | Registered Name | References |
|---|---|---|---|---|
| Alternaria cassiae | Cassia obtusifolia L. | Soy | Recipe development— “CASST” | [54] |
| Alternaria destruens | Cuscuta spp. | Cranberry field | Assessment—Smolder | [11] |
| C. purpura | P. Serotina | Forest | Commercialized—Biochon TM | [61] |
| C. purpura | Populus euramericana | Guinier forest | Commercialized—Chontrol® | [57] |
| Cephalospprium diospyri | Diospyras virginiana L. | Pastures, pastures | Oklahoma | [53] |
| Chondrostereum purpureum (Fr.) | Pouz Prunus serotina Ehrh. | Forest, mountains | Commercialized—Mycotech™ | [54] |
| Citrus lime (L.) Osbeck | D. Sanguinalis | Cultivated areas | Commercial herbicide—Avenger® | [51] |
| Citrus sinensis (L.) Osbeck | Solanum nigrum L. | Crop land, roadside | Commercialized—Green Match™ | [62] |
| Colletotrichum gloeosporioides | Hakea sericea Schrad. & J.C. Wendl. | Mountain meadows | Commercialized—Hakak | [55] |
| Colletotrichum gloeosporioides | Malvae, Malva Pusilla Sm. | Flax, lentils, horticultural crops | Commercialized—BioMal® [56] | [56] |
| Colletotrichum gloeosporioidesaeschynomene | Aeschynomene virginica L. | Rice, soybeans | Commercialized—Colle™ | [54] |
| Cylindrobasidium | leave Acacia spp. | Forest, pasture | Commercialized—Stump-Out™ | [61] |
| Cymbopogon citratus (DC.) | Stapf. spp. | Agricultural land | Commercialized—Green Match™ EX | [65] |
| Phoma macrostoma | Reynoutria japonica Houtt. | Golf courses, agriculture, and agroforestry | Commercialized—Phoma | [59] |
| Phytophthora palmivora | Morrenia odorata (Hook. & Arn.) Lindl. | Citrus groves | Commercialized—Devine™ | [54] |
| Puccinia thlaspeos C. Shub. | Isatis tinctoria L. | Forest, pastures | -Beloukha® [62] | [64] |
| S. aromaticum | E. crus-galli | Farmland, rice | Commercialized—Weed Slayer® | [59] |
| Sclerotinia minor Jagger. | Taraxacum sp. | Turf | Commercialized—Sarritor® [60] | [61] |
| Streptomyces acidi scabies | Taraxacum officinale L. | Turf | Commercialized—Opportune® | [59] |
| Syzygium aromaticum (L.) Merr. & LM Perry & Presl. Cinnamomum verum J. | E. crus-galli | Rice, farmland | Commercialized—WeedZap® | [53] |
| Xanthomonas campestris | Poa annua L. | Turf, athletic fields | Commercialized—Camperico | [55] |
| Microbial Technology | Impact on Product Safety (Measurable Criteria) | Impact on Production/Process Efficiency (Measurable Criteria) |
|---|---|---|
| Bioherbicides | Increases: -Reduction in chemical residues in product: Target: ≥90% reduction compared to synthetic herbicides. -Toxicity to non-target organisms: Target: ≤5% mortality/damage compared to control. | Potentially increases: -Weed control efficacy: Target: ≥80% reduction in weed biomass. -Crop yield: Target: ≥5% yield increase. -Challenges: Resistance development (monitoring), commercialization metrics (market share). |
| Bioinsecticides | Increases: -Absence/reduction of chemical residues in product: Target: Complete absence or >95% reduction. -Pest specificity: Target: ≤5% impact on beneficial insects. -Safety for humans/animals: Target: WHO Toxicity Class IV (lowest hazard). | Increases: -Pest control efficacy: Target: ≥70% reduction in pest population. -Crop loss reduction: Target: Reduction of losses by ≥10%. -Challenges: Resistance development (monitoring), reliance on environmental conditions (e.g., optimal temp.). |
| Effective Microorganisms (EMs) | Increases: -Reduction in chemical fertilizer use: Target: ≥15% reduction. -Food quality indicators: Target: ≥5% increase in vitamin/nutrient content (e.g., Vit. C, protein). | Increases (with variable results): -Increase in soil organic matter content: Target: Increase by ≥0.1% annually. -Nutrient availability (P, K, N): Target: ≥10% increase in soil analyses. -Yield increase: Target: Variable (ranging from −5% to +20% depending on crop and conditions). -Controversial issues: Repeatability of results under different conditions. |
| Mycorrhizal Preparations | Increases: -Absence of chemical residues in product: Target: Complete absence. -Natural symbiosis: Target: Confirmed root colonization (e.g., ≥50% of roots colonized). | Increases: -Nutrient uptake (P, N): Target: ≥20% increase. -Water uptake: Target: Increased drought tolerance (e.g., ≤10% yield reduction under water deficit). -Stress resistance: Target: ≥15% reduction in stress symptoms (e.g., salinity). -Long-lasting effects: Effect maintained for ≥2 seasons. |
| Microorganisms in Nitrification/Denitrification (Wastewater) | Enhances environmental safety: -Reduction in total nitrogen (TN) concentration in wastewater: Target: ≥90% TN removal efficiency. -Compliance with environmental standards: Target: TN levels fall below permissible limits. | Increases process efficiency: -Nitrogen (N) removal efficacy: Target: ≥95% N removal efficiency. -Treatment cost: Target: Reduction in energy/chemical costs by ≥10%. -Hydraulic retention time (HRT): Target: Shortening of HRT by ≥15%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicka, B.; Barbaś, P.; Vambol, V.; Skiba, D.; Pszczółkowski, P.; Niazi, P.; Bienia, B. Applied Microbiology for Sustainable Agricultural Development. Appl. Microbiol. 2025, 5, 78. https://doi.org/10.3390/applmicrobiol5030078
Sawicka B, Barbaś P, Vambol V, Skiba D, Pszczółkowski P, Niazi P, Bienia B. Applied Microbiology for Sustainable Agricultural Development. Applied Microbiology. 2025; 5(3):78. https://doi.org/10.3390/applmicrobiol5030078
Chicago/Turabian StyleSawicka, Barbara, Piotr Barbaś, Viola Vambol, Dominika Skiba, Piotr Pszczółkowski, Parwiz Niazi, and Bernadetta Bienia. 2025. "Applied Microbiology for Sustainable Agricultural Development" Applied Microbiology 5, no. 3: 78. https://doi.org/10.3390/applmicrobiol5030078
APA StyleSawicka, B., Barbaś, P., Vambol, V., Skiba, D., Pszczółkowski, P., Niazi, P., & Bienia, B. (2025). Applied Microbiology for Sustainable Agricultural Development. Applied Microbiology, 5(3), 78. https://doi.org/10.3390/applmicrobiol5030078










