Abstract
This study aimed to evaluate the production of xylose reductase (XR), an enzyme responsible for converting xylose into xylitol, by Candida tropicalis ATCC 750 using hemicellulosic hydrolysate from cashew apple bagasse (CABHM) as a low-cost carbon source. The effects of temperature, aeration, and fluid dynamics on XR biosynthesis were also investigated. The highest XR production (1.53 U mL−1) was achieved at 30 °C, with 8.3 g·L−1 of xylitol produced by the yeast under microaerobic conditions, demonstrating that aeration and fluid dynamics are important factors in this process. Cellular metabolism and enzyme production decreased at temperatures above 35 °C. The maximum enzymatic activity was observed at pH 7.0 and 50 °C. XR is a heterodimeric protein with a molecular mass of approximately 30 kDa. These results indicate that CABHM is a promising substrate for XR production by C. tropicalis, contributing to the development of enzymatic bioprocesses for xylitol production from lignocellulosic biomass. This study also demonstrates the potential of agro-industrial residues as sustainable feedstocks in biorefineries, aligning with the principles of a circular bioeconomy.
1. Introduction
Xylitol is a polyol obtained from D-xylose with a wide range of application in the food, pharmaceutical, beverage, and nutraceuticals industries [1,2,3,4]. Xylitol is a product with an annual market value of approximately USD 340 million. The global xylitol market in personal care and cosmetics is expected to reach USD 48.8 million by 2025, according to a new report by Grand View Research, Inc. San Francisco CA, USA (2021) [5]. Currently, xylitol is produced on a large scale through chemical processes; however, due to high production costs [6], non-conventional routes, such as biotechnological processes, have been investigated [4,7,8,9]. Studies report that several microorganisms, including filamentous fungi [10], bacteria [4,11], and yeast [3,8,12,13], are capable of metabolizing xylose into xylitol, with yeasts being considered the most promising. However, xylitol production via fermentation processes is limited by low conversion rates and productivity [14]. Therefore, one interesting alternative is the enzymatic production of xylitol, which may allow for higher yields [4,15,16].
The enzyme that catalyzes this reaction is xylose reductase (EC 1.1.1.21), an intracellular enzyme commonly found in yeast and fungi. This intracellular enzyme is localized in the cytoplasm of xylose-assimilating microorganisms, where it catalyzes the first step of xylose metabolism by reducing xylose to xylitol with the concomitant oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) in its reduced form [3,17]. Analyses of xylose reductase (XR) genes from different sources have shown a predominant specificity for NADPH [8,16,18], but some XR produced by Candida intermedia were capable of utilizing both NADH (nicotinamide adenine dinucleotide) and NADPH [19].
For the biotechnological production of the xylose reductase enzyme, the yeast Candida tropicalis was selected due to its ability to synthesize XR, which is involved in the process of converting xylose into xylitol [8,15,16,18,19].
The high cost of commercial xylose limits large-scale XR production and its industrial application in the manufacture of xylitol and other value-added bioproducts [14]. Then, it is necessary to research new/alternative sources of xylose for XR production and characterization using potential xylose-fermenting yeast. In this context, lignocellulosic agro-industrial residues represent a low-cost and abundant alternative feedstock. One such material is cashew apple bagasse [7,20].
Cashew apple bagasse (CAB) is composed of cellulose, hemicellulose, and lignin [21]. Its hemicellulosic fraction can be hydrolyzed using dilute acid to obtain a hemicellulosic hydrolysate with high xylose concentration [7,13,20]. Efficient xylose utilization is essential in bioprocesses aimed at converting lignocellulosic material into valuable bioproducts in the biorefinery concepts. However, hydrolysates-derived lignocellulosic biomass is composed mainly of glucose and xylose, and the presence of glucose can influence the metabolic expression of the xylose reductase and, consequently, the production of xylitol. Another factor that may influence XR production is the aeration and the mechanism of oxygen action in the culture medium containing xylose and glucose, yet this effect remains poorly understood.
In this context, the present study aimed to evaluate an ecofriendly strategy to produce xylose reductase by Candida tropicalis ATCC750, through biotechnological processes using hemicellulosic hydrolysate from cashew apple bagasse. The influence of temperature on the kinetics of fermentation was assessed, along with the effects of aeration and fluid dynamics, and the produced XR enzyme was characterized.
The production of value-added biochemicals such as xylose reductase from low-cost agro-industrial residues represents a sustainable alternative for both waste valorization and industrial bioprocess development, with potential to improve xylitol production efficiency and reduce environmental impact.
2. Results and Discussion
2.1. Cashew Apple Bagasse and Cashew Apple Bagasse Hydrolysate Composition
Dilute-acid hydrolysis was effective in releasing glucose, xylose, and arabinose from the cellulose and hemicellulose present in the CAB, providing these carbohydrates for microbial assimilation and xylitol production. Furthermore, inhibitory compounds were also generated during dilute-acid hydrolysis. Cashew apple bagasse hydrolysate (CABH) contained 6.8 g L−1 of cellobiose, 28.6 g L−1 of glucose, 18.3 g L−1 of xylose, 11.7 g L−1 of arabinose, 0.9 g L−1 of formic acid, and 1.6 g L−1 of acetic acid (see Table 1). The total concentration of fermentable sugars obtained by dilute-acid hydrolysis, including glucose, xylose, and arabinose, was approximately 58.6 g L−1.

Table 1.
Composition of cashew apple bagasse hydrolysate.
After detoxification and treatment with activated carbon, the composition of CABHM was 3.7 g L−1 of cellobiose, 22.7 g L−1 of glucose, 15.2 g L−1 of xylose, 11.0 g L−1 of arabinose, 0.26 g L−1 of formic acid, and 1.2 g L−1 of acetic acid (Table 1). After the pH adjustment with Ca(OH)2, the total amount of carbohydrates was reduced (from 58.6 to 53 g L−1) and there was no significant variation in the carbohydrates’ concentration (48.9 g L−1) after treatment with activated carbon. The concentration of xylose decreased by 2.0 g L−1, and this low loss of carbohydrates during the treatment step is important to establish a viable process to detoxify the culture medium.
Furfural and 5-HMF were not detected in the CABH. Regarding acetic acid and formic acid, only a slight reduction in their concentrations was observed after treatments with Ca(OH)2 and activated carbon. The most suitable detoxification process to improve hemicellulosic hydrolysate fermentation depends on the origin of the raw material. The ideal method should eliminate the greatest number of toxic compounds while minimizing loss of fermentable sugars. However, to ensure efficient fermentation, once the process parameters are evaluated, it must be defined if a total or partial reduction in the inhibitory compounds is necessary to avoid a negative impact on the yeast’s cellular metabolism.
2.2. The Production of Xylitol and Xylose Reductase Enzyme by Candida tropicalis
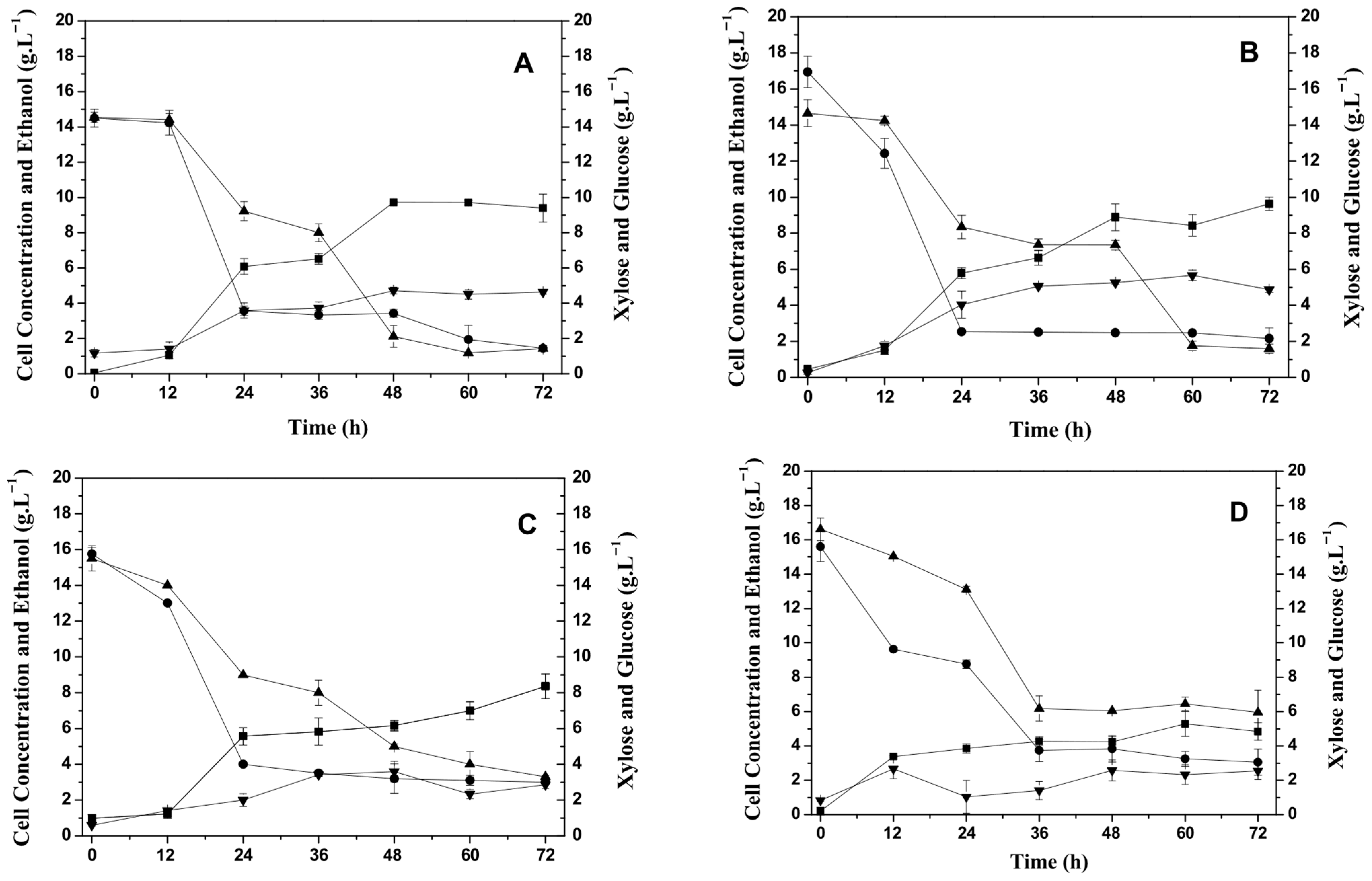
Initially, the influence of temperature on the production of the xylose reductase enzyme (XR) and xylitol by C. tropicalis ATCC750 yeast was evaluated using cashew apple bagasse hemicellulosic hydrolysate medium (CABHM) and the profile of glucose and xylose consumption, cell growth, and ethanol production are presented in Figure 1A–D.
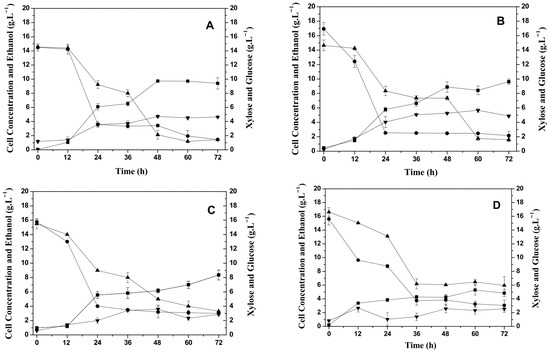
Figure 1.
Effect of temperature on cell growth, carbohydrate consumption, and ethanol production by Candida tropicalis ATCC750 cultivated at 150 rpm in the hemicellulosic hydrolysate from cashew apple bagasse (CABHM). (A) 25 °C, (B) 30 °C, (C) 35 °C, and (D) 40 °C. (■) Biomass (g L−1); (▲) Xylose (g L−1); (▼) Ethanol (g L−1); and (●) Glucose (g L−1).
Cell growth was slightly affected by increasing the temperature from 25 °C to 30 °C, but it declined considerably at temperatures above 35 °C. The greatest cell growth was at temperatures of 25 °C (9.4 g L−1) and 30 °C (9.6 g L−1), while the lowest growth occurred at 40 °C, obtaining 4.8 g L−1 of biomass. Cell growth, based on the initial xylose concentration, was higher compared to the study by Arruda et al. [22], who evaluated the production of XR and XDH enzymes by Candida guilliermondii in a medium composed of sugarcane bagasse hydrolysate (with 75 g L−1 of xylose) and noted a maximum production of biomass 10.6 g L−1 in 120 h of fermentation at 30 °C.
C. tropicalis ATCC750 consumed glucose and xylose, but the yeast preferentially metabolized glucose. The yeast did not produce xylitol when cultivated in the CABHM. These results can possibly be explained by the presence of glucose and inhibitory substances, i.e., acetic acid and formic acid, that decrease the metabolism of xylose. However, it produced ethanol at all temperatures, and the highest ethanol concentration was achieved at 30 °C (5.7 g L−1)—see Figure 1. Then, the glucose consumed influenced the metabolic pathway favoring the production of ethanol.
The ethanol yield at each temperature was calculated based on both the consumption of glucose alone and the combined consumption of glucose and xylose. When considering only glucose, the ethanol yields were 0.30, 0.35, 0.16, and 0.21 gethanol·gglucose−1 at 25, 30, 35, and 40 °C, respectively. However, when calculated based on the total consumption of glucose and xylose, the yields decreased to 0.15, 0.19, 0.08, and 0.09 gethanol·gglucose+xylose−1, respectively. These lower values are likely due to the incomplete assimilation of xylose by Candida tropicalis under the tested conditions, as the yeast preferentially consumes glucose, and xylose metabolism may be repressed in its presence (catabolite repression). Moreover, no xylitol production was observed in these assays, despite the detected activity of xylose reductase. This suggests that the presence of glucose and potential inhibitors in the hydrolysate may have redirected the carbon flux toward ethanol production while limiting the reductive conversion of xylose to xylitol.
Also, the operational conditions may have influenced cellular metabolism. The process was carried out under natural aeration with an agitation of 150 rpm, and this condition may have favored the synthesis of the NAD-linked xylitol dehydrogenase enzyme that converts xylitol to xylulose, which then becomes integrated into the main metabolic pathways (i.e., glycolysis and the pentose phosphate pathways) [23], resulting in ethanol as one of the main products of metabolism. Also, the conditions did not favor a high consumption of xylose (Figure 1) due to repression by the presence of glucose [24].
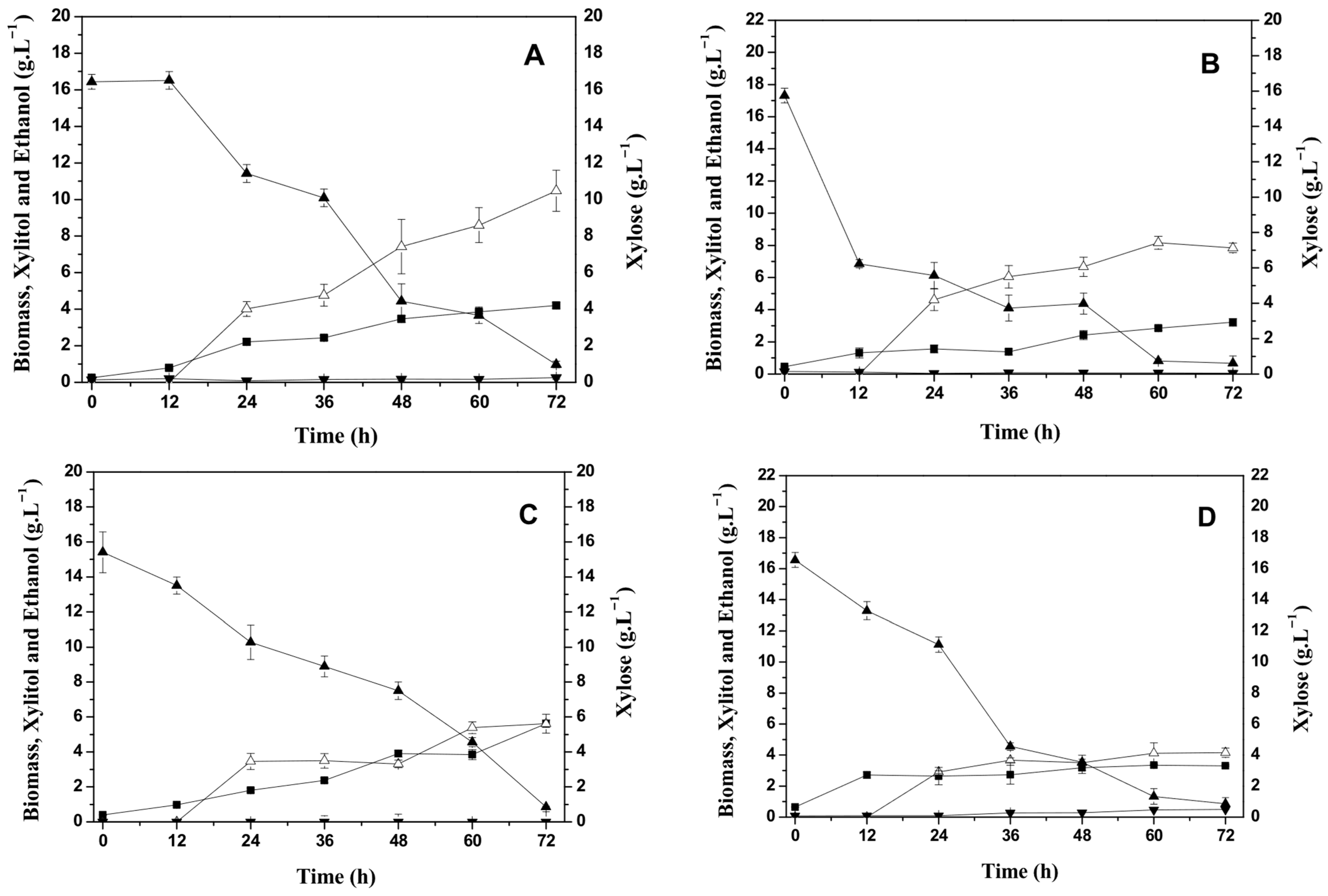
To better understand the inhibitory role of glucose on xylitol production observed in CABHM, additional bioprocesses were carried out using a formulated medium (FM) with a similar composition to CABHM but containing only xylose as the carbon source. This strategy allowed us to isolate the effect of glucose and assess whether its presence represses xylose metabolism and xylitol biosynthesis. The experimental results of the production of xylitol by C. tropicalis ATCC750 using the formulated medium (FM) are shown in Figure 2A–D.
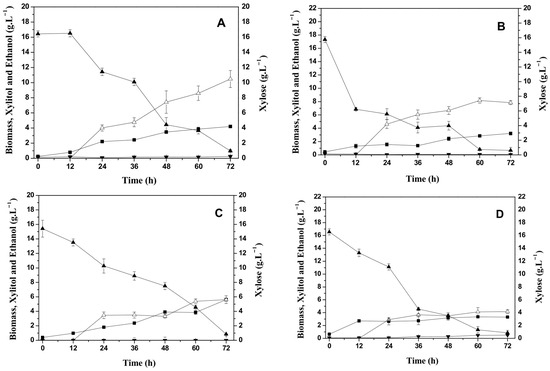
Figure 2.
Effect of temperature on cell growth, carbohydrate consumption, and xylitol and ethanol production by Candida tropicalis ATCC750 cultivated at 150 rpm in the formulated medium (FM). (A) 25 °C, (B) 30 °C, (C) 35 °C, and (D) 40 °C. (■) Biomass (g L−1); (▲) Xylose (g L−1); (▼) Ethanol (g L−1); and (∆) Xylitol (g L−1).
The C. tropicalis ATCC750 yeast metabolized xylose at all temperatures evaluated, obtaining final concentrations below 1 g L−1 with 72 h of bioprocess. The greatest cell growth was observed at 35 °C (5.62 g L−1), with a slight increase in culture time from 64 h to 72 h. The microbial growth decreased with increasing temperature, resulting in 3.3 g. L−1 of cells at 40 °C. Similar cell growth behavior was observed using the CABHM.
The yeast produced xylitol, obtaining 10 g L−1 of xylitol at 25 °C, corresponding to a yield of 0.68 gxylitol·gxylose−1 and productivity of 0.15 gL−1 h−1, as shown in Table 2. However, in the bioprocess conducted at 40 °C, a xylitol concentration of 4.2 g L−1 was also obtained, corresponding to a yield of 0.26 gxylitol·gxylose−1 and productivity of 0.06 g L−1 h−1. This could be justified by the hypothesis that under high temperature conditions, the production of xylitol is favored, since a greater proportion of xylose is converted into xylitol under limited cell growth [24].

Table 2.
Influence of temperature on cell growth and xylitol production by Candida tropicalis ATCC 750 using the formulated medium (FM). Data were obtained after 72 h of cultivation at 150 rpm under each temperature condition.
Although the microorganism does not synthesize xylitol when cultivated in CABHM, the production of the XR enzyme was observed through the determination of the enzymatic activity of the crude extract (Table 3) in both media.

Table 3.
Effect of temperature on xylose reductase production by Candida tropicalis ATCC 750 cultivated in hemicellulosic hydrolysate from cashew apple bagasse medium (CABHM) or in formulated medium (FM). Data were obtained after 72 h of cultivation at 150 rpm under each temperature condition. Means followed by the same letter do not differ significantly according to Tukey’s test (p < 0.05).
The influence of temperature on xylose reductase production was statistically evaluated through analysis of variance (ANOVA) for each culture medium. In the formulated medium (FM), temperature significantly affected all evaluated parameters: enzymatic activity expressed as U mL−1 of extract (F = 22.076; p = 0.000317), U g−1 of cells (F = 23.544; p = 0.000253), and U mg−1 of protein (F = 6.109; p = 0.018246). A similar effect was observed in the cashew apple bagasse hydrolysate medium (CABHM), with significant differences also found for enzymatic activity in U mL−1 of extract (F = 100.935; p = 0.000001) and in U g−1 of cells (F = 100.937; p = 0.000001). However, the temperature did not significantly affect the enzymatic activity expressed in U mg−1 of protein (F = 0.851; p = 0.504), likely due to the low expression or limited extraction of the enzyme under these conditions, which resulted in minimal variation in specific activity across temperatures. These results confirm the significant role of temperature in modulating enzyme productivity under both nutrient conditions. After, Tukey’s test (p < 0.05) was applied, and statistically homogeneous groups are indicated by identical letters in Table 3.
In bioprocessing using CABHM, the highest XR activity was obtained at 25 °C (0.265 U mL−1), corresponding to the enzymatic activity per gram of cell of 0.530 U g−1 and a specific activity of 0.071 U mg−1. This temperature coincides with one of the temperatures that favored cell growth (25 °C and 30 °C).
Although cell concentrations are similar at temperatures of 25 °C and 30 °C, there was a decrease in activity of XR enzyme of 30% at 30 °C (0.181 U mL−1) compared to that at 25 °C (0.265 U mL−1) when the yeast was cultivated in the CABHM, possibly due to a metabolic shift toward the production of ethanol. Overall, the XR enzymatic activity decreased as the temperature increased (for 35 °C and 40 °C), correlating with the reduced cell growth observed at these temperatures.
The evaluated yeast synthesized xylose reductase enzyme and it produced xylitol when cultivated in FM, obtaining a crude enzymatic extract with an activity of 0.365 U mL−1 (Table 3). The XR enzyme activity obtained in the CABHM was lower than the XR activity obtained in the FM at all temperatures evaluated, with a difference of 27% in the activity obtained in the production conducted at 25 °C. However, the specific activities obtained in the process conducted at 25 °C were similar, indicating that the enzymatic extract obtained in the process using FM had a higher concentration of proteins. Also, the CABHM is more viable than the FM, due to the cost of xylose used in the preparation of the culture medium.
The specific XR activity from C. tropicalis ATCC750 produced at 25 °C is similar or superior to the results reported in the literature. Cortez et al. [25] studied the production of XR by Candida guilliermondii using the sugarcane bagasse hemicellulosic hydrolysate, and the specific activity obtained was 0.38 U mg−1. Kim et al. [26] evaluated the production of XR enzyme by Kluyveromyces marxianus ATCC36907 in synthetic medium, and the author reported an activity of 0.37 U mg−1. Rafiqul and Sakinah [27] evaluated in their study the use of the Meranti wood sawdust hydrolysate in the production of XR by C. tropicalis, and the highest specific activity obtained was 0.91 U mg−1.
The highest values of enzymatic activity per grams of cell (0.730 U g−1) and specific activity (0.06 U mg−1) were obtained at 25 °C using the FM to produce the XR enzyme, and this temperature provided the highest cell growth. Ethanol production was not observed, indicating that yeast used the pentose pathway to produce xylitol (see Figure 2), since glucose was not added in this medium. Then, there was an induction of metabolism in the production of the enzyme xylose reductase and xylitol in the formulated medium, in which xylose was the only substrate added to the medium.
The results indicate that the microorganism evaluated, C. tropicalis ATCC750, may be promising in the production of xylose reductase. Yablochkova, Bolotnikova, and Mikhailova [17] studied the activity of XR and XDH in different species of yeasts (including the genera Candida, Kluyveromyces, Pichia, Torulopsis, and Pachysolen) and observed that the strain C. tropicallis Y-456 had the highest specific XR activity. In view of what the literature reports, microorganisms with high XR and NADPH-dependent activity are potentially producers of xylitol from D-xylose [3,8].
In order to increase the production of the enzyme xylose reductase using the CABHM, experiments with different aeration conditions (obtained by varying the volume of the reaction medium and the flask) were carried out—see Section 3.3.
2.3. Study of the Production of Xylose Reductase Enzyme in Different Aeration and Dynamic Fluid Conditions
The yeast Candida tropicalis is known for its ability to produce both xylitol and ethanol in comparable amounts. The predominant metabolic route, toward polyol or ethanol production, is mainly determined by the types of carbohydrates available in the culture medium and the aeration conditions [23]. This helps explain the results observed in Section 2.3. As shown in Table 4, variations in aeration levels had a significant impact on the activity of xylose reductase (XR) during cultivation using the CABHM.

Table 4.
Influence of aeration and fluid dynamics on enzyme production of xylose reductase by Candida tropicalis ATCC750 using the hemicellulosic hydrolysate from cashew apple bagasse medium (CABHM). Data reported with 24 h of processing at 25 °C and 150 rpm.
XR activity was highest under microaerobic conditions (Table 4, Experiment 03), reaching 1.530 ± 0.182 U mL−1. Under these conditions, ethanol concentration remained below 2 g L−1. Notably, this new set of operating conditions also enabled xylitol production from the CABHM, yielding 8.3 g L−1. The XR activity obtained in this study is higher than that reported in previous works [8,28,29,30].
In the pentose phosphate pathway, xylitol can be oxidized to xylulose by the NAD+-dependent xylitol dehydrogenase (XDH) enzyme, which then enters the ethanol production pathway. However, under oxygen-limited (microaerobic/anaerobic) conditions, xylulose formation is hindered, resulting in xylitol accumulation and its subsequent excretion into the extracellular medium [8]. According to Zhang et al. [28], a major limiting factor is the redox imbalance caused by the coenzyme preferences of XR (NADPH) and XDH (NAD+). Thus, Experiment 03, characterized by a low oxygen transfer rate, promoted both higher XR activity and xylitol production.
The results can also be attributed to fluid hydrodynamics, which determine mass transfer across the liquid film on the flask walls and base, as well as within the bulk rotating liquid. The interfacial area (a) and the mass transfer coefficient (kL) varied among the experiments. These parameters are critical for evaluating gas–liquid mass transfer, hydromechanical stress, and effective shear rate in bioprocess development using shake flasks [29].
The largest liquid–air contact area was observed in Experiment 03 (approximately 150 cm2); however, in this experiment, the liquid height was also greater (11 cm), which affected the fluid movement. In Experiment 02, the contact area and liquid height were approximately 90 cm2 and 2.7 cm, respectively. These conditions provided better aeration of the culture medium. The smallest contact area was recorded in Experiment 01, at 42 cm2, with a liquid height similar to that of Experiment 02 (h = 2.4 cm). A similar xylose reductase (XR) enzyme production was observed in both assays (Experiments 01 and 02, see Table 4).
Based on these results, it is evident that aeration and fluid dynamics are critical parameters for efficient xylose reductase production by Candida tropicalis ATCC 750. Therefore, the selected operating conditions for XR enzyme production using CABHM were 2000 mL Erlenmeyer flasks containing 1000 mL of reaction medium, incubated at 30 °C and 150 rpm.
To estimate the product yields at a larger scale, a mass balance was performed considering the use of 1 kg of cashew apple bagasse as the initial raw material. According to the experimental conditions, 200 g of dry bagasse yielded approximately 750 mL of detoxified hemicellulosic hydrolysate (CABHM). Therefore, scaling proportionally, 1 kg of bagasse generated 3.75 L of hydrolysate after dilute acid treatment, separation, and detoxification processes. This hydrolysate contained approximately 85.13 g of glucose and 57.00 g of xylose. When this entire volume was used under the bioprocess conditions described in Experiment 3 (aerobic system at 30 °C for 24 h using 1000 mL of reaction medium in 2000 mL Erlenmeyer flasks), the bioprocess using C. tropicalis ATCC 750 resulted in the production of 31.13 g of xylitol, 6.38 g of ethanol, and 5.74 units of xylose reductase activity. These results underscore the biotechnological potential of using cashew apple bagasse as a carbon source for simultaneous enzyme production and the generation of value-added bioproducts.
Compared to conventional synthetic media, the use of cashew apple bagasse hydrolysate demonstrated comparable enzymatic productivity, while promoting waste reduction and adding value to a by-product often discarded by the juice industry.
The main advantage of the present study lies in the use of cashew apple bagasse hydrolysate (CABHM) as a renewable and low-cost carbon source for xylose reductase production, eliminating the need for synthetic media or expensive inducers commonly used in previous studies. While Lugani and Sooch [4], Dasgupta et al. [8], and Zhang et al. [18] reported high enzymatic yields, their systems typically relied on enriched media or controlled induction strategies. In contrast, our process employed a non-detoxified lignocellulosic hydrolysate and a non-genetically modified strain (Candida tropicalis ATCC 750), simplifying implementation and reducing production costs. Notably, other authors, such as Zhang et al. [28], engineered Kluyveromyces marxianus to improve xylose reductase expression, whereas our results were obtained using a wild-type strain under natural fermentation conditions. Additionally, the evaluation of fluid dynamics and aeration effects adds originality to our approach, as these operational parameters are seldom explored in enzyme-focused bioprocess studies. Limitations of this work include the moderate enzyme yields compared to genetically enhanced strains and the reduced xylitol productivity under CABHM conditions, likely due to the presence of inhibitors (as noted by Yablochkova et al. [17]). Nevertheless, the integrated and low-input strategy demonstrated here aligns with biorefinery and circular economy principles, contributing to the valorization of agricultural waste streams through dual production of biocatalysts and biofuels.
2.4. Characterization of Xylose Reductase Enzyme Produced Using CABHM
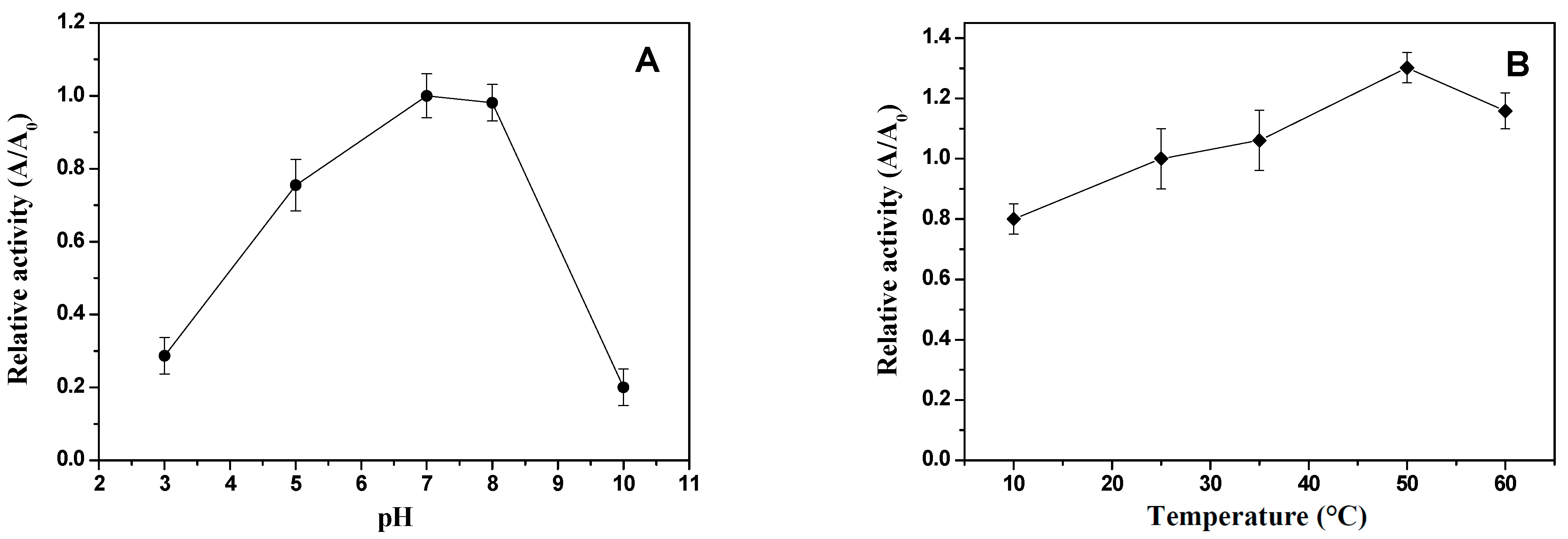
Figure 3A,B present the effects of pH and temperature, respectively, on the activity of the xylose reductase enzyme produced under bioprocess conditions at 25 °C for 24 h in 250 mL Erlenmeyer flasks containing 100 mL of culture medium.
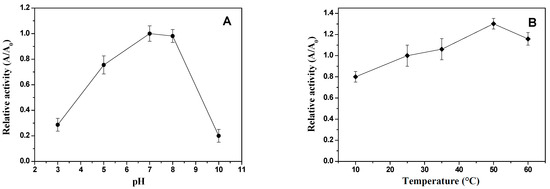
Figure 3.
Influence of pH at 25 °C (A) and temperature (B) on enzymatic activity of xylose reductase from Candida tropicalis ATCC750, produced using the hemicellulosic hydrolysate from cashew apple bagasse (CABHM) as medium, under bioprocessing conditions at 25 °C for 24 h in 250 mL Erlenmeyer flasks with 100 mL of the culture.
The optimum pH for XR activity was determined by measuring enzymatic activity at different pH values while maintaining the temperature at 25 °C, and the results are shown in Figure 3A. The XR enzyme from C. tropicalis ATCC750 exhibited optimal activity at pH 7.0 and 8.0. This behavior was similar to that of XR enzymes obtained from C. antarctica [30], C. intermedia [30], and C. parapsilosis [31].
XR activity was low at pH 3 and 10, as these more acidic or basic pH values did not favor the oxidation–reduction reactions catalyzed by this enzyme. This behavior might be attributed to changes in the protonation states of active site residues involved in the reduction reaction, indicating that the XR enzyme required a pH near the neutrality region to catalyze reactions with efficiency. Additionally, the decrease in activity is possibly due to the ionization of functional groups that hinder the binding of the enzyme to its substrate (S) or the formation of the enzyme–coenzyme complex necessary for catalysis [16]. Furthermore, extreme pH values may cause partial denaturation or inactivation of the enzyme by altering its native conformation [16]. This is corroborated by the literature, in which most studied XRs have shown optimal pH in the range of 5 to 7 [16,32].
The XR enzymatic activity at different temperatures is shown in Figure 3B. The optimum temperature for XR activity was 50 °C using xylose as the substrate. At the highest temperature (60 °C), there was a decrease in enzymatic activity, likely due to enzyme inactivation. This result is similar to the optimum temperature ranges reported in XR from Pichia stipitis, which showed an optimum temperature above 38 °C [33], and XR from Neurospora crossa, with optimum temperatures ranging from 45 °C to 55 °C [32].
However, other studies have reported the optimum temperature of XR enzymes from C. intermedia [30], C. guilliermondii [25], and C. tropicalis [27] at 25 °C, and at this temperature, the XR activity obtained in this study at 25 °C showed a 15% difference compared to that at 50 °C. According to Dasgupta et al. [8], most characterized XRs exhibit optimal activity between 30 and 35 °C, with denaturation occurring at higher temperatures. The higher optimum temperature observed for XR from C. antarctica ATCC750, cultivated in CABHM, might be attributed to the presence of certain amino acid residues, such as glutamate and proline [34], and leucine [35], in the enzyme structure.
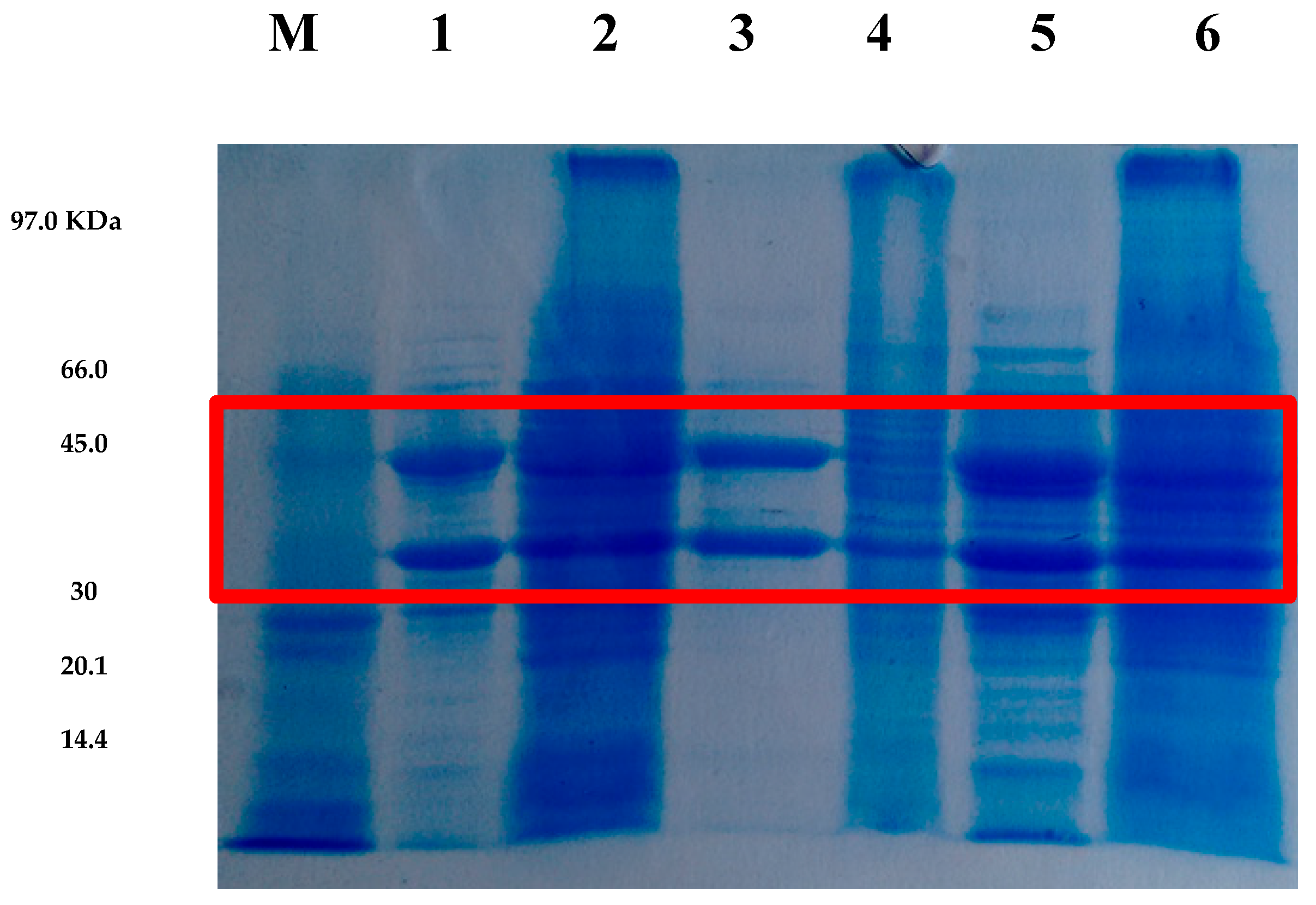
According to the SDS-PAGE analysis (Figure 4), a molecular mass in the range of approximately 30–45 kDa was identified for the XR produced under all tested conditions, which is consistent with the molecular weight range reported in the literature. Additionally, the enzymatic extracts obtained from cultures grown in CABHM appear sharper compared to the extracts obtained from cultures grown in FM, possibly due to differences in protein concentration (Figure 4).
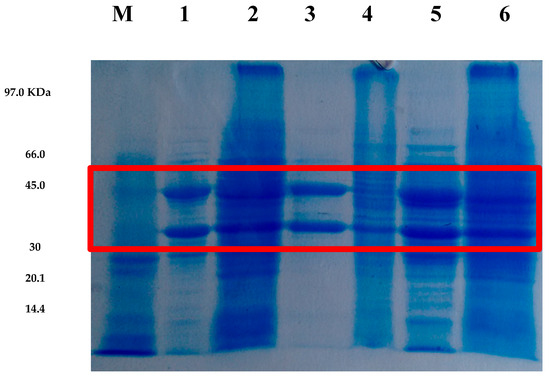
Figure 4.
SDS-PAGE electrophoresis of xylose reductase (XR) enzymatic extracts from Candida tropicalis ATCC 750 produced in different media and at various temperatures. Lane M shows the molecular weight markers. Lanes 1 and 2 correspond to XR produced in CABHM and formulated medium (FM) at 25 °C, respectively. Lanes 3 and 4 show XR produced in CABHM and FM at 30 °C, respectively, while lanes 5 and 6 represent XR produced in CABHM and FM at 40 °C, respectively. The red square highlights the region corresponding to the estimated molecular weight range of XR.
The molecular mass of xylose reductase can vary widely depending on the microorganism of origin and can reach values of 30 to 70 kDa. According to Cortez et al. [25], the XR of C. guilliermondii FTI 20037 is composed of one or two units of 30–60 kDa. Ho et al. [36] reported in their studies an XR of C. shehatae with 33 kDa. Thus, the XR produced by C. tropicalis ATCC750 presented a molecular mass similar to those reported in the literature.
Two intense bands were also observed in the analysis, suggesting that XR from C. tropicalis ATCC750 is heterodimeric, consisting of distinct subunits for each catalytic domain, one binding to NADPH and the other to the xylose substrate. Studies have reported that XR enzymes can be monomeric or dimeric (homodimeric or heterodimeric) [37], and that their structure depends on the microorganism used in the synthesis.
3. Materials and Methods
3.1. Microorganism, Material Lignocellulosic, and Chemicals
The microorganism C. tropicalis ATCC750 was obtained from the André Tosellos Foundation (Campinas, São Paulo, Brazil). The culture was stored at −4 °C on YEPD agar medium (Yeast Extract Peptone Dextrose, composed of 5 g L−1 yeast extract; 5 g L−1 peptone; 20 g L−1 dextrose; 1 g L−1 monobasic potassium phosphate (KH2PO4); 5 g L−1 magnesium sulfate monohydrate (MgSO4·7H2O); and 20 g L−1 agar), supplemented with glycerol (50%).
Cashew apple bagasse (Anacardium occidentale L.), without any pretreatment, was kindly provided by Jandaia Industry of Juice (Ceará, Brazil). Initially, the cashew apple bagasse was pretreated according to Correia et al. [38] and was referred to as CAB. The chemical composition of CAB was 17.73% w/w cellulose, 19.22% w/w hemicellulose, 33.41% w/w lignin, 6.41% w/w extractives, and 1.50% w/w ashes.
Xylose, xylitol, and a reduced form of nicotinamide adenine dinucleotide phosphate (NADPH) were bought from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Preparation of Cashew Apple Bagasse Hydrolysate
Cashew apple bagasse hydrolysate (CABH) was obtained according to the methodology proposed by Albuquerque et al. [39]. CAB was hydrolyzed with dilute sulfuric acid (0.6 mol L−1 H2SO4) at 121 °C (in autoclave) for 30 min, in 250 mL Erlenmeyer flasks with 100 mL of reaction volume and a solid percentage of 20% w/v. After hydrolysis, the liquid fraction was recovered by vacuum filtration, and the pH was adjusted to 6.0 ± 0.2 with Ca(OH)2. The solution was then filtrated to separate the precipitate. After, the hydrolysate was detoxified using activated carbon (3% w/v) under the agitation at 200 rpm and 30 °C for 2 h [20]. The detoxified hemicellulosic hydrolysate was recovered by vacuum filtration. The liquid fractions obtained in the three stages were characterized.
The detoxified hemicellulosic hydrolysate was supplemented with 3 g L−1 yeast extract, 3 g L−1 K2HPO4, and 1 g L−1 MgSO4·7H2O, and this medium was sterilized at 110 °C for 10 min and designated as CABHM. The CABHM was evaluated for the production of xylose reductase enzyme and xylitol.
3.3. Batch Fermentation for Production of Xylose Reductase Enzyme and Xylitol
In this step, the production of the enzyme xylose reductase and xylitol was evaluated by C. tropicalis ATCC750 in batch culture. Two production media were evaluated, hemicellulosic hydrolysate medium (CABHM) and formulated medium (FM). The formulated medium was composed of 3 g L−1 yeast extract, 3 g L−1 K2HPO4, 1 g L−1 MgSO4·7H2O, and 18.8 g L−1 xylose. The formulated medium composition was similar for CABHM (see composition of CABHM in Table 1), except for the absence of glucose.
For inoculum preparation, the microorganism was inoculated on Petri dishes containing YEPD agar and incubated at 30 °C for 48 h. After that, three isolated colonies were transferred to the inoculum medium (CABHM or FM), which was maintained in an orbital shaker at 30 °C and 150 rpm for 24 h. Samples were taken from the culture media of inoculum, and cell concentration was measured by absorbance (at a wavelength of 600 nm) and the dry cell concentration was determined with a calibration curve. After, the cells were centrifuged and washed with sterile distilled water and used as inoculum (approx. 0.3 ± 0.1 g L−1 cell concentration).
The influence of temperature on the kinetics of growth of Candida tropicalis and enzyme production was evaluated under isothermal conditions at 25 °C, 30 °C, 35 °C, or 40 °C. All experiments were carried out in 250 mL Erlenmeyer flasks containing 100 mL of culture medium (CABHM or FM) on a rotary shaker at 150 rpm for 72 h, with 0.3 ± 0.1 g L−1 initial cell concentration. All assays were performed in triplicate. Samples were withdrawn at predefined intervals to analyze cell growth, substrate (xylose and glucose), and product (xylitol and ethanol) concentrations. The cells were used to extract the XR enzyme as described in Topic 3.5.
3.4. Influence of Fluid Dynamics and Aeration on the Production of the Xylose Reductase Enzyme and Xylitol
The bioprocesses were conducted at 25 °C for 24 h under three modes using CABHM: (1) in 250 mL Erlenmeyer flasks with 100 mL of the culture; (2) in 1000 mL Erlenmeyer flasks with 300 mL of the culture medium; and (3) in 2000 mL Erlenmeyer flasks with 1000 mL of the culture medium. All experiments were conducted under orbital shaking at 150 rpm. All experiments were conducted in triplicate using 0.3 ± 0.1 g L−1 initial cell concentration. The XR enzyme was extracted from cells according to Section 3.5.
3.5. Extraction of XR Enzyme
The cells were washed and suspended in potassium phosphate buffer (0.1 mol L−1, pH 7.0) at biomass cell/buffer ratio of 1:2 (w/v). The cell suspension was disrupted by ultrasonication (QSonica—Sonicators, Newtown, CT, USA) at 20 kHz using pulsing/resting cycles of 5 s for 15 min, while maintaining the suspension in an ice bath. The resulting cell lysate was centrifuged to obtain the supernatant solution. For further clarification, the supernatant was centrifuged again at 6500 rpm for 20 min. The clarified supernatant was used as crude enzyme extract.
3.6. XR Activity Determination
XR activity was determined by measuring the amount of NADPH oxidized during the reduction of xylose to xylitol at 25 °C, following the method described by Yokoyama et al. [15]. The reaction mixture consisted of 1.2 mL of 0.1 mol L−1 phosphate buffer (pH 7), 0.2 mL of 0.1 mol L−1 2-mercaptoethanol (2-ME), 0.1 mL crude XR, and 0.1 mL of 3.4 mmol. L−1 NADPH.
The reaction mixture was allowed to stand for 1 min, and then the reaction was initiated by adding 0.2 mL of 0.5 mol L−1 D-xylose. For the control, pre-boiled XR was used instead of active enzyme. The rate of NADPH oxidation was measured at 340 nm using a UV–vis spectrophotometer, with readings taken every minute over 5 min intervals. One unit (U) of XR activity was defined as the amount of enzyme required to catalyze the oxidation of 1 μmol of NADPH per minute at pH 7 and 25 °C. XR activity was calculated using the equation proposed by Rafiqul et al. [16]. The slope of endogenous oxidation of NADPH was subtracted from the slope of samples to determine the enzyme activity.
Total protein was measured using the Bradford method [40], with bovine serum albumin (BSA) as standard.
3.7. Characterization of the XR Enzyme
3.7.1. Determination of Optimum pH and Temperature for XR Activity
A partial characterization of the crude enzymatic extract was conducted to determine the optimum pH and temperature for xylose reductase (XR) activity. The enzyme used in this assay was produced under bioprocess conditions at 25 °C for 24 h in 250 mL Erlenmeyer flasks containing 100 mL of culture medium.
The optimum pH was assessed by measuring enzymatic activity at different pH values: 3.0 (acetate buffer); 5.0, 6.0, and 8.0 (potassium phosphate buffer); and 10.0 (carbonate–bicarbonate buffer), maintaining the temperature constant at 25 °C. The optimum temperature was evaluated by incubating the reaction mixture at pH 7.0 for 5 min at various temperatures ranging from 10 °C to 60 °C.
All other assay conditions followed the procedure described in Section 3.6, with pH and temperature being the only variables adjusted for this analysis.
3.7.2. Electrophoresis and Molecular Mass Determination
SDS-PAGE analysis of the XR enzymatic extracts produced using the hemicellulosic hydrolysate (CABHM) or formulated medium (FM) was performed using the Bio-Rad Mini-PROTEAN® TGX ™ (São Paulo, Brazil). Electrophoresis was carried out on polyacrylamide slab gels according to the Laemmli method [41], with a 5% stacking gel overlaid on the separating gel of 12% polyacrylamide. Enzymatic extracts, 40 µL, were mixed with 10 µL rupture buffer and heated at 100 °C for 5 min. The denatured proteins, 10 µL, were separated at 180 V/20 mA for approximately 1 h in SDS running buffer (25 mM Tris base, 192 mM glycine, 0.1% w/v SDS). The gels were stained by the Coomassie brilliant blue method and used low molecular weight marker (SDS Marker—GE Healthcare Life Sciences, Marlborough, MA, USA) as standard.
3.8. Analytical Methods and Statistical Analysis
Cell growth (biomass) was determined by measuring the optical density of samples using a UV–visible spectrophotometer (20 Genesis, Brazil) at 600 nm, and biomass concentration (in g L−1) was determined by a calibration curve of dry weight (g L−1) versus optical density (600 nm). Glucose, xylose, ethanol, xylitol, and inhibitors (organic acids, furfural, and hydroxymethylfurfural (HMF)) were analyzed by high-performance liquid chromatography (HPLC) using a Waters HPLC system (Waters, Milford, MA, USA) following the methodology described by Marques Jr and Rocha [20]. Samples were identified by comparing their retention times with those of standard carbohydrates, xylitol, inhibitors, and ethanol standards.
Data analysis for statistical significance was conducted by a one-way analysis of variance (ANOVA) at a significance level of 95%. The data were also analyzed using the Tukey test using Microcal Origin 8.1 software (Microcal Software Inc., Northampton, MA, USA) at a significant level of 95%.
4. Conclusions
The hemicellulosic hydrolysate of cashew apple bagasse represents a promising xylose source for the production of xylose reductase (XR) enzyme by Candida tropicalis, achieving an enzymatic activity of 1.53 U mL−1. Aeration and fluid dynamics are key factors in this bioprocess, and the glucose present in the hydrolysate influences the metabolic pathway. XR from C. tropicalis ATCC 750 exhibited optimal activity at pH values of 7.0 and 8.0, with an optimum temperature of 50 °C when using xylose as the substrate. The synthesized enzyme has an approximate molecular mass of 30 kDa and displays a heterodimeric structure.
This study highlights the advantage of using a non-genetically modified strain and a low-cost agro-industrial residue, simplifying the production process compared to other works that employ genetically engineered microorganisms or refined substrates. However, enzymatic yields were lower than those obtained with induced or synthetic media, and the presence of inhibitory compounds in the hydrolysate limited xylitol production. Despite these challenges, the integrated approach contributes to sustainable bioprocess development and valorization of agro-industrial residues, aligning with current trends in industrial biotechnology and circular bioeconomy.
Xylose reductase from C. tropicalis can serve as a biocatalyst for xylitol production from hemicellulosic hydrolysate waste. These findings contribute to the development of sustainable bioprocesses for enzyme production and the valorization of agro-industrial residues, aligning with current trends in industrial biotechnology and circular bioeconomy.
Author Contributions
Conceptualization, M.V.P.R. and J.d.F.S.; methodology, M.V.P.R., J.d.F.S. and F.D.d.S.; validation, M.V.P.R. and B.C.P.; formal analysis, M.V.P.R., J.d.F.S., C.E.A.S. and B.C.P.; investigation, M.V.P.R., J.d.F.S., F.D.d.S., C.E.A.S. and B.C.P.; resources, M.V.P.R., C.E.A.S. and B.C.P.; data curation, M.V.P.R., J.d.F.S., F.D.d.S., C.E.A.S. and B.C.P.; writing—original draft preparation, M.V.P.R.; writing—review and editing, M.V.P.R. and B.C.P.; visualization, M.V.P.R. and J.d.F.S.; supervision, M.V.P.R. and B.C.P.; project administration, M.V.P.R.; funding acquisition, M.V.P.R., C.E.A.S. and B.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian research agency: Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (under grant number 316373/2021-4), Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico—FUNCAP (grant number PROEX PR2-0101-00012.01.00/15), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, and Banco Santander and Universidade Federal do Ceará, by Ibero-American Scholarship Program Young Teachers and Researchers Santander Universities 2015.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The corresponding author thanks at the Instituto de Investigación en Ciencias de la Alimentación (CIAL), Universidad Autónoma de Madrid (UAM), for the opportunity to carry out this Exchange Program and for providing all the infrastructure to carry out the experiments. To Gloria Fernandez-Lorente and Paz García for the support during the internship at CIAL. Also, the authors thank the Jandaia Juices Processing Industry (Ceará, Brazil) for has kindly provided the cashew apple bagasse used in this study. All those acknowledged have consented to be included in this section.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Antonio, A.G.; Pierro, V.S.D.S.; Maia, L.C. Caries preventive effects of xylitol-based candies and lozenges: A systematic review. J. Public Health Dent. 2011, 71, 117–124. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, B.K.; Kim, Y.J. The cariogenic characters of xylitol-resistant and xylitol-sensitive Streptococcus mutans in biofilm formation with salivary bacteria. Arch. Oral Biol. 2012, 57, 697–703. [Google Scholar] [CrossRef]
- Albuquerque, T.L.; Silva Junior, I.J.; Macedo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: A review. Process Biochem. 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Lugani, Y.; Sooch, B.S. Fermentative production of xylitol from a newly isolated xylose reductase producing Pseudomonas putida BSX-46. LWT 2020, 134, 109988. [Google Scholar] [CrossRef]
- Grand View Research, Inc. 2021. Available online: https://www.prnewswire.com (accessed on 11 March 2022).
- Hongzhi, L.; Cheng, K.; Ge, J.; Ping, W. Statistical optimization of xylitol production from corncob hemicellulose hydrolysate by Candida tropicalis HDY-02. New Biotechnol. 2011, 28, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.L.; Gomes, S.D.L.; Marques, J.E.; Silva, I.J.D.; Rocha, M.V.P. Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal. Today 2015, 255, 33–40. [Google Scholar] [CrossRef]
- Dasgupta, D.; Ghosh, D.; Bandhu, S.; Agrawal, D.; Suman, S.K.; Adhikari, D.K. Purification, characterization and molecular docking study of NADPH dependent xylose reductase from thermotolerant Kluyveromyces sp. IIPE453. Process Biochem. 2016, 51, 124–133. [Google Scholar] [CrossRef]
- Jin, L.Q.; Yang, B.; Xu, W.; Chen, X.X.; Jia, D.X.; Liu, Z.Q.; Zheng, Y.G. Immobilization of recombinant Escherichia coli whole cells harboring xylose reductase and glucose dehydrogenase for xylitol production from xylose mother liquor. Bioresour. Technol. 2019, 285, 121344. [Google Scholar] [CrossRef]
- Okamoto, K.; Kanawaku, R.; Masumoto, M.; Yanase, H. Efficient xylose fermentation by the brown rot fungus Neolentinus lepideus. Enz. Microb. Technol. 2012, 50, 96–100. [Google Scholar] [CrossRef]
- Suzuki, S.; Sugiyama, M.; Mihara, Y.; Hashiguchi, K.; Yokozeki, K. Novel enzymatic method for the production of xylitol from D-arabitol by Gluconobacter oxydans. Biosc. Biotechnol. Biochem. 2002, 66, 2614–2620. [Google Scholar] [CrossRef][Green Version]
- Misra, S.; Raghuwanshi, S.; Gupta, P.; Dutt, K.; Saxena, R.K. Fermentation behavior of osmophilic yeast Candida tropicalis isolated from the nectar of Hibiscus rosa sinensis flowers for xylitol production. Antonie Leeuwenhoek 2012, 101, 393–402. [Google Scholar] [CrossRef]
- Rocha, M.V.P.; Rodrigues, T.H.S.; Albuquerque, T.L.; Gonçalves, L.R.B.; Macedo, G.R. Evaluation of dilute acid pretreatment on cashew apple bagasse for ethanol and xylitol production. Chem. Eng. J. 2014, 243, 234–243. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M.M. Kinetic studies on acid hydrolysis of Meranti wood sawdust for xylose production. Chem. Eng. Sci. 2012, 71, 431–437. [Google Scholar] [CrossRef]
- Yokoyama, S.; Suzuki, T.; Kawai, K.; Horitsu, H.; Takamizawa, K. Purification, characterization and structure analysis of NADPH-dependent d-xylose reductases from Candida tropicalis. J. Ferment. Bioeng. 1995, 79, 217–223. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M.M.; Zularisam, A.W. Evaluation of sawdust hemicellulosic hydrolysate for bioproduction of xylitol by enzyme xylose reductase. Food Bioprod. Process. 2015, 94, 82–89. [Google Scholar] [CrossRef]
- Yablochkova, E.N.; Bolotnikova, O.I.; Mikhailova, N.P.; Nemova, N.N.; Ginak, A.I. The activity of xylose reductase and xylitol dehydrogenase in yeasts. Microbiology 2003, 72, 414–417. [Google Scholar] [CrossRef]
- Zhang, M.; Puri, A.K.; Wang, Z.; Singh, S.; Permaul, K. A unique xylose reductase from Thermomyces lanuginosus: Effect of lignocellulosic substrates and inhibitors and applicability in lignocellulosic bioconversion. Bioresour. Technol. 2019, 281, 374–381. [Google Scholar] [CrossRef]
- Mayr, P.; Brüggler, K.; Kulbe, K.D.; Nidetzky, B. D-Xylose metabolism by Candida intermedia: Isolation and characterization of two forms of aldose reductase with different coenzyme specificities. J. Chromatogr. B Biomed. Sci. Appl. 2000, 737, 195–202. [Google Scholar] [CrossRef]
- Marques Junior, J.E.; Rocha, M.V.P. Development of a purification process via crystallization of xylitol produced for bioprocess using a hemicellulosic hydrolysate from the cashew apple bagasse as feedstock. Bioprocess Biosyst. Eng. 2021, 44, 713–725. [Google Scholar] [CrossRef]
- Marques Junior, J.E.; de Queiroz, L.P.; Albuquerque, T.L.; Zampieri, D.S.; Melo, V.M.M.; Rocha, M.V.P. Lactic acid production from cashew apple bagasse, an agro-industrial waste, and its application in the enzymatic synthesis of polylactic acid. Biocatal. Agric. Biotechnol. 2024, 56, 102987. [Google Scholar] [CrossRef]
- de Arruda, P.V.; Rodrigues, R.d.C.; da Silva, D.D.V.; Felipe, M.d.G.A. Evaluation of hexose and pentose in pre-cultivation of Candida guilliermondii on the key enzymes for xylitol production in sugarcane hemicellulosic hydrolysate. Biodegradation 2011, 22, 815–822. [Google Scholar] [CrossRef]
- Yablochkova, E.N.; Bolotnikova, O.I.; Mikhailova, N.P.; Nemova, N.N.; Ginak, A.I. The activity of key enzymes in xylose-assimilating yeasts at different rates of oxygen transfer to the fermentation medium. Microbiology 2004, 73, 129–133. [Google Scholar] [CrossRef]
- Kastner, J.R.; Eiteman, M.A.; Lee, S.A. Glucose repression of xylitol production in Candida tropicalis mixed-sugar fermentations. Biotechnol. Lett. 2001, 23, 1663–1667. [Google Scholar] [CrossRef]
- Cortez, E.V.; Pessoa-Jr, A.; Felipe, M.G.A.; Roberto, I.C.; Vitolo, M. Characterization of xylose reductase extracted by CTAB-reversed micelles from Candida guilliermondii homogenate. Braz. J. Pharm. Sci. 2006, 42, 251–257. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, J.B.; Jang, S.W.; Ha, S.J. Enhanced xylitol production by mutant Kluyveromyces marxianus 36907–FMEL1 due to improved xylose reductase activity. Appl. Biochem. Biotechnol. 2015, 176, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Rafiqul, I.S.M.; Sakinah, A.M.M. Production of xylose reductase from adapted Candida tropicalis grown in sawdust hydrolysate. Biocatal. Agric. Biotechnol. 2014, 3, 227–235. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Wang, D.; Gao, X.; Hong, J. Identification of a xylose reductase gene in the xylose metabolic pathway of Kluyveromyces marxianus NBRC1777. J. Ind. Microbiol. Biotechnol. 2011, 38, 2001–2010. [Google Scholar] [CrossRef]
- Azizan, A.; Büchs, J. Three-dimensional (3D) evaluation of liquid distribution in shake flask using an optical fluorescence technique. J. Biol. Eng. 2017, 11, 28. [Google Scholar] [CrossRef]
- Nidetzky, B.; Brüggler, K.; Kratzer, R.; Mayr, P. Multiple forms of xylose reductase in Candida intermedia: Comparison of their functional properties using quantitative structure–activity relationships, steady-state kinetic analysis, and pH studies. J. Agric. Food Chem. 2003, 51, 7930–7935. [Google Scholar] [CrossRef]
- Lee, J.K.; Koo, B.S.; Kim, S.Y. Cloning and characterization of the xyl1 gene, encoding an NADH-Preferring xylose reductase from Candida parapsilosis, and its functional expression in Candida tropicalis. Appl. Environ. Microbiol. 2003, 69, 6179–6188. [Google Scholar] [CrossRef]
- Woodyer, R.; Simurdiak, M.; van der Donk, W.A.; Zhao, H. Heterologous expression, purification and characterization of a highly active xylose redutase from Neurospora crassa. Appl. Biochem. Microbiol. 2005, 71, 1642–1647. [Google Scholar] [CrossRef]
- Verduyn, C.; Kleef, R.V.; Frank, J.; Schreuder, H.; Van Dijken, J.P.; Scheffers, W.A. Properties of the NAD(P)H- dependent xylose reductase from the xylose-fermenting yeast Pichia stipitis. Biochem. J. 1985, 226, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Luo, H.; Tian, J.; Turunen, O.; Huang, H.; Shi, P.; Hua, H.; Wang, C.; Wang, S.; Yao, B. Thermostability improvement of a Streptomyces xylanase by introducing proline and glutamic acid residues. Appl. Environ. Microb. 2014, 80, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.; Sharma, N.; Bhalla, T.C. Computational analysis of amino acid sequences in relation to thermostability of interspecific nitrile degrading enzyme (amidase) from various thermophiles/hyperthermophiles. Sci. Rep. 2012, 1, 556. [Google Scholar]
- Ho, N.W.Y. Purification, characterization and amino terminal sequence of xylose reductase from Candida shehatae. Enzyme Microb. Technol. 1990, 12, 33–39. [Google Scholar] [CrossRef]
- Wilson, D.K.; Kavanagh, K.L.; Klimacek, M.; Nidetzky, B. The xylose reductase (AKR2B5) structure: Homology and divergence from other aldo-keto reductase and opportunities for protein engineering. Chem. Biol. Interact. 2003, 143–144, 515–521. [Google Scholar] [CrossRef]
- Correia, J.A.C.; Silva, J.S.; Gonçalves, L.R.B.; Rocha, M.V.P. Different design configurations of simultaneous saccharification and fermentation to enhance ethanol production from cashew apple bagasse pretreated with alkaline hydrogen peroxide applying the biorefinery concept. Biomass Convers. Biorefin. 2022, 12, 2767–2780. [Google Scholar] [CrossRef]
- Albuquerque, T.L.; Gomes, S.D.L.; da Silva Junior, I.J.; Gonçalves, L.R.B.; Rocha, M.V.P. Xylitol production by different yeasts: Kinetic study and biosynthesis from cashew apple bagasse hydrolysate. Can. J. Chem. Eng. 2023, 101, 3668–3679. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).