Abstract
Schizophrenia is a complex psychiatric disorder traditionally linked to neurotransmitter dysregulation, particularly within dopamine and glutamate pathways. However, recent evidence implicates the gut–brain axis as a potential contributor to its pathophysiology. This perspective article proposes a systems-level understanding of schizophrenia that incorporates the role of gut microbial dysbiosis specifically, reductions in short-chain fatty acid (SCFA)-producing taxa, and elevations in pro-inflammatory microbes. These imbalances may compromise gut barrier integrity, stimulate systemic inflammation, and disrupt neurochemical signaling in the brain. We synthesize findings from animal models, clinical cohorts, and microbial intervention trials, highlighting mechanisms such as SCFA regulation, altered tryptophan–kynurenine metabolism, and microbial impacts on neurotransmitters. We also explore microbiome-targeted interventions like probiotics, prebiotics, dietary strategies, and fecal microbiota transplantation (FMT) and their potential as adjunctive therapies. While challenges remain in causality and translation, integrating gut–brain axis insights may support more personalized and biologically informed models of schizophrenia care.
1. Introduction
Schizophrenia is a multifaceted psychiatric disorder affecting approximately 1% of the global population and is marked by a wide spectrum of symptoms and an elusive etiology [1]. Prevailing models used to understand the pathology and symptoms of schizophrenia have long emphasized neurotransmitter dysregulation, particularly dopamine imbalance and overactivity [2,3]. While foundational, this perspective captures only a fragment of the disorder’s biological complexity. Contemporary research increasingly points to schizophrenia as the product of dynamic interactions between genetic predisposition, epigenetic modulation, environmental exposures, and systemic inflammatory processes [4]. Clinically, it disrupts cognition, emotion, and behaviour, and often manifests through hallucinations, delusions, and impairments in motivation, memory, and daily functioning [5]. There are both positive and negative symptoms to schizophrenia. Any addition to a person’s mental experience, like delusions, hallucinations, disorganized thinking, excitement or agitation, suspiciousness, grandiosity, and hostility, among many, are classified as positive symptoms. Negative symptoms of schizophrenia refer to symptoms that cause reductions or losses in normal functioning, like lacking emotional expression, emotional withdrawal, poor rapport, social withdrawal, difficulty in abstract thinking, and stereotyped thinking [5]. Although antipsychotic medications can mitigate acute psychotic symptoms, long-term outcomes frequently depend on early intervention and sustained psychosocial support.
Parallel with these developments, a growing body of research has turned to the gut microbiome as a novel frontier in psychiatric science [6,7]. Alterations in gut microbial composition are now believed to influence brain function through immune modulation, neuroinflammatory pathways, and the regulation of neurotransmitter synthesis. Immune modulation differs from neuroinflammatory pathways, which involve direct inflammatory activity within the central nervous system (CNS). Immune modulation involves systemic changes mediated by microbial metabolites such as SCFAs, which are produced when gut microbes ferment dietary fibers [8]. SCFAs can regulate immune cell proliferation and cytokine production, and they also exert anti-inflammatory effects by binding to G-protein-coupled receptors found on the surface of many cells throughout the body, playing a key role in transmitting signals inside the cell. SCFAs can inhibit enzymes like histone deacetylases (HDACs), which control how certain genes involved in inflammation are expressed. Within this framework, schizophrenia may be partially rooted in disruptions along the gut–brain axis, a connection that repositions the disorder within a broader system-level understanding of mental health.
This article argues that current models of schizophrenia, although biologically grounded, remain incomplete without consideration of the gut–brain axis. We propose that the findings from emerging microbiome studies of schizophrenia warrant serious consideration as a critical component of a more comprehensive framework for understanding and treating schizophrenia. Repositioning the disorder within a systems-level, bidirectional model of brain–body interaction invites new therapeutic possibilities, including microbiome-based interventions that could enhance outcomes when used alongside traditional psychiatric approaches.
This perspective draws on a curated body of recent studies including clinical cohorts, animal models, multi-omic profiling, and microbial intervention trials. While not itself an experimental study, all articles were searched in databases like PubMed, Scopus, ScienceDirect, and the Cochrane Library for this paper. Reference lists of the retrieved articles were systematically searched for the various combinations of the following terms: schizophrenia, gut microbiome or gut-brain-axis, SCFAs, blood-brain barrier or BBB, and targeted therapy. One investigator independently reviewed the full texts of eligible studies and verified selections in consultation with two additional authors. Findings from these diverse methodologies were synthesized to develop a system-level conceptual framework linking gut microbiota to schizophrenia.
2. The Gut–Brain Axis and Its Implications for Broad Mental Health Conditions
Gut microbes can modulate systemic inflammation and help shape immune responses by producing signaling molecules that enter the bloodstream and interact with the brain [9]. These interactions occur through three primary mechanisms, the first being through the modulation of systemic inflammation. Microbes produce metabolites that can enter the bloodstream and travel to the brain, either triggering inflammatory pathways or reducing them [9,10]. The second mechanism involves direct communication via the vagus nerve, which conveys sensory information from the gut to the brain. Vagal sensory neurons receive input from enteric neurons, which coordinate digestive functions, as well as enteroendocrine cells that sense nutrients and microbial byproducts and release signaling molecules in response [11]. Although the vagus nerve does not produce neurotransmitters itself, it transmits signals shaped by gut microbial activity, including those related to the presence of gamma-aminobutyric acid (GABA), dopamine, and serotonin [9,10,12]. The third mechanism includes the action of metabolites produced by gut microbes. Gut microbes metabolize food ingested and synthesize neuroactive compounds—particularly SCFAs, which are beneficial [9,10]. The SCFA produced upon digestion of dietary fiber, butyrate, helps regulate neuroinflammation and maintain the BBB, protecting the brain from harmful agents [10,13]. Other tryptophan-derived metabolites, like kynurenine, increase neuroinflammation and cognitive decline [14].
Together, these findings challenge the dominant notion that psychiatric disorders originate solely in the brain. The gut–brain axis reveals a more intricate biological reality, in which microbial, immune, and neural systems also regulate mental health. As evidence continues to accumulate, it becomes increasingly clear that any comprehensive model of psychiatric illness must account for this broader physiological network.
3. Microbiome Dysbiosis and Its Role in Schizophrenic Pathophysiology
To understand this section, the concept of microbiome dysbiosis should be explained, as it carries multiple definitions in contemporary literature. For this paper, dysbiosis refers to a disruption in the composition, diversity, or function of the gut microbiome that deviates from an individuals’ unique healthy baseline. Operationally, this includes a reduction in beneficial butyrate-producing taxa (Faecalibacterium and Roseburia), an increase in pro-inflammatory or potentially pathogenic microbes (Eggerthella and Escherichia/Shigella), and associated alterations in metabolic byproducts like SCFAs, glutamate, or lipopolysaccharides (LPSs) that have been linked to neurological or immunological function [15]. In the context of schizophrenia specifically, the gut–brain axis offers a framework for explaining dimensions of the disorder that traditional neurotransmitter-based models do not fully capture. Dopamine, glutamate, and GABA-related microbial genes’ dysregulation remain central to prevailing theories due to their elevated levels in body for those with schizophrenia [16,17].
A growing body of preclinical and clinical research has revealed consistent alterations in gut microbiota composition among individuals with schizophrenia when compared to healthy controls. Although total microbial counts. known as alpha diversity, do not appear markedly different, there are reproducible shifts in the microbial community structure, known as beta diversity [4,18,19]. A reduction was found in bacteria like Faecalibacterium, Coprococcus, and Roseburia, which synthesize SCFAs crucial for maintaining gut barrier integrity, regulating neuroinflammation, and supporting brain health [19,20,21]. Alongside this reduction, an increase in bacteria associated with inflammation and disrupted neurotransmission was found in those with schizophrenia. For example, Eggerthella, a microbe associated with disrupted glutamate metabolism, tends to be elevated, as does Lactobacillus [22], which produces lactic acid that leaks through the gut and alters brain pH, promotes inflammation, and affects neurotransmission while influencing GABA signaling [4,18,19]. Additionally, Escherichia/Shigella species are more abundant in this population and are known to activate inflammatory responses and interfere with GABA pathways [4,18,19]. See Table A1 for a summary of gut microbial composition in schizophrenia compared to healthy participants. These compositional imbalances correlate with heightened systemic inflammation, disrupted metabolic profiles, and increased symptom severity in schizophrenia patients. While causality has not been established, these patterns suggest a pathway that merits investigation.
This connection becomes more compelling when viewed through metabolic outcomes. In a study by Peng et al. (2022), researchers examined three groups: the healthy, those at-risk of schizophrenia, and those with schizophrenia [23]. Due to lacking SCFA-producing bacteria, as noted from past studies, it was found that levels of two SCFAs, valeric acid and caproic acid, were significantly lower in both the schizophrenia group and in the at-risk population. These SCFAs specifically are known to support brain health, reduce inflammation, and protect nerve cells, which makes their deficiency correlated with symptom severity [23]. The directionality of this relationship remains unclear.
Taken together, these findings reinforce the proposed perspective, that schizophrenia may not arise solely from disruptions within the brain, but also from signals originating in the gut. Microbial dysbiosis may serve as a modifiable risk factor, and exploring this relationship introduces the possibility of complementary therapeutic strategies targeting the microbiome.
4. Mechanistic Pathways Linking Gut Microbiota to Schizophrenia
Several mechanisms exist to explain how gut microbiota might influence brain function in the context of schizophrenia. Three key interrelated pathways involving SCFAs, tryptophan–kynurenine metabolism, and neurotransmitter signaling best explain how microbiome disturbances influence schizophrenia symptoms. See Figure 1 for a summary of these pathways.
Ju et al. (2023) explain that SCFAs, particularly butyrate, acetate, and propionate, are microbial fermentation products that cross the BBB and regulate neuroinflammation, BBB integrity, and synaptic plasticity [24]. Butyrate, for instance, inhibits HDACs, upregulates brain-derived neurotrophic factor (BDNF), and promotes anti-inflammatory signaling, all of which are relevant to cognitive and affective symptoms in schizophrenia [24]. Shi et al. (2023) remark that a reduced abundance of SCFA-producing genera such as Faecalibacterium, Roseburia, Dialister, and Megasphaera has been observed in patients with schizophrenia and correlates with symptom severity [25].
The tryptophan–kynurenine pathway is another critical interface between the gut and brain. Hare et al. (2023) and Pedraz-Petrozzi et al. (2020) explain that inflammatory activation of indoleamine 2,3-dioxygenase diverts tryptophan metabolism away from serotonin synthesis and toward kynurenine [26,27]. This leads to elevated levels of kynurenic acid, an N-methyl-D-aspartate (NMDA) receptor and α7-nicotinic receptor antagonist, and quinolinic acid, a neurotoxic NMDA receptor agonist. Both metabolites have been implicated in cognitive impairment and psychotic symptoms [26,27]. This shift can lead to the accumulation of neurotoxic metabolites and altered glutamate signaling, both of which are implicated in schizophrenia’s cognitive and affective disturbances [4,21,28].
Finally, Kamath et al. (2024) and Munawar et al. (2021) explain that gut microbes influence neurotransmitter signaling both directly, by producing GABA (Lactobacillus), dopamine (Bacillus), and serotonin precursors, and indirectly, by modulating precursor availability and immune signaling [4,29]. Dysbiosis may disrupt dopaminergic, glutamatergic, and GABAergic tone, contributing to the positive, negative, and cognitive symptoms of schizophrenia like hallucinations, delusions, or cognitive deficits [1,4].
Although not a primary mechanistic pathway itself, intestinal permeability, or “leaky gut”, is a consequence of microbiome dysbiosis [28,29]. A study by Maes et al. (2019) noted that tight junction proteins, like occludin, and claudin-5, and adherens junction proteins, like E-cadherin and β-catenin, help maintain the integrity of the gut barrier [30]. In schizophrenia, patients show elevated immunoglobin A antibodies against these proteins, suggesting the immune system is reacting to their breakdown. This breakdown in the gut barrier allows microbial products like LPSs to enter circulation, breach the BBB, and activate the brain’s immune cells, microglia [4,20,30]. This may trigger systemic inflammation and BBB breakdown, which results in neuroinflammation, which could contribute to the negative symptoms of schizophrenia and cognitive decline [4,20], though the causal mechanisms are being investigated. This pathway is particularly relevant given the elevated inflammatory markers observed in many individuals with schizophrenia [19,21].
Qi et al. (2024) demonstrated that gut dysbiosis in schizophrenia is closely linked to altered levels of glutamate, nicotinamide, and arachidonic acid, three metabolites involved in neurotransmission and inflammation [17]. These changes were associated with microbial genera such as Clostridium and Lactobacillus, suggesting that gut microbial imbalance may affect systemic signaling. Building on this, Ghorbani et al. (2024) provided findings indicative of gut–brain interactions at the systemic level [31]. In a pilot study measuring plasma biomarkers, patients with schizophrenia exhibited elevated serotonin and dopamine levels, and moderate reductions in BDNF. These neurochemical shifts correlated with the relative abundance of gut bacteria such as Roseburia intestinalis, Dorea longicatena, and Parabacteroides goldsteinii. The microbial profile also showed an increase in succinate and acetate-producing bacteria, commonly associated with inflammation and metabolic disturbance [31]. Together, these findings suggest that dysbiosis may be associated with shifts in circulating neurotransmitter levels, possibly mediated by metabolites that cross the gut barrier. This supports the hypothesis that intestinal permeability may act as a gateway for inflammatory or neuroactive compounds, initiating or worsening brain dysfunction in schizophrenia [17].
While these mechanisms remain under investigation, recent experimental research has begun to test for causality. Wei et al. (2024) showed that FMT from individuals with schizophrenia into specific-pathogen-free mice led to schizophrenia-like behaviors, including hyperactivity, anxiety, and social deficits [32]. Moreover, recipient mice exhibited dysregulated gene expression in pathways related to neuroactive ligand–receptor interactions, GABA signaling, and immune function. Notably, four genes implicated in both human and mouse brain transcriptomes—Gabre, Baiap3, Magel2, and Cybb—were correlated with specific microbial taxa, reinforcing the hypothesis that gut microbiota may shape brain function via immune and neurochemical pathways. These findings offer compelling support for a mechanistic role of the gut–brain axis in schizophrenia’s pathophysiology and suggest that gut microbes may actively modulate central processes involved in psychosis [32]. These results offer a biologically plausible association between microbial alterations and CNS dysfunction, suggesting that the gut microbiome is an active participant in schizophrenia’s neurobiology, though causality has yet to be firmly established.
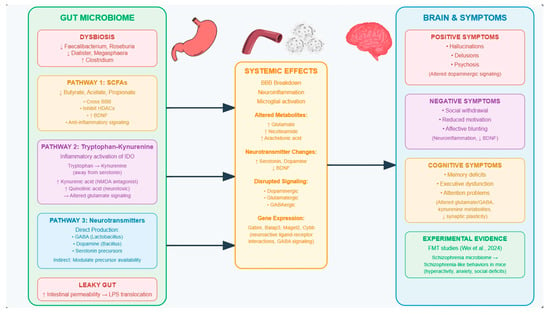
Figure 1.
Schematic overview of gut microbiome-related pathways implicated in schizophrenia. Dysbiosis is characterized by a reduction in SCFA-producing bacteria and increased pathogenic taxa, leading to systemic effects such as neuroinflammation, altered neurotransmitter synthesis, and disrupted signaling. These changes may contribute to the manifestation of schizophrenia symptoms across positive, negative, and cognitive domains. Arrows represent directional influence and communication between subsystems. Specifically, signaling cascades and physiological interactions originating in the gut can influence systemic immune or metabolic responses, which in turn modulate neural activity in the brain [32].
5. Therapeutic Implications
Building on these mechanistic insights, targeting the microbiome may offer a potential therapeutic opportunity. Preliminary studies have explored interventions such as probiotics, prebiotics, dietary modulation, and FMT with varying degrees of success. See Table A2 for a summary of all microbiome-based interventions.
5.1. Probiotics
Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits to the host. In schizophrenia, formulations containing Lactobacillus and Bifidobacterium species have been studied. Small clinical trials suggest that probiotic supplementation may help alleviate GI symptoms and modestly reduce psychiatric symptom severity [33]. Multiple clinical trials controlling for placebo found that probiotic supplementation significantly reduced overall symptom severity, particularly in general psychopathology domains. The findings suggest that probiotics have been proposed to modulate neuroinflammatory pathways, oxidative stress, and neurotransmitter systems via the microbiota–gut–brain axis, offering a biologically plausible mechanism of action [33]. Definitive mechanisms remain under investigation.
A particular study by Tomasik et al. (2015) demonstrated that probiotic supplementation with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB12 in individuals for 14 weeks with chronic schizophrenia led to immunomodulatory effects [34]. While no changes in the severity of core psychiatric symptoms were observed, as measured by the total Positive and Negative Syndrome Scale (PANSS), the probiotic group showed a significant reduction in von Willebrand factor (vWF), a marker of inflammation and cardiovascular risk. There were also borderline increases in immune-regulating molecules, including BDNF, monocyte chemoattractant protein-1 (MCP-1), and inflammatory cytokines such as RANTES and MIP-1β, which are known to influence neuroplasticity and immune signaling. Pathway analysis indicated these shifts were related to the IL-17 family cytokine pathways, which play a key role in intestinal and immune function [34]. Severance et al. (2017) provided a supplement of Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB12 over 14 weeks [35]. It was found that males had lower Candida albicans antibodies, but females did not due to potential baseline differences in genitourinary yeast exposure. C. albicans was important for their study, as its overgrowth can contribute to worse GI and psychiatric symptoms in schizophrenia. As a result, they found significantly higher PANSS scores for positive and general symptoms, and suggested C. albicans seropositivity can be a biomarker for a subgroup of patients who can benefit from microbiome-targeted interventions [35]. In a more recent study, Ghaderi et al. (2019) provided a supplement of Lactobacillus acidophilus, Bifidobacterium bifidium, Limosilactobacillus reuteri, and Limosilactobacillus fermentum over 12 weeks [36]. In this experiment, there was a significant improvement in the PANSS total score and general psychopathology PANSS subscale [36]. In a study by Jamilian and Ghaderi (2021), a combination of Lactobacillus aciphilus, Bifidobacterium lactis, Bifidobacterium bifidum, Bifidobacterium longum, and Selenium was administered over 12 weeks to a group of patients with schizophrenia [37]. Selenium is given with probiotics as a trace mineral, which plays a critical role in antioxidant defense through its involvement in glutathione peroxidase, which is an enzyme that protects the brain from oxidative stress [29,38,39]. Its deficiency, which is often seen in patients with schizophrenia, may allow for an increase in oxidative damage, decreased glutathione activity, and heightened immune responses, all of which are relevant to the pathophysiology of schizophrenia. Upon probiotic supplementation with selenium, a significant improvement in the clinical symptoms of the disorder was found [37]. They also noted a significant improvement in the GI system of patients, as schizophrenia may be accompanied by symptoms of diarrhea, constipation, and celiac disease [40,41]. Mujahid et al. (2022) provided patients with risperidone, an antipsychotic medicine used for the treatment of schizophrenia, alongside probiotics, which were undisclosed in the article, for a duration of 6 weeks [42]. They too found significant reduction in the total PANSS score in the group treated with probiotics compared to the control group [42]. In a final study by Mohammadi et al. (2024), participants diagnosed with schizophrenia received a probiotic supplement containing Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus paracasei, Bifidobacterium longum, and Bacillus coagulans [43]. This was given with vitamin D every day for 12 weeks due to their supposed synergistic action. The authors found a decrease in the PANSS total score between the study groups, but these results were not statistically significant according to their established criteria. They did, however, report better cognitive function as measured by the MoCA test, as more participants reached a MoCA score of above 26, which is considered a normal cognition threshold. Significant reductions in C-reactive protein, total cholesterol, and fasting blood sugar in the probiotic and vitamin D group were also found [43]. C-reactive protein, a marker for systemic inflammation, has been indicated to be associated with the pathogenesis of schizophrenia, as its concentration in the blood is often found to be moderately increased in people with schizophrenia [44]. Tamtaji et al. (2019) combined probiotic supplementation with selenium in their study, which resulted in a significant reduction in serum C-reactive protein, showcasing similar effects as other studies [45]. Given that the potential side effects of probiotic treatment are minimal and are generally tolerated well among patients [46], they may serve as possible adjuncts to traditional pharmacological solutions. These findings offer preliminary evidence that probiotics may influence systemic inflammation and intestinal permeability, though further research is required to establish consistent therapeutic effects.
5.2. Prebiotics
Prebiotics are nondigestible dietary fibers that selectively stimulate the growth or activity of beneficial gut bacteria. Unlike probiotics, prebiotics do not introduce live microbes but instead enhance the environment for existing commensals. Compounds like galactooligosaccharides and fructooligosaccharides have been studied in relation to psychiatric conditions for their ability to support SCFA-producing bacteria. Clinical studies in schizophrenia are limited, but preclinical studies suggest that prebiotics may modulate neuroinflammation, restore microbial diversity, and influence stress responses and cognitive function. Kao et al. (2018) found that a galactooligosaccharide, called B-GOS, could reduce olanzapine-induced weight gain and neuroinflammatory markers in mice [47]. Furthermore, prebiotics did not interfere with the intended action of olanzepine on the brain; rather, they increased proteins linked to cognition in the brain’s cortex. B-GOS and olanzepine did increase plasma acetate levels individually, which is an SCFA linked to appetite and brain signaling. But, when taken together, acetate levels normalized, suggesting a potential adjunctive therapy to reduce side effects of commonly prescribed antipsychotic medications [47]. Trials in human populations with schizophrenia are still needed to clarify efficacy and optimal formulations.
5.3. Dietary Interventions
Dietary interventions like the Mediterranean diet for schizophrenia have been proposed as a means of helping treat immune and metabolic dysfunction in schizophrenia by increasing SCFA production in the gut. Studies of dietary intake in schizophrenia suggest that most patients have poor diet largely characterized by fast and processed foods [48], increased sodium and cholesterol intake [49], and high saturated fats and low fiber [50]. The high content of fiber, polyphenols, and omega-3 fatty acids promotes the growth of SCFA-producing bacteria like Faecalbacterium and Roseburia [51,52]. Noted prior, these microbes were found to be reduced in those with schizophrenia. There remains a lack of evidence pertaining to this topic, necessitating controlled trials to understand the validity of these claims in human models. Other diets include the ketogenic and gluten-free diet. A ketogenic diet is a high-fat, very-low-carbohydrate diet that induces ketosis, a metabolic state where the body uses ketone bodies instead of glucose for energy [53]. When on the ketogenic diet, rodent models of schizophrenia reduced hyperactivity and stereotyped behaviours, improved working memory and cognition, and normalized hippocampal function and glutamatergic signaling. Authors suggest that these results were comparable to or synergistic with antipsychotic drugs like olanzepine [53]. During clinical trials, remission of symptoms, deductions in the PANSS score, weight loss, and improved social functioning was reported while on the ketogenic diet [53,54]. The exact mechanism for why the ketogenic diet is effective is still debated, but speculations involve NMDA receptor modulation through its counteraction against glutamatergic hypofunction. Mitochondrial support is also debated, as the diet improves energy metabolism and reduces oxidative stress. Finally, microbiome modulation is also considered, but this is still speculative [53,54].
The final diet is a gluten-free diet. This diet is used particularly, as research suggests that one in three people with schizophrenia have elevated AGA, and immunoglobin G—inflammatory markers that suggest gluten sensitivity [55]. Some people with schizophrenia have anti-gliadin antibodies (AGAs) or anti-transglutaminase 6 tTG6—markers of gluten sensitivity that may be linked to psychiatric symptoms [56]. Gluten sensitivity may trigger immune activation and inflammation, which are implicated in schizophrenia. Removing gluten may reduce cytokine levels, improve gut barrier integrity, and lower neuroinflammation, especially in those with AGA immunoglobin G [55]. Participants on a gluten-free diet for 5 weeks showed moderate improvements in negative symptoms, enhanced attention, and significant reductions in gastrointestinal symptoms compared to a gluten-containing control. AGA Immunoglobin G levels also dropped more in the gluten-free group, suggesting reduced immune activation. The intervention was well tolerated, indicating potential benefit for a biologically defined subgroup [55].
5.4. FMT
Beyond probiotic solutions, alternative mechanisms like FMT and dietary changes are also being researched as potential effective therapeutic. FMT involves the transfer of stool from a healthy donor into the gastrointestinal (GI) tract of a recipient, with the aim of restoring microbial diversity and function. The study by Wei et al. (2024) involved the transplantation of microbiota from individuals with schizophrenia into healthy mice [32]. The transplant induced schizophrenia-like behaviors, providing experimental support to a potential causal role in symptom development. Restoring microbial balance through interventions, like FMT, and dietary changes that promote beneficial bacteria could offer new symptom-relief pathways. Moreover, mice exposed to schizophrenia-associated microbiota showed the altered expression of genes tied to neurotransmission, immunity, and stress response (e.g., Gabre, Baiap3, Magel2, and Cybb), opening doors to molecular therapies or biomarker-driven personalization. While these strategies remain experimental, they invite a shift in psychiatric treatment paradigms from narrowly targeting dopamine to considering inflammation, gut–brain communication, and early intervention.
Though not a replacement for antipsychotics, microbiome-focused therapies like these could meaningfully enhance outcomes when used adjunctively. In the future, therapies should be personalized and microbiome-informed to consider symptom clusters, drug response profiles, and individual dysbiosis patterns. Personalized treatments will enable healthcare practitioners to predict drug responses in the body. Given that up to 30% of patients fail to respond to antipsychotic therapy [57], personalized therapy may be able to reduce the percentage of individuals that do not benefit from the current broad-spectrum options available. It may also minimize unwanted side effects of antipsychotic medication, as this personalized approach will take into account specific microbial patterns, like high Firmicutes/Bacteroidetes ratios, which are linked to weight gain and metabolic dysfunction in those with schizophrenia [29]. This approach requires further research and time to develop, which is why current evidence advocates for microbiome-informed prescribing. Gut-neutral antipsychotic medications like lurasidone are given emphasis for metabolically vulnerable patients [29]. This would help reduce side effects like weight gain and insulin resistance that are common with common antipsychotics available today [58,59,60] while reducing the gut’s influence on schizophrenic symptoms. Currently, research associates certain microbes to metabolize common antipsychotic drugs like risperidone, reducing their efficacy on schizophrenic symptoms [61,62,63]. This necessitates the consideration of the gut microbiota in therapeutic intervention alongside novel pharmacotherapy.
6. Challenges in Implementation
Despite growing interest in microbiome-based interventions, translating these approaches into mainstream psychiatric care remains complex. One of the key challenges is the considerable variability among individuals’ microbiomes and response to treatment. Effects of probiotics, dietary interventions, or fecal microbiota transplantation tend to be modest, and many clinical trials lack rigorous psychiatric endpoints or long-term follow-up [20,28].
A further complication lies in the term “dysbiosis” itself, which remains loosely defined [20,28]. Though we have a general hypothesis, a deep understanding of what the changes in gut microbiome composition do to impact mental health symptoms requires further exploration. For example, there was a noted abundance of Lactobacilli found in the gut microbiome of those with schizophrenia, but some argue that these microbes are beneficial, which adds to the complexity of understanding the impact an imbalanced gut microbiome on schizophrenic symptoms [28].
Furthermore, the question of causality remains unresolved as to whether dysbiosis contributes to schizophrenia, or if it is a downstream consequence of the disorder itself or confounding variables like antipsychotic medication, poor diet, chronic stress, and a high body mass index (BMI). It is important to note that while a strong association exists, the definitive cause-and-effect relationship between gut dysbiosis and schizophrenia has not yet been conclusively established. Some shifts, particularly increases in Lactobacillus, were more common in patients taking the antipsychotic medications olanzapine and risperidone, suggesting traditional treatment itself may be creating a feedback loop of metabolic and psychiatric burden [18,29,63]. This is particularly relevant given the metabolic side effects of these antipsychotic medications such as weight gain and altered appetite regulation [29,58,60,64,65,66]. These medications affect brain pathways that regulate appetite, metabolism, and fat storage, which increases cravings for carbohydrates, reduces satiety signals, and slows down metabolism, forming optimal conditions for the accumulation of body fat. Research highlights the potential of shared microbiome signature between schizophrenia and obesity, including microbial changes, low-grade inflammation, altered BDNF levels, and tryptophan metabolism shifts [28]. This overlap suggests that the microbiome may be a point linking metabolic and psychiatric disturbances. Moreover, many of the microbiome alterations observed in schizophrenia, like reduced butyrate-producing bacteria and elevated inflammatory genera, were also found in other disorders like major depressive disorder (MDD), bipolar disorder, and anxiety [18]. This suggests a transdiagnostic microbiome profile, in which there may be a shared inflammatory or metabolic pathway across conditions.
The role of Lactobacillus species in schizophrenia remains uncertain, with studies showing mixed results. While some of the research reports improvement in inflammation or symptoms, others find minimal or no therapeutic benefit. We think that these inconsistencies may reflect the influence of contextual factors like diet, medication, and bacterial strain differences between individuals. Individual dietary habits could affect how well probiotic strains colonize or survive in the gut, and antipsychotic medications may alter the microbial environment in ways that impact treatment outcomes. Moreover, different studies use different subtypes of Lactobacillus which may vary in their effects on the immune system and neurotransmitter signalling.
Methodological inconsistencies further complicate interpretation. Differences in sample collection, sequencing platforms, bioinformatic pipelines, and statistical analysis limit reproducibility and comparability across studies. To advance the field, future research must adopt standardized protocols, longitudinal study designs, and integrative multi-omic approaches (e.g., metagenomics, metabolomics, and immunophenotyping). Additionally, developing translational models with clinically relevant endpoints will be critical to validating microbiome-based therapies and understanding their true potential in psychiatric care [20].
While findings remain mixed and sample sizes small, current evidence strengthens the case for expanding schizophrenia treatment beyond traditional dopamine-targeted approaches. Although some studies report no significant improvements in core psychiatric symptoms, probiotics have positively affected biological processes relevant to general mental health—including gut permeability, systemic inflammation, and potentially cognition [33,67]. These results underscore the need for larger, well-controlled trials to clarify the therapeutic potential and identify which patient subgroups may benefit most.
7. Conclusions
The gut–brain axis is reshaping how neurology, psychiatry, and microbial biology intersect. In schizophrenia, a disorder marked by complexity and biological ambiguity, this framework provides an integrative perspective, linking immune, metabolic, and neurochemical pathways under a unifying biological model. While discovery is underway, early microbiome research suggests that gut dysbiosis may contribute to neuroinflammation, altered neurotransmission, and symptom severity in schizophrenia. Recognizing the microbiome as a potential contributor to schizophrenia’s pathophysiology raises important questions, particularly regarding how this research may be translated into therapeutic products. When used adjunctively with traditional pharmacological solutions, microbiome-targeted strategies hold promise for mitigating inflammation, improving cognition, and enhancing treatment response. However, variability in microbial signatures, methodological inconsistencies, and unresolved questions of causality challenge their clinical implementation. Despite these obstacles, the gut microbiome remains a compelling adjunctive target in schizophrenia and requires further longitudinal and interventional studies to clarify whether observed microbiome shifts are causal drivers or secondary consequences of schizophrenia pathology. This will require rigorous clinical trials, mechanistic clarity, and a more precise understanding of how microbiome shifts relate to psychiatric outcomes. As research progresses, a systems-level approach to psychiatric illness, one that accounts for microbial influences, may contribute to more biologically informed and personalized therapeutic strategies for those with schizophrenia.
Author Contributions
Conceptualization, A.M. and H.C.; validation, J.H.; formal analysis, H.C., A.M. and C.M.; investigation, H.C. and J.B.; resources, E.H., J.-L.L.-S. and J.B.; writing—original draft preparation, H.C.; writing—review and editing, A.M. and J.H.; supervision, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BMI | Body mass index |
| CNS | Central nervous system |
| FMT | Fecal microbiota transplantation |
| GI | Gastrointestinal |
| GABA | Gamma-aminobutyric acid |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDD | Major depressive disorder |
| SCFA | Short-chain fatty acid |
| vWF | Von Willebrand factor |
| HDACs | Histone deacetylases |
| PANSS | Positive and Negative Syndrome Scale |
| NMDA | N-methyl-D-aspartate |
Appendix A

Table A1.
Microbial composition in schizophrenia compared to healthy controls.
Table A1.
Microbial composition in schizophrenia compared to healthy controls.
| Microbial Genus | Change in Schizophrenia | Functional Relevance |
|---|---|---|
| Faecalibacterium | Decreased | Produces butyrate (SCFA); anti-inflammatory; supports gut barrier and brain health |
| Roseburia | Decreased | Butyrate producer; anti-inflammatory; supports gut–brain axis |
| Coprococcus | Decreased | SCFA-producing; linked to cognitive function |
| Dialister/Megasphaera | Decreased | SCFA-producing; neuroprotective and anti-inflammatory roles |
| Eggerthella | Increased | Linked to pro-inflammatory signaling; altered glutamate metabolism |
| Escherichia/Shigella | Increased | Elevates inflammation; affects GABAergic signaling |
| Lactobacillus | Increased | Produces lactic acid; strain-dependent effects on neurotransmission and pH |
| Clostridium | Variable | Associated with neurotransmitter shifts (glutamate, nicotinamide, etc.) |
| Parabacteroides goldsteinii | Increased | Correlation with altered serotonin/dopamine; associated with inflammation markers |

Table A2.
Summary of clinical trials using microbiome-based interventions.
Table A2.
Summary of clinical trials using microbiome-based interventions.
| Intervention Type | Study (Author, Year) | Intervention | Results |
|---|---|---|---|
| Probiotic | Tomasik et al. (2015) [34] | L. rhamnosus GG, B. animalis (14 weeks) | Decreased von Willebrand factor; no PANSS change |
| Ghaderi et al. (2019) [36] | Multi-strain probiotic (12 weeks) | Decreased PANSS total and general subscale | |
| Severance et al. (2017) [35] | Same strains as above (14 weeks) | Decreased C. albicans antibodies in males; Decreased PANSS in positive/general domains | |
| Jamilian & Ghaderi (2021) [37] | Probiotics + Selenium (12 weeks) | Decreased PANSS; improved GI symptoms; antioxidant support via glutathione | |
| Mujahid et al. (2022) [42] | Probiotics + Risperidone (6 weeks) | Decreased PANSS significantly | |
| Mohammadi et al. (2024) [43] | Probiotics + Vitamin D (12 weeks) | Increased MoCA cognition; Decreased CRP, cholesterol, blood sugar; non-significant PANSS decrease | |
| Romero-Ferreiro et al. (2025) [33] | Meta-analysis | Modest improvements in general psychopathology | |
| Tamtaji et al. (2019) [45] | Probiotics + Selenium | Decreased CRP; systemic inflammation improved | |
| Prebiotic | Kao et al. (2018) [47] | B-GOS with Olanzapine (mice) | Decreased Weight gain, Decreased neuroinflammatory markers; Increased cortical cognition-related proteins |
| Dietary | Sarnyai & Palmer (2020) [53]; Sethi et al. (2024) [54] | Ketogenic diet | Decreased PANSS, improved memory, social function; remission reported |
| Kelly et al. (2019) [55] | Gluten-free diet (5 weeks) | Decreased AGA IgG, GI symptoms, and negative symptoms; Increased attention | |
| Joseph et al. (2017) [51]; Akerele et al. (2025) [52] | Mediterranean diet (proposed) | Improves SCFA production; underexplored in clinical trials yet | |
| FMT | Wei et al. (2024) [32] | FMT from schizophrenia patients to mice | Induced schizophrenia-like behaviors and altered gene expression linked to neurotransmission |
References
- Schoretsanitis, G. The role of neurotransmitters in schizophrenia. Neurosci. Psychiatry 2024, 7, 239–241. [Google Scholar]
- Hirvonen, J.; Hietala, J. Dysfunctional brain networks and genetic risk for schizophrenia: Specific neurotransmitter systems. CNS Neurosci. Ther. 2010, 17, 89–96. [Google Scholar] [CrossRef]
- Grace, A.A. Dopamine system dysregulation and the pathophysiology of schizophrenia: Insights from the methylazoxymethanol acetate model. Biol. Psychiatry 2017, 81, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Munawar, N.; Ahsan, K.; Muhammad, K.; Ahmad, A.; Anwar, M.A.; Shah, I.; Al Ameri, A.K.; Al Mughairbi, F. Hidden role of gut microbiome dysbiosis in schizophrenia: Antipsychotics or psychobiotics as therapeutics? Int. J. Mol. Sci. 2021, 22, 7671. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- Generoso, J.S.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz. J. Psychiatry 2020, 43, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The microbiota–gut–brain axis in psychiatric disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Bostick, J.W.; Schonhoff, A.M.; Mazmanian, S.K. Gut microbiome-mediated regulation of neuroinflammation. Curr. Opin. Immunol. 2022, 76, 102177. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.D.; Xu, Q.J.; Chang, R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020, 62, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693 Pt B, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S. Roles of diet-associated gut microbial metabolites on brain health: Cell-to-cell interactions between gut bacteria and the central nervous system. Adv. Nutr. 2024, 15, 100136. [Google Scholar] [CrossRef] [PubMed]
- Missiego-Beltrán, J.; Beltrán-Velasco, A.I. The role of microbial metabolites in the progression of neurodegenerative diseases—Therapeutic approaches: A comprehensive review. Int. J. Mol. Sci. 2024, 25, 10041. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gong, X.; Zhou, D.; Hong, Z. Perturbations in gut microbiota composition in patients with autoimmune neurological diseases: A systematic review and meta-analysis. Front. Immunol. 2025, 16, 1513599. [Google Scholar] [CrossRef] [PubMed]
- Gründer, G.; Cumming, P. The dopamine hypothesis of schizophrenia. Neurobiol. Schizophr. 2016, 109–124. [Google Scholar] [CrossRef]
- Qi, D.; Liu, P.; Wang, Y.; Tai, X.; Ma, S. Unveiling the gut microbiota blueprint of schizophrenia: A multilevel omics approach. Front. Psychiatry 2024, 15, 1452604. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; O’Hely, M.; Simpson, C.A.; Green, J.; Marx, W.; Hair, C.; et al. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kosciolek, T.; Eyler, L.T.; Knight, R.; Jeste, D.V. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J. Psychiatr. Res. 2018, 99, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Theleritis, C.; Stefanou, M.-I.; Demetriou, M.; Alevyzakis, E.; Triantafyllou, K.; Smyrnis, N.; Spandidos, D.; Rizos, E. Association of gut dysbiosis with first episode psychosis. Mol. Med. Rep. 2024, 30, 130. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Maukonen, J.; Hyytiäinen, T.; Kieseppä, T.; Orešič, M.; Sabunciyan, S.; Mantere, O.; Saarela, M.; Yolken, R.; Suvisaari, J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr. Res. 2018, 192, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ouyang, L.; Li, D.; Li, Z.; Yuan, L.; Fan, L.; Liao, A.; Li, J.; Wei, Y.; Yang, Z.; et al. Short-chain fatty acids in patients with schizophrenia and ultra-high risk population. Front. Psychiatry 2022, 13, 977538. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.T.; Shin, Y.; Han, S.; Kwon, J.S.; Choi, T.G.; Kang, I.; Kim, S.S. The gut–brain axis in schizophrenia: The implications of the gut microbiome and SCFA production. Nutrients 2023, 15, 4391. [Google Scholar] [CrossRef]
- Shi, L.; Ju, P.; Meng, X.; Wang, Z.; Yao, L.; Zheng, M.; Cheng, X.; Li, J.; Yu, T.; Xia, Q.; et al. Intricate role of intestinal microbe and metabolite in schizophrenia. BMC Psychiatry 2023, 23, 856. [Google Scholar] [CrossRef] [PubMed]
- Hare, S.M.; Adhikari, B.M.; Mo, C.; Chen, S.; Wijtenburg, S.A.; Seneviratne, C.; Kane-Gerard, S.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Schwarcz, R.; et al. Tryptophan challenge in individuals with schizophrenia and healthy controls: Acute effects on circulating kynurenine and kynurenic acid, cognition and cerebral blood flow. Neuropsychopharmacology 2023, 48, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Pedraz-Petrozzi, B.; Elyamany, O.; Rummel, C.; Mulert, C. Effects of inflammation on the kynurenine pathway in schizophrenia—A systematic review. J. Neuroinflamm. 2020, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Szeligowski, T.; Yun, A.L.; Lennox, B.R.; Burnet, P.W.J. The gut microbiome and schizophrenia: The current state of the field and clinical applications. Front. Psychiatry 2020, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Sokolenko, E.; Collins, K.; Chan, N.S.L.; Mills, N.; Clark, S.R.; Marques, F.Z.; Joyce, P. IUPHAR themed review: The gut microbiome in schizophrenia. Pharmacol. Res. 2024, 211, 107561. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Sirivichayakul, S.; Kanchanatawan, B.; Vodjani, A. Breakdown of the paracellular tight and adherens junctions in the gut and blood brain barrier and damage to the vascular barrier in patients with deficit schizophrenia. Neurotox. Res. 2019, 36, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Benedict, G.; Mei, T.M.; Ramly, S.S.; Arif, M.; Croft, L.; Parimannan, S.; Rajandas, H.; Lee, S.Y.; Rasat, M. Functional associations of the gut microbiome with dopamine, serotonin, and BDNF in schizophrenia: A pilot study. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 123. [Google Scholar] [CrossRef]
- Wei, N.; Ju, M.; Su, X.; Zhang, Y.; Huang, Y.; Rao, X.; Cui, L.; Lin, Z.; Dong, Y. Transplantation of gut microbiota derived from patients with schizophrenia induces schizophrenia-like behaviors and dysregulated brain transcript response in mice. Schizophrenia 2024, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ferreiro, V.; García-Fernández, L.; Romero, C.; De la Fuente, M.; Diaz-del Cerro, E.; Scala, M.; González-Soltero, R.; Álvarez-Mon, M.A.; Peñuelas-Calvo, I.; Rodriguez-Jimenez, R. Impact of probiotic treatment on clinical symptom reduction in schizophrenia: A systematic review and meta-analysis. J. Psychiatr. Res. 2025, 182, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, J.; Yolken, R.H.; Bahn, S.; Dickerson, F.B. Immunomodulatory effects of probiotic supplementation in schizophrenia patients: A randomized, placebo-controlled trial. Biomark. Insights 2015, 10, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.G.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav. Immun. 2017, 62, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; Banafshe, H.R.; Mirhosseini, N.; Moradi, M.; Karimi, M.-A.; Mehrzad, F.; Bahmani, F.; Asemi, Z. Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry 2019, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, H.; Ghaderi, A. The effects of probiotic and selenium co-supplementation on clinical and metabolic scales in chronic schizophrenia: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2021, 199, 4430–4438. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, I.; Niczyporuk, P.; Urbaniak, A.; Tomaszek, N.; Modzelewski, S.; Waszkiewicz, N. Investigating the impacts of diet, supplementation, microbiota, gut–brain axis on schizophrenia: A narrative review. Nutrients 2024, 16, 2228. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, F.; Liu, X.; Song, M.; Grosso, G.; Battino, M.; Boesch, C.; Li, H.; Liu, X. Effect of supplementation with probiotics in patients with schizophrenia: Systematic review and meta-analysis of randomized controlled clinical trials. Foods 2025, 14, 1773. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Stallings, C.; Origoni, A.; Vaughan, C.; Khushalani, S.; Leister, F.; Yang, S.; Krivogorsky, B.; Alaedini, A.; Yolken, R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol. Psychiatry 2010, 68, 100–104. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Dockx, L.; Bernagie, C.; Peuskens, B.; Sweers, K.; Leucht, S.; Tack, J.; Van de Straete, S.; Wampers, M.; Peuskens, J. Prevalence and severity of antipsychotic related constipation in patients with schizophrenia: A retrospective descriptive study. BMC Gastroenterol. 2011, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, E.H.; Limoa, E.; Syamsuddin, S.; Bahar, B.; Renaldi, R.; Aminuddin, A.; Lisal, S.T. Effect of probiotic adjuvant therapy on improvement of clinical symptoms interleukin 6 levels in patients with schizophrenia. Psychiatry Investig. 2022, 19, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Sadighi, G.; Nazeri Astaneh, A.; Tajabadi-Ebrahimi, M.; Dejam, T. Co-administration of probiotic and vitamin D significantly improves cognitive function in schizophrenic patients: A double-blinded randomized controlled trial. Neuropsychopharmacol. Rep. 2024, 44, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, P.; Chi, D.; Wu, T.; Mei, Z.; Cui, G. Association between C-reactive protein and risk of schizophrenia: An updated meta-analysis. Oncotarget 2017, 8, 75445–75454. [Google Scholar] [CrossRef] [PubMed]
- Tamtaji, O.R.; Heidari-soureshjani, R.; Mirhosseini, N.; Kouchaki, E.; Bahmani, F.; Aghadavod, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: A randomized, double-blind, controlled trial. Clin. Nutr. 2019, 38, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.C.-C.; Spitzer, S.; Anthony, D.C.; Lennox, B.; Burnet, P.W.J. Prebiotic attenuation of olanzapine-induced weight gain in rats: Analysis of central and peripheral biomarkers and gut microbiota. Transl. Psychiatry 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Strassnig, M.; Brar, J.S.; Ganguli, R. Nutritional assessment of patients with schizophrenia: A preliminary study. Schizophr. Bull. 2003, 29, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Eskinazi, B.; Camboim Rockett, F.; Delgado, V.B.; Schweigert Perry, I.D. Nutritional status, food intake and cardiovascular disease risk in individuals with schizophrenia in southern Brazil: A case-control study. Rev. De Psiquiatr. Y Salud Ment. 2014, 7, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.C.; Borba, C.P.; Daley, T.B.; Boxill, R.; Nguyen, D.D.; Culhane, M.A.; Louie, P.; Cather, C.; Evins, A.E.; Freudenreich, O.; et al. Dietary intake profile of patients with schizophrenia. Ann. Clin. Psychiatry 2006, 18, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Depp, C.; Shih, P.B.; Cadenhead, K.S.; Schmid-Schönbein, G. Modified mediterranean diet for enrichment of short chain fatty acids: Potential adjunctive therapeutic to target immune and metabolic dysfunction in schizophrenia? Front. Neurosci. 2017, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Akerele, C.A.; Koralnik, L.R.; Lafont, E.; Gilman, C.; Walsh-Messinger, J.; Malaspina, D. Nutrition and brain health: Implications of Mediterranean diet elements for psychiatric disorders. Schizophr. Res. 2025, 281, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Sarnyai, Z.; Palmer, C.M. Ketogenic therapy in serious mental illness: Emerging evidence. Int. J. Neuropsychopharmacol. 2020, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Wakeham, D.; Ketter, T.; Hooshmand, F.; Bjorstead, J.; Richards, B.; Westman, E.; Krauss, R.M.; Saslow, L. Ketogenic diet intervention on metabolic and psychiatric health in bipolar and schizophrenia: A pilot trial. Psychiatry Res. 2024, 335, 115866. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.L.; Demyanovich, H.K.; Rodriguez, K.M.; Čiháková, D.; Talor, M.V.; McMahon, R.P.; Richardson, C.M.; Vyas, G.; Adams, H.A.; August, S.M.; et al. Randomized controlled trial of a gluten-free diet in patients with schizophrenia positive for antigliadin antibodies (AGA IgG): A pilot feasibility study. J. Psychiatry Neurosci. 2019, 44, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Levinta, A.; Mukovozov, I.; Tsoutsoulas, C. Use of a gluten-free diet in schizophrenia: A systematic review. Adv. Nutr. 2018, 9, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Stürup, A.E.; Hjorthøj, C.; Jensen, H.D.; Melau, M.; Davy, J.W.; Nordentoft, M.; Albert, N. Self-reported reasons for discontinuation or continuation of antipsychotic medication in individuals with first-episode schizophrenia. Early Interv. Psychiatry 2023, 17, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Murashita, M.; Kusumi, I.; Inoue, T.; Takahashi, Y.; Hosoda, H.; Kangawa, K.; Koyama, T. Olanzapine increases plasma ghrelin level in patients with schizophrenia. Psychoneuroendocrinology 2005, 30, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Lindenmayer, J.-P.; Davis, J.M.; Kelly, E.; Viviano, T.F.; Cornwell, J.; Hu, Q.; Khan, A.; Vaidhyanathaswamy, S. Effects of olanzapine and risperidone on glucose metabolism and insulin sensitivity in chronic schizophrenic patients with long-term antipsychotic treatment: A randomized 5-month study. J. Clin. Psychiatry 2009, 70, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Zhou, Y.; Zhang, X.; Jiang, H. Gut microbiome profiles may be related to atypical antipsychotic associated overweight in Asian children with psychiatric disorder: A preliminary study. Front. Cell. Infect. Microbiol. 2023, 13, 1124846. [Google Scholar] [CrossRef] [PubMed]
- Meuldermans, W.; Hendrickx, J.; Mannens, G.; Lavrijsen, K.; Janssen, C.; Bracke, J.; Jeune, L.L.; Lauwers, W.; Heykants, J. The metabolism and excretion of risperidone after oral administration in rats and dogs. Drug Metab. Dispos. 1994, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. J. Lab. Clin. Med. 2017, 179, 204–222. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. The gut microbiome and antipsychotic treatment response. Behav. Brain Res. 2020, 396, 112886. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Xia, L.; Xu, L.; Deng, L.; Jin, G. A comparative study to determine the association of gut microbiome with schizophrenia in Zhejiang, China. BMC Psychiatry 2022, 22, 731. [Google Scholar] [CrossRef] [PubMed]
- Wathen, A.B.; West, E.S.; Lydic, R.; Baghdoyan, H.A. Olanzapine causes a leptin-dependent increase in acetylcholine release in mouse prefrontal cortex. SLEEP 2012, 35, 315–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, S.; Lin, Q.; Xiong, W.; Li, L.; Straub, L.; Zhang, D.; Zapata, R.; Zhu, Q.; Sun, X.-N.; Zhang, Z.; et al. Hyperleptinemia contributes to antipsychotic drug–associated obesity and metabolic disorders. Sci. Transl. Med. 2023, 15, eade8460. [Google Scholar] [CrossRef] [PubMed]
- Borkent, J.; Ioannou, M.; Neijzen, D.; Haarman, B.C.M.; Sommer, I.E.C. Probiotic formulation for patients with bipolar or schizophrenia spectrum disorder: A double-blind, randomized placebo-controlled trial. Schizophr. Bull. 2024, sbae188. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).