Characterizing Aqueous Extracts of Native Plants in Northeastern Mexico: Prospects for Quorum-Sensing Inhibition Against Gram-Negative Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Bacterial Strains
2.3. Preparation of Aqueous Extracts
2.4. Antibacterial and Anti-QS Activity
2.5. Violacein Quantification by Flask Incubation
2.6. Minimum Inhibitory Concentration (MIC)
2.7. Biofilm Inhibition Assay
2.8. UPLC-MS Analysis of Extracts
2.9. Statistical Analysis
3. Results
3.1. Extract Yields and Processing Efficiency
3.2. Anti-QS Activity in Disk Diffusion Assays
3.3. Violacein Quantification (Flask Assay)
3.4. Antibacterial Activity and Minimum Inhibitory Concentration
3.5. Biofilm Inhibition (Initial Cell Attachment)
3.6. UPLC-MS Phytochemical Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Committee on Antimicrobial Susceptibility Testing. Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method. 2017. Available online: https://www.eucast.org (accessed on 1 January 2021).
- Rahman, M.R.T.; Lou, Z.; Yu, F.; Wang, P.; Wang, H. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko on foodborne pathogens. Saudi J. Biol. Sci. 2017, 24, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Ramírez, G.; Zamilpa, A.; Zavala, M.; Pérez, J.; Morales, D.; Tortoriello, J. Chrysoeriol and other polyphenols from Tecoma stans with lipase inhibitory activity. J. Ethnopharmacol. 2016, 185, 1–8. [Google Scholar] [CrossRef]
- Gómez-Flores, R.; Espinosa-Ramos, D.; Quintanilla-Licea, R.; Barrón-González, M.P.; Tamez-Guerra, P.; Tamez-Guerra, R.; Rodríguez-Padilla, C. Antimicrobial activity of Gymnosperma glutinosum methanol extracts against Helicobacter pylori. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 55–59. [Google Scholar] [CrossRef][Green Version]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.J.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea) leaves. Molecules 2007, 12, 1153–1163. [Google Scholar] [CrossRef]
- Castro-López, C.; Bautista-Hernández, I.; González-Hernández, M.D.; Martínez-Ávila, G.C.G.; Rojas, R.; Gutiérrez-Díez, A.; Medina-Herrera, N.; Aguirre-Arzola, V.E. Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars. Molecules 2019, 24, 173. [Google Scholar] [CrossRef]
- Gómez-Flores, R.; Quintanilla-Licea, R.; Verde-Star, M.J.; Morado-Castillo, R.; Vázquez-Díaz, D.; Tamez-Guerra, R.; Tamez-Guerra, P.; Rodríguez-Padilla, C. Long-chain alkanes and ent-labdane-type diterpenes from G. glutinosum with cytotoxic activity. Phytother. Res. 2012, 26, 1632–1636. [Google Scholar] [CrossRef]
- Delgadillo-Ruíz, L.; Bañuelos-Valenzuela, R.; Delgadillo-Ruíz, O.; Silva-Vega, M.; Gallegos-Flores, P. Composición química y efecto antibacteriano de extractos de L. tridentata. Nova Sci. 2017, 9, 273–290. [Google Scholar] [CrossRef]
- Lira-Saldivar, F.D.; Hernández-Suárez, R.H.; Hernández-Castillo, M. Activity of L. tridentata and chitosan against fungi. Rev. Chapingo Ser. Hortic. 2006, 12, 211–216. [Google Scholar] [CrossRef]
- Morado-Castillo, R.; Quintanilla-Licea, R.; Gómez-Flores, R.; Blaschek, W. Total phenolic and flavonoid contents of G. glutinosum. Eur. J. Med. Plants 2016, 15, 1–8. [Google Scholar] [CrossRef]
- González-Chávez, M.M.; Arana-Argaéz, V.; Zapata-Morales, J.R.; Avila-Venegas, A.K.; Alonso-Castro, A.J.; Isiordia-Espinoza, M.; Martínez, R. Pharmacological evaluation of 2-angeloyl ent-dihydrotucumanoic acid. Pharm. Biol. 2017, 55, 873–879. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; González-Chávez, M.M.; Zapata-Morales, J.R.; Verdinez-Portales, A.K.; Sánchez-Recillas, A.; Ortiz-Andrade, R.; Isiordia-Espinoza, M.; Martínez-Gutiérrez, F.; Ramírez-Morales, M.A.; Domínguez, F.; et al. Antinociceptive activity of ent-dihydrotucumanoic acid isolated from G. glutinosum. Drug Dev. Res. 2017, 78, 340–348. [Google Scholar] [CrossRef]
- Salazar-Aranda, R.; Perez-Lopez, L.A.; Lopez-Arroyo, J.; Alanis-Garza, B.A.; Waksman de Torres, N. Antimicrobial and antioxidant activities of plants from northeast Mexico. Evid. Based Complement. Alternat. Med. 2011, 2011, 536139. [Google Scholar] [CrossRef]
- Bashyal, B.; Li, L.; Bains, T.; Debnath, A.; LaBarbera, D.V. L. tridentata: A novel source for anti-parasitic agents. PLoS Negl. Trop. Dis. 2017, 11, e0005832. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Damte, D. Evaluation of anti-quorum sensing activity of 97 indigenous plant extracts. J. Microb. Biochem. Technol. 2013, 5, 42–46. [Google Scholar] [CrossRef]
- Abudoleh, S.M.; Mahasneh, A.M. Anti-quorum sensing activity of substances from wild berry–associated bacteria. Avicenna J. Med. Biotechnol. 2017, 9, 23–30. [Google Scholar]
- Jesudhasan, P.R.; Cepeda, M.L.; Widmer, K.; Dowd, S.E.; Soni, K.A.; Hume, M.E.; Zhu, J.; Pillai, S.D. Transcriptome analysis of genes regulated by luxS/autoinducer-2 in Salmonella enterica serovar Typhimurium. Foodborne Pathog. Dis. 2010, 7, 399–410. [Google Scholar] [CrossRef]
- Rammo, R.N.N. Bactericidal and anti-biofilm formation of aqueous plant extracts against pathogenic bacteria. Asian J. Pharm. Res. 2017, 7, 25–29. [Google Scholar] [CrossRef]
- Ilk, S.; Saglam, N.; Ozgen, M.; Korkusuz, F. Chitosan nanoparticles enhance the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2017, 94, 653–662. [Google Scholar] [CrossRef]
- Liang, L.; Sproule, A.; Haltli, B.; Marchbank, D.H.; Berrué, F.; Overy, D.P.; McQuillan, K.; Lanteigne, M.; Duncan, N.; Correa, H.; et al. A new natural product and quorum sensing deactivation by culturing with heat-killed inducer. Front. Microbiol. 2019, 9, 3351. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Wang, L.; Zeng, W.; Sun, Y.; Zhou, C.; Zhou, T.; Shen, M. Effect of chlorogenic acid on the quorum-sensing system of clinically isolated multidrug-resistant Pseudomonas aeruginosa. J. Appl. Microbiol. 2022, 132, 1008–1017. [Google Scholar] [CrossRef]
- Majumdar, G.; Mandal, S. Antibacterial activity analysis of kaempferol and its derivatives targeting virulence and quorum sensing associated proteins by in silico methods. Microbe 2025, 6, 100259. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Gao, D.; Zang, Y.; Zhang, X.; Bao, G. Chlorogenic acid inhibits quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2019, 103, 9039–9051. [Google Scholar] [CrossRef]
- Vega-Menchaca, M.C.; Morales, C.R.; Star, J.V.; Or, A.; Morales, M.E.R.; Nuntilde, M.A.; Gallardo, L.B.S. Antimicrobial activity of five plants from Northern Mexico. Afr. J. Microbiol. Res. 2013, 7, 5011–5017. [Google Scholar] [CrossRef]
- Pires, F.B.; Dolwitsch, C.B.; Prá, V.D.; Faccin, H.; Monego, D.L.; de Carvalho, L.M.; Viana, C.; Lameira, O.; Lima, F.O.; Bressan, L.; et al. Qualitative and quantitative analysis of phenolic content by HPLC-DAD and UHPLC-ESI-MS/MS. Rev. Bras. Farmacogn. 2017, 27, 426–433. [Google Scholar] [CrossRef]
- Pires, F.B.; Dolwitsch, C.B.; Prá, V.D.; Faccin, H.; Monego, D.L.; de Carvalho, L.M.; Viana, C.; Lameira, O.; Lima, F.O.; Bressan, L.; et al. Polyphenol–protein complexes in biofilm prevention. Sci. Total Environ. 2019, 658, 1098–1105. [Google Scholar] [CrossRef]
- Bin Zaman, S.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Willyard, C. Drug-resistant bacteria ranked. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-M.; Chang, C.-C.; Lin, C.-W.; Ko, S.-Y.; Hsu, Y.-C. Cucurbitacin E as inducer of apoptosis in oral cancer. Int. J. Mol. Sci. 2013, 14, 17147–17156. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.M.; Mohamed, M.A.; Ibrahim, M.T. Cytotoxic activity of secondary metabolites from Tecomaria capensis. Int. J. Pharm. Phytochem. Res. 2016, 8, 1173–1182. [Google Scholar]

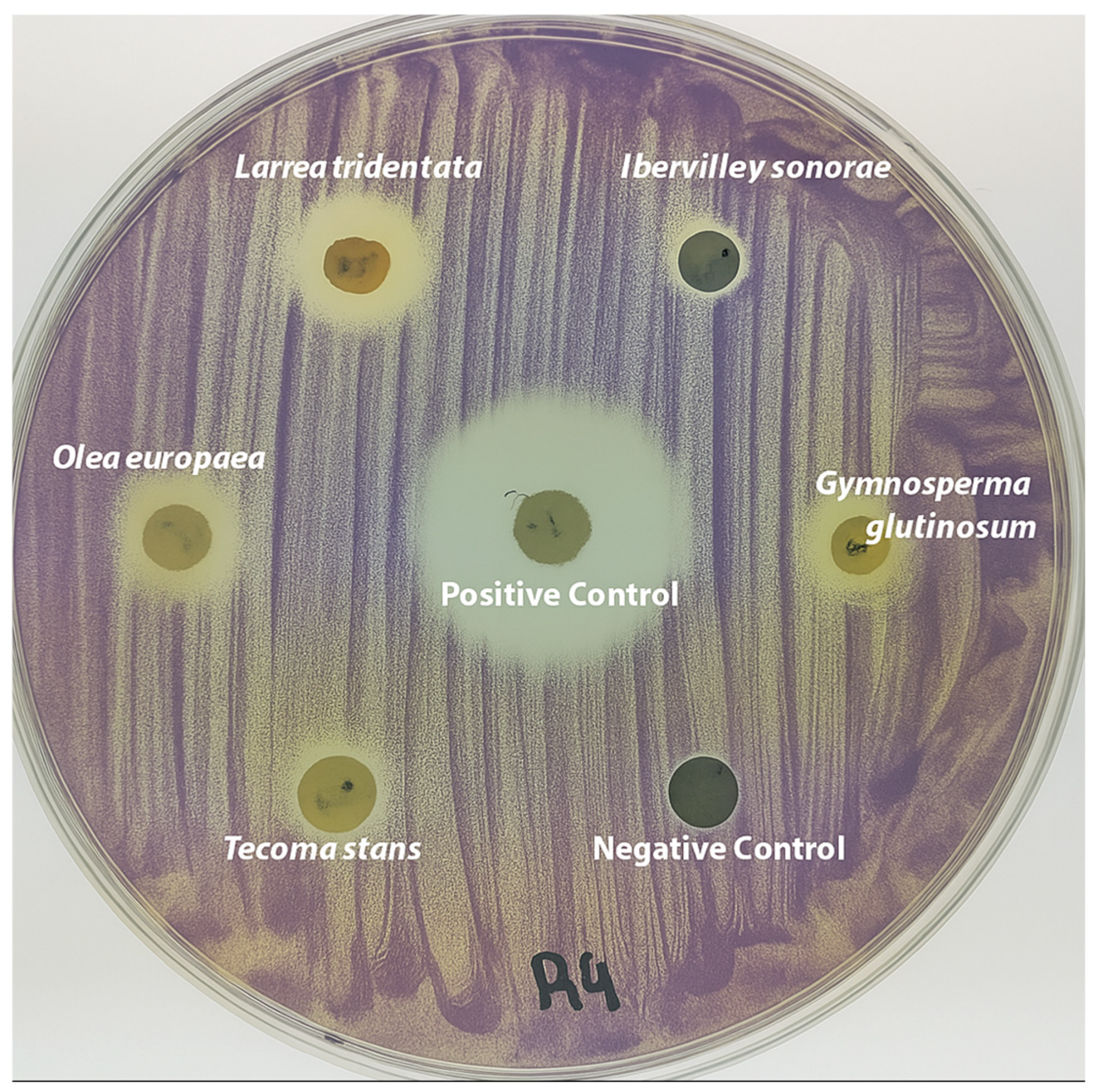
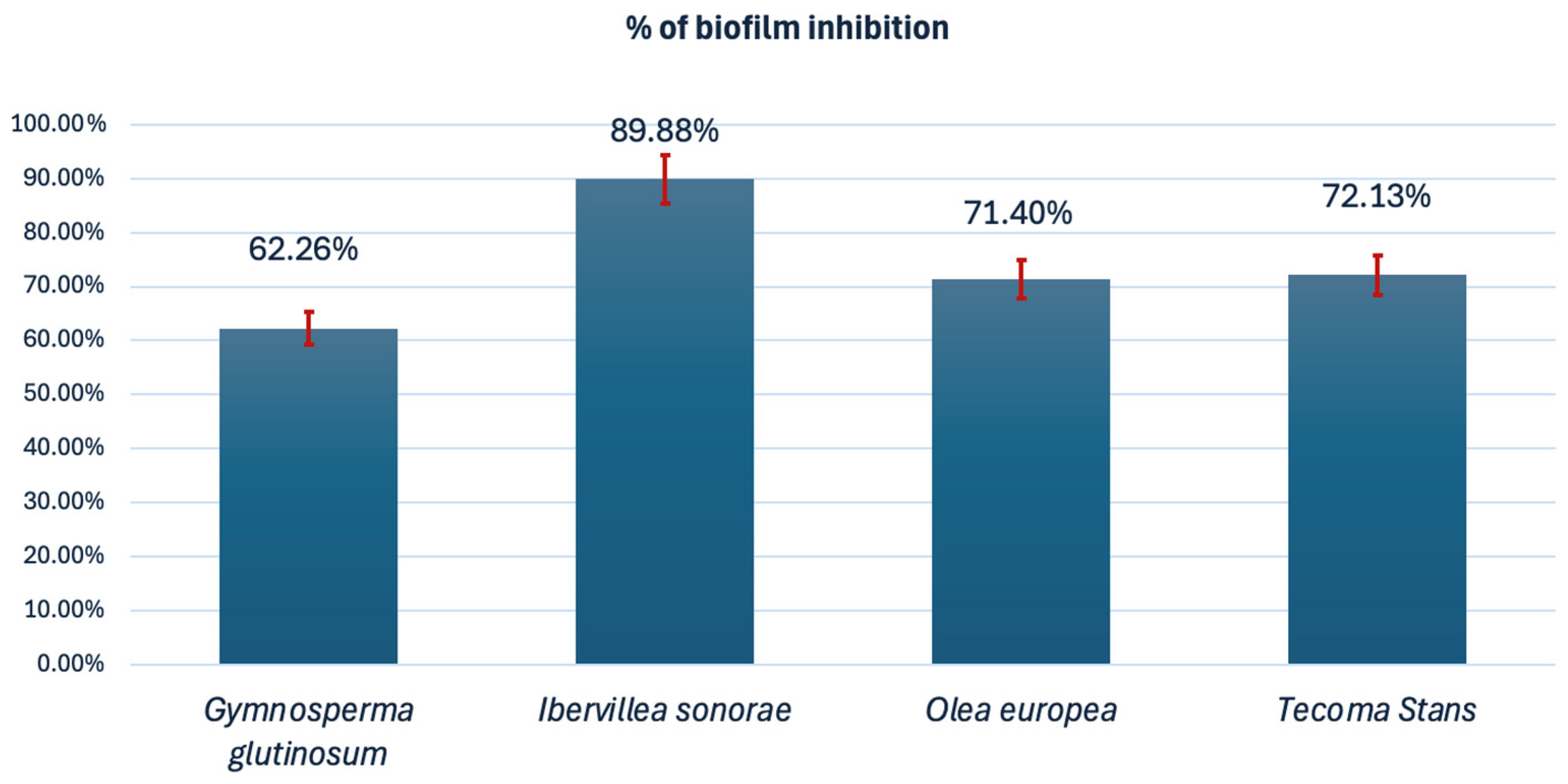
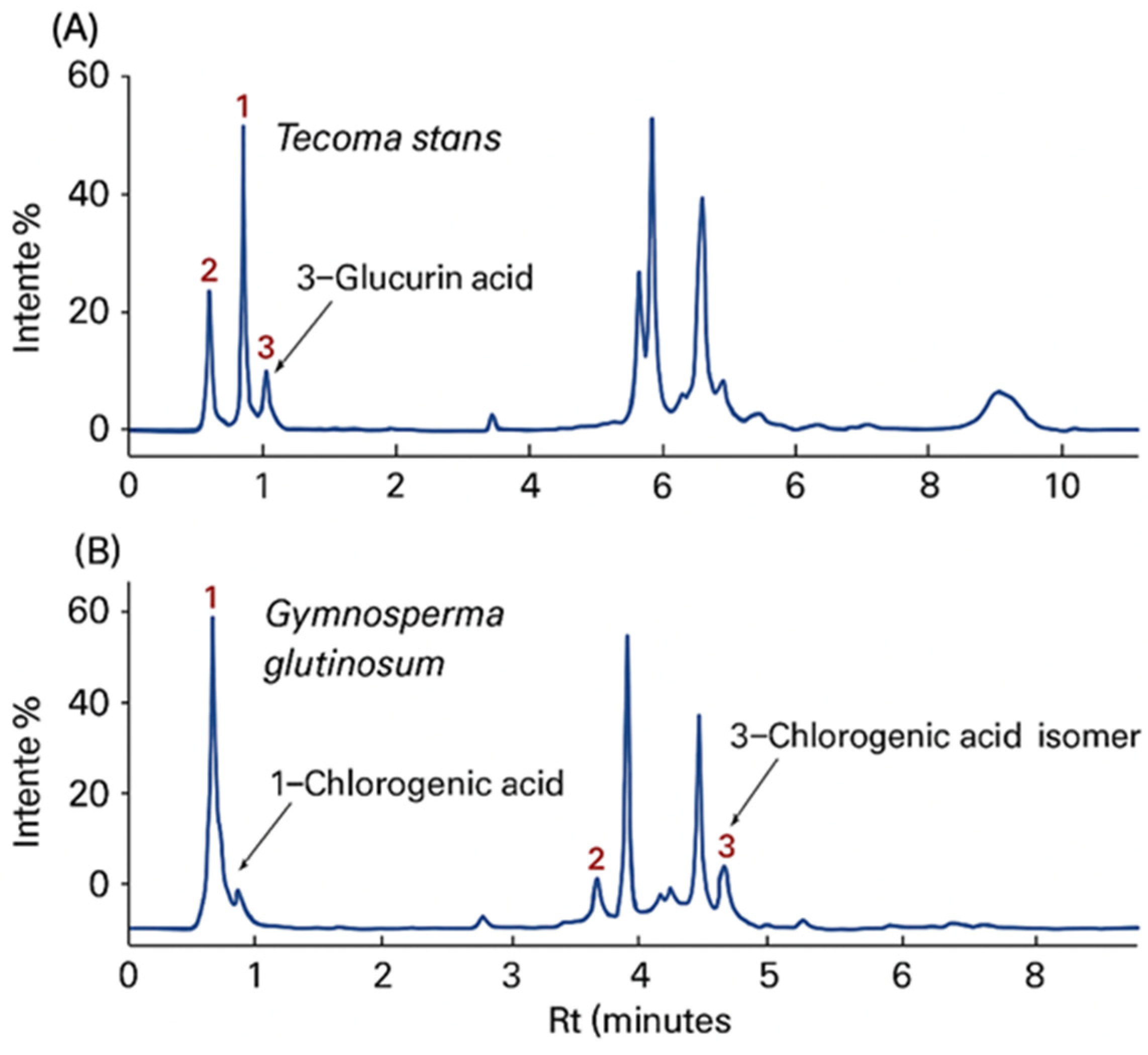
| Scientific Name (Author) | Family | Spanish Common Name | English Common Name | Voucher No. | Plant Part Used | Yield (% w/w) |
|---|---|---|---|---|---|---|
| Gymnosperma glutinosum (Spreng.) Less. | Asteraceae | Tata Lencho, Pegajosa | Sticky Bush/Resin Bush | UANL-26,671 | Aerial parts (AP) | 22.6 |
| Ibervillea sonorae (S. Watson) Greene | Cucurbitaceae | Wereke, Wareque | Wereke/Desert Gourd | UANL-25,589 | Root (RO) | 22.1 |
| Larrea tridentata (Sessé & Moc. ex DC.) Coville | Zygophyllaceae | Gobernadora | Creosote Bush | UANL-26,696 | Leaves (LE) | 16.3 |
| Olea europaea L. | Oleaceae | Olivo | Olive | UANL-26,697 | Leaves (LE) | 16.7 |
| Tecoma stans (L.) Juss. ex Kunth | Bignoniaceae | Tronadora | Yellow Bells/Trumpet Bush | UANL-26,702 | Leaves (LE) | 25.8 |
| Extract | Inhibition Zone (mm) | MIC (mg/mL) |
|---|---|---|
| G. glutinosum | None | 6.8 ± 0.55 |
| I. sonorae | None | 9.8 ± 0.45 |
| L. tridentata | 10.0 ± 1.15 | 11.8 ± 0.89 |
| O. europaea | 10.0 ± 1.41 | 14.4 ± 0.84 |
| T. stans | None | >40.0 |
| Positive Control (Gentamicin) | 22.3 ± 0.7 (10 µg/disc) | 0.0016 ± 0.0002 |
| Peak | Retention Time (min) | Tentative Compound |
|---|---|---|
| 1 | 0.0829 | D-(−)-Quinic acid |
| 2 | 0.727 | Glucuronic acid |
| 3 | 0.660 | Kaempferol |
| Peak | Retention Time (min) | Tentative Compound |
|---|---|---|
| 1 | 3.095 | Chlorogenic acid |
| 2 | 3.569 | Kaempferol |
| 3 | 4.245 | Chlorogenic acid isomer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiroz-Hernandez, J.E.; Hernandez-Vidal, G.; Perez-Gonzalez, O.; Castillo-Velazquez, U.; Aguirre-Arzola, V.E. Characterizing Aqueous Extracts of Native Plants in Northeastern Mexico: Prospects for Quorum-Sensing Inhibition Against Gram-Negative Bacteria. Appl. Microbiol. 2025, 5, 61. https://doi.org/10.3390/applmicrobiol5030061
Quiroz-Hernandez JE, Hernandez-Vidal G, Perez-Gonzalez O, Castillo-Velazquez U, Aguirre-Arzola VE. Characterizing Aqueous Extracts of Native Plants in Northeastern Mexico: Prospects for Quorum-Sensing Inhibition Against Gram-Negative Bacteria. Applied Microbiology. 2025; 5(3):61. https://doi.org/10.3390/applmicrobiol5030061
Chicago/Turabian StyleQuiroz-Hernandez, Jose E., Gustavo Hernandez-Vidal, Orquidea Perez-Gonzalez, Uziel Castillo-Velazquez, and Victor E. Aguirre-Arzola. 2025. "Characterizing Aqueous Extracts of Native Plants in Northeastern Mexico: Prospects for Quorum-Sensing Inhibition Against Gram-Negative Bacteria" Applied Microbiology 5, no. 3: 61. https://doi.org/10.3390/applmicrobiol5030061
APA StyleQuiroz-Hernandez, J. E., Hernandez-Vidal, G., Perez-Gonzalez, O., Castillo-Velazquez, U., & Aguirre-Arzola, V. E. (2025). Characterizing Aqueous Extracts of Native Plants in Northeastern Mexico: Prospects for Quorum-Sensing Inhibition Against Gram-Negative Bacteria. Applied Microbiology, 5(3), 61. https://doi.org/10.3390/applmicrobiol5030061











