A Potent Fluorescent Derivative of 8-Hydroxyquinoline Suggests Cell Wall Damage as a Possible Cellular Action of the 5-Triazole 8-Hydroxyquinoline Class
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Synthesis of 5-Nitro-8-Hydroxyquinoline (2)
2.1.2. Synthesis of 5-Azido-8-Hydroxyquinoline (4)
2.1.3. Synthesis of 1-Chloro-4-Ethynylbenzene (8)
2.1.4. Synthesis of 5-(4-(4-Chlorophenyl)-1H-1,2,3-Triazol-1-yl)Uinoline-8-ol (10)
2.2. Fungal Strains
Susceptibility Tests
2.3. Cellular Action Studies
2.3.1. Sorbitol Protection Assays
2.3.2. Ergosterol Effect Assay
2.3.3. Confocal Fluorescence Microscopy of Fungal Strains Treated with Compound 10
2.3.4. Confocal Fluorescence Microscopy with Calcofluor White (CFW)
2.3.5. Scanning Electron Microscopy
3. Results
3.1. Chemistry
3.2. Biological Assays
3.2.1. Fungal Susceptibility Test
3.2.2. Cellular Action Assays
Sorbitol Protection Assay
Exogenous Ergosterol Effect Assay
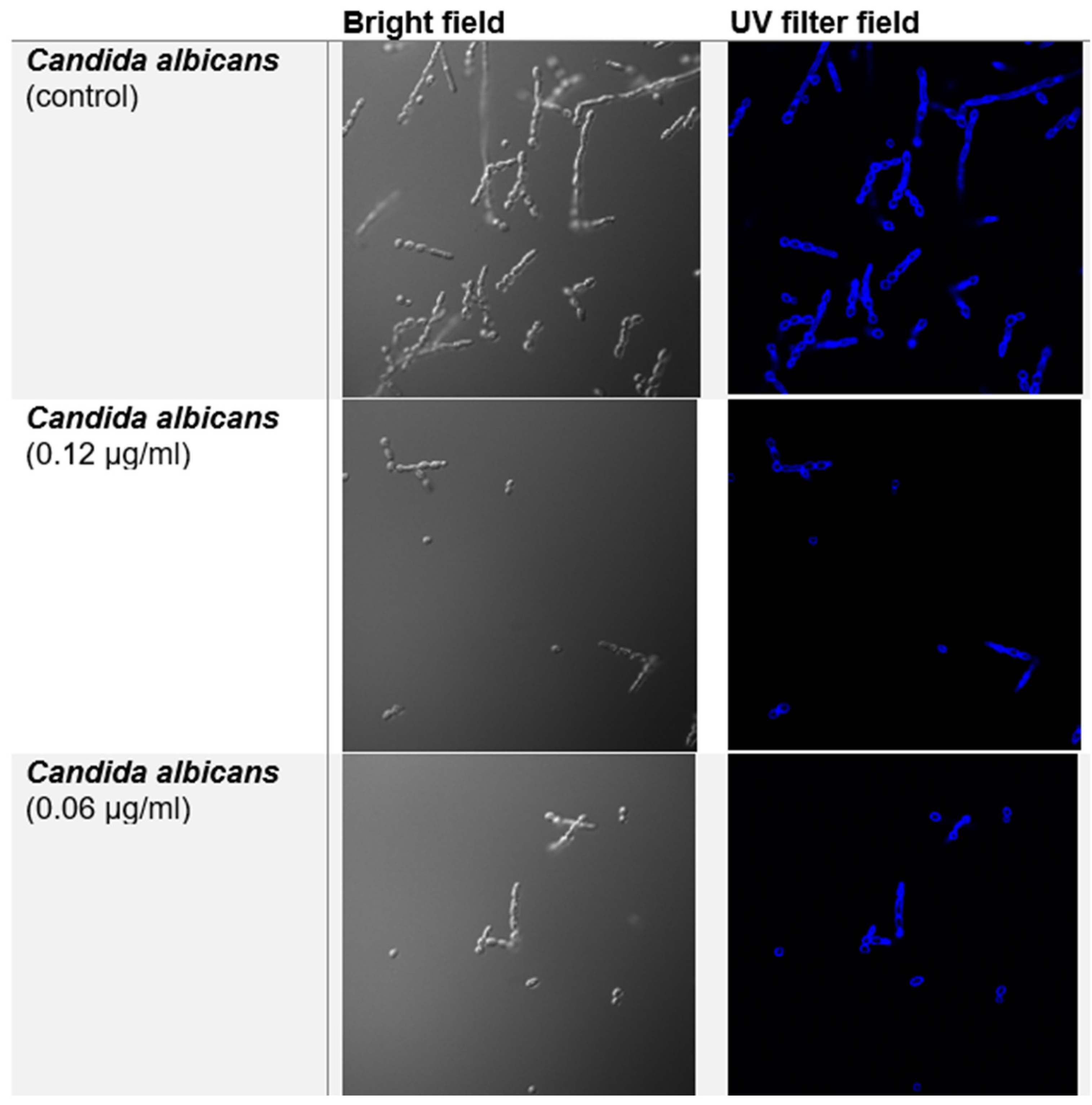
Confocal Fluorescence Microscopy
Confocal Fluorescence Microscopy with Calcofluor White (CFW)
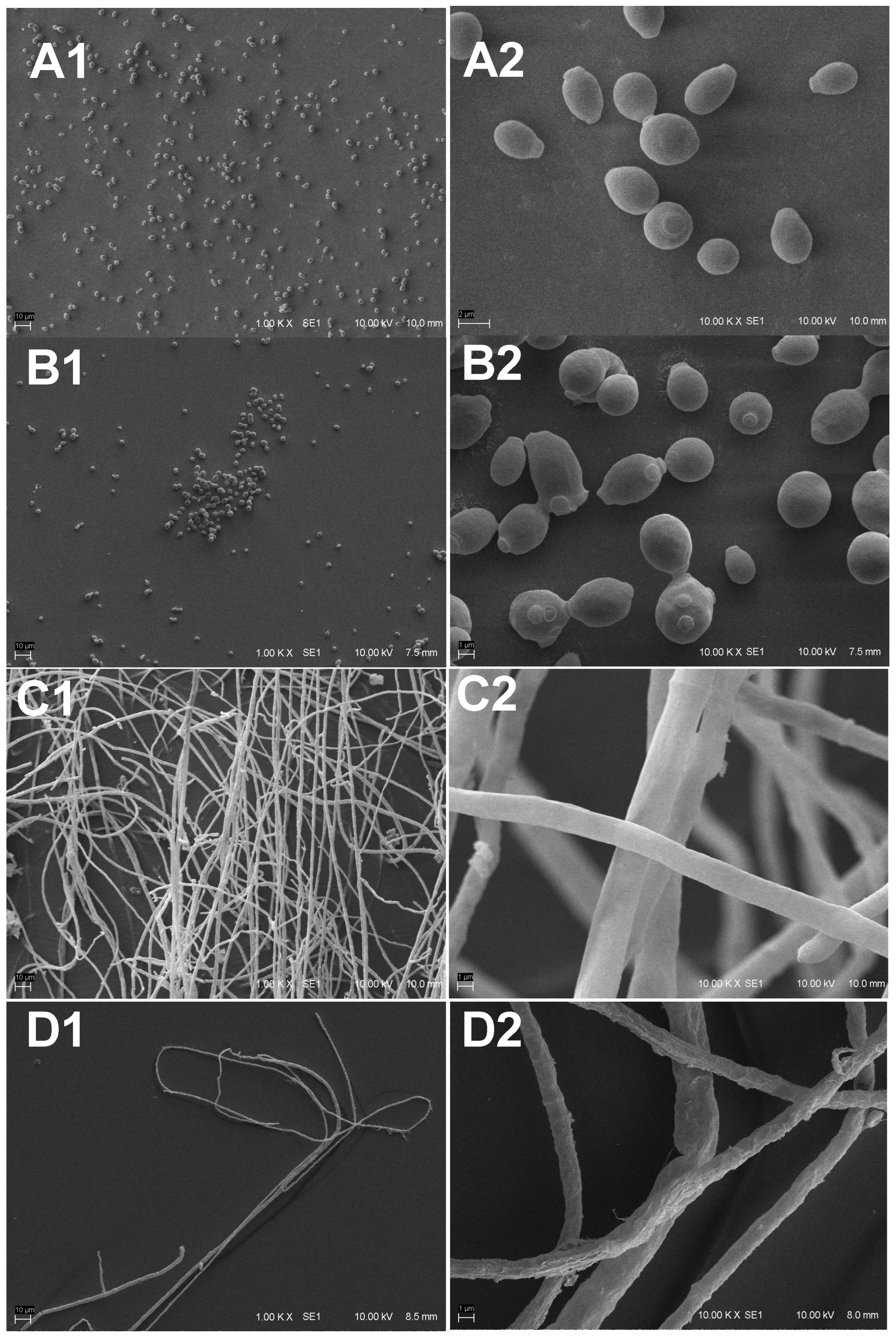
Scanning Electron Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giacobbe, D.R.; Maraolo, A.E.; Simeon, V.; Magnè, F.; Pace, M.C.; Gentile, I.; Chiodini, P.; Viscoli, C.; Sanguinetti, M.; Mikulska, M.; et al. Changes in the relative prevalence of candidaemia due to non-albicans Candida species in adult in-patients: A systematic review, meta-analysis and meta-regression. Mycoses 2020, 63, 334–342. [Google Scholar] [CrossRef]
- de Albuquerque Maranhão, F.C.; Oliveira-Júnior, J.B.; Araújo, M.A.D.S.; Silva, D.M.W. Mycoses in northeastern Brazil: Epidemiology and prevalence of fungal species in 8 years of retrospective analysis in Alagoas. Braz. J. Microbiol. 2019, 50, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Tóth, R.; Nosek, J.; Mora-Montes, H.M.; Gabaldon, T.; Bliss, J.M.; Nosanchuk, J.D.; Turner, S.A.; Butler, G.; Vágvölgyi, C.; Gácser, A. Candida parapsilosis: From Genes to the Bedside. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Faure-Cognet, O.; Fricker-Hidalgo, H.; Pelloux, H.; Leccia, M.T. Superficial Fungal Infections in a French Teaching Hospital in Grenoble Area: Retrospective Study on 5470 Samples from 2001 to 2011. Mycopathologia 2016, 181, 59–66. [Google Scholar] [CrossRef]
- Xie, F.; Peng, F. Anti-Prostate Cancer Activity of 8-Hydroxyquinoline-2-Carboxaldehyde–Thiosemicarbazide Copper Complexes by Fluorescent Microscopic Imaging. J. Fluoresc. 2017, 27, 1937–1941. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.M.; Li, S.S.; Liu, D.M.; Lv, X.H.; Sun, X.L. Synthesis of Electron-Deficient Borinic Acid Polymers with Multiresponsive Properties and Their Application in the Fluorescence Detection of Alizarin Red S and Electron-Rich 8-Hydroxyquinoline and Fluoride Ion: Substituent Effects. Macromolecules 2017, 50, 6872–6879. [Google Scholar] [CrossRef]
- Rosa, P.D.; Ramirez-Castrillon, M.; Valente, P.; Fuentefria, A.M.; Van Diepeningen, A.D.; Goldani, L.Z. Fusarium riograndense sp. nov. a new species in the Fusarium solani species complex causing fungal rhinosinusitis. J. Mycol. Med. 2018, 28, 29–35. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Zhang, J.; Chen, R.; Zhong, X.; Wu, X.; Zheng, L.; Zhao, J. In vitro evaluation of photodynamic effects against biofilms of dermatophytes involved in onychomycosis. Front. Microbiol. 2019, 10, 1228. [Google Scholar] [CrossRef]
- Gauthier, G.M.; Keller, N.P. Crossover fungal pathogens: The biology and pathogenesis of fungi capable of crossing kingdoms to infect plants and humans. Fungal Genet. Biol. 2013, 61, 146–157. [Google Scholar] [CrossRef]
- Herkert, P.F.; Al-Hatmi, A.M.S.; De Oliveira Salvador, G.L.; Muro, M.D.; Pinheiro, R.L.; Nucci, M.; Queiroz-Telles, F.; De Hoog, G.S.; Meis, J.F. Molecular Characterization and Antifungal Susceptibility of Clinical Fusarium Species From Brazil. Front. Microbiol. 2019, 10, 737. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, T.; Shu, D.; Zhang, W.; Luan, F.; Shi, L.; Guo, D. Synthesis and luminescence properties of novel 8-hydroxyquinoline derivatives and their Eu(III) complexes. Luminescence 2018, 33, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.D.; Sheid, K.; Locatelli, C.; Marinho, D.; Goldani, L. Fusarium solani keratitis: Role of antifungal susceptibility testing and identification to the species level for proper management. Braz. J. Infect. Dis. 2019, 23, 197–199. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte resistance to antifungal drugs: Mechanisms and prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Scheel, C.M.; Hurst, S.F.; Barreiros, G.; Akiti, T.; Nucci, M.; Balajee, S.A. Molecular analyses of Fusarium isolates recovered from a cluster of invasive mold infections in a Brazilian hospital. BMC Infect. Dis. 2013, 13, 49. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.S.; Van Den Ende, A.H.G.G.; Stielow, J.B.; Van Diepeningen, A.D.; Seifert, K.A.; McCormick, W.; Assabgui, R.; Gräfenhan, T.; De Hoog, G.S.; Levesque, C.A. Evaluation of two novel barcodes for species recognition of opportunistic pathogens in Fusarium. Fungal Biol. 2016, 120, 231–245. [Google Scholar] [CrossRef]
- De Aguiar Peres, N.T.; Maranhão, F.C.A.; Rossi, A.; Martinez-Rossi, N.M. Dermatophytes: Host-pathogen interaction and antifungal resistance. An. Bras. Dermatol. 2010, 85, 657–667. [Google Scholar] [CrossRef]
- Patel, D.; Castelo-Soccio, L.A.; Rubin, A.I.; Streicher, J.L. Laboratory Monitoring During Systemic Terbinafine Therapy for Pediatric Onychomycosis. JAMA Dermatol. 2017, 153, 1326–1327. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Pastuch-Gawolek, G.; Mrozek-Wilczkiewicz, A.; Kuczak, M.; Skonieczna, M.; Musiol, R. Synthesis of 8-hydroxyquinoline glycoconjugates and preliminary assay of their β1,4-GalT inhibitory and anti-cancer properties. Bioorg. Chem. 2019, 84, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Cherdtrakulkiat, R.; Boonpangrak, S.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Derivatives (halogen, nitro and amino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochem. Biophys. Rep. 2016, 6, 135–141. [Google Scholar] [CrossRef]
- Kadri, D.; Crater, A.K.; Lee, H.; Solomon, V.R.; Ananvoranich, S. The potential of quinoline derivatives for the treatment of Toxoplasma gondii infection. Exp. Parasitol. 2014, 145, 135–144. [Google Scholar] [CrossRef]
- Kassem, E.M.; El-Sawy, E.R.; Abd-Alla, H.I.; Mandour, A.H.; Abdel-Mogeed, D.; El-Safty, M.M. Synthesis, antimicrobial, and antiviral activities of some new 5-sulphonamido-8-hydroxyquinoline derivatives. Arch. Pharm. Res. 2012, 35, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, J.; Li, W.; Sun, J.; Peng, M. Direct root penetration and rhizome vascular colonization by Fusarium oxysporum f. sp. cubense are the key steps in the successful infection of Brazil cavendish. Plant Dis. 2017, 101, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef]
- Zuo, R.; Garrison, A.T.; Basak, A.; Zhang, P.; Huigens, R.W.; Ding, Y. In vitro antifungal and antibiofilm activities of halogenated quinoline analogues against Candida albicans and Cryptococcus neoformans. Int. J. Antimicrob. Agents 2016, 48, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Joaquim, A.R.; Gionbelli, M.P.; Gosmann, G.; Fuentefria, A.M.; Lopes, M.S.; de Andrade, S.F. Novel Antimicrobial 8-Hydroxyquinoline-Based Agents: Current Development, Structure–Activity Relationships, and Perspectives. J. Med. Chem. 2021, 64, 16349–16379. [Google Scholar] [CrossRef]
- Joaquim, A.R.; Pippi, B.; de Cesare, M.A.; Rocha, D.A.; Boff, R.T.; Staudt, K.J.; Ruaro, T.C.; Zimmer, A.R.; de Araújo, B.V.; Silveira, G.P.; et al. Rapid tools to gain insights into the interaction dynamics of new 8-hydroxyquinolines with few fungal lines. Chem. Biol. Drug Des. 2019, 93, 1186–1196. [Google Scholar] [CrossRef]
- Fuentefria, A.M.; Pippi, B.; Lana, D.F.D.; Donato, K.K.; de Andrade, S.F. Antifungals discovery: An insight into new strategies to combat antifungal resistance. Lett. Appl. Microbiol. 2018, 66, 2–13. [Google Scholar] [CrossRef]
- Pippi, B.; Reginatto, P.; Machado, G.D.R.M.; Bergamo, V.Z.; Lana, D.F.D.; Teixeira, M.L.; Franco, L.L.; Alves, R.J.; Andrade, S.F.; Fuentefria, A.M. Evaluation of 8-Hydroxyquinoline Derivatives as Hits for Antifungal Drug Design. Med. Mycol. 2017, 55, 763–773. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Gershon, H.; Clarke, D.D.; Gershon, M. Preparation and Fungitoxicity of Some Trichloro-, Tribromo-, Tetrachloro-, and Tetrabromo-8-Quinolinols. Monatsh. Chem. 2001, 132, 1075–1080. [Google Scholar] [CrossRef]
- da Silva, N.M.; Gentz, C.d.B.; Reginatto, P.; Fernandes, T.H.M.; Kaminski, T.F.A.; Lopes, W.; Quatrin, P.M.; Vainstein, M.H.; Abegg, M.A.; Lopes, M.S.; et al. 8-Hydroxyquinoline 1,2,3-triazole derivatives with promising and selective antifungal activity. Med. Mycol. 2020, 59, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Ezabadi, I.R.; Camoutsis, C.; Zoumpoulakis, P.; Geronikaki, A.; Soković, M.; Glamočilija, J.; Ćirić, A. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg. Med. Chem. 2008, 16, 1150–1161. [Google Scholar] [CrossRef]
- Pippi, B.; Lopes, W.; Reginatto, P.; Silva, F.É.K.; Joaquim, A.R.; Alves, R.J.; Silveira, G.P.; Vainstein, M.H.; Andrade, S.F.; Fuentefria, A.M. New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 2019, 27, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pippi, B.; Joaquim, A.R.; Lopes, W.; Machado, G.R.M.; Bergamo, V.Z.; Giuliani, L.M.; Abegg, M.A.; Cruz, L.; Vainstein, M.H.; Fuentefria, A.M.; et al. 8-Hydroxyquinoline-5-sulfonamides are promising antifungal candidates for the topical treatment of dermatomycosis. J. Appl. Microbiol. 2020, 128, 1038–1049. [Google Scholar] [CrossRef]
- Mazumder, U.K.; Gupta, M.; Karki, S.S.; Bhattacharya, S.; Rathinasamy, S.; Thangavel, S. Synthesis, anticancer and antibacterial activity of some novel mononuclear Ru(II) complexes. Chem. Pharm. Bull. 2004, 52, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Kuang, C.; Yang, Q.; Cheng, X. Cs2CO3-mediated synthesis of terminal alkynes from 1,1-dibromo-1-alkenes. Tetrahedron. Lett. 2011, 52, 992–994. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Hu, Y.; Yuan, L.; Chen, S.; Wu, P.; Wang, W.; Zhang, S.; Zhang, W. An Efficient Method for the Production of Terminal Alkynes from 1,1-Dibromo-1-alkenes and its Application in the Total Synthesis of Natural Product Dihydroxerulin. Adv. Synth. Catal. 2015, 357, 553–560. [Google Scholar] [CrossRef]
- Shen, T.; Xu, Y.; Jiang, C.; Lai, Y.; Liu, S.; Zhang, L.; Qian, C.; Zhou, S. Electrochemical Synthesis of Aryl Chlorides Using HCl as the Chlorine Source. ACS Sustain. Chem. Eng. 2024, 12, 3289–3297. [Google Scholar] [CrossRef]
- Singh, R.M.; Nandini, D.; Bharadwaj, K.C.; Gupta, T.; Singh, R.P. Na2S-mediated synthesis of terminal alkynes from gem-dibromoalkenes. Org. Biomol. Chem. 2017, 15, 9979–9982. [Google Scholar] [CrossRef]
- M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous fungi. Approved Standard-Second Edition. Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2008.
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard-Third Edition. Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2008.
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the Mechanism of Action of the Antifungal Phytolaccoside B Isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cheng, L.W.; Land, K.M. Advances in Antifungal Development: Discovery of New Drugs and Drug Repurposing. Pharmaceuticals 2022, 15, 787. [Google Scholar] [CrossRef] [PubMed]


| CA 10 | CK ATCC 6258 | CG M28 | CP 10 | CT 04 | TME ATCC | MCA 01 | MGY ATCC | TRU 51 | FS ATCC 36031 | FO HCF46 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 1 | 1 | 1 | 1 | 2 | 0.5 | 1 | 2 | 0.5 | 4 | >64 |
| FCZ | 16 | >32 | 4 | 0.5 | 4 | - | - | - | - | - | - |
| CICLO | - | - | - | - | - | <0.125 | >1 | 0.25 | 1 | - | - |
| VCZ | - | - | - | - | - | - | - | - | - | 8 | 4 |
| Fungi Strains | Readings | 10 | MCF | ||
|---|---|---|---|---|---|
| −/Sorbitol | +/Sorbitol | −/Sorbitol | +/Sorbitol | ||
| CAMS5 | Day 2 | 2 | 4 | 0.015 | 0.03 |
| Day 7 | 4 | 8 | 0.03 | >2 | |
| MCA 01 | Day 4 | 1 | 2 | 0.125 | 2 |
| Day 7 | 2 | 2 | 0.125 | 2 | |
| TME 40 | Day 4 | 2 | 4 | 0.125 | 2 |
| Day 7 | 4 | 4 | 0.125 | 0.5 | |
| Compounds | Fungi Strains | MIC1 | MIC2 | MIC3 | MIC4 | MIC5 |
|---|---|---|---|---|---|---|
| 10 | CAMS5 | 2 | 2 | 2 | 2 | 2 |
| MCA 01 | 2 | 1 | 2 | 2 | 2 | |
| TME 40 | 2 | 1 | 2 | 2 | 2 | |
| AFB | CAMS5 | 2 | >8 | >8 | >8 | >8 |
| MCA 01 | 8 | >8 | >8 | >8 | >8 | |
| TME 40 | >8 | >8 | >8 | >8 | >8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentz, C.d.B.; Lopes, M.S.; Quatrin, P.M.; Gionbelli, M.P.; de Cesare, M.A.; Perin, A.P.; Lopes, W.; Fuentefria, A.M.; Vainstein, M.H.; Andrade, S.F.d. A Potent Fluorescent Derivative of 8-Hydroxyquinoline Suggests Cell Wall Damage as a Possible Cellular Action of the 5-Triazole 8-Hydroxyquinoline Class. Appl. Microbiol. 2025, 5, 38. https://doi.org/10.3390/applmicrobiol5020038
Gentz CdB, Lopes MS, Quatrin PM, Gionbelli MP, de Cesare MA, Perin AP, Lopes W, Fuentefria AM, Vainstein MH, Andrade SFd. A Potent Fluorescent Derivative of 8-Hydroxyquinoline Suggests Cell Wall Damage as a Possible Cellular Action of the 5-Triazole 8-Hydroxyquinoline Class. Applied Microbiology. 2025; 5(2):38. https://doi.org/10.3390/applmicrobiol5020038
Chicago/Turabian StyleGentz, Caroline de Bem, Marcela Silva Lopes, Priscilla Maciel Quatrin, Mariana Pies Gionbelli, Maycon Antonio de Cesare, Ana Paula Perin, William Lopes, Alexandre Meneghello Fuentefria, Marilene Henning Vainstein, and Saulo Fernandes de Andrade. 2025. "A Potent Fluorescent Derivative of 8-Hydroxyquinoline Suggests Cell Wall Damage as a Possible Cellular Action of the 5-Triazole 8-Hydroxyquinoline Class" Applied Microbiology 5, no. 2: 38. https://doi.org/10.3390/applmicrobiol5020038
APA StyleGentz, C. d. B., Lopes, M. S., Quatrin, P. M., Gionbelli, M. P., de Cesare, M. A., Perin, A. P., Lopes, W., Fuentefria, A. M., Vainstein, M. H., & Andrade, S. F. d. (2025). A Potent Fluorescent Derivative of 8-Hydroxyquinoline Suggests Cell Wall Damage as a Possible Cellular Action of the 5-Triazole 8-Hydroxyquinoline Class. Applied Microbiology, 5(2), 38. https://doi.org/10.3390/applmicrobiol5020038






