Abstract
This study evaluated the antimicrobial potential of a Bacillus subtilis spore-based probiotic cocktail to reduce foodborne pathogens in both nutrient-rich laboratory media and a complex food matrix (hummus). Three common foodborne pathogens—Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella Typhimurium—were cultured individually in full-strength, half-strength, and quarter-strength tryptic soy broth (TSB) with or without the probiotic spores (~7 log CFU/mL). Additionally, a commercial hummus formulation was inoculated with L. monocytogenes (~3 log CFU/g) and B. subtilis spores (~7 log CFU/g) and stored at 30 °C to simulate temperature abuse. In TSB, E. coli and Salmonella grew to ~8.2 log CFU/mL in full-strength media, with no significant inhibition by the probiotics. However, L. monocytogenes showed substantial suppression: in nutrient-limited TSB, viable counts dropped below the detection limit of 1.48 log CFU/mL by 24 h in the presence of probiotics. In hummus, L. monocytogenes grew to an average of 8.22 log CFU/g in the absence of probiotics but remained significantly lower at an average of 5.03 log CFU/g when co-inoculated with B. subtilis (p < 0.05). Germination of probiotic spores was confirmed within 6 h under all conditions. These findings suggest that B. subtilis spores selectively inhibit Listeria, particularly under nutrient stress or abuse conditions. While the probiotic had limited impact on Gram-negative pathogens, its application may serve as a clean-label strategy for suppressing L. monocytogenes in ready-to-eat (RTE) foods. This dual-model approach provides insights into both mechanistic activity and practical limitations of spore probiotics in complex food matrices.
1. Introduction
Ready-to-eat (RTE) refrigerated foods have emerged as notable vehicles for foodborne pathogens, especially when minimal processing and mild formulations allow microbial survival [1]. Hummus, a popular chickpea-based dip, exemplifies this risk. In the United States, Listeria monocytogenes contamination of hummus has been linked to multiple outbreaks and recalls in recent years [2,3]. Between 2002 and 2017, at least 17 hummus-associated outbreaks were reported nationally, resulting in approximately 1105 illnesses, 64 hospitalizations, and 4 deaths [1]. Notably, two listeriosis outbreaks in 2013 accounted for all four fatalities, including one California incident that sickened 28 people [1]. Even in cases without confirmed illnesses, contamination has prompted large-scale product recalls—for instance, Sabra recalled over 30,000 cases of hummus in 2015 after routine testing found L. monocytogenes [3]. These events underscore the food safety vulnerability of minimally processed dips, such as hummus, and the critical need for effective control measures.
Controlling pathogens in RTE dips is challenging because their intrinsic properties often permit bacterial growth under refrigeration [1]. Hummus typically has water activity above 0.92 and pH above 4.4—conditions that support the growth of many foodborne pathogens. Unlike many cooked foods that undergo a final lethal heat treatment, hummus is typically consumed without such treatment after preparation, making it susceptible to post-processing contamination. While ingredients, such as chickpeas, are cooked during preparation, other components, such as tahini, may not be heat treated, and improper sanitation, poor water quality, or contaminated packaging materials can introduce pathogens. L. monocytogenes, in particular, is a psychrotrophic pathogen capable of proliferating at refrigeration temperatures. Previous studies have shown that L. monocytogenes not only survives but may increase in hummus during storage, particularly when mild acidification and salt levels are used [2]. Under temperature-abuse conditions (e.g., 30 °C), Listeria can grow rapidly, with levels exceeding 8 log CFU/g within 48 h in some formulations, highlighting the importance of additional safety barriers.
Modern consumer preferences further complicate formulation strategies. There is growing demand for “clean-label” foods—products with short ingredient lists free from synthetic additives or preservatives [4]. In response, manufacturers are pursuing natural food safety interventions to meet regulatory and market expectations. These trends have increased interest in bio-preservation strategies that utilize naturally occurring microorganisms or their metabolites to inhibit pathogens [5]. Among these, protective cultures and probiotic microorganisms have shown potential to maintain microbial stability in minimally processed foods without altering flavor or texture [6,7].
Probiotics are traditionally defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host” [8,9]. Beyond their health effects, certain strains also exert antimicrobial activity through nutrient competition, pH reduction, or secretion of bacteriocins. While lactic acid bacteria have been widely studied for this purpose, their viability and functional performance are often compromised in non-fermented, refrigerated, or high-pH foods [5,10]. In contrast, spore-forming Bacillus species—including Bacillus subtilis—are exceptionally resilient and able to withstand diverse processing and storage conditions [11,12,13].
Bacillus subtilis produces endospores—highly resistant, dormant structures capable of surviving heat, desiccation, acid, and other harsh food environments [11,12]. Unlike vegetative probiotic cells, spores can remain stable for months, only germinating when triggered by favorable environmental conditions, such as elevated temperature or nutrient availability [6,13,14]. This dormancy makes them ideal for use in RTE foods; they remain inactive under refrigerated storage, posing minimal risk to product quality, and become metabolically active only when the food is exposed to conditions that would also promote pathogen growth—such as temperature abuse [4,6,15]
Once germinated, B. subtilis can inhibit pathogens through several mechanisms: rapid nutrient uptake, production of siderophores (e.g., bacillibactin), competitive exclusion of colonization sites, and secretion of narrow-spectrum antimicrobials, such as subtilosin A and subtilin, which are particularly effective against Gram-positive bacteria, such as Listeria monocytogenes [15,16,17,18]. Because Gram-negative organisms, such as Salmonella and E. coli, have protective outer membranes, they are less sensitive to these peptide-based antimicrobials, making Gram-positive pathogens more susceptible to inhibition by Bacillus-based probiotics [5].
This selective activity has been supported in multiple studies and was also observed in the preliminary broth experiments reported here, where Bacillus subtilis spores significantly inhibited L. monocytogenes under nutrient-limited conditions, yet had a limited impact on E. coli O157:H7 and Salmonella Typhimurium. However, whether this efficacy translates to complex food matrices, such as hummus, remains unclear. Hummus presents a high-viscosity, particulate-rich environment that may hinder probiotic–pathogen interactions and create micro-niches in which Listeria can persist undisturbed [2,19].
To address this knowledge gap, the present study was designed to investigate the suitability of Bacillus subtilis spores as biocontrol agents in both simplified and complex food systems. Specifically, the objectives were (1) to assess the ability of a B. subtilis spore cocktail to inhibit L. monocytogenes, E. coli O157:H7, and S. Typhimurium under varying nutrient conditions in tryptic soy broth, and (2) to evaluate the probiotic’s effectiveness in controlling L. monocytogenes in a temperature-abused hummus matrix. The following sections detail the experimental design, microbial enumeration methods, and observed results under both laboratory and food system conditions.
2. Materials and Methods
2.1. Preparation of Microbial Cultures
2.1.1. Listeria monocytogenes
For this study, four strains of L. monocytogenes were used and included: Scott A-2, V7-2, PMM39-2, and PMM383-2. Scott A and V7 are well-known strains; PMM383 and PMM39 were isolated from raw and ready-to-eat (RTE) meat products, respectively) These strains were previously adapted to streptomycin (100 μg/mL), through spontaneous mutation. The L. monocytogenes cultures were grown individually in tryptic soy broth (TSB; Difco, Becton Dickinson, Sparks, MD, USA), supplemented with 100 μg/mL streptomycin and 100 μg/mL rifamycin. After approximately 18 h of incubation at 30 °C, they were centrifuged (3000× g for 15 min), the supernatant was then disposed of, and the pellets were resuspended in saline solution (9 mg/mL sodium chloride solution). A four-strain cocktail was made for L. monocytogenes by combining 2 mL of each strain. The final concentration of the bacterial cocktail was adjusted to approximately 5.1 ± 0.2 log CFU/mL.
2.1.2. Escherichia coli O157:H7
Five strains of E. coli O157:H7 used in this study: 1 (Beef isolate), 5 (human isolate), 932 (human isolate), E009 (Beef isolate) and E0122 (cattle isolate). Each E. coli strain used had previously been adapted to 50 mg/L nalidixic acid [20] through spontaneous mutation. These strains were individually grown in TSB supplemented with 50 mg/L nalidixic acid for approximately 18 h at 37 °C. At the end of the incubation period, bacterial strains were washed by centrifugation as described above. A five-strain cocktail was made for E. coli by mixing an equal amount of each strain in a 15 mL capacity sterile centrifuge tube. The final concentration of the cocktail was adjusted to 5.2 ± 0.3 log CFU/mL.
2.1.3. Salmonella Typhimurium DT104
Three strains of S. Typhimurium DT 104 were used and included: H2662 (cattle isolate), 13068A (cattle isolate), and H3279 (human isolate). S. Typhimurium strains were grown individually in TSB, which had been supplemented with 32 mg/L ampicillin, 16 mg/L tetracycline, and 64 mg/L streptomycin. After the strains grew for approximately 18 h at 37 °C, they were washed by centrifugation and a five-strain bacterial cocktail containing a pathogen population of 5.1 ± 0.2 log CFU/mL was prepared using the method described above.
All bacterial cocktails were centrifuged at 4000× g for 10 min, and then the pellets were resuspended in phosphate-buffered saline solution (PBS) to create experiment-ready cultures.
2.1.4. Probiotic Strains
Two Bacillus subtilis strains were used in this study: Bacillus subtilis 1 and Bacillus subtilis Novonesis HU58™. Both strains are commercially available spore-forming probiotics that have been designated as Generally Recognized as Safe (GRAS) by the United States Food and Drug Administration (FDA) as well as by an independent expert panel. The spore preparations were obtained from commercial suppliers in spray-dried powder form. The initial concentrations of the powdered products were 11.47 log CFU/g for B. subtilis 1 and 10.18 log CFU/g for HU58™. For all experimental assays, a mixed spore cocktail was prepared by combining 50 mg of B. subtilis 1 and 950 mg of HU58™ powder, thereby reflecting a formulation dominated by the HU58™ strain.
2.2. Impact of Bacillus spores on the Growth of Foodborne Pathogens
This study assessed the influence of the Bacillus subtilis spore cocktail on the growth of three foodborne pathogens: Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes. The objective was to determine whether the probiotic spores could inhibit the proliferation of these pathogens in nutrient-rich and nutrient-limited environments.
To simulate varying nutrient availability, three formulations of tryptic soy broth (TSB) were prepared: full-strength (100%), half-strength (50%), and quarter-strength (25%). Each TSB formulation (10 mL per tube) was dispensed into sterile 15 mL polypropylene centrifuge tubes. All media were autoclaved at 121 °C for 15 min and cooled to room temperature prior to inoculation.
Each tube was individually inoculated with one of the target pathogens to achieve an initial concentration of approximately 3 log CFU/mL. The probiotic spore cocktail was then added to achieve a final concentration of approximately 7 log CFU/mL, in accordance with WHO recommendations suggesting a probiotic threshold between 6 to 9 log CFU/mL to confer health benefits [8,9]. The higher probiotic dose was selected to mimic typical probiotic supplementation levels used in commercial and therapeutic applications.
The tubes were incubated aerobically at 37 °C for E. coli O157:H7 and S. Typhimurium, and at 30 °C for L. monocytogenes, which better reflects its psychrotrophic growth characteristics. At 0, 3, 6, 12, and 24 h time points, 2 mL of each culture was sampled. One milliliter was used for the enumeration of pathogens and total viable counts, and the remaining 1 mL was used for spore germination analysis.
For microbial enumeration, 1 mL of the samples wereserially diluted in 0.1% buffered peptone water and surface-plated onto selective and non-selective media. E. coli O157:H7 was enumerated on MacConkey agar (BD Difco, Sparks, MD, USA) supplemented with 50 mg/L nalidixic acid. S. Typhimurium was plated on Xylose Lysine Deoxycholate (XLD) agar (BD Difco, Sparks, MD, USA) supplemented with 32 mg/L ampicillin, 16 mg/L tetracycline, and 64 mg/L streptomycin. L. monocytogenes was enumerated using Oxford agar base (Oxoid, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 100 μg/mL streptomycin and 100 μg/mL rifamycin. For total bacterial counts, samples were plated on tryptic soy agar (TSA; BD Difco, Sparks, MD, USA). The survival of probiotics was analyzed by subtracting the colony counts received on selective media from total bacterial counts on TSA.
The second portion of the sample (1 mL) was used to assess spore germination over the incubation period. Samples were transferred to sterile 15 mL borosilicate glass tubes and subjected to a thermal shock by immersion in a pre-heated water bath (Thermo Scientific Precision GP, Waltham, MA, USA) at 80 °C for 10 min using a previously published method [13]. This treatment selectively inactivated vegetative cells while preserving heat-resistant spores. The thermally treated samples were then plated on TSA to enumerate remaining spore populations.
2.3. Effectiveness of Bacillus spores to Control L. monocytogenes in Hummus
2.3.1. Hummus Preparation
Hummus was prepared using a standardized protocol to ensure batch-to-batch consistency and reproducibility for experimental use. Chickpeas (Cicer arietinum) served as the primary ingredient. For each batch, 453 g of dry chickpeas (approximately one 16 oz packet) were weighed and soaked overnight in potable water supplemented with 0.5 g of baking soda per 450 g of chickpeas [19]. Sodium bicarbonate has been shown to enhance textural properties by promoting seed coat breakdown and reducing cooking time (Figure 1).

Figure 1.
Preparation of Hummus.
Following hydration, the chickpeas were drained and cooked in a domestic pressure cooker until fully softened—typically for six whistles, equivalent to approximately 25 min of cooking time. This step ensured complete gelatinization of starches and optimal softening. After cooking, chickpeas were drained and weighed, yielding a final cooked mass of approximately 800 g. Cooked chickpeas not used immediately were stored in sterile, airtight containers at −20 °C to maintain product integrity.
Tahini was prepared in-house by blending toasted white sesame seeds and extra virgin olive oil in a 70:30 (w/w) ratio. A total of 700 g of sesame seeds were lightly toasted to enhance flavor development and reduce moisture content. The seeds were then ground with olive oil using a high-speed blender until a smooth, uniform paste was obtained. Prepared tahini was aliquoted into 65 g portions and stored at 4 °C in sterile containers to ensure consistency across trials.
The final hummus formulation consisted of 70% (w/w) mashed chickpeas, 10% tahini, 7% water, 7% olive oil, 5% freshly squeezed lemon juice, and 1% salt. All ingredients were sourced from a commercial online retailer (Amazon.com, Seattle, WA, USA) to ensure consistency across batches. The components were combined and homogenized using a laboratory-grade blender (Waring Commercial Laboratory Blender, Model 7011HS, Waring Products, Stamford, CT, USA) until a smooth, creamy consistency was achieved. To ensure uniformity in sample texture across replicates, exactly 200.61 g of blended hummus was portioned for each experiment, and 15.1 mL of sterile deionized water was added to standardize moisture content and product viscosity.
All prepared hummus samples were stored at 4 °C in sterile, sealed containers and used within 24 h of preparation. This approach ensured microbial stability and maintained the physicochemical properties essential for downstream experimental analyses.
2.3.2. Inoculation of Hummus with L. monocytogenes and Bacillus spores
To assess the survival dynamics of L. monocytogenes and probiotic Bacillus subtilis spores in a refrigerated hummus matrix, the prepared hummus (as described above) was aseptically portioned into sterile polypropylene containers, each containing 25 g of product. The hummus samples were then inoculated with a cocktail of L. monocytogenes strains to achieve a final concentration of approximately 3 log CFU/g. Simultaneously, a mixed probiotic spore cocktail (comprising B. subtilis 1 and HU58™ strains) was added to reach a final concentration of 7 log CFU/g.
Following inoculation, all samples were mixed thoroughly within their respective containers to ensure homogeneous distribution of both target organisms. The containers were then stored at 30 ± 1 °C to simulate temperature-abuse conditions. Sampling was conducted at 0, 12, 24, and 48 h post-inoculation to monitor changes in microbial populations over time.
For microbial enumeration, one full 25 g sample container was removed at each sampling point. The entire hummus sample was transferred to a sterile stomacher bag and diluted in phosphate-buffered saline (PBS; pH 7.2) at a 1:3 ratio (w/v). The samples were homogenized using a stomacher (Seward Stomacher 400 Circulator, Model 0400/CLR, Seward Laboratory Systems Inc., Bohemia, NY, USA) for 1 min.
Following homogenization, aliquots were serially diluted in 0.1% buffered peptone water. L. monocytogenes was enumerated by surface plating on Oxford agar base with antibiotics as described above to inhibit background flora. Total viable bacterial counts (including probiotics and pathogens) were determined by plating on TSA.
To assess the persistence and germination of probiotic spores in the hummus matrix, a portion of each homogenized sample (1 mL) was subjected to thermal treatment. The sample was transferred to a sterile 15 mL glass tube and heated in a water bath at 80 °C for 15 min to eliminate vegetative cells. Surviving spores were enumerated by surface plating on TSA.
2.4. Statistical Analysis
All experiments were performed in triplicate. Pathogen and probiotic population data (log CFU/mL or log CFU/g) were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s Honest Significant Difference (HSD) post-hoc test using JMP Pro version 14 to determine significant differences between treatment groups. Mean values were reported with standard deviations, and statistical significance was set at p < 0.05. Data analysis and graphical presentation were performed using GraphPad Prism (version 10.0, GraphPad Software, San Diego, CA, USA). Letter groupings (A, B, C) were used on graphs to denote statistically distinct groups at the final time point.
3. Results and Discussion
3.1. Impact of Bacillus spores on the Growth of Foodborne Pathogens
3.1.1. Escherichia coli O157:H7
E. coli O157:H7 demonstrated consistent growth across all TSB concentrations, with significantly greater growth observed in full-strength TSB (100%) compared to the half-strength (50%) and quarter-strength (25%) formulations (p < 0.05). At 24 h, E. coli reached 8.2 log CFU/mL in full-strength TSB, whereas final counts were 7.39 log CFU/mL and 7.3 log CFU/mL in half- and quarter-strength media, respectively. There was no significant difference between the half- and quarter-strength TSB treatments (p > 0.05). The Bacillus subtilis spore cocktail, introduced at 7.1 log CFU/mL, showed differential growth patterns depending on nutrient availability. In full-strength TSB, spore counts increased to 9.4 log CFU/mL, while in half- and quarter-strength TSB, the final concentrations were 8.2 log CFU/mL and 8.1 log CFU/mL, respectively, which were not significantly different from each other yet lower than full-strength (p < 0.05). Despite high spore concentrations, the probiotic cocktail did not significantly inhibit E. coli growth under the tested conditions (Figure 2).
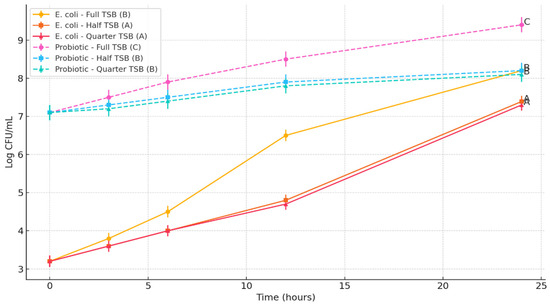
Figure 2.
Impact of Bacillus subtilis Spore Cocktail on the Growth of Escherichia coli O157:H7 under varying nutrient enrichment levels. Lines bearing different letters at the 24 h time point are significantly different (p < 0.05).
3.1.2. Salmonella Typhimurium DT 104
S. Typhimurium exhibited a growth trend similar to E. coli, with significantly higher populations recorded in full-strength TSB (p < 0.05) (Figure 3). After 24 h, Salmonella reached 8.2 log CFU/mL in full-strength TSB, while populations in half-strength and quarter-strength TSB were 7.39 and 7.3 log CFU/mL, respectively. The difference between half- and quarter-strength media was not statistically significant (p > 0.05). The Bacillus subtilis spore cocktail initiated at 7.0 log CFU/mL increased to 9.3 log CFU/mL in full-strength TSB, whereas in half- and quarter-strength media, growth plateaued at 8.2 and 8.1 log CFU/mL, respectively. These results confirm that although the probiotic spores proliferated under all nutrient conditions, the reduced nutrient concentrations significantly restricted their final population levels (p < 0.05). However, the Bacillus subtilis cocktail did not exhibit an inhibitory effect on S. Typhimurium.
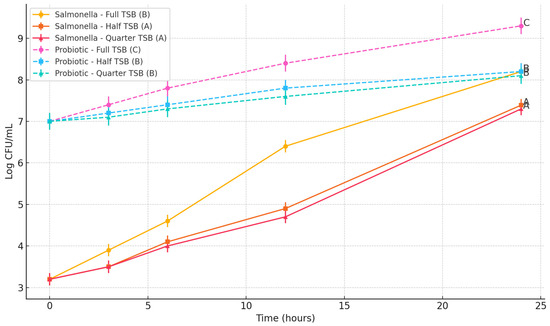
Figure 3.
Impact of Bacillus subtilis spore cocktail on the growth of Salmonella Typhimurium DT104 under varying nutrient enrichment levels. Lines bearing different letters at the 24 h time point are significantly different (p < 0.05).
3.1.3. Listeria monocytogenes
L. monocytogenes displayed a unique growth pattern compared to E. coli and Salmonella, particularly under nutrient-limited conditions. In full-strength TSB, Listeria grew from an initial 3.2 log CFU/mL to 8.2 log CFU/mL at 24 h. However, in half-strength TSB, viable counts declined from 3.2 to 1.48 log CFU/mL by 24 h, and a similar decrease was observed in quarter-strength TSB (final count 1.48 log CFU/mL), falling below the detection threshold. The probiotic Bacillus subtilis cocktail initiated at 7.2 log CFU/mL and reached 9.4 log CFU/mL in full-strength TSB, while half- and quarter-strength TSB showed final probiotic levels of 8.2 and 8.1 log CFU/mL, respectively. This result indicates a statistically significant suppression of L. monocytogenes under nutrient-limited conditions when co-cultured with the Bacillus subtilis spore cocktail (p < 0.05), potentially due to antimicrobial activity or competitive exclusion. The data clearly demonstrate that Listeria is more susceptible to inhibition by the probiotic under nutrient stress compared to the other pathogens evaluated (Figure 4).
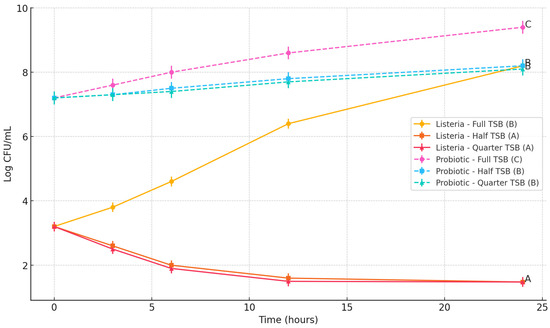
Figure 4.
Impact of Bacillus subtilis spore cocktail on the growth of Listeria monocytogenes under varying nutrient enrichment levels. Lines bearing different letters at the 24 h time point are significantly different (p < 0.05).
The rate of spore germination was evaluated under all nutrient enrichment conditions (full-strength, half-strength, and quarter-strength TSB). In all cases, nearly complete germination of Bacillus subtilis spores was observed within 6 h of incubation. This was confirmed using the heat resistance assay described in the methods, where no viable spores were recovered following treatment at 80 °C for 10 min, indicating a loss of heat resistance and successful germination regardless of nutrient availability.
The results of this study demonstrate that Bacillus subtilis spore probiotics exhibit stronger antagonistic effects against the Gram-positive pathogen L. monocytogenes than against Gram-negative pathogens, such as S. Typhimurium and E. coli O157:H7. This observation aligns with previous findings that B. subtilis—a spore-forming, transient probiotic—employs multiple mechanisms to suppress microbial competitors, including competitive exclusion, nutrient competition, and the production of antimicrobial peptides [9]. The significantly reduced Listeria levels, particularly under nutrient-limited conditions, suggest that B. subtilis is most effective when its competitive advantages—such as rapid germination and nutrient scavenging—are maximized. Gram-positive bacteria, lacking an outer membrane, are also more susceptible to bacteriocins and other antimicrobial compounds produced by B. subtilis [15,18].
One of the primary mechanisms contributing to pathogen suppression by B. subtilis is competitive exclusion, particularly in nutrient-limited environments. The bacterium’s ability to outcompete pathogens for essential nutrients, including carbon sources and iron, can restrict the growth of susceptible organisms, such as L. monocytogenes [21]. In nutrient-scarce environments, B. subtilis produces siderophores, such as bacillibactin, further depriving other bacteria of iron [22]. This study’s observation that Listeria populations declined below detectable limits in half- and quarter-strength media supports the theory that nutrient deprivation plays a critical role in inhibition. Gram-negative organisms, such as Salmonella and E. coli, possess more adaptive nutrient acquisition systems, potentially explaining their persistence despite co-culture with the probiotic [21].
Antimicrobial production is another significant contributor to B. subtilis’ antagonistic activity, especially against Gram-positive bacteria. The bacterium is known to produce a range of antimicrobial compounds, including subtilosin A, subtilin, and other lipopeptides and polyketides [18,22]. These compounds target the peptidoglycan layer of Gram-positive organisms, leading to cell lysis and death. Gram-negative bacteria, by contrast, are inherently more resistant due to their outer membrane, which limits access of large peptide antibiotics to the cell wall and membrane [15]. The differential susceptibility observed in this study—marked inhibition of Listeria and sustained growth of Salmonella and E. coli—is consistent with these mechanistic differences.
While B. subtilis may not directly inhibit Gram-negative pathogens through bactericidal activity, its role in competitive exclusion remains important, particularly in complex ecosystems, such as the gastrointestinal tract. La Ragione and Woodward (2003) [23] showed that oral administration of B. subtilis spores to chicks significantly reduced Salmonella Enteritidis colonization without directly killing the pathogen, likely due to competition for adhesion sites and nutrients. This suggests that in real-world applications, B. subtilis may still help control Gram-negative pathogens indirectly, especially when used in combination with other interventions or delivered in environments that support its colonization and metabolic activity.
The findings of this study contribute to a growing body of evidence demonstrating the selective antimicrobial activity of Bacillus subtilis spores. While Listeria monocytogenes is highly susceptible to B. subtilis inhibition through nutrient competition and antimicrobial peptide production, S. Typhimuriumand E. coli O157:H7 are less affected due to their structural defenses and metabolic versatility. These findings highlight the potential application of B. subtilis probiotics for targeted control of Gram-positive pathogens, particularly in nutrient-limited systems or food environments where traditional interventions may be limited.
3.2. Efficacy of Bacillus spores to Reduce L. monocytogenes from Hummus in a Temperature-Abuse Scenario
The impact of Bacillus subtilis probiotic spores on the growth of L. monocytogenes in a hummus matrix stored at 30 °C was evaluated over a 48 h period. In samples without probiotics, L. monocytogenes populations increased from an initial level of 2.6–3.0 log CFU/g to between 7.72 and 8.82 log CFU/g by 48 h (Figure 5). In contrast, hummus samples co-inoculated with a probiotic spore cocktail (including B. subtilis 1 and HU58™ strains) exhibited significantly reduced Listeria growth, reaching only 4.33 to 5.65 log CFU/g at the same time point (p < 0.05). The initial inoculum levels were similar across all treatments, and standard deviation bars reflect biological triplicates. The substantial inhibition of Listeria in the presence of probiotics suggests effective competitive exclusion and/or antimicrobial activity by B. subtilis spores under temperature-abuse storage conditions.
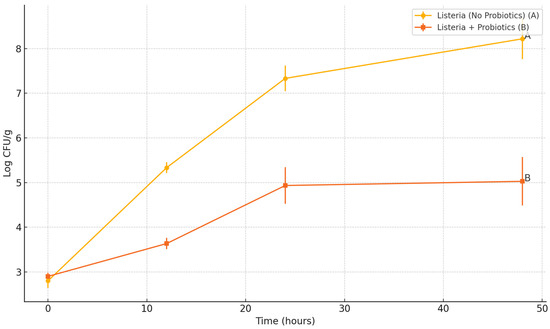
Figure 5.
Impact of Bacillus subtilis Spore Cocktail on the Growth of Listeria monocytogenes in Hummus. Lines bearing different letters at the 24 h time point are significantly different (p < 0.05).
L. monocytogenes contamination in ready-to-eat (RTE) dips, such as hummus, is a well-documented food safety concern, linked to multiple recalls and outbreaks in recent years [2,3]. The study evaluated the potential of Bacillus subtilis spore-based probiotics to suppress L. monocytogenes in a hummus matrix stored under temperature-abuse conditions (30 °C), which reflect a worst-case scenario for product handling and simulate optimal conditions for Listeria proliferation. While the B. subtilis spores were able to reduce pathogen growth compared to the control without probiotics, the reduction was less dramatic than observed in laboratory media. This moderate level of control highlights the real-world challenges of applying probiotic interventions in complex food systems. Given that L. monocytogenes is capable of robust growth in nutrient-rich RTE foods under warm storage conditions [1], even modest reductions achieved through natural, clean-label approaches, such as probiotics, can meaningfully improve food safety.
The comparatively reduced probiotic effectiveness observed in hummus is likely due to the structural and physical complexity of the matrix. Hummus is a high-viscosity, emulsion-based product containing particulates, such as mashed chickpeas and tahini. This dense structure likely creates isolated microenvironments that restrict the diffusion of probiotic spores and metabolites, limiting direct contact with Listeria cells. Such compartmentalization may allow L. monocytogenes to proliferate within protected niches, even in the presence of probiotics. These limitations are compounded by the fact that B. subtilis spores must germinate into metabolically active vegetative cells to exert antimicrobial effects. Although storage at 30 °C favors germination more than refrigeration, the lack of free water and the heterogeneous distribution of nutrients in the hummus matrix may still limit uniform germination and activity. Bhunia (2012) [5] and Blaiotta et al. (2023) [4] both highlight that probiotic efficacy in food systems is strongly influenced not just by temperature, but by food structure and water activity, which affect microbial behavior and interactions. In our study, the less pronounced Listeria reduction compared to broth cultures suggests that physical barriers within the hummus limited probiotic–pathogen contact, thereby weakening competitive exclusion and antimicrobial action.
It is also important to note that Listeria growth was not inherently inhibited by the hummus formulation itself. Control samples (hummus with L. monocytogenes only) showed significant pathogen growth over 48 h at 30 °C, confirming that the combination of ingredients—including lemon juice, salt, and oil—did not prevent pathogen proliferation. This finding is consistent with prior studies that have reported Listeria growth in hummus and similar dips under temperature-abuse conditions [1,2]. In contrast, Tran et al. (2020) [22] showed that Bacillus amyloliquefaciens could effectively inhibit Listeria on cantaloupe surfaces—an open, lower-viscosity environment that likely allowed more direct probiotic–pathogen interactions. Our findings suggest that successful probiotic applications in complex foods, such as hummus, require not only strain selection and appropriate temperature conditions, but also consideration of the physical food matrix characteristics that may hinder probiotic distribution and function. Strategies, such as improving homogenization, optimizing spore germination, or co-applying diffusible antimicrobials, may enhance probiotic efficacy in dense RTE foods. Nevertheless, the observed pathogen suppression demonstrates that B. subtilis spores hold promise as a supplemental control strategy under temperature-abuse conditions, where risk of Listeria outgrowth is highest.
4. Conclusions
This study demonstrates that Bacillus subtilis spores exhibit targeted antimicrobial activity against Listeria monocytogenes under both laboratory and food matrix conditions, with enhanced effectiveness observed under nutrient-limited and temperature-abuse environments. While their impact on Gram-negative pathogens was minimal, the probiotic spores substantially suppressed Listeria in broth and hummus systems, supporting their potential role as a clean-label, natural intervention strategy in ready-to-eat products. These findings highlight the importance of environmental context, such as nutrient availability and matrix structure, in determining probiotic–pathogen dynamics. Further research is warranted to optimize delivery systems and explore synergistic combinations with other natural antimicrobials for comprehensive food safety protection.
5. Limitations
Although this study successfully demonstrated the selective antimicrobial activity of Bacillus subtilis spores against Listeria monocytogenes in both broth and hummus models, several limitations should be acknowledged. First, while hummus was chosen for its relevance as a high-risk RTE product, its dense, heterogeneous structure likely limited probiotic–pathogen interactions. These physical constraints may not reflect conditions in more open or homogeneous food systems, limiting the generalizability of the findings. Second, the study focused on short-term temperature abuse (48 h at 30 °C), and did not evaluate longer-term storage or fluctuating temperatures commonly encountered in real-world distribution and retail environments. Addressing these limitations in future studies will help refine the practical application of spore-based probiotics as natural antimicrobials in RTE foods.
Author Contributions
Conceptualization, A.D. and R.J.; Methodology, A.D., R.J., X.M.T. and J.P.; Investigation, A.D. and X.M.T.; Data curation, A.D.; Writing, review and editing, A.D., R.J., J.P. and X.M.T.; Supervision, R.J.; Project administration, R.J.; Funding Acquisition, R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the USDA NIFA award number 2023-67018-39830.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Jessie Payne was employed by the company Nestle-Purina. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| CFU | Colony forming unit |
| GRAS | Generally Recognized as Safe |
| PBS | Phosphate buffered saline |
| RTE | Ready to eat |
| TSA | Tryptic soy agar |
| TSB | Tryptic soy broth |
References
- Alali, W.Q.; Mann, D.A.; Beuchat, L.R. Viability of Salmonella and Listeria Monocytogenes in Delicatessen Salads and Hummus as Affected by Sodium Content and Storage Temperature. J. Food Prot. 2012, 75, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Abu Ghoush, M.; Al-Nabulsi, A.A.; Holley, R.A. Control of Salmonella Enterica and Listeria Monocytogenes in Hummus Using Allyl Isothiocyanate. Int. J. Food Microbiol. 2018, 278, 73–80. [Google Scholar] [CrossRef]
- What Is Listeria and How Did It Get in My Hummus? Available online: https://www.pbs.org/newshour/health/listeria-get-hummus (accessed on 6 May 2025).
- Blaiotta, G.; De Sena, M.; De Girolamo, F.; Aponte, M.; Romano, R. Probiotic Bacilli Incorporation in Foods: Is Really so Easy? Food Microbiol. 2023, 115, 104342. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.R.; Bhunia, A.K. Modern Approaches in Probiotics Research to Control Foodborne Pathogens. Adv. Food Nutr. Res. 2012, 67, 185–239. [Google Scholar] [CrossRef]
- Williams, N.; Weir, T.L. Spore-Based Probiotic Bacillus Subtilis: Current Applications in Humans and Future Perspectives. Fermentation 2024, 10, 78. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Payne, J.; Bellmer, D.; Jadeja, R.; Muriana, P. The Potential of Bacillus Species as Probiotics in the Food Industry: A Review. Foods 2024, 13, 2444. [Google Scholar] [CrossRef]
- Todorov, S.D.; Ivanova, I.V.; Popov, I.; Weeks, R.; Chikindas, M.L. Bacillus Spore-Forming Probiotics: Benefits with Concerns? Crit. Rev. Microbiol. 2022, 48, 513–530. [Google Scholar] [CrossRef]
- Zhang, C.; Li, B.; Jadeja, R.; Fang, J.; Hung, Y.-C. Effects of Bacterial Concentrations and Centrifugations on Susceptibility of Bacillus Subtilis Vegetative Cells and Escherichia Coli O157:H7 to Various Electrolyzed Oxidizing Water Treatments. Food Control 2016, 60, 440–446. [Google Scholar] [CrossRef]
- Payne, J.; Bellmer, D.; Jadeja, R. Determining the Viability and Stability of Bacillus in Baked Products. LWT 2025, 218, 117519. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic Characteristics of Bacillus Coagulans and Associated Implications for Human Health and Diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic Bacilli Inhibit Salmonella Biofilm Formation Without Killing Planktonic Cells. Front. Microbiol. 2021, 12, 615328. [Google Scholar] [CrossRef]
- Nannan, C.; Vu, H.Q.; Gillis, A.; Caulier, S.; Nguyen, T.T.T.; Mahillon, J. Bacilysin within the Bacillus Subtilis Group: Gene Prevalence versus Antagonistic Activity against Gram-Negative Foodborne Pathogens. J. Biotechnol. 2021, 327, 28–35. [Google Scholar] [CrossRef]
- Erega, A.; Stefanic, P.; Dogsa, I.; Danevčič, T.; Simunovic, K.; Klančnik, A.; Smole Možina, S.; Mandic Mulec, I. Bacillaene Mediates the Inhibitory Effect of Bacillus Subtilis on Campylobacter Jejuni Biofilms. Appl. Environ. Microbiol. 2021, 87, e02955-20. [Google Scholar] [CrossRef]
- Zheng, G.; Hehn, R.; Zuber, P. Mutational Analysis of the sbo-alb. Locus of Bacillus Subtilis: Identification of Genes Required for Subtilosin Production and Immunity. J. Bacteriol. 2000, 182, 3266–3273. [Google Scholar] [CrossRef]
- Salazar, J.K.; Natarajan, V.; Stewart, D.; Fay, M.; Gonsalves, L.J.; Mhetras, T.; Sule, C.; Tortorello, M.L. Listeria Monocytogenes Growth Kinetics in Refrigerated Ready-to-Eat Dips and Dip Components. PLoS ONE 2020, 15, e0235472. [Google Scholar] [CrossRef]
- Jadeja, R.; Hung, Y.-C. Influence of Nalidixic Acid Adaptation on Sensitivity of Various Shiga Toxin-Producing Escherichia Coli to EO Water Treatment. LWT Food Sci. Technol. 2013, 54, 298–301. [Google Scholar] [CrossRef]
- Podnar, E.; Erega, A.; Danevčič, T.; Kovačec, E.; Lories, B.; Steenackers, H.; Mandic-Mulec, I. Nutrient Availability and Biofilm Polysaccharide Shape the Bacillaene-Dependent Antagonism of Bacillus Subtilis against Salmonella Typhimurium. Microbiol. Spectr. 2022, 10, e01836-22. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Imre, K.; Arslan-Acaroz, D.; Istanbullugil, F.R.; Fang, Y.; Ros, G.; Zhu, K.; Acaroz, U. Mechanisms of Probiotic Bacillus against Enteric Bacterial Infections. One Health Adv. 2023, 1, 21. [Google Scholar] [CrossRef]
- La Ragione, R.M.; Woodward, M.J. Competitive Exclusion by Bacillus Subtilis Spores of Salmonella Enterica Serotype Enteritidis and Clostridium Perfringens in Young Chickens. Vet. Microbiol. 2003, 94, 245–256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).