Microbiota-Induced Radioprotection: A Novel Approach to Enhance Human Radioresistance with In-Situ Genetically Engineered Gut Bacteria
Abstract
:1. Introduction
2. Keynote Approaches to Microbiota-Mediated Human Radioresistance
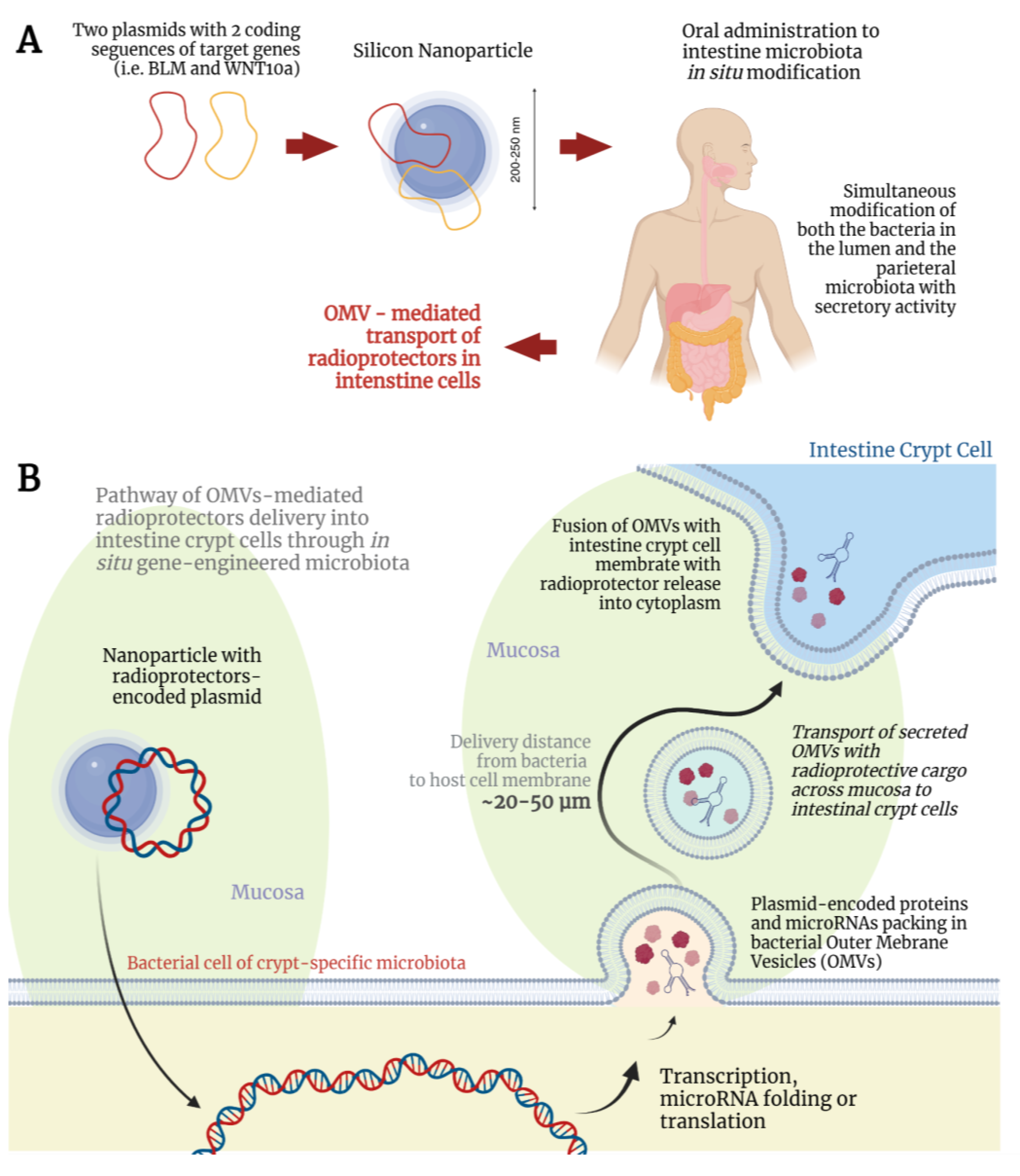
2.1. Modification of the Human Microbiota
2.2. Targeted Delivery of Plasmids to Crypt-Specific Microbiota
2.3. Interaction of Modified Microbiota with Intestinal Crypts and Enhancement of Overall Radioresistance
3. Microbiome-Induced Space Suit
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Microbiome Induced Space Suit. Available online: https://2021.igem.org/Team:MEPhI (accessed on 30 September 2024).
- Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Panou, E.; Graikou, K.; Tsafantakis, N.; Sakellarakis, F.-N.; Chinou, I. Phytochemical Profiling and Biological Activities of Two Helianthemum Species Growing in Greece. Sci. Pharm. 2024, 92, 42. [Google Scholar] [CrossRef]
- Khodamoradi, E.; Hoseini-Ghahfarokhi, M.; Amini, P.; Motevaseli, E.; Shabeeb, D.; Musa, A.E.; Najafi, M.; Farhood, B. Targets for protection and mitigation of radiation injury. Cell. Mol. Life Sci. 2020, 77, 3129–3159. [Google Scholar] [CrossRef]
- McLaughlin, M.F.; Donoviel, D.B.; Jones, J.A. Novel Indications for Commonly Used Medications as Radiation Protectants in Spaceflight. Aerosp. Med. Hum. Perform. 2017, 88, 665–676. [Google Scholar] [CrossRef]
- Langell, J.; Jennings, R.; Clark, J.; Ward, J.B., Jr. Pharmacological agents for the prevention and treatment of toxic radiation exposure in spaceflight. Aviat. Space Environ. Med. 2008, 79, 651–660. [Google Scholar] [CrossRef]
- Cortese, F.; Klokov, D.; Osipov, A.; Stefaniak, J.; Moskalev, A.; Schastnaya, J.; Cantor, C.; Aliper, A.; Mamoshina, P.; Ushakov, I.; et al. Vive la radiorésistance!: Converging research in radiobiology and biogerontology to enhance human radioresistance for deep space exploration and colonization. Oncotarget 2018, 9, 14692–14722. [Google Scholar] [CrossRef]
- Everett, W.H.; Curiel, D.T. Gene therapy for radioprotection. Cancer Gene Ther. 2015, 22, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Delhove, J.; Osenk, I.; Prichard, I.; Donnelley, M. Public Acceptability of Gene Therapy and Gene Editing for Human Use: A Systematic Review. Hum. Gene Ther. 2020, 31, 20–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Xu, J.Y.; Xing, Y.; Wu, P.; Jin, Y.W.; Wei, W.; Zhao, L.; Yang, J.; Chen, G.C.; Qin, L.Q. Lactobacillus rhamnosus GG alleviates radiation-induced intestinal injury by modulating intestinal immunity and remodeling gut microbiota. Microbiol. Res. 2024, 286, 127821. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.M.; Guo, H.X.; Cai, J.W.; Meng, X.C. Bifidobacterium breve alleviates DSS-induced colitis in mice by maintaining the mucosal and epithelial barriers and modulating gut microbes. Nutrients 2022, 14, 3671. [Google Scholar] [CrossRef]
- Teng, G.; Liu, Z.; Liu, Y.; Wu, T.; Dai, Y.; Wang, H.; Wang, W. Probiotic Escherichia coli Nissle 1917 expressing Elafin protects against inflammation and restores the gut microbiota. Front. Microbiol. 2022, 13, 819336. [Google Scholar] [CrossRef] [PubMed]
- Barra, M.; Danino, T.; Garrido, D. Engineered probiotics for detection and treatment of inflammatory intestinal diseases. Front. Bioeng. Biotechnol. 2020, 8, 265. [Google Scholar] [CrossRef]
- Meena, S.K.; Joriya, P.R.; Yadav, S.M.; Kumar, R.; Meena, P.; Patel, D.D. Modulation of radiation-induced intestinal injury by radioprotective agents: A cellular and molecular perspectives. Rev. Environ. Health 2022, 38, 295–311. [Google Scholar] [CrossRef]
- Venkidesh, B.S.; Shankar, S.R.; Narasimhamurthy, R.K.; Rao, S.B.S.; Mumbrekar, K.D. Radioprotective potential of probiotics against gastrointestinal and neuronal toxicity: A preclinical study. Clin. Transl. Oncol. 2023, 25, 3165–3173. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A.K.; Verma, V.; Singh, B.; Mal, G.; Nagpal, R.; Hemalatha, R. Bioengineered probiotics as a new hope for health and diseases: An overview of potential and prospects. Future Microbiol. 2016, 11, 585–600. [Google Scholar] [CrossRef]
- Bubnov, D.M.; Yuzbashev, T.V.; Khozov, A.A.; Melkina, O.E.; Vybornaya, T.V.; Stan, G.B.; Sineoky, S.P. Robust counterselection and advanced λRed recombineering enable markerless chromosomal integration of large heterologous constructs. Nucleic Acids Res. 2022, 50, 8947–8960. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles-A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous silica nanoparticle nanocarriers: Biofunctionality and biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef]
- Saffarian, A.; Mulet, C.; Regnault, B.; Amiot, A.; Tran-Van-Nhieu, J.; Ravel, J.; Sobhani, I.; Sansonetti, P.J.; Pédron, T. Crypt- and Mucosa-Associated Core Microbiotas in Humans and Their Alteration in Colon Cancer Patients. mBio 2019, 10, e01315-19. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Lee, Y.S.; Lee, S.H.; Kim, M.N.; O, E.; Kim, K.S.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef] [PubMed]
- Pédron, T.; Mulet, C.; Dauga, C.; Frangeul, L.; Chervaux, C.; Grompone, G.; Sansonetti, P.J. A crypt-specific core microbiota resides in the mouse colon. mBio 2012, 3, e00116-12. [Google Scholar] [CrossRef]
- Peck, B.C.E.; Shanahan, M.T.; Singh, A.P.; Sethupathy, P. Gut Microbial Influences on the Mammalian Intestinal Stem Cell Niche. Stem Cells Int. 2017, 2017, 5604727. [Google Scholar] [CrossRef] [PubMed]
- Bonis, V.; Rossell, C.; Gehart, H. The intestinal epithelium–fluid fate and rigid structure from crypt bottom to villus tip. Front. Cell Dev. Biol. 2021, 9, 661931. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Foley, E. Microbial recognition regulates intestinal epithelial growth in homeostasis and disease. FEBS J. 2022, 289, 3666–3691. [Google Scholar] [CrossRef]
- Dobrzyński, L.; Fornalski, K.W.; Feinendegen, L.E. Cancer Mortality Among People Living in Areas With Various Levels of Natural Background Radiation. Dose Response 2015, 13, 1559325815592391. [Google Scholar] [CrossRef] [PubMed]
- Kasianchuk, N.; Rzymski, P.; Kaczmarek, Ł. The biomedical potential of tardigrade proteins: A review. Biomed. Pharmacother. 2023, 158, 114063. [Google Scholar] [CrossRef]
- Wang, K.Y.; Yamada, S.; Izumi, H.; Tsukamoto, M.; Nakashima, T.; Tasaki, T.; Guo, X.; Uramoto, H.; Sasaguri, Y.; Kohno, K. Critical in vivo roles of WNT10A in wound healing by regulating collagen expression/synthesis in WNT10A-deficient mice. PLoS ONE 2018, 13, e0195156. [Google Scholar] [CrossRef]
- Kumagai, M.; Guo, X.; Wang, K.Y.; Izumi, H.; Tsukamoto, M.; Nakashima, T.; Tasaki, T.; Kurose, N.; Uramoto, H.; Sasaguri, Y.; et al. Depletion of WNT10A Prevents Tumor Growth by Suppressing Microvessels and Collagen Expression. Int. J. Med. Sci. 2019, 16, 416–423. [Google Scholar] [CrossRef]
- Ababou, M. Bloom syndrome and the underlying causes of genetic instability. Mol. Genet. Metab. 2021, 133, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Mondragón-Palomino, O.; Poceviciute, R.; Lignell, A.; Griffiths, J.A.; Takko, H.; Ismagilov, R.F. Three-dimensional imaging for the quantification of spatial patterns in microbiota of the intestinal mucosa. Proc. Natl. Acad. Sci. USA 2022, 119, e2118483119. [Google Scholar] [CrossRef] [PubMed]
- Westover, C.; Najjar, D.; Meydan, C.; Grigorev, K.; Veling, M.Y.; Chang, R.L.; Chin, C.; Butler, D.; Afshin, E.E.; Silver, P.A.; et al. Multi-omics Analysis of Dsup Expressing Human Cells Reveals Open Chromatin Architectural Dynamics Underyling Radioprotection. BioRxiv. 2020. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kunieda, T. DNA protection protein, a novel mechanism of radiation tolerance: Lessons from tardigrades. Life 2017, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Hesgrove, C.; Boothby, T.C. The biology of tardigrade disordered proteins in extreme stress tolerance. Cell Commun. Signal. 2020, 18, 178. [Google Scholar] [CrossRef]
- Nishad, S.; Chauhan, P.K.; Sowdhamini, R.; Ghosh, A. Chronic exposure of humans to high level natural background radiation leads to robust expression of protective stress response proteins. Sci. Rep. 2021, 11, 1777. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Jaiswal, S.; Kumar, R.; Arora, R.; Sharma, R.K. Himalayan Bioresource Rhodiola imbricata as a promising radioprotector for nuclear and radiological emergencies. J. Pharm. Bioallied. Sci. 2010, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, L.J.; Mancinelli, R.L. Life in extreme environments. Nature 2001, 409, 1092–1101. [Google Scholar] [CrossRef]
- Espinal, A.; Epperly, M.W.; Mukherjee, A.; Fisher, R.; Shields, D.; Wang, H.; Huq, M.S.; Hamade, D.F.; Vlad, A.M.; Coffman, L.; et al. Intestinal radiation protection and mitigation by second-generation probiotic Lactobacillus-reuteri engineered to deliver Interleukin-22. Int. J. Mol. Sci. 2022, 23, 5616. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Jiang, Y.; Shen, W.; Pei, H.; Wang, G.; Pei, P.; Yang, K. The role of bacteria and its derived biomaterials in cancer radiotherapy. Acta Pharm. Sin. B 2023, 13, 4149–4171. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Ting, J.P.Y. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Crawford, P.A.; Gordon, J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA 2005, 102, 132254–132259. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Yue, J. Radiotherapy and the gut microbiome: Facts and fiction. Rad. Oncol. 2021, 16, 9. [Google Scholar] [CrossRef]
- Xie, L.W.; Lu, H.Y.; Tang, L.F.; Tang, F.L.; Zhu, R.Q.; Wang, D.F.; Cai, S.; Tian, Y.; Li, M. Probiotic consortia protect the intestine against radiation injury by improving intestinal epithelial homeostasis. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, J.; Pan, H.; Zhang, X.; Zhang, Y. Genetically engineered bacterium: Principles, practices, and prospects. Front. Microbiol. 2022, 13, 997587. [Google Scholar] [CrossRef] [PubMed]
- Caetano, M.A.F.; Castelucci, P. Role of short chain fatty acids in gut health and possible therapeutic approaches in inflammatory bowel diseases. World J. Clin. Cases 2022, 10, 9985. [Google Scholar] [CrossRef]
- Kerry, R.G.; Das, G.; Golla, U.; del Pilar Rodriguez-Torres, M.; Shin, H.S.; Patra, J.K. Engineered probiotic and prebiotic nutraceutical supplementations in combating non-communicable disorders: A review. Curr. Pharm. Biotechnol. 2022, 23, 72–97. [Google Scholar] [CrossRef] [PubMed]
- Westover, C.; Najjar, D.; Meydan, C.; Grigorev, K.; Veling, M.T.; Iosim, S.; Colon, R.; Yang, S.; Restrepo, U.; Chin, C.R.; et al. Engineering Radioprotective Human Cells Using the Tardigrade Damage Suppressor Protein, DSUP 2. Spaceflight 2020, 50, 51. [Google Scholar]
- Shaba, E.; Landi, C.; Marzocchi, C.; Vantaggiato, L.; Bini, L.; Ricci, C.; Cantara, S. Proteomics reveals how the tardigrade damage suppressor protein teaches transfected human cells to survive UV-C stress. Int. J. Mol. Sci. 2023, 24, 11463. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Defense in the Toughest Animals on the Earth: Its Contribution to the Extreme Resistance of Tardigrades. Int. J. Mol. Sci. 2024, 25, 8393. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakimova, A.O.; Nikolaeva, A.; Galanova, O.; Shestakova, V.A.; Smirnova, E.I.; Levushkina, A.; Baranovskii, D.S.; Smirnova, A.N.; Stepanenko, V.N.; Kudlay, D.A.; et al. Microbiota-Induced Radioprotection: A Novel Approach to Enhance Human Radioresistance with In-Situ Genetically Engineered Gut Bacteria. Appl. Microbiol. 2025, 5, 1. https://doi.org/10.3390/applmicrobiol5010001
Yakimova AO, Nikolaeva A, Galanova O, Shestakova VA, Smirnova EI, Levushkina A, Baranovskii DS, Smirnova AN, Stepanenko VN, Kudlay DA, et al. Microbiota-Induced Radioprotection: A Novel Approach to Enhance Human Radioresistance with In-Situ Genetically Engineered Gut Bacteria. Applied Microbiology. 2025; 5(1):1. https://doi.org/10.3390/applmicrobiol5010001
Chicago/Turabian StyleYakimova, Anna O., Anastasiia Nikolaeva, Olesya Galanova, Victoria A. Shestakova, Ekaterina I. Smirnova, Alina Levushkina, Denis S. Baranovskii, Anna N. Smirnova, Vasiliy N. Stepanenko, Dmitry A. Kudlay, and et al. 2025. "Microbiota-Induced Radioprotection: A Novel Approach to Enhance Human Radioresistance with In-Situ Genetically Engineered Gut Bacteria" Applied Microbiology 5, no. 1: 1. https://doi.org/10.3390/applmicrobiol5010001
APA StyleYakimova, A. O., Nikolaeva, A., Galanova, O., Shestakova, V. A., Smirnova, E. I., Levushkina, A., Baranovskii, D. S., Smirnova, A. N., Stepanenko, V. N., Kudlay, D. A., Shegay, P. V., Kaprin, A. D., Sosin, D. V., & Klabukov, I. D. (2025). Microbiota-Induced Radioprotection: A Novel Approach to Enhance Human Radioresistance with In-Situ Genetically Engineered Gut Bacteria. Applied Microbiology, 5(1), 1. https://doi.org/10.3390/applmicrobiol5010001






