An Exploratory Study on the Growth Dynamics of Alkalihalophilus marmarensis Using a Model-Based Approach
Abstract
1. Introduction
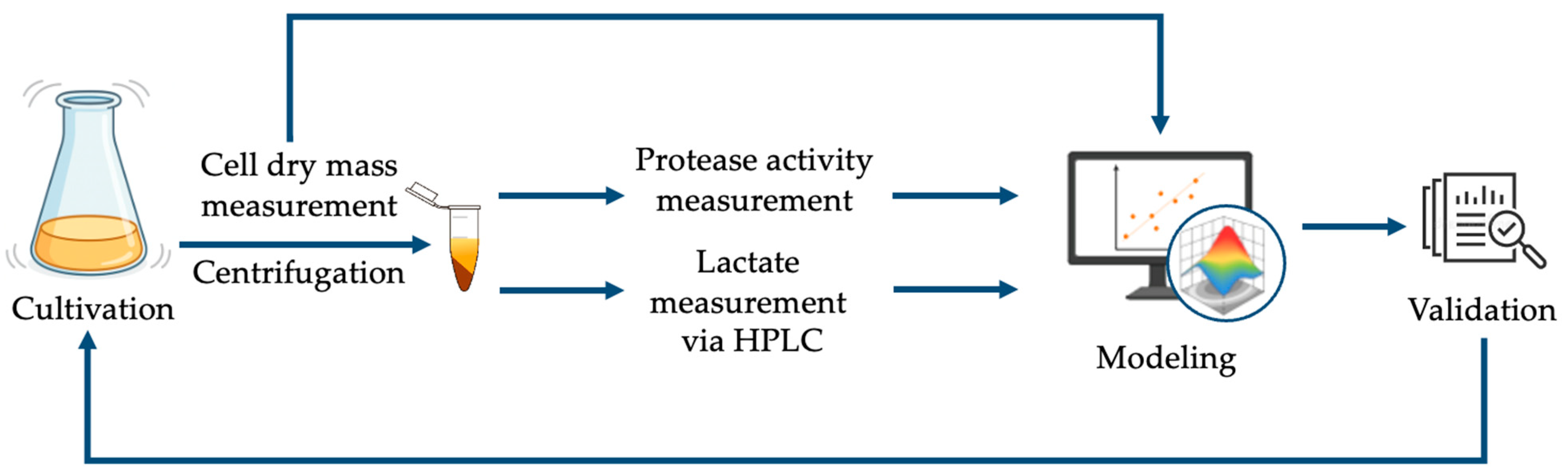
2. Materials and Methods
2.1. Bacterial Growth Conditions and Media Preparations
2.2. Protease Activity Assay
2.3. Lactate Detection and Quantification via HPLC
2.4. Statistical Modeling and Optimization Framework
2.4.1. Design of Experiments
2.4.2. Modeling
2.4.3. Investigation of Growth and Product Yields Through Optimization
2.4.4. Validation of Model Outputs
2.4.5. Statistical Analyses
3. Results and Discussion
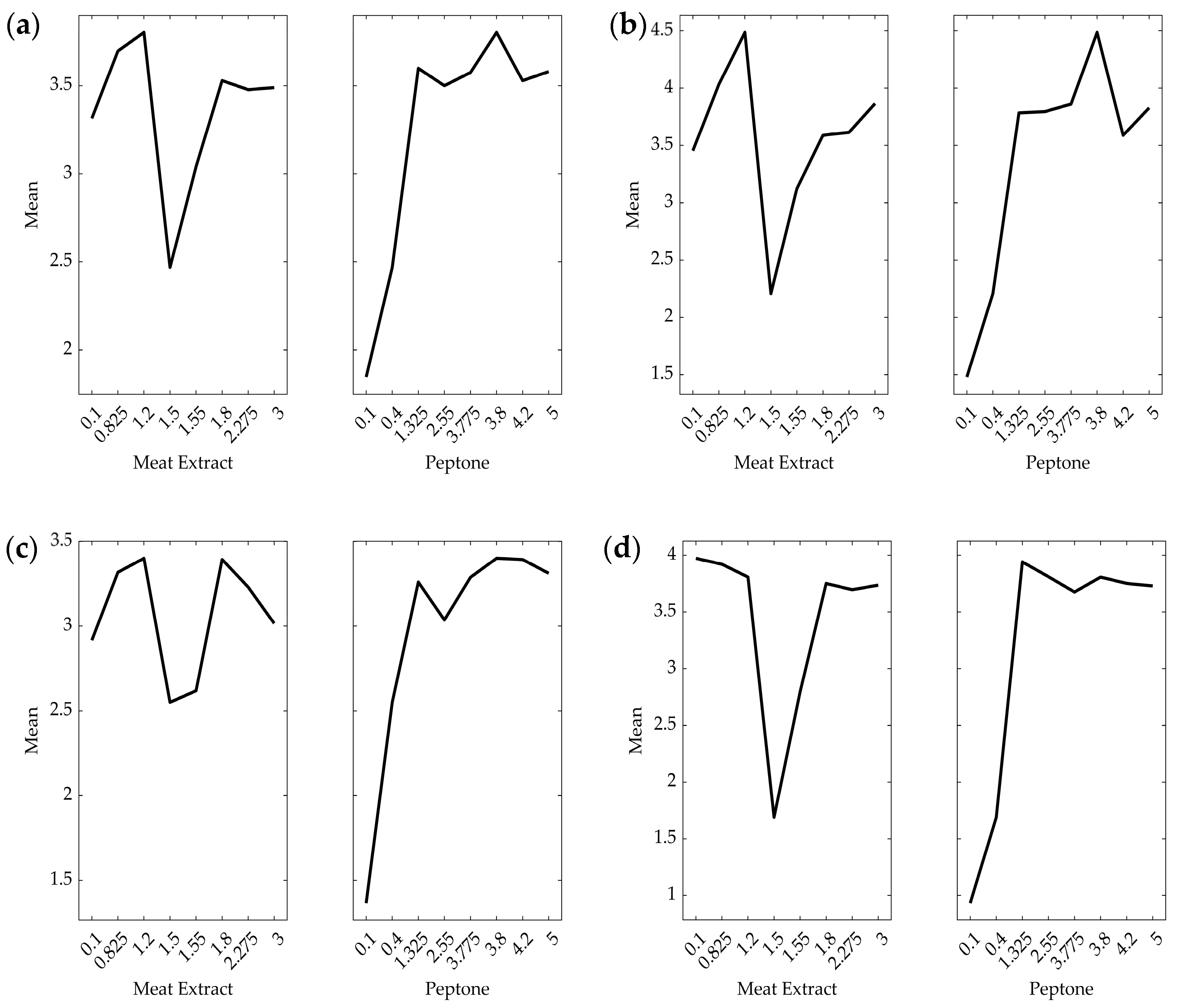
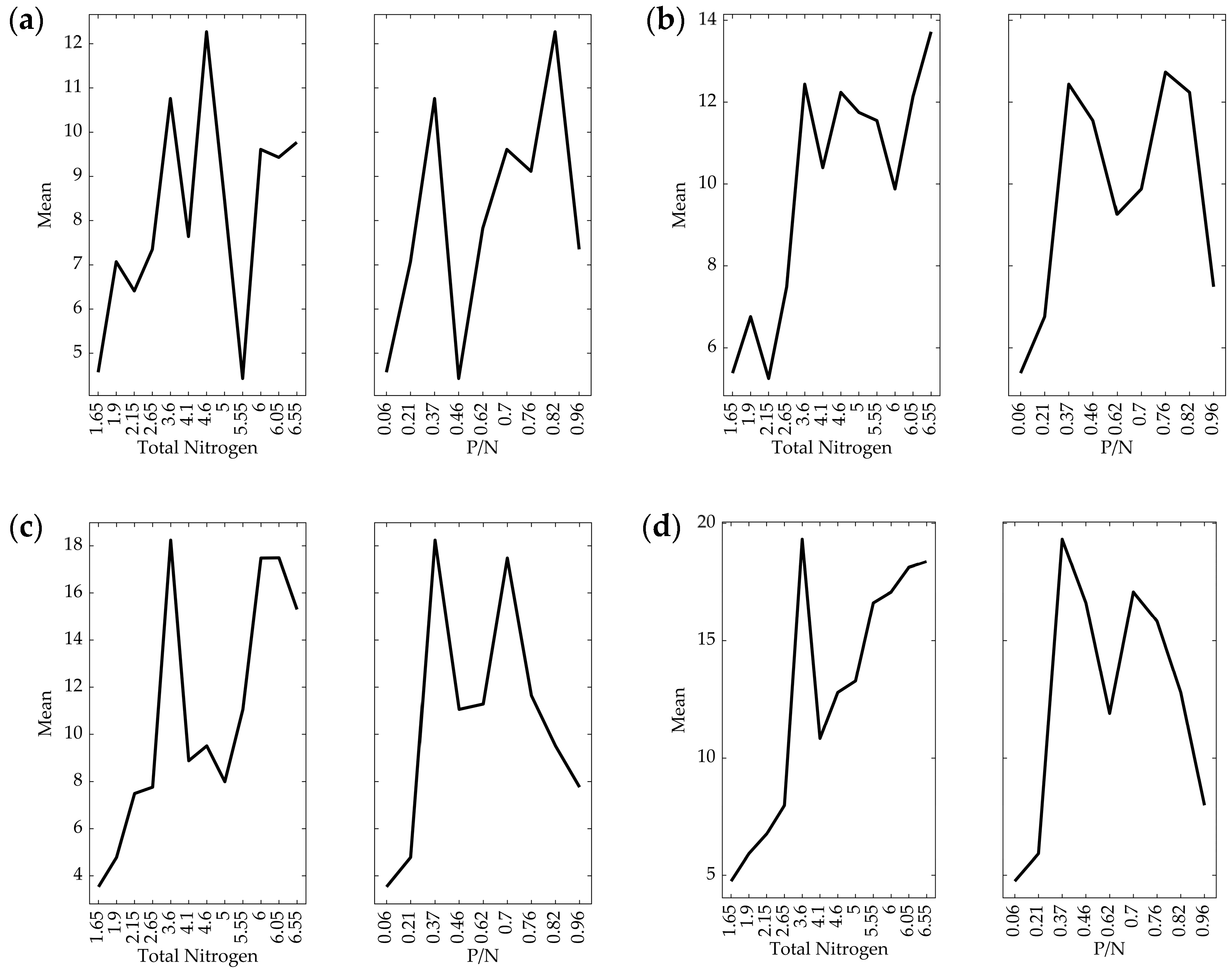
3.1. Effect of Nitrogen Source on Growth
3.2. Effect of Nitrogen Source on Lactate Production
3.3. Effect of Nitrogen Source on Protease Activity
3.4. Modeling of Growth and Metabolite Production
3.5. Experimental Validation of the Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RSM | Response Surface Methodology |
| CDM | Cell Dry Mass |
| TCA | Trichloroacetic Acid |
| h | Hours |
Appendix A
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| Cell Dry Mass | Corrected Model | 82.56 a | 47 | 1.76 | 10.20 | 0.00 |
| Intercept | 1580.16 | 1 | 1580.16 | 9173.60 | 0.00 | |
| Condition | 50.00 | 11 | 4.55 | 26.39 | 0.00 | |
| Time | 2.55 | 1 | 2.55 | 14.78 | 0.00 | |
| pH | 1.83 | 1 | 1.83 | 10.62 | 0.00 | |
| Media * Time | 4.84 | 11 | 0.44 | 2.55 | 0.01 | |
| Media * pH | 9.35 | 11 | 0.85 | 4.93 | 0.00 | |
| Time * pH | 0.33 | 1 | 0.33 | 1.94 | 0.17 | |
| Media * Time * pH | 13.67 | 11 | 1.24 | 7.22 | 0.00 | |
| Error | 16.54 | 96 | 0.17 | |||
| Total | 1679.26 | 144 | ||||
| Corrected Total | 99.10 | 143 | ||||
| a. R Squared = 0.833 (Adjusted R Squared = 0.751) | ||||||
| Lactate Concentration | Corrected Model | 1698.79 a | 47 | 36.14 | 15.77 | 0.00 |
| Intercept | 10,511.297 | 1 | 10,511.30 | 4586.72 | 0.00 | |
| Condition | 1145.825 | 11 | 104.17 | 45.45 | 0.00 | |
| pH | 151.851 | 1 | 151.85 | 66.26 | 0.00 | |
| Time | 64.567 | 1 | 64.57 | 28.17 | 0.00 | |
| Media * Time | 257.952 | 11 | 23.45 | 10.23 | 0.00 | |
| Media * pH | 63.195 | 11 | 5.74 | 2.51 | 0.01 | |
| Time * pH | 1.136 | 1 | 1.14 | 0.50 | 0.48 | |
| Media * Time * pH | 14.267 | 11 | 1.30 | 0.57 | 0.85 | |
| Error | 110.001 | 48 | 2.29 | |||
| Total | 12,320.091 | 96 | ||||
| Corrected Total | 1808.794 | 95 | ||||
| a. R Squared = 0.939 (Adjusted R Squared = 0.880) | ||||||
| Protease Activity | Corrected Model | 22,361.27 a | 47 | 475.77 | 59.93 | 0.00 |
| Intercept | 9931.75 | 1 | 9931.75 | 1251.02 | 0.00 | |
| Condition | 19,611.55 | 11 | 1782.87 | 224.57 | 0.00 | |
| Time | 844.38 | 1 | 844.38 | 106.36 | 0.00 | |
| pH | 15.56 | 1 | 15.56 | 1.96 | 0.17 | |
| Media * Time | 1031.28 | 11 | 93.75 | 11.81 | 0.00 | |
| Media * pH | 297.09 | 11 | 27.01 | 3.40 | 0.00 | |
| Time * pH | 230.28 | 1 | 230.28 | 29.01 | 0.00 | |
| Media * Time * pH | 331.13 | 11 | 30.10 | 3.79 | 0.00 | |
| Error | 762.13 | 96 | 7.94 | |||
| Total | 33,055.16 | 144 | ||||
| Corrected Total | 23,123.41 | 143 | ||||
| a. R Squared = 0.967 (Adjusted R Squared = 0.951) | ||||||
| Type of Model | R2 Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDM (g/L) | Lactate Concentration (g/L) | Protease Activity (U/mL) | ||||||||||
| pH 8.8 | pH 10.5 | pH 8.8 | pH 10.5 | pH 8.8 | pH 10.5 | |||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| Linear Model | 0.48 | 0.48 | 0.49 | 0.42 | 0.35 | 0.73 | 0.57 | 0.81 | 0.61 | 0.64 | 0.64 | 0.76 |
| Quadratic Model | 0.81 | 0.83 | 0.73 | 0.79 | 0.62 | 0.92 | 0.58 | 0.85 | 0.79 | 0.81 | 0.85 | 0.91 |
| Pure Quadratic Model | 0.81 | 0.82 | 0.73 | 0.79 | 0.47 | 0.74 | 0.58 | 0.81 | 0.74 | 0.71 | 0.8 | 0.85 |
| Pareto Solution No. | Dependent | Output Variables | |||
|---|---|---|---|---|---|
| Meat Extract (%, w/v) | Peptone (%, w/v) | CDM (g/L) | Lactate (g/L) | Protease (U/mL) | |
| 1 | 0.1002 | 3.0331 | 4.174 | 9.294 | 10.555 |
| 2 | 2.1996 | 2.0086 | 3.625 | 15.024 | 5.462 |
| 3 | 2.7717 | 0.1176 | 1.166 | 16.859 | 10.853 |
| 4 | 2.278 | 0.1022 | 1.266 | 12.607 | 22.567 |
| 5 | 2.1084 | 0.7552 | 2.334 | 12.464 | 17.863 |
| 6 | 1.0752 | 0.7596 | 2.551 | 4.883 | 38.222 |
| 7 | 2.9855 | 0.1008 | 1.080 | 18.686 | 6.095 |
| 8 | 0.6918 | 0.5653 | 2.361 | 1.223 | 50.441 |
| 9 | 2.6442 | 2.013 | 3.565 | 17.332 | 1.004 |
| 10 | 0.3833 | 2.9759 | 4.159 | 9.962 | 9.926 |
References
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context. Front. Microbiol. 2019, 10, 447–668. [Google Scholar] [CrossRef] [PubMed]
- Marzban, G.; Tesei, D. The Extremophiles: Adaptation Mechanisms and Biotechnological Applications. Biology 2025, 14, 412. [Google Scholar] [CrossRef] [PubMed]
- Preiss, L.; Hicks, D.B.; Suzuki, S.; Meier, T.; Krulwich, T.A. Alkaliphilic Bacteria with Impact on Industrial Applications, Concepts of Early Life Forms, and Bioenergetics of ATP Synthesis. Front. Bioeng. Biotechnol. 2015, 3, 146008. [Google Scholar] [CrossRef] [PubMed]
- Denizci, A.A.; Kazan, D.; Erarslan, A. Bacillus marmarensis Sp. Nov., an Alkaliphilic, Protease-Producing Bacterium Isolated from Mushroom Compost. Int. J. Syst. Evol. Microbiol. 2010, 60, 1590–1594. [Google Scholar] [CrossRef] [PubMed]
- Wernick, D.G. Toward Nitrogen-Neutral and Contamination-Resistant Biofuels and Chemicals for a Sustainable Future. Ph.D. Thesis, The University of California, Berkeley, CA, USA, 2015. [Google Scholar]
- Joshi, A.; Thite, S.; Karodi, P.; Joseph, N.; Lodha, T. Alkalihalobacterium elongatum Gen. Nov. Sp. Nov.: An Antibiotic-Producing Bacterium Isolated from Lonar Lake and Reclassification of the Genus Alkalihalobacillus into Seven Novel Genera. Front. Microbiol. 2021, 12, 722369. [Google Scholar] [CrossRef] [PubMed]
- Wernick, D.G.; Pontrelli, S.P.; Pollock, A.W.; Liao, J.C. Sustainable Biorefining in Wastewater by Engineered Extreme Alkaliphile Bacillus marmarensis. Sci. Rep. 2016, 6, 20224. [Google Scholar] [CrossRef] [PubMed]
- Kerimak Öner, M.N. Purification and Characterization of Alkaline Protease from Obligate Alkaliphilic Bacillus marmariensis GMBE 72. Ph.D. Thesis, The Univesity of Kocaeli, Kocaeli, Türkiye, 2009. [Google Scholar]
- Canpulat, M. Co-Production of Enzymes and Nanoparticles by Extremophilic Microorganisms. M.Sc. Thesis, Marmara University, Istanbul, Türkiye, 2022. [Google Scholar]
- Sethi, S.; Bhatti, G.S. Trends in Biotechnology of Polyextremophiles; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 417–440. ISBN 3031550323. [Google Scholar]
- Atakav, Y.; Pinar, O.; Kazan, D. Investigation of the Physiology of the Obligate Alkaliphilic Bacillus marmarensis Gmbe 72t Considering Its Alkaline Adaptation Mechanism for Poly(3-Hydroxybutyrate) Synthesis. Microorganisms 2021, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, S.; Fujisawa, M. Industrial Applications of Alkaliphiles and Their Enzymes-Past, Present and Future. Environ. Technol. 2010, 31, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, K. Alkaliphiles-Genetic Properties and Applications of Enzymes; Kodansha: Tokyo, Japan, 2006; pp. 125–141. ISBN 4-06-211513-1. [Google Scholar]
- Mamo, G.; Mattiasson, B. Biotechnology of Extremophiles: Advances and Challenges; Rampelotto, P.H., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 243–272. ISBN 978-3-319-13521-2. [Google Scholar]
- Mesbah, N.M. Industrial Biotechnology Based on Enzymes from Extreme Environments. Front. Bioeng. Biotechnol. 2022, 10, 870083. [Google Scholar] [CrossRef] [PubMed]
- Özgören, T. Production of Polyhydroxyalkanoate from Extreme Obligate Alkaliphilic Strain Bacillus marmarensis Gmbe 72 T Isolated from Mushroom Compost. M.Sc. Thesis, Marmara University, Istanbul, Türkiye, 2017. [Google Scholar]
- Verma, J.; Pandey, S.; Kumar, C.; Saxena, S. A Detergent-Compatible Alkaline Metalloprotease from Bacillus pseudofirmus BBAU-19: Characterization and Application. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 499–510. [Google Scholar] [CrossRef]
- Fu, Y.; Rao, Y.; Liao, Y.; Zhang, Q.; Ma, X.; Cai, D.; Chen, S. Protein Engineering, Expression Optimization, and Application of Alkaline Protease from Alkalihalobacillus clausii FYX. Int. J. Biol. Macromol. 2025, 307, 141891. [Google Scholar] [CrossRef] [PubMed]
- Saberianpour, S.; Abkhooie, L.; Elyasifar, B.; Dilmaghani, A. Screening and Optimization of Protease Enzyme Produced by Strains of Alkalihalobacillus sp. and Bacillus sp. Curr. Biotechnol. 2020, 9, 40–45. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. Breakthroughs in Statistics: Methodology and Distribution; Springer: New York, NY, USA, 1992; pp. 270–310. ISBN 978-1-4612-4380-9. [Google Scholar]
- Breig, S.J.M.; Luti, K.J.K. Response Surface Methodology: A Review on Its Applications and Challenges in Microbial Cultures. Mater. Today Proc. 2021, 42, 2277–2284. [Google Scholar] [CrossRef]
- Suriya, P.; Sangeetha, S.P. Optimization of Microbial Fermentation Processes Using RSM Approach for Soil Improvement. J. Build. Pathol. Rehabil. 2025, 10, 72. [Google Scholar] [CrossRef]
- Amaradio, M.N.; Ojha, V.; Jansen, G.; Gulisano, M.; Costanza, J.; Nicosia, G. Pareto Optimal Metabolic Engineering for the Growth-coupled Overproduction of Sustainable Chemicals. Biotechnol. Bioeng. 2022, 119, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chattopadhyay, R.; Sinha, S.K.; Regar, M.L. Applications of Selected Response Surface Design of Experiments and Advanced Control Charts in Textile Engineering. In Textile Calculation; Woodhead Publishing: Cambridge, UK, 2023; pp. 13–55. [Google Scholar] [CrossRef]
- Veza, I.; Spraggon, M.; Fattah, I.M.R.; Idris, M. Response Surface Methodology (RSM) for Optimizing Engine Performance and Emissions Fueled with Biofuel: Review of RSM for Sustainability Energy Transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Sigg, A.; Klimacek, M.; Nidetzky, B. Pushing the Boundaries of Phosphorylase Cascade Reaction for Cellobiose Production II: Model-based Multiobjective Optimization. Biotechnol. Bioeng. 2024, 121, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Claes, E.; Heck, T.; Coddens, K.; Sonnaert, M.; Schrooten, J.; Verwaeren, J. Bayesian Cell Therapy Process Optimization. Biotechnol. Bioeng. 2024, 121, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Pogodaev, A.; Hernández Rodríguez, T.; Li, M.; García Münzer, D.G. Modeling of Bioprocess Pre-Stages for Optimization of Perfusion Profiles and Increased Process Understanding. Biotechnol. Bioeng. 2024, 121, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Takami, H.; Akiba, T.; Horikoshi, K. Production of Extremely Thermostable Alkaline Protease from Bacillus sp. No. AH-101. Appl. Microbiol. Biotechnol. 1989, 30, 120–124. [Google Scholar] [CrossRef]
- Kishore, G.; Karthik, A.; Gopal, S.; Kumar, A.R.; Bhat, M.; Udupa, N. Development of RP-HPLC Method for Simultaneous Estimation of Lactic Acid and Glycolic Acid. Pharma Chem. 2013, 5, 335–340, ISSN 0975-413X. [Google Scholar]
- Fitlm. Available online: https://www.mathworks.com/help/stats/fitlm.html (accessed on 21 April 2025).
- Kula, C.; Sayar, N.A. Multi-Objective Optimization of a Novel Crude Lipase-Catalyzed Fatty Acid Methyl Ester (FAME) Production Using Low-Order Polynomial and Kriging Models. Int. J. Green Energy 2019, 16, 657–665. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. An Overview of Biodegradable Poly (Lactic Acid) Production from Fermentative Lactic Acid for Biomedical and Bioplastic Applications. Biomass Convers. Biorefin. 2024, 14, 3057–3076. [Google Scholar] [CrossRef]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial Proteases and Their Applications. Front. Microbiol. 2023, 14, 1236368. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of Nitrogen Status in Wine Alcoholic Fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Soini, J.; Ukkonen, K.; Neubauer, P. High Cell Density Media for Escherichia Coli Are Generally Designed for Aerobic Cultivations-Consequences for Large-Scale Bioprocesses and Shake Flask Cultures. Microb. Cell Factories 2008, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Konzock, O.; Nielsen, J. TRYing to Evaluate Production Costs in Microbial Biotechnology. Trends Biotechnol. 2024, 42, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.S. Proteases: History, Discovery, and Roles in Health and Disease. J. Biol. Chem. 2019, 294, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; John Martin, J.J.; Li, X.; Zhou, L.; Li, R.; Li, Q.; Zhang, J.; Fu, D.; Cao, H. Optimization of the Fermentation Culture Conditions of Bacillus amyloliquefaciens Ck-05 Using Response Surface Methodology. Front. Microbiol. 2025, 16, 1552645. [Google Scholar] [CrossRef] [PubMed]
- Lorca, G.L.; Chung, Y.J.; Barabote, R.D.; Weyler, W.; Schilling, C.H.; Saier, M.H. Catabolite Repression and Activation in Bacillus Subtilis: Dependency on CcpA, HPr, and HprK. J. Bacteriol. 2005, 187, 7826–7839. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parecha, D.; Alfano, A.; Cimini, D.; Schiraldi, C. Vegan Grade Medium Component Screening and Concentration Optimization for the Fermentation of the Probiotic Strain Lactobacillus paracasei IMC 502® Using Design of Experiments. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae016. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Sharma, V. Physiological and Biotechnological Aspects of Extremophiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–22, ISBN-10 0128183225. [Google Scholar]
- Nguyen, P.Y.; Marques, R.; Wang, H.; Reis, M.A.M.; Carvalho, G.; Oehmen, A. The Impact of pH on the Anaerobic and Aerobic Metabolism of Tetrasphaera-Enriched Polyphosphate Accumulating Organisms. Water Res. X 2023, 19, 100177. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, C.; Vishali, A.; Nagendran, N.A.; Baskar, K.; Hashem, A.; Abd_Allah, E.F. Optimization of Protease Production from Bacillus Halodurans under Solid State Fermentation Using Agrowastes. Saudi J. Biol. Sci. 2021, 28, 4263–4269. [Google Scholar] [CrossRef] [PubMed]
- Janto, B.; Ahmed, A.; Ito, M.; Liu, J.; Hicks, D.B.; Pagni, S.; Fackelmayer, O.J.; Smith, T.-A.; Earl, J.; Elbourne, L.D.H.; et al. Genome of Alkaliphilic Bacillus pseudofirmus OF4 Reveals Adaptations That Support the Ability to Grow in an External pH Range from 7.5 to 11.4. Environ. Microbiol. 2011, 13, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, K. Alkaliphiles: Some Applications of Their Products for Biotechnology. Microbiol. Mol. Biol. Rev. 1999, 63, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Beg, Q.K.; Gupta, R. Optimization of Alkaline Protease Production from Bacillus sp. by Response Surface Methodology. Curr. Microbiol. 2002, 44, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Oskouie, S.F.G.; Tabandeh, F.; Yakhchali, B.; Eftekhar, F. Response Surface Optimization of Medium Composition for Alkaline Protease Production by Bacillus clausii. Biochem. Eng. J. 2008, 1, 37–42. [Google Scholar] [CrossRef]
- Gupta, R.; Beg, Q.K.; Khan, S.; Chauhan, B. An Overview on Fermentation, Downstream Processing and Properties of Microbial Alkaline Proteases. Appl. Microbiol. Biotechnol. 2002, 60, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Habicher, T.; John, A.; Scholl, N.; Daub, A.; Klein, T.; Philip, P.; Büchs, J. Introducing Substrate Limitations to Overcome Catabolite Repression in a Protease Producing Bacillus licheniformis Strain Using Membrane-Based Fed-Batch Shake Flasks. Biotechnol. Bioeng. 2019, 116, 1326–1340. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, Y.R.; El-Enshasy, H.A.; Soliman, N.A.; El-Gendi, H. Bioprocess Development for Production of Alkaline Protease by Bacillus pseudofirmus Mn6 through Statistical Experimental Designs. J. Microbiol. Biotechnol. 2009, 19, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dodia, M.; Singh, S. Extracellular Alkaline Protease from a Newly Isolated Haloalkaliphilic Bacillus sp.: Production and Optimization. Process Biochem. 2005, 40, 3569–3575. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhang, Q.; Cai, D.; Chen, S.; Wang, Y. Enhancement of Alkaline Protease Production in Recombinant Bacillus licheniformis by Response Surface Methodology. Bioresour. Bioprocess. 2023, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gupta, R.S. A Phylogenomic and Comparative Genomic Framework for Resolving the Polyphyly of the Genus Bacillus: Proposal for Six New Genera of Bacillus Species, Peribacillus Gen. Nov., Cytobacillus Gen. Nov., Mesobacillus Gen. Nov., Neobacillus Gen. Nov., Metabacillus Gen. Nov. and Alkalihalobacillus Gen. Nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 406–438. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.M.; Kumar, R.; Panwar, S.; Kumar, A. Microbial Alkaline Proteases: Optimization of Production Parameters and Their Properties. J. Genet. Eng. Biotechnol. 2017, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Patané, A.; Jansen, G.; Conca, P.; Carapezza, G.; Costanza, J.; Nicosia, G. Multi-Objective Optimization of Genome-Scale Metabolic Models: The Case of Ethanol Production. Ann. Oper. Res. 2019, 276, 211–227. [Google Scholar] [CrossRef]
- de Medeiros, E.M.; Noorman, H.; Maciel Filho, R.; Posada, J.A. Multi-Objective Sustainability Optimization of Biomass Residues to Ethanol via Gasification and Syngas Fermentation: Trade-Offs Between Profitability, Energy Efficiency, and Carbon Emissions. Fermentation 2021, 7, 201. [Google Scholar] [CrossRef]
- Snoeck, S.; Guidi, C.; De Mey, M. “Metabolic Burden” Explained: Stress Symptoms and Its Related Responses Induced by (over)Expression of (Heterologous) Proteins in Escherichia Coli. Microb. Cell Factories 2024, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Evers, T.M.J.; Hochane, M.; Tans, S.J.; Heeren, R.M.A.; Semrau, S.; Nemes, P.; Mashaghi, A. Deciphering Metabolic Heterogeneity by Single-Cell Analysis. Anal. Chem. 2019, 91, 13314–13323. [Google Scholar] [CrossRef] [PubMed]


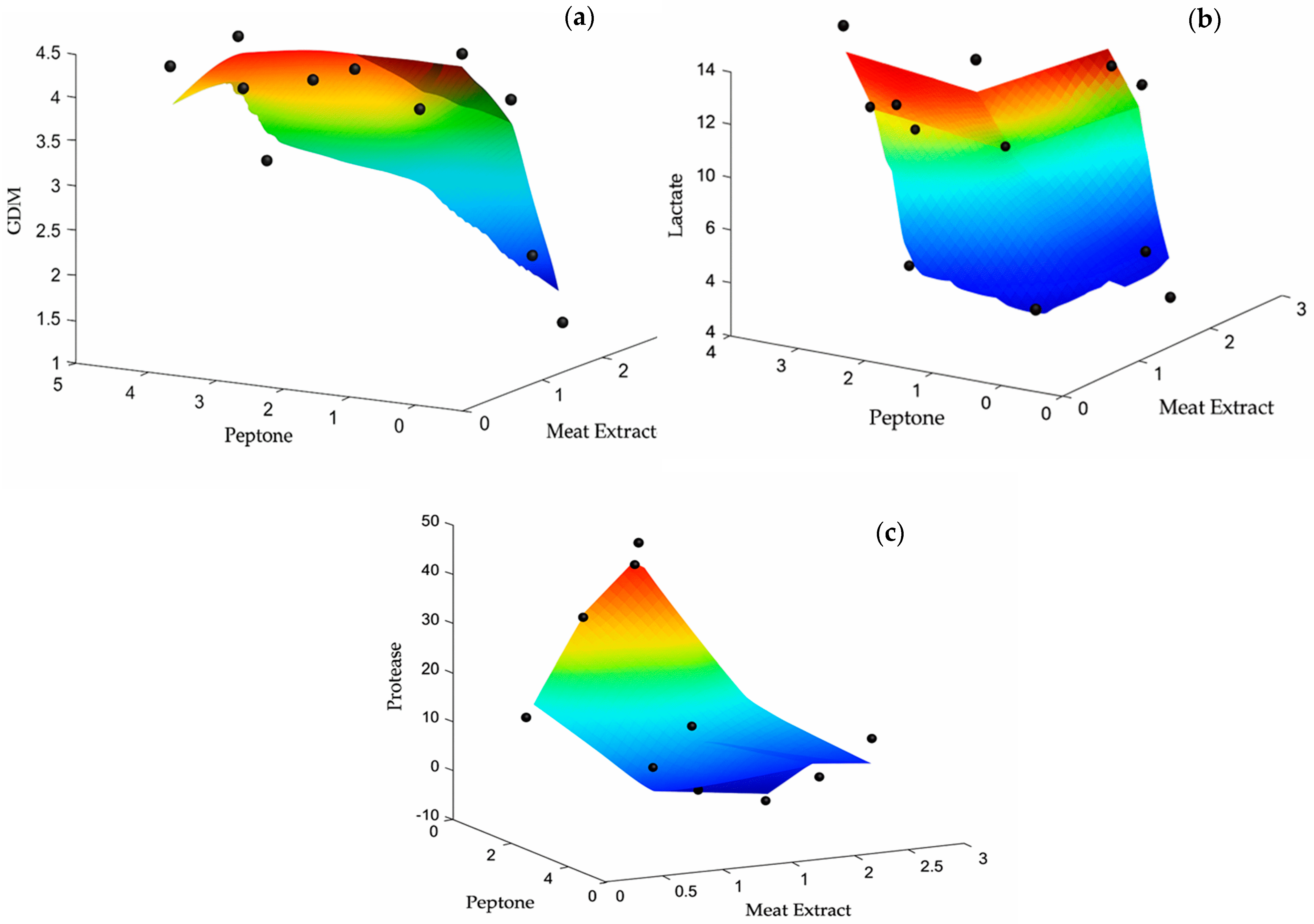
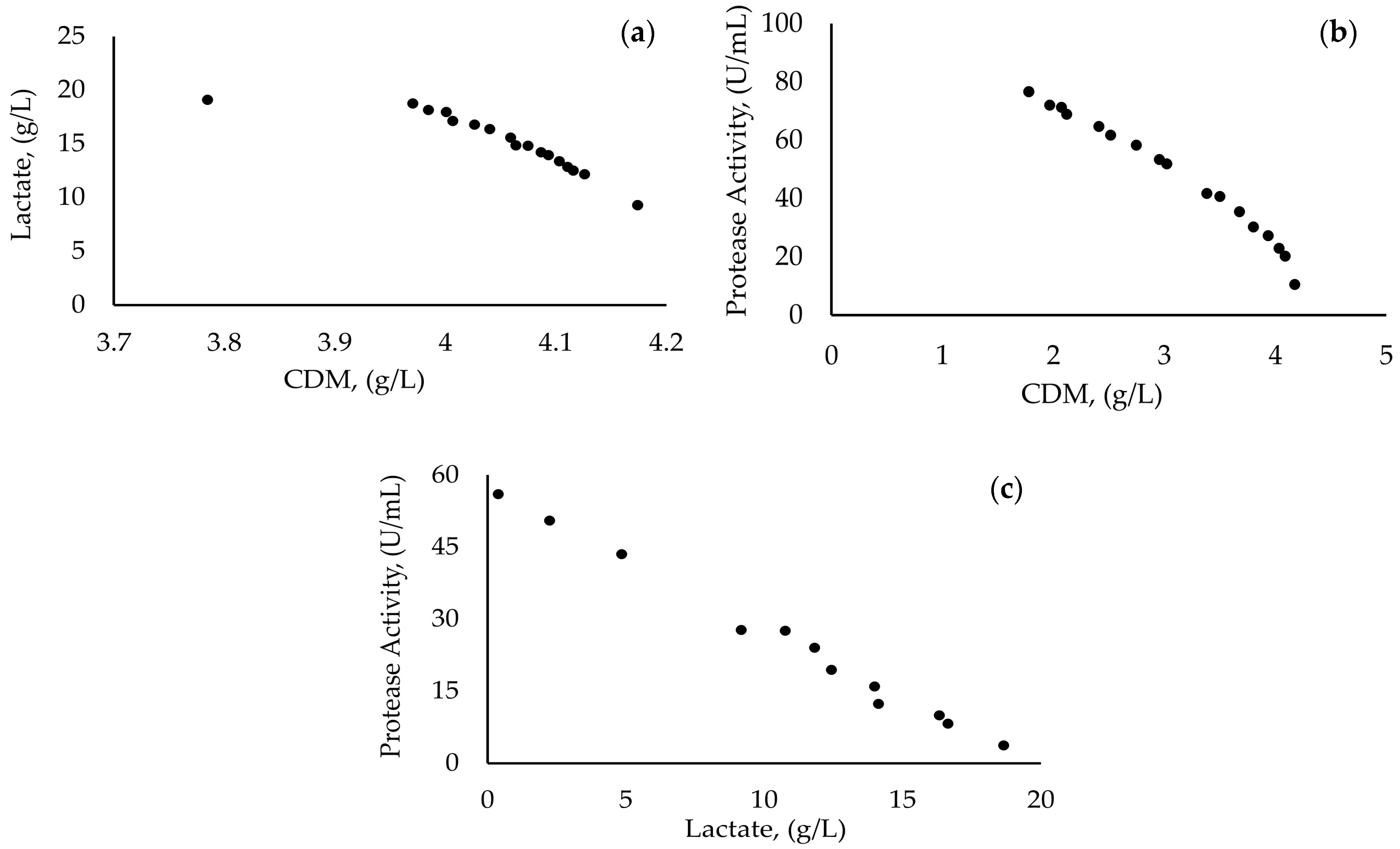
| Sample | Meat Extract | Peptone | Cell Dry Mass (g/L) | |||
|---|---|---|---|---|---|---|
| pH 8.8 | pH 10.5 | |||||
| 24 h | 48 h | 24 h | 48 h | |||
| 1 | 0.10 | 2.55 | 3.3 ± 1.3 | 3.5 ± 1.4 | 2.9 ± 0.1 | 4.0 ± 0.2 |
| 2 | 0.825 | 1.325 | 3.7 ± 0.1 | 4.0 ± 0.1 | 3.4 ± 0.2 | 4.1 ± 0.7 |
| 3 | 0.825 | 3.775 | 3.7 ± 0.2 | 4.1 ± 0.3 | 3.3 ± 0.1 | 3.7 ± 0.1 |
| 4 | 1.20 | 3.80 | 3.8 ± 0.1 | 4.5 ± 0.4 | 3.4 ± 0.1 | 3.8 ± 0.1 |
| 5 | 1.50 | 0.40 | 2.5 ± 0.2 | 2.2 ± 0.1 | 2.6 ± 0.4 | 1.7 ± 0.2 |
| 6 | 1.55 | 0.10 | 1.9 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.3 | 0.9 ± 0.2 |
| 7 | 1.55 | 2.55 | 3.7 ± 0.2 | 4.1 ± 0.2 | 3.2 ± 0.2 | 3.7 ± 0.4 |
| 8 | 1.55 | 5.00 | 3.6 ± 0.2 | 3.8 ± 0.3 | 3.3 ± 0.1 | 3.7 ± 0.1 |
| 9 | 1.80 | 4.20 | 3.5 ± 0.1 | 3.6 ± 0.1 | 3.4 ± 0.1 | 3.8 ± 0.1 |
| 10 | 2.275 | 1.325 | 3.5 ± 0.1 | 3.6 ± 0.2 | 3.2 ± 0.2 | 3.8 ± 0.1 |
| 11 | 2.275 | 3.775 | 3.5 ± 0.2 | 3.6 ± 0.1 | 3.3 ± 0.1 | 3.6 ± 0.1 |
| 12 | 3.00 | 2.55 | 3.5 ± 0.2 | 3.9 ± 0.1 | 3.0 ± 0.1 | 3.7 ± 0.1 |
| Sample | Meat Extract | Peptone | Lactate Concentration (g/L) | |||
|---|---|---|---|---|---|---|
| pH 8.8 | pH 10.5 | |||||
| 24 h | 48 h | 24 h | 48 h | |||
| 1 | 0.10 | 2.55 | 7.4 ± 1.7 | 7.5 ± 0.4 | 7.8± 0.7 | 8.0 ± 0.0 |
| 2 | 0.825 | 1.325 | 6.4 ± 0.6 | 5.2 ± 0.6 | 7.5± 0.0 | 6.8 ± 1.0 |
| 3 | 0.825 | 3.775 | 12.3 ± 4.3 | 12.2 ± 0.1 | 9.5 ± 0.0 | 12.8 ± 0.9 |
| 4 | 1.20 | 3.80 | 8.5 ± 0.6 | 11.8 ± 1.6 | 8.0 ± 1.1 | 13.3 ± 0.5 |
| 5 | 1.50 | 0.40 | 7.1 ± 0.2 | 6.8 ± 0.3 | 4.8 ± 0.3 | 5.9 ± 0.5 |
| 6 | 1.55 | 0.10 | 4.6 ± 0.3 | 5.4 ± 0.1 | 3.5 ± 0.6 | 4.8 ± 1.0 |
| 7 | 1.55 | 2.55 | 7.6 ± 0.4 | 10.4 ± 0.8 | 8.9 ± 0.4 | 10.8 ± 0.3 |
| 8 | 1.55 | 5.00 | 9.8 ± 0.1 | 13.7 ± 0.6 | 15.3 ± 1.1 | 18.4 ± 0.3 |
| 9 | 1.80 | 4.20 | 9.6 ± 0.8 | 9.9 ± 1.0 | 17.5 ± 1.4 | 17.1 ± 0.1 |
| 10 | 2.275 | 1.325 | 10.8 ± 0.9 | 12.4 ± 1.2 | 18.2 ± 1.9 | 19.3 ± 0.3 |
| 11 | 2.275 | 3.775 | 9.4 ± 0.2 | 12.1 ± 1.7 | 17.5 ± 0.3 | 18.1 ± 0.9 |
| 12 | 3.00 | 2.55 | 4.7 ± 0.3 | 11.6 ± 1.5 | 11.1 ± 0.5 | 16.6 ± 2.4 |
| Sample | Meat Extract | Peptone | Protease Activity (U/mL) | |||
|---|---|---|---|---|---|---|
| pH 8.8 | pH 10.5 | |||||
| 24 h | 48 h | 24 h | 48 h | |||
| 1 | 0.10 | 2.55 | 8.3 ± 1.6 | 12.7 ± 7.4 | 4.6 ± 1.9 | 14.9 ± 0.1 |
| 2 | 0.825 | 1.325 | 14.6 ± 1.4 | 25.4 ± 9.0 | 12.2 ± 0.9 | 31.7 ± 5.5 |
| 3 | 0.825 | 3.775 | 0 | 0 | 0 | 6.7 ± 0.9 |
| 4 | 1.20 | 3.80 | 0 | 0 | 0 | 0 |
| 5 | 1.50 | 0.40 | 36.4 ± 2.7 | 41.7 ± 5.0 | 26.3 ± 5.7 | 43.5 ± 1.6 |
| 6 | 1.55 | 0.10 | 18.2 ± 2.1 | 19.8 ± 4.6 | 16.4 ± 5.1 | 37.7 ± 4.2 |
| 7 | 1.55 | 2.55 | 0 | 8.4 ± 4.9 | 0 | 9.5 ± 4.5 |
| 8 | 1.55 | 5.00 | 0 | 3.2 ± 0.5 | 0 | 0 |
| 9 | 1.80 | 4.20 | 0 | 0 | 0 | 0 |
| 10 | 2.275 | 1.325 | 0 | 0 | 0 | 0 |
| 11 | 2.275 | 3.775 | 0 | 0 | 0 | 0 |
| 12 | 3.00 | 2.55 | 0 | 0 | 0 | 3.7 ± 2.6 |
| pH | Time (h) | Meat Extract | Peptone | |
|---|---|---|---|---|
| CDM (g/L) | 8.8 | 48 | 0.10 | 3.03 |
| Lactate (g/L) | 8.8 | 48 | 3.00 | 1.61 |
| Protease (U/mL) | 10.5 | 48 | 0.10 | 0.10 |
| Meat Extract | Peptone | CDM | Lactate Concentration | Protease Activity | |||
|---|---|---|---|---|---|---|---|
| Prediction | Validation | Prediction | Validation | Prediction | Validation | ||
| 0.10 | 3.03 | - | 3.7 ± 0.4 | - | - | ||
| 0.10 | 0.10 | 16.2 ± 1.0 | |||||
| 3.00 | 1.61 | - | 16.6 ± 1.4 | - | |||
| 1.8 | 3.0 | 4.1 | 4.0 ± 0.7 | 14.9 | 19.3 ± 0.9 | - | - |
| 0.2 | 1.40 | 3.4 | 3.4 ± 0.1 | - | - | 41.8 | 30.9 ± 3.0 |
| 2.0 | 0.20 | - | - | 11.8 | 12.2 ± 2.0 | 24.0 | 21.2 ± 4.7 |
| 2.6 | 2.0 | 2.6 | 2.4 ± 0.7 | 4.9 | 4.9 ± 0.9 | 38.2 | 32.1 ± 7.6 |
| 1.0 | 0.75 | 3.6 | 3.3 ± 0.6 | 17.3 | 20.6 ± 2.9 | 1 | 1.1 ± 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atakav, Y.; Kurpejović, E.; Kazan, D.; Sayar, N.A. An Exploratory Study on the Growth Dynamics of Alkalihalophilus marmarensis Using a Model-Based Approach. Appl. Microbiol. 2025, 5, 69. https://doi.org/10.3390/applmicrobiol5030069
Atakav Y, Kurpejović E, Kazan D, Sayar NA. An Exploratory Study on the Growth Dynamics of Alkalihalophilus marmarensis Using a Model-Based Approach. Applied Microbiology. 2025; 5(3):69. https://doi.org/10.3390/applmicrobiol5030069
Chicago/Turabian StyleAtakav, Yağmur, Eldin Kurpejović, Dilek Kazan, and Nihat Alpagu Sayar. 2025. "An Exploratory Study on the Growth Dynamics of Alkalihalophilus marmarensis Using a Model-Based Approach" Applied Microbiology 5, no. 3: 69. https://doi.org/10.3390/applmicrobiol5030069
APA StyleAtakav, Y., Kurpejović, E., Kazan, D., & Sayar, N. A. (2025). An Exploratory Study on the Growth Dynamics of Alkalihalophilus marmarensis Using a Model-Based Approach. Applied Microbiology, 5(3), 69. https://doi.org/10.3390/applmicrobiol5030069






