The Effect of Gut Microbiome, Neurotransmitters, and Digital Insights in Autism
Abstract
1. Introduction
1.1. Gut Microbiome
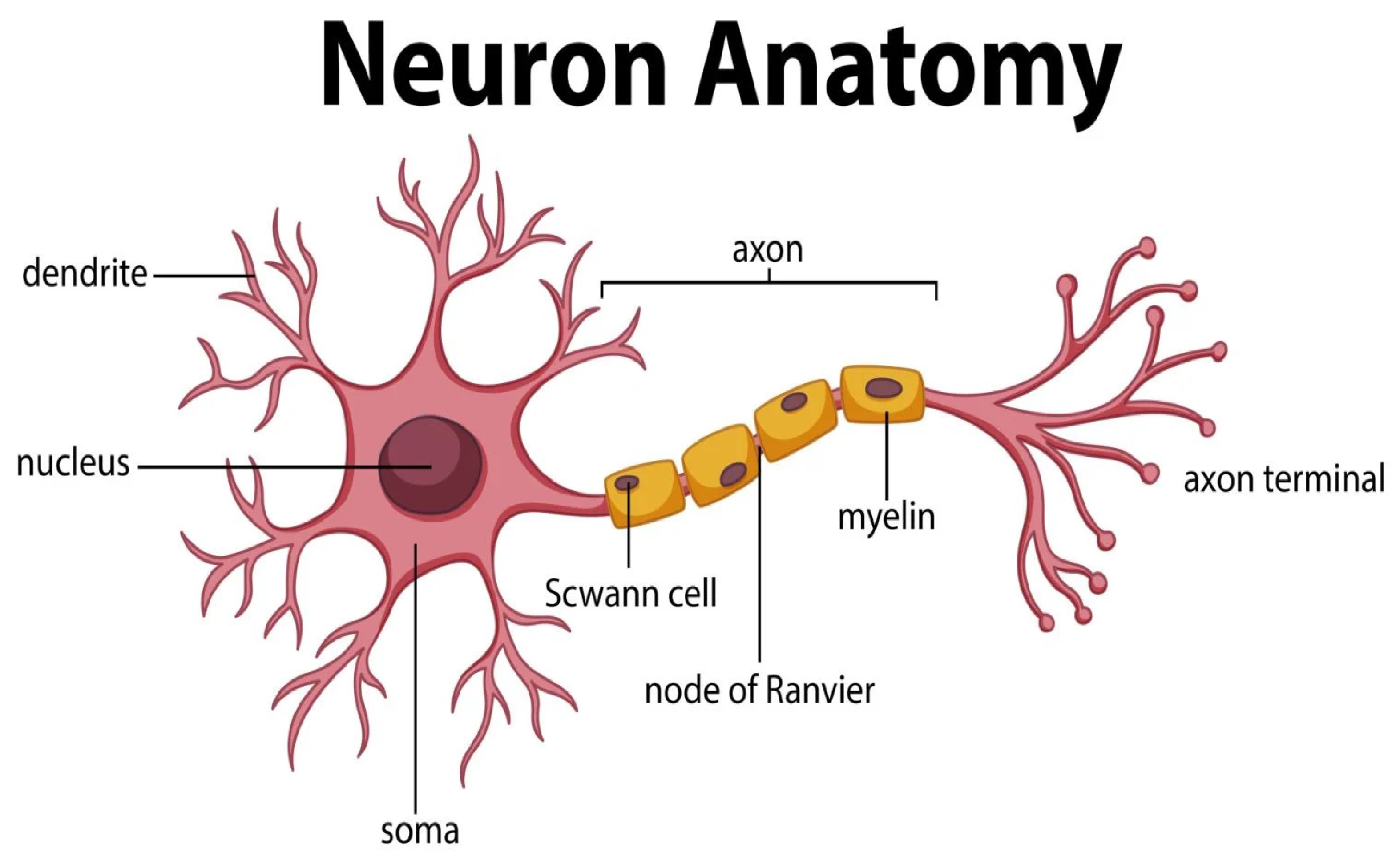
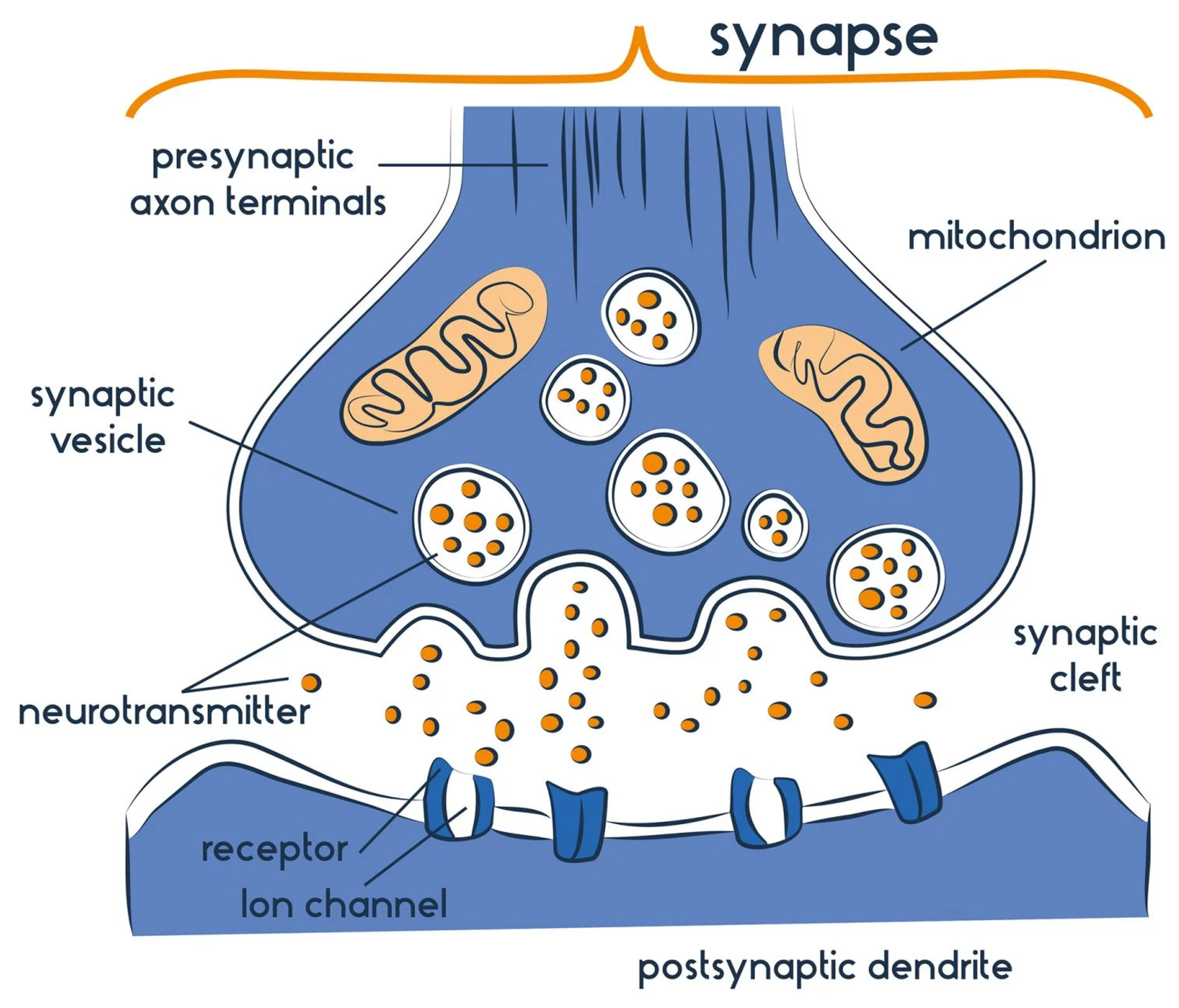
1.2. Neurotransmitters
1.3. Autism Spectrum Disorders
2. Gut Microflora and Its Connection to NTs in ASD
2.1. Eating Habits in ASD
2.2. Effects of Selective Nutrition of Children with Autism on NTs
2.3. Dysbiosis Prevention–Treatment in ASD
2.4. Gut Microbiome and Neurotransmitters
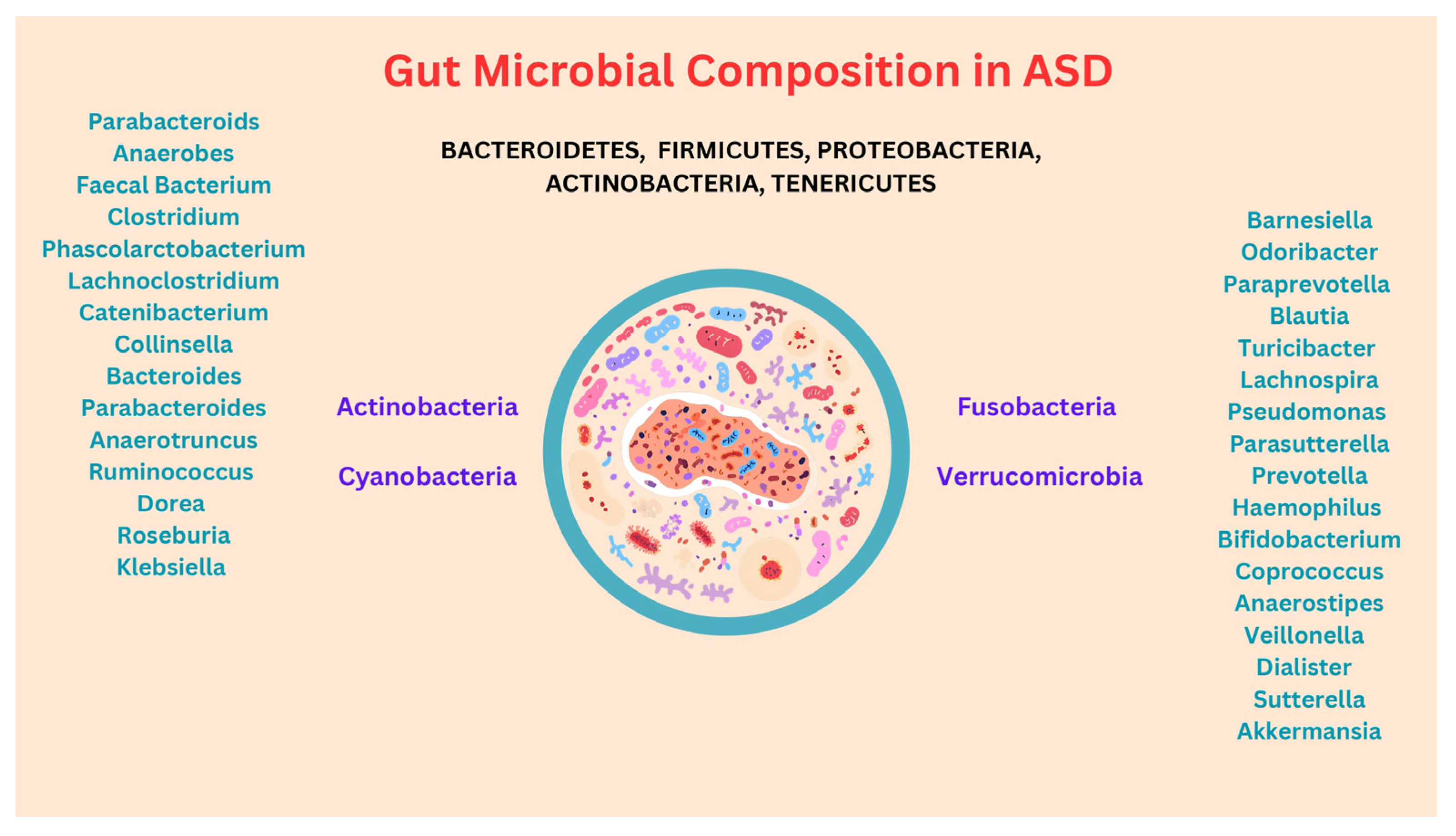
3. Composition of the Gut Microbiota in ASD
4. ASD and NTs
4.1. Serotonin
4.2. Norepinephrine or Noradrenaline
4.3. Dopamine
4.4. Oxytocin
4.5. GABA–Glutamate
5. The Contribution of Digital Technology
6. General Discussion
7. Conclusions
Limitations and Suggestions for Future Research
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez, A.; Stombaugh, J.; Lozupone, C.; Turnbaugh, P.J.; Gordon, J.I.; Knight, R. The mind-body-microbial continuum. Dialogues Clin. Neurosci. 2011, 13, 55–62. [Google Scholar] [CrossRef]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Shakya, A.; Husain, G.M.; Emerald, M.; Kumar, V. Gut-Microbiota and Mental Health: Current and Future Perspectives. J. Pharmacol. Clin. Toxicol. 2014, 2, 1016. Available online: https://www.researchgate.net/publication/260425062 (accessed on 28 October 2024).
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.; Basiri, R. Amino Acids, B Vitamins, and Choline May Independently and Collaboratively Influence the Incidence and Core Symptoms of Autism Spectrum Disorder. Nutrients 2022, 14, 2896. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Margolis, K.G. Reprint of: Serotonin as a Link between the Gut-Brain-Microbiome Axis in Autism Spectrum Disorders. Pharmacol. Res. 2019, 140, 115–120. [Google Scholar] [CrossRef] [PubMed]
- De Sales-Millán, A.; Aguirre-Garrido, J.F.; González-Cervantes, R.M.; Velázquez-Aragón, J.A. Microbiome–Gut–Mucosal–Immune–Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology. Behav. Sci. 2023, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Li, J.; O’Hara, B.; Alberts, I.; Xiong, L.; Li, J.; Li, X. The Role of GABAergic Neural Circuits in the Pathogenesis of Autism Spectrum Disorder. Int. J. Dev. Neurosci. 2020, 80, 73–85. [Google Scholar] [CrossRef]
- Shannon, J.; Salomon, C.; Chettiath, T.; Abbas, H.; Taraman, S. Autism spectrum disorder and the promise of artificial intelligence. J. Child Adolesc. Behav. 2022, 10, 428. [Google Scholar]
- Surianarayanan, C.; Lawrence, J.J.; Chelliah, P.R.; Prakash, E.; Hewage, C. Convergence of Artificial Intelligence and Neuroscience towards the Diagnosis of Neurological Disorders—A Scoping Review. Sensors 2023, 23, 3062. [Google Scholar] [CrossRef] [PubMed]
- Bamicha, V.; Drigas, A. ToM & ASD: The Interconnection of Theory of Mind with the Social-Emotional, Cognitive Development of Children with Autism Spectrum Disorder. The Use of ICTs as an Alternative Form of Intervention in ASD. Tech. Soc. Sci. J. 2022, 33, 42–72. [Google Scholar] [CrossRef]
- Hussain, S.J.; Cohen, L.G. Exploratory studies: A crucial step towards better hypothesis-driven confirmatory research in brain stimulation. J. Physiol. 2017, 595, 1013–1014. [Google Scholar] [CrossRef]
- Swedberg, R. Exploratory Research. In The Production of Knowledge: Enhancing Progress in Social Science; Cambridge University Press: Cambridge, UK, 2020; pp. 17–41. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Basic Definitions and Concepts: Organization of the Gut Microbiome. Gastroenterol. Clin. N. Am. 2017, 46, 1–8. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut Feelings: The Emerging Biology of Gut–Brain Communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Narbad, A.; Rodríguez, J.M. Autism Spectrum Disorder Associated with Gut Microbiota at Immune, Metabolomic, and Neuroactive Level. Front. Neurosci. 2020, 14, 578666. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, A.; Alhazmi, S.; Alburae, N.; Bahieldin, A. The Human Gut Microbiome as a Potential Factor in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 1363. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Microbiome Disturbances and Autism Spectrum Disorders. Drug Metab. Dispos. 2015, 43, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Sideraki, A.; Papageorgiou, E.; Tsiava, M.; Drigas, A. Stress, Hormones & the role of ICT in autism. Tech. BioChemMed 2022, 3, 42–59. [Google Scholar]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Bamicha, V.; Salapata, Y. LLLT Applications May Enhance ASD Aspects Related to Disturbances in the Gut Microbiome, Mitochondrial Activity, and Neural Network Function. Braz. J. Sci. 2023, 3, 140–158. [Google Scholar] [CrossRef]
- Duman, J.G.; Tu, Y.-K.; Tolias, K.F. Emerging Roles of BAI Adhesion-GPCRs in Synapse Development and Plasticity. Neural Plast. 2016, 2016, 8301737. [Google Scholar] [CrossRef] [PubMed]
- Barnfield, A. Role of Neurotransmitters. In Encyclopedia of Personality and Individual Differences; Zeigler-Hill, V., Shackelford, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Poornimai Abirami, G.P.; Radhakrishnan, R.K.; Johnson, E.; Roshan, S.A.; Yesudhas, A.; Parveen, S.; Biswas, A.; Ravichandran, V.R.; Muthuswamy, A.; Kandasamy, M. The Regulation of Reactive Neuroblastosis, Neuroplasticity, and Nutraceuticals for Effective Management of Autism Spectrum Disorder. Adv. Neurobiol. 2020, 24, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Guy-Evans, O. An Easy Guide to Neuron Anatomy with Diagrams. Available online: https://www.simplypsychology.org/neuron.html# (accessed on 1 September 2024).
- Teleanu, R.I.; Niculescu, A.-G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, T.; Prajapati, A.; Sethi, P. Nutrition, Neurotransmitters, and Behavior. In Nutrition and Psychiatric Disorders; Mohamed, W., Kobeissy, F., Eds.; Springer: Singapore, 2022; pp. 89–108. [Google Scholar] [CrossRef]
- Guy-Evans, O. What Happens at The Synapse Between Two Neurons? Available online: https://www.simplypsychology.org/synapse.html (accessed on 1 September 2024).
- Dhailappan, A.; Samiappan, S. Impact of Diet on Neurotransmitters. In Role of Nutrients in Neurological Disorders; Rajagopal, S., Ramachandran, S., Sundararaman, G., Gadde Venkata, S., Eds.; Springer: Singapore, 2022; pp. 363–383. [Google Scholar] [CrossRef]
- LaGreca, M.; Hutchinson, D.R.; Skehan, L. The Microbiome and Neurotransmitter Activity. J. Sci. Med. 2021, 3. [Google Scholar] [CrossRef]
- Drigas, A.; Mitsea, E. Metacognition, Stress—Relaxation Balance & Related Hormones. Int. J. Recent Contrib. Eng. Sci. IT 2021, 9, 4. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A.; Rogovsky, V.S. Role of Neurochemicals in the Interaction between the Microbiota and the Immune and the Nervous System of the Host Organism. Probiotics Antimicrob. Proteins 2017, 9, 215–234. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and Mental Health: A Review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Mehra, A.; Arora, G.; Sahni, G.; Kaur, M.; Singh, H.; Singh, B.; Kaur, S. Gut Microbiota and Autism Spectrum Disorder: From Pathogenesis to Potential Therapeutic Perspectives. J. Tradit. Complement. Med. 2022, 13, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed]
- Bragg, M.; Chavarro, J.E.; Hamra, G.B.; Hart, J.E.; Tabb, L.P.; Weisskopf, M.G.; Volk, H.E.; Lyall, K. Prenatal Diet as a Modifier of Environmental Risk Factors for Autism and Related Neurodevelopmental Outcomes. Curr. Environ. Health Rep. 2022, 9, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Bamicha, V.; Drigas, A. The Evolutionary Course of Theory of Mind—Factors That Facilitate or Inhibit Its Operation & the Role of ICTs. Tech. Soc. Sci. J. 2022, 30, 138–158. [Google Scholar] [CrossRef]
- Horn, J.; Mayer, D.E.; Chen, S.; Mayer, E.A. Role of Diet and Its Effects on the Gut Microbiome in the Pathophysiology of Mental Disorders. Transl. Psychiatry 2022, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.G.; Berry, R.C.; McElhanon, B.O.; Jaquess, D.L. Dietary Diversity in Children with Autism. In Comprehensive Guide to Autism; Patel, V., Preedy, V., Martin, C., Eds.; Springer: New York, NY, USA, 2014; pp. 2077–2097. [Google Scholar] [CrossRef]
- Leader, G.; Tuohy, E.; Chen, J.L.; Mannion, A.; Gilroy, S.P. Feeding Problems, Gastrointestinal Symptoms, Challenging Behavior and Sensory Issues in Children and Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Malhi, P.; Saini, S.; Bharti, B.; Attri, S.; Sankhyan, N. Sensory Processing Dysfunction and Mealtime Behavior Problems in Children with Autism. Indian Pediatr. 2021, 58, 842–845. [Google Scholar] [CrossRef]
- Mahmoud, N.F.; Abdelhameed, R.S.; Abdelmonam, S.A.; Abdelmonem, A.A.; Khalil, D.M.; Bakia, S.A.S. Parent-Reported Feeding Characteristics in Children with ASD vs. Children Who Are Typically Developing. Egypt. J. Otolaryngol. 2021, 37, 110. [Google Scholar] [CrossRef]
- Peretti, S.; Mariano, M.; Mazzocchetti, C.; Mazza, M.; Pino, M.C.; Verrotti Di Pianella, A.; Valenti, M. Diet: The Keystone of Autism Spectrum Disorder? Nutr. Neurosci. 2018, 22, 825–839. [Google Scholar] [CrossRef]
- Kawicka, A.; Regulska-Ilow, B. How nutritional status, diet and dietary supplements can affect autism. A review. Rocz. Panstw. Zakl. Hig. 2013, 64, 1–12. [Google Scholar] [PubMed]
- Kaur, H.; Singh, Y.; Singh, S.; Singh, R.B. Gut Microbiome Mediated Epigenetic Regulation of Brain Disorder and Application of Machine Learning for Multi-Omics Data Analysis. Genome 2020, 64, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Fouquier, J.; Moreno Huizar, N.; Donnelly, J.; Glickman, C.; Kang, D.-W.; Maldonado, J.; Jones, R.A.; Johnson, K.; Adams, J.B.; Krajmalnik-Brown, R.; et al. The Gut Microbiome in Autism: Study-Site Effects and Longitudinal Analysis of Behavior Change. mSystems 2021, 6, e00848-20. [Google Scholar] [CrossRef]
- Rodop, B.B.; Başkaya, E.; Altuntaş, İ.; Erbaş, O. Nutrition Effect on Autism Spectrum Disorders. J. Exp. Basic Med. Sci. 2021, 2, 7–17. [Google Scholar] [CrossRef]
- MacFabe, D.F. Enteric Short-Chain Fatty Acids: Microbial Messengers of Metabolism, Mitochondria, and Mind: Implications in Autism Spectrum Disorders. Microb. Ecol. Health Dis. 2015, 26, 28177. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Donovan, S.M. Microbiome and Nutrition in Autism Spectrum Disorder: Current Knowledge and Research Needs. Nutr. Rev. 2016, 74, 723–736. [Google Scholar] [CrossRef]
- Shanmugam, H.; Ganguly, S.; Priya, B. Plant Food Bioactives and Its Effects on Gut Microbiota Profile Modulation for Better Brain Health and Functioning in Autism Spectrum Disorder Individuals: A Review. Food Front. 2022, 3, 124–141. [Google Scholar] [CrossRef]
- Shah, F.; Dwivedi, M. Pathophysiological Role of Gut Microbiota Affecting Gut–Brain Axis and Intervention of Probiotics and Prebiotics in Autism Spectrum Disorder. In Probiotic Research in Therapeutics; Deol, P.K., Sandhu, S.K., Eds.; Springer: Singapore, 2022; pp. 69–115. [Google Scholar] [CrossRef]
- Cohen, Y.; Valdés-Mas, R.; Elinav, E. The Role of Artificial Intelligence in Deciphering Diet–Disease Relationships: Case Studies. Annu. Rev. Nutr. 2023, 43, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Moos, W.H.; Faller, D.V.; Harpp, D.N.; Kanara, I.; Pernokas, J.; Powers, W.R.; Steliou, K. Microbiota and Neurological Disorders: A Gut Feeling. BioResearch Open Access 2016, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Drigas, A.; Bamicha, V. PoM & ToM—Harnessing the Power of Mind in Theory of Mind by Shaping Beneficial Mental States in Preschoolers and the ICT’s Role. Res. Soc. 2023, 12, e13212541590. [Google Scholar] [CrossRef]
- Davies, C.; Mishra, D.; Eshraghi, R.S.; Mittal, J.; Sinha, R.; Bulut, E.; Mittal, R.; Eshraghi, A.A. Altering the Gut Microbiome to Potentially Modulate Behavioral Manifestations in Autism Spectrum Disorders: A Systematic Review. Neurosci. Biobehav. Rev. 2021, 128, 549–557. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Zavitsanou, A.; Salapata, Y.; Stathopoulou, A. Special Nutrition for Students with Special Education Needs, and the ICT’s Role for Their Health Education. TechHub J. 2022, 2, 82–97. Available online: https://techhubresearch.com/index.php/journal/article/view/77 (accessed on 2 November 2024).
- Zavitsanou, A.; Drigas, A. Nutrition in Mental and Physical Health. Tech. Soc. Sci. J. 2021, 23, 67–77. [Google Scholar] [CrossRef]
- Driga, A.M.; Zavitsanou, A.; Drigas, A. Heavy Metals, Halogens, Carbon and Nitrogen Oxides, and Autism the Important Role of Digital Technologies in Health Education, Prevention and Digital Health. GSC Adv. Res. Rev. 2023, 16, 50–60. [Google Scholar] [CrossRef]
- Javadpour, P.; Askari, S.; Ghasemi, R. Nutrition, Cognitive Functions, and Emotions. In Nutrition and Psychiatric Disorders. Nutritional Neurosciences; Mohamed, W., Kobeissy, F., Eds.; Springer: Singapore, 2022; pp. 27–50. [Google Scholar] [CrossRef]
- Sahay, A.S.; Sundrani, D.P.; Joshi, S.R. Neurotrophins. In Neurotrophins: Role in Placental Growth and Development. Vitamins and Hormones; Gerald, L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 243–261. [Google Scholar] [CrossRef]
- Hennessy, M.; Hamblin, M.R. Photobiomodulation and the Brain: A New Paradigm. J. Opt. 2017, 19, 013003. [Google Scholar] [CrossRef] [PubMed]
- De Luca, F. Endocrinological Abnormalities in Autism. Semin. Pediatr. Neurol. 2020, 35, 100582. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Gao, X.; Wang, Z.; Cao, S.; Liang, G.; He, D.; Lv, Z.; Wang, L.; Xu, P.; Zhang, Q. Comparison of Gut Microbiota in Autism Spectrum Disorders and Neurotypical Boys in China: A Case-Control Study. Synth. Syst. Biotechnol. 2021, 6, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Xu, F.; Wang, Y.; Duan, M.; Guo, M.; Zhang, Q.; Zhao, H.; Zheng, H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020, 13, 1614–1625. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef]
- Jendraszak, M.; Gałęcka, M.; Kotwicka, M.; Regdos, A.; Pazgrat-Patan, M.; Andrusiewicz, M. Commercial Microbiota Test Revealed Differences in the Composition of Intestinal Microorganisms between Children with Autism Spectrum Disorders and Neurotypical Peers. Sci. Rep. 2021, 11, 24274. [Google Scholar] [CrossRef]
- Kang, D.-W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in Fecal Microbial Metabolites and Microbiota of Children with Autism Spectrum Disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef]
- Mihailovich, M.; Tolinački, M.; Bajić, S.S.; Lestarevic, S.; Pejovic-Milovancevic, M.; Golić, N. The Microbiome–Genetics Axis in Autism Spectrum Disorders: A Probiotic Perspective. Int. J. Mol. Sci. 2024, 25, 12407. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Xu, Y.; Zhang, X.; Zhang, L.; Duan, G.; Song, C.; Li, Z.; Yang, Y.; Wang, Y.; Wang, X.; et al. Gut Microbiota Changes in Patients with Autism Spectrum Disorders. J. Psychiatr. Res. 2020, 129, 149–159. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Lozupone, C.; Kang, D.-W.; Adams, J.B. Gut Bacteria in Children with Autism Spectrum Disorders: Challenges and Promise of Studying How a Complex Community Influences a Complex Disease. Microb. Ecol. Health Dis. 2015, 26, 26914. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Ristori, M.V.; Guerrera, S.; Guarrasi, V.; Conte, F.; Russo, A.; Lupi, E.; Albitar-Nehme, S.; Gardini, S.; Paci, P.; et al. Gut Microbiota Ecology and Inferred Functions in Children with ASD Compared to Neurotypical Subjects. Front. Microbiol. 2022, 13, 871086. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Marzal, L.N.; Rojas-Velazquez, D.; Rigters, D.; Prince, N.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P.; Lopez-Rincon, A. A Robust Microbiome Signature for Autism Spectrum Disorder across Different Studies Using Machine Learning. Sci. Rep. 2024, 14, 814. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.T.; Jin, D.-M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-Level Analysis of the Gut–Brain Axis Shows Autism Spectrum Disorder-Associated Molecular and Microbial Profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Yin, X.; Rutherford, E.M.; Wee, B.; Choi, J.; Chrisman, B.S.; Dunlap, K.L.; Hannibal, R.L.; Hartono, W.; Lin, M.; et al. Multi-Angle Meta-Analysis of the Gut Microbiome in Autism Spectrum Disorder: A Step toward Understanding Patient Subgroups. Sci. Rep. 2022, 12, 17034. [Google Scholar] [CrossRef]
- Nakazawa-Miklasevica, M.; Daneberga, Z.; Murmane, D.; Kroica, J.; Cupane, L.; Isarova, D.; Berga-Svitina, E.; Masinska, M.; Miklasevics, E. Alterations of Gut Microbiota among Children with Autism Spectrum Disorder. Mol. Genet. Microbiol. Virol. 2021, 36, S29–S36. [Google Scholar] [CrossRef]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xiao, H.; Zhu, H.; Du, Y.; Wang, L. Revealing the Gut Microbiome Mystery: A Meta-Analysis Revealing Differences between Individuals with Autism Spectrum Disorder and Neurotypical Children. BioSci. Trends 2024, 18, 233–249. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Larroya-García, A.; Navas-Carrillo, D.; Orenes-Piñero, E. Impact of Gut Microbiota on Neurological Diseases: Diet Composition and Novel Treatments. Crit. Rev. Food Sci. Nutr. 2019, 59, 3102–3116. [Google Scholar] [CrossRef] [PubMed]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Kong, Q.; Tian, P.; Zhai, Q.; Wang, G.; Liu, X.; Zhao, J.; Zhang, H.; Lee, Y.K.; Chen, W. Targeting Gut Microbiota Dysbiosis: Potential Intervention Strategies for Neurological Disorders. Engineering 2020, 6, 415–423. [Google Scholar] [CrossRef]
- Fernández, M.; Mollinedo-Gajate, I.; Peñagarikano, O. Neural Circuits for Social Cognition: Implications for Autism. Neuroscience 2018, 370, 148–162. [Google Scholar] [CrossRef]
- Cetin, F.H.; Tunca, H.; Güney, E.; Iseri, E. Neurotransmitter Systems in Autism Spectrum Disorder; InTech: Houston, TX, USA, 2015; pp. 15–30. [Google Scholar] [CrossRef]
- Florea, T.; Palimariciuc, M.; Cristofor, A.C.; Dobrin, I.; Chiriță, R.; Bîrsan, M.; Dobrin, R.P.; Pădurariu, M. Oxytocin: Narrative Expert Review of Current Perspectives on the Relationship with Other Neurotransmitters and the Impact on the Main Psychiatric Disorders. Medicina 2022, 58, 923. [Google Scholar] [CrossRef] [PubMed]
- Mitsea, E.; Drigas, A.; Skianis, C. Mindfulness for Anxiety Management and Happiness: The Role of VR, Metacognition, and Hormones. Technium BioChemMed 2022, 3, 37–52. [Google Scholar] [CrossRef]
- Rodnyy, A.Y.; Kondaurova, E.M.; Tsybko, A.S.; Popova, N.K.; Kudlay, D.A.; Naumenko, V.S. The Brain Serotonin System in Autism. Rev. Neurosci. 2023, 35, 1–20. [Google Scholar] [CrossRef]
- Yang, C.-J.; Tan, H.-P.; Du, Y.-J. The Developmental Disruptions of Serotonin Signaling May Involved in Autism during Early Brain Development. Neuroscience 2014, 267, 1–10. [Google Scholar] [CrossRef]
- Beversdorf, D.Q. The Role of the Noradrenergic System in Autism Spectrum Disorders, Implications for Treatment. Semin. Pediatr. Neurol. 2020, 35, 100834. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Belardo, A.; Zolla, L. A Metabolomics Approach to Investigate Urine Levels of Neurotransmitters and Related Metabolites in Autistic Children. Biochim. Biophys. Acta 2020, 1866, 165859. [Google Scholar] [CrossRef]
- Saha, S.; Chatterjee, M.; Dutta, N.; Sinha, S.; Mukhopadhyay, K. Analysis of Neurotransmitters Validates the Importance of the Dopaminergic System in Autism Spectrum Disorder. World J. Pediatr. 2023, 19, 770–781. [Google Scholar] [CrossRef]
- Pavăl, D. A dopamine hypothesis of autism spectrum disorder. Dev. Neurosci. 2017, 39, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Hollestein, V.; Poelmans, G.; Forde, N.J.; Beckmann, C.F.; Ecker, C.; Mann, C.; Schäfer, T.; Moessnang, C.; Baumeister, S.; Banaschewski, T.; et al. Excitatory/Inhibitory Imbalance in Autism: The Role of Glutamate and GABA Gene-Sets in Symptoms and Cortical Brain Structure. Transl. Psychiatry 2023, 13, 18. [Google Scholar] [CrossRef]
- Maier, S.; Düppers, A.L.; Runge, K.; Dacko, M.; Lange, T.; Fangmeier, T.; Riedel, A.; Ebert, D.; Endres, D.; Domschke, K.; et al. Increased Prefrontal GABA Concentrations in Adults with Autism Spectrum Disorders. Autism Res. 2022, 15, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, I.; Dionísio, A.; Violante, I.R.; Monteiro, R.; Castelo-Branco, M. Motor Cortex Excitation/Inhibition Imbalance in Young Adults with Autism Spectrum Disorder: A MRS-TMS Approach. Front. Psychiatry 2022, 13, 860448. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, D. GABA Receptors in Brain Development, Function, and Injury. Metab. Brain Dis. 2014, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Cellot, G.; Cherubini, E. Functional Role of Ambient GABA in Refining Neuronal Circuits Early in Postnatal Development. Front. Neural Circuits 2013, 7, 136. [Google Scholar] [CrossRef]
- Begum, P.S.; Razak, M.A.; Rajagopal, S. Influence of Amino Acids on Autism and Attention-Deficit Hyperactive Disorder. In Proteins Associated with Neurodevelopmental Disorders. Nutritional Neurosciences; Qoronfleh, M.W., Essa, M.M., Saravana Babu, C., Eds.; Springer: Singapore, 2022; pp. 257–276. [Google Scholar] [CrossRef]
- Meskó, B.; Drobni, Z.; Bényei, É.; Gergely, B.; Győrffy, Z. Digital Health Is a Cultural Transformation of Traditional Healthcare. mHealth 2017, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, K.; He, X.; Zhou, J.; Jin, C.; Shen, L.; Gao, Y.; Tian, M.; Zhang, H. Structural, Functional, and Molecular Imaging of Autism Spectrum Disorder. Neurosci. Bull. 2021, 37, 1051–1071. [Google Scholar] [CrossRef]
- Al-Biltagi, M.; Saeed, N.K.; Qaraghuli, S. Gastrointestinal Disorders in Children with Autism: Could Artificial Intelligence Help? AIG Artif. Intell. Gastroenterol. 2022, 3, 1–12. [Google Scholar] [CrossRef]
- Green, J.; Leadbitter, K.; Ainsworth, J.; Bucci, S. An Integrated Early Care Pathway for Autism. Lancet Child Adolesc. Health 2022, 6, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, A.; Loukeris, D.; Karabatzaki, Z.; Politi, E.; Salapata, Y.; Drigas, A. Evaluation of Mobile Apps Effectiveness in Children with Autism Social Training via Digital Social Stories. Int. J. Interact. Mob. Technol. 2020, 14, 5. [Google Scholar] [CrossRef]
- Kyriakaki, E.; Karabatzaki, Z.; Salapata, Y. Mobile Applications for Autism. Eximia 2023, 8, 51–66. [Google Scholar]
- Drigas, A.; Karyotaki, M. E-Learning and ICTs Applications in Nutrition Science. Int. J. Recent Contrib. Eng. Sci. IT 2013, 1, 4–10. [Google Scholar] [CrossRef]
- Bravou, V.; Driga, A.M.S.; Drigas, A. Emotion Regulation, the Function of Stress Hormones & Digital Technologies. Tech. BioChemMed 2022, 3, 27–33. [Google Scholar] [CrossRef]
- Bamicha, V.; Drigas, A. Consciousness Influences in ToM and Metacognition Functioning—An Artificial Intelligence Perspective. Res. Soc. Dev. 2023, 12, e13012340420. [Google Scholar] [CrossRef]
- Bamicha, V.; Drigas, A. Theory of Mind in Relation to Metacognition and ICTs. A Metacognitive Approach to ToM. Sci. Electron. Arch. 2023, 16. [Google Scholar] [CrossRef]
- Kourtesis, P.; Kouklari, E.-C.; Roussos, P.; Mantas, V.; Papanikolaou, K.; Skaloumbakas, C.; Pehlivanidis, A. Virtual Reality Training of Social Skills in Adults with Autism Spectrum Disorder: An Examination of Acceptability, Usability, User Experience, Social Skills, and Executive Functions. Behav. Sci. 2023, 13, 336. [Google Scholar] [CrossRef] [PubMed]
- Mitsea, E.; Drigas, A.; Skianis, C. Digitally Assisted Mindfulness in Training Self-Regulation Skills for Sustainable Mental Health: A Systematic Review. Behav. Sci. 2023, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Mitsea, E.; Drigas, A.; Skianis, C. Effects of Digital Games Training on Hormonal Responses and Brain Plasticity. Res. Soc. Dev. 2023, 12, e20512239568. [Google Scholar] [CrossRef]
- Vacca, R.A.; Augello, A.; Gallo, L.; Caggianese, G.; Malizia, V.; La Grutta, S.; Murero, M.; Valenti, D.; Tullo, A.; Balech, B.; et al. Serious Games in the New Era of Digital-Health Interventions: A Narrative Review of Their Therapeutic Applications to Manage Neurobehavior in Neurodevelopmental Disorders. Neurosci. Biobehav. Rev. 2023, 149, 105156. [Google Scholar] [CrossRef] [PubMed]
- Moraiti, I.; Fotoglou, A.; Drigas, A. Digital and Mobile Applications for Autism Inclusion. Int. J. Online Biomed. Eng. 2023, 19, 83. [Google Scholar] [CrossRef]
- Ghazali, A.S.; Ham, J.; Barakova, E.; Markopoulos, P. Assessing the Effect of Persuasive Robots Interactive Social Cues on Users’ Psychological Reactance, Liking, Trusting Beliefs and Compliance. Adv. Robot. 2019, 33, 325–337. [Google Scholar] [CrossRef]
- Papanastasiou, G.; Drigas, A.; Skianis, C. Serious Games: How Do They Impact Special Education Needs Children. Tech. Educ. Humanit. 2022, 2, 41–58. [Google Scholar] [CrossRef]
- Chaidi, I.; Drigas, A. Digital Games & Special Education. Tech. Soc. Sci. J. 2022, 34, 214–236. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Shamim, M.; Clement, C.K. Trends and issues to integrate ICT in teaching learning for the future world of education. Int. J. Eng. Sci. Technol. 2011, 11, 114–119. [Google Scholar]
- Pergantis, P.; Drigas, A. Sensory Integration Therapy as Enabler for Developing Emotional Intelligence in Children with Autism Spectrum Disorder and the ICT’s Role. Braz. J. Sci. 2023, 2, 53–65. [Google Scholar] [CrossRef]
- Pergantis, P.; Drigas, A. Assistive Technology for Autism Spectrum Disorder Children That Experiences Stress and Anxiety. Braz. J. Sci. 2023, 2, 77–93. [Google Scholar] [CrossRef]
- Taniya, M.A.; Chung, H.-J.; Al Mamun, A.; Alam, S.; Aziz, M.A.; Emon, N.U.; Islam, M.M.; Hong, S.-T.; Podder, B.R.; Ara Mimi, A.; et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 915701. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, H.; Cheng, Y.; Xu, F.; Ruan, G.; Ying, S.; Tang, W.; Chen, L.; Chen, M.; Lv, L.; et al. Fecal Microbiota Transplantation Relieves Gastrointestinal and Autism Symptoms by Improving the Gut Microbiota in an Open-Label Study. Front. Cell. Infect. Microbiol. 2021, 11, 759435. [Google Scholar] [CrossRef]
- Halverson, T.; Alagiakrishnan, K. Gut Microbes in Neurocognitive and Mental Health Disorders. Ann. Med. 2020, 52, 423–443. [Google Scholar] [CrossRef]
- Yang, J.; Fu, X.; Liao, X.; Li, Y. Effects of Gut Microbial-Based Treatments on Gut Microbiota, Behavioral Symptoms, and Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: A Systematic Review. Psychiatry Res. 2020, 293, 113471. [Google Scholar] [CrossRef] [PubMed]
- Sideraki, A.; Drigas, A. The Role of Cortisol and Microbiome in the Anxiety of People with ASD and the Use of ICTs for Regulation. World J. Biol. Pharm. Health Sci. 2023, 14, 137–148. [Google Scholar] [CrossRef]
- Kapalka, G.M. Substances Involved in Neurotransmission. In Practical Resources for the Mental Health Professional, Nutritional and Herbal Therapies for Children and Adolescents; Kapalka, G.M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: San Diego, CA, USA, 2010; pp. 71–99. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The Contribution of Environmental Exposure to the Etiology of Autism Spectrum Disorder. Cell Mol. Life Sci. 2018, 76, 1275–1297. [Google Scholar] [CrossRef]
- Garg, S.; Sharma, S. Impact of Artificial Intelligence in Special Need Education to Promote Inclusive Pedagogy. Int. J. Inf. Educ. Technol. 2020, 10, 523–527. [Google Scholar] [CrossRef]
- Mitrofanis, J.; Henderson, L. How and Why Does Photobiomodulation Change Brain Activity? Neural Regen. Res. 2020, 15, 2243. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.; Liebert, A.; Pang, V.; Magistretti, P.; Mitrofanis, J. Lights on for Autism: Exploring Photobiomodulation as an Effective Therapeutic Option. Neurol. Int. 2022, 14, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Guang, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamicha, V.; Pergantis, P.; Drigas, A. The Effect of Gut Microbiome, Neurotransmitters, and Digital Insights in Autism. Appl. Microbiol. 2024, 4, 1677-1701. https://doi.org/10.3390/applmicrobiol4040114
Bamicha V, Pergantis P, Drigas A. The Effect of Gut Microbiome, Neurotransmitters, and Digital Insights in Autism. Applied Microbiology. 2024; 4(4):1677-1701. https://doi.org/10.3390/applmicrobiol4040114
Chicago/Turabian StyleBamicha, Victoria, Pantelis Pergantis, and Athanasios Drigas. 2024. "The Effect of Gut Microbiome, Neurotransmitters, and Digital Insights in Autism" Applied Microbiology 4, no. 4: 1677-1701. https://doi.org/10.3390/applmicrobiol4040114
APA StyleBamicha, V., Pergantis, P., & Drigas, A. (2024). The Effect of Gut Microbiome, Neurotransmitters, and Digital Insights in Autism. Applied Microbiology, 4(4), 1677-1701. https://doi.org/10.3390/applmicrobiol4040114







