Therapeutic Potential of Endophytic Microbes: Emphasizing Both Fungal and Bacterial Endophytes
Abstract
1. Introduction
2. Endophytic Fungi
3. Endophytic Bacteria
4. Fungi and Bacteria Endophytes: Natural Allies in Controlling Plant Pathogens
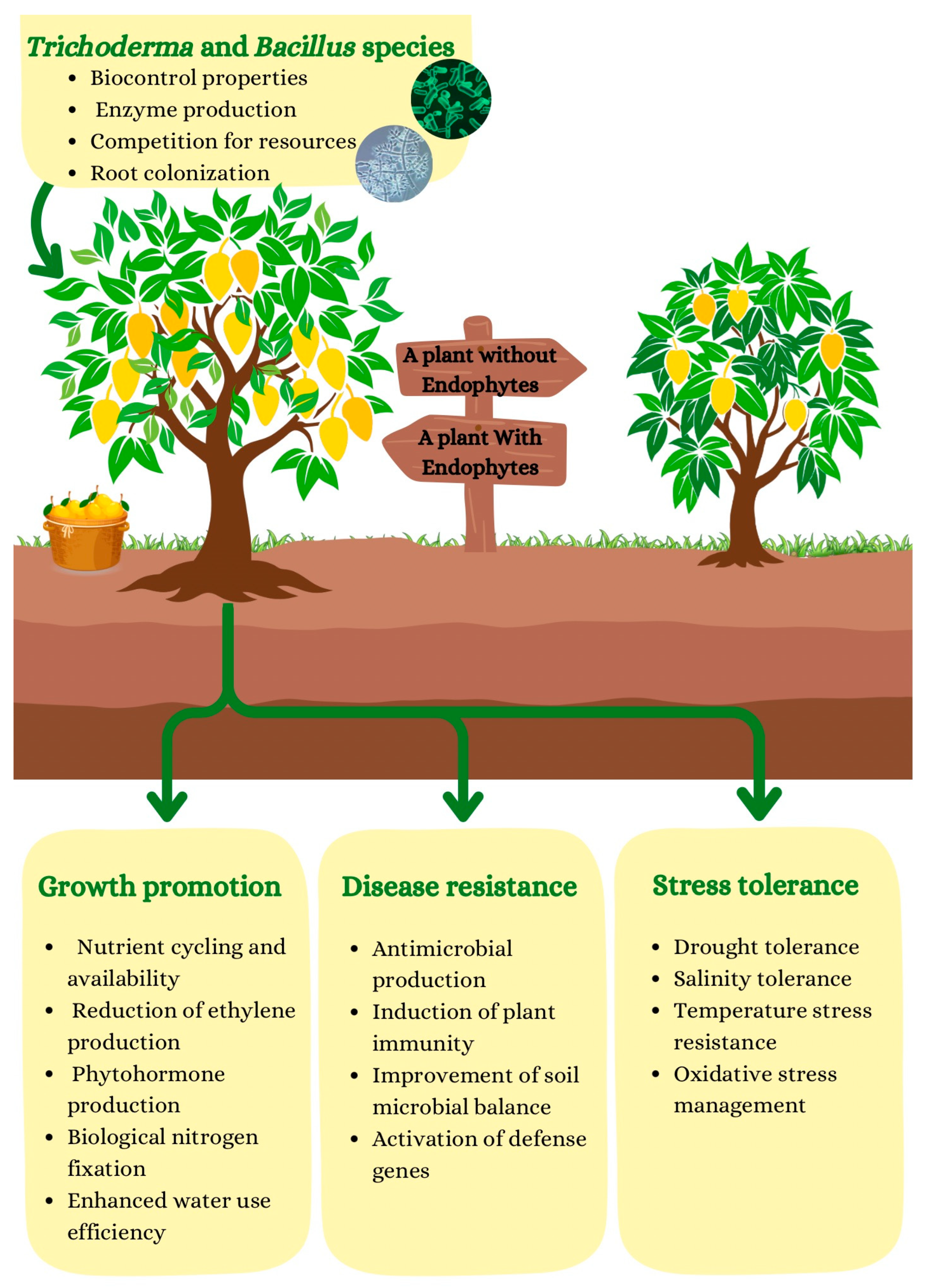
4.1. Mechanisms of Pathogen Control
- Antibiosis: is a biological phenomenon where one organism produces substances that inhibit or kill another organism, often as a means of competing for resources or territory. In the context of plant-microbe interactions, antibiosis typically refers to the production of bioactive compounds, such as antibiotics, that target and suppress the growth of harmful pathogens. These compounds can be produced by various microorganisms, including bacterial endophytes, which reside within plant tissues and contribute to plant health by protecting against pathogens [67,68]. Bacterial endophytes, such as P. fluorescens, A. brasilense, and B. subtilis, are known to produce a variety of bioactive compounds, including antibiotics, siderophores, and enzymes, which directly inhibit pathogen growth. For example, siderophores are molecules that bind to iron, sequestering it from the environment. Since iron is essential for pathogen growth, the production of siderophores by beneficial bacteria can limit pathogen growth by making iron unavailable. This mechanism of competition for iron is a form of antibiosis, as it prevents pathogens from thriving. Studies, such as those by [69], have demonstrated the role of bacterial endophytes in controlling plant pathogens through antibiosis, highlighting their potential in sustainable agriculture by reducing reliance on chemical pesticides. By promoting plant health and resisting infections, endophytic bacteria can contribute to more resilient crops.
- Competition for Resources: Endophytes help protect plants from pathogens by outcompeting them for vital resources, including nutrients, water, and space within plant tissues. These beneficial microbes colonize areas where pathogens would normally thrive, blocking access and preventing pathogen establishment. One example is A. brasilense, which colonizes the root systems of grasses like maize, outcompeting pathogens such as Rhizoctonia solani [70]. By depleting available nutrients and occupying ecological niches, Azospirillum limits pathogen growth and prevents infections.Another example is Bacillus amyloliquefaciens, which has been shown to outcompete pathogens like Colletotrichum and Botrytis in tomatoes and other crops [71]. These endophytes produce antimicrobial compounds, including lipopeptides, which inhibit pathogen growth. Additionally, T. harzianum is an endophytic fungus that can protect plants like tomatoes and cucumbers from soil-borne pathogens such as Fusarium oxysporum by colonizing the root zone and reducing pathogen access to resources [72]. Trichoderma also prevents pathogen establishment by outcompeting harmful microbes for nutrients and by producing enzymes that break down pathogen cell walls. This competitive exclusion strategy is widely used in integrated pest management (IPM), where beneficial microbes are introduced to enhance plant protection, reduce pathogen populations, and decrease reliance on chemical pesticides, promoting sustainability in agriculture [73].
- Induced Systemic Resistance (ISR): is a defense mechanism where endophytes activate a plant’s immune system, priming it to respond more quickly and effectively to future pathogen attacks. This process involves the production of signaling molecules such as jasmonic acid (JA), salicylic acid (SA), and ethylene by the endophyte [74]. These molecules trigger the plant’s defense pathways, which include the activation of pathogenesis-related proteins, enhanced production of antimicrobial compounds, and the fortification of plant cell walls. One of the key aspects of ISR is that it “primes” the plant’s defense system, meaning the plant’s immune response is heightened and ready to respond faster when a pathogen challenge occurs. For instance, P. fluorescens, a well-studied endophyte, induces ISR in plants by producing acetoin and other volatile compounds that trigger the plant’s immune system [75]. In rice and wheat, these compounds have been shown to increase resistance to fungal pathogens like Rhizoctonia and Pythium [76]. Unlike traditional resistance mechanisms, ISR is typically more generalized and can protect plants against a wide range of pathogens. This makes it a sustainable method of crop protection, reducing the need for chemical pesticides while enhancing plant health and resilience to environmental stressors.
- Parasitism of Pathogens: certain endophytic fungi and bacteria can parasitize or directly attack plant pathogens, providing an effective means of pathogen control [77]. These parasitic relationships are often established through the production of enzymes that degrade the cell walls or other essential components of the pathogen. For example, species of Trichoderma, such as T. harzianum, are well known for their ability to parasitize pathogenic fungi by secreting chitinases and glucanases, enzymes that break down the cell walls of the fungi [78]. This parasitism effectively kills the pathogens, preventing them from establishing infections in plants. Trichoderma can colonize plant roots and other tissues, forming a protective barrier against soil-borne pathogens like Fusarium and Verticillium spp. In addition bacteria like Bacillus subtilis also exhibit parasitic behavior. They can attack pathogenic bacteria and fungi by competing for space and resources or by producing bacteriocins and lytic enzymes that directly kill the pathogen. For instance, Bacillus strains have been shown to control bacterial wilt in tomatoes and other crops by directly attacking the pathogen Ralstonia solanacearum [79]. The parasitic ability of endophytes offers a promising biological control strategy, reducing the need for chemical pesticides and supporting sustainable farming practices.
4.2. Examples of Fungal and Bacterial Endophytes in Pathogen Control
- Trichoderma spp.: Trichoderma is one of the most studied fungal endophytes for its biocontrol properties. These fungi are known to colonize plant roots and protect against soil-borne pathogens like R. solani and F. oxysporum. Trichoderma produces a range of enzymes, such as chitinases and glucanases, that degrade the cell walls of pathogenic fungi. Additionally, Trichoderma can outcompete pathogens for nutrients and space, reducing their ability to infect the plant [80].
- Piriformospora spp.: This root-colonizing endophytic fungus is known for its ability to enhance plant growth and confer resistance to a variety of pathogens. P. indica has been shown to protect plants like barley and maize from root rot caused by Fusarium species. The fungus induces systemic resistance in the host plant and produces antimicrobial compounds that inhibit the growth of the pathogen [81].
- Epichloë spp.: These fungal endophytes are found in grasses, where they form mutualistic relationships. Epichloë species produce alkaloids that deter herbivores and inhibit the growth of fungal pathogens. In tall fescue grass, for example, E. coenophiala has been shown to protect against pathogens such as Bipolaris sorokiniana, which causes leaf spot diseases [82].
- Bacillus spp.: Bacillus species are widely recognized for their role in biocontrol. These bacteria produce a variety of antimicrobial compounds, including lipopeptides and antibiotics, that inhibit the growth of fungal and bacterial pathogens. B. subtilis, for example, has been used to control diseases like bacterial wilt in tomatoes and rice blast caused by Magnaporthe oryzae. The bacterium also induces systemic resistance in plants, making them more resilient to future pathogen attacks [83].
- Pseudomonas spp.: P. fluorescens is a well-known bacterial endophyte that provides protection against a range of soil-borne pathogens. It produces antibiotics like pyoluteorin and 2,4-diacetylphloroglucinol, which inhibit the growth of pathogens such as Pythium and Fusarium. Pseudomonas species also promote plant growth by producing phytohormones and enhancing nutrient availability [84].
- Streptomyces spp.: These endophytic bacteria are notable for their ability to produce a wide range of antibiotics, making them effective against various plant pathogens. Streptomyces griseoviridis, for example, has been used to control diseases like potato scab caused by Streptomyces scabies and damping-off in seedlings caused by R. solani. In addition to producing antibiotics, Streptomyces species can also degrade pathogen cell walls and induce systemic resistance in plants [85].
5. Applications in Sustainable Agriculture
6. Challenges and Future Prospects
7. Conclusions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current scenario and future prospects of endophytic microbes: Promising candidates for abiotic and biotic stress management for agricultural and environmental sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Garcia, L.; Liu, H. Plant growth promotion by endophytes: Mechanisms and applications. Plant Growth Regul. 2022, 60, 315–329. [Google Scholar]
- Gakuubi, M.M.; Munusamy, M.; Liang, Z.-X.; Ng, S.B. Fungal endophytes: A promising frontier for discovery of novel bioactive compounds. J. Fungi 2021, 7, 786. [Google Scholar] [CrossRef]
- Waghund, R.R.; Shelake, R.M.; Shinde, M.S.; Hayashi, H. Endophyte microbes: A weapon for plant health management. In Microorganisms for Green Revolution; Springer: Singapore, 2017; pp. 303–325. [Google Scholar]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2019, 68, 247–255. [Google Scholar] [CrossRef]
- Nguyen, T.; Brown, M.; Wilson, D. Plant stress tolerance enhanced by endophytic microbes. J. Plant Biol. 2020, 77, 142–156. [Google Scholar]
- Wilson, T.; Thomas, C. Sustainable agriculture practices with endophytic microbes. J. Sustain. Farming 2023, 48, 56–69. [Google Scholar]
- Bugti, G.A.; Chen, H.; Bin, W.; Rehman, A.; Ali, F. Pathogenic Response of Entomopathogenic Fungal Strains on Larvae of Fall Armyworm (Spodoptera frugiperda). Entomol. Appl. Sci. Lett. 2024, 11, 48–55. [Google Scholar] [CrossRef]
- Hawksworth, D.L. Endocytosis in Filamentous Fungal Hyphae. Mycol. Res. 2004, 108, 2–3. [Google Scholar]
- Brown, J.; Smith, L.; Patel, A. Nutrient solubilization by endophytic fungi and its impact on plant growth. J. Agric. Sci. 2020, 58, 123–134. [Google Scholar]
- Alghamdi, R.G.; Zabermawi, N.M.; Altihani, F.A.; Bokhari, F.M.; Makki, R.M.; Hassoubah, S.A.; Najjar, A.A. Diversity and Density of Fungi Isolated from Dried Fruits. J. Biochem. Technol. 2023, 14, 45–55. [Google Scholar] [CrossRef]
- Husein, N.; Qaralleh, H.; Al-Tarawneh, A.; AlSarayreh, A.; Qaisi, Y.A.; Al-limoun, M.; Shadid, K.; Qaralleh, I.; Al-Jaafreh, A.; Majali, I. Modeling phenol biodegradation with Pantoea agglomerans as plant-growth-promoting bacteria. J. Adv. Pharm. Educ. Res. 2024, 14, 63–71. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J. Osmoprotectant production by endophytic fungi under drought conditions. J. Plant Physiol. 2022, 79, 190–203. [Google Scholar]
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.S.; Barrett, G.A.; Jackson, R.W. Endophytes vs. tree pathogens and pests: Can they be used as biological control agents to improve tree health? Eur. J. Plant Pathol. 2019, 155, 711–729. [Google Scholar] [CrossRef]
- Sharma, I.; Dhar, M.K.; Raina, A.; Choudhary, M.; Apra; Kaul, S. Fungal endophyte bioinoculants as a green alternative towards sustainable agriculture. Heliyon 2023, 1, 48–93. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.; Zhang, Y. The future of endophytic microbes in agriculture: Challenges and opportunities. Future Agric. J. 2024, 32, 85–101. [Google Scholar]
- Patel, R.; Thomas, J.; Zhao, Q. Environmental impacts of endophyte integration in agriculture. Environ. Sci. Policy 2021, 52, 33–45. [Google Scholar]
- Akram, S.; Ahmed, A.; He, P.; Liu, Y.; Wu, Y.; Munir, S.; He, Y. Uniting the role of endophytic fungi against plant pathogens and their interaction. J. Fungi 2023, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, H.; Xu, L.; Cui, H.; Xin, A.; Liu, H.; Qin, B. Diversity and functions of endophytic fungi associated with roots and leaves of Stipa purpurea in an alpine steppe at Qinghai-Tibet Plateau. J. Microbiol. Biotechnol. 2020, 30, 1027–1036. [Google Scholar] [CrossRef]
- Maheshwari, A.; Mmbaga, M.T. Endophytic fungi residing within Cornus florida L. in Mid-Tennessee: Phylogenetic diversity, enzymatic properties, and potential role in plant health. Plants 2024, 13, 1250. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.Y.; Cui, J.L.; Wang, M.L.; Wang, J.H. Biotransformation ability of endophytic fungi: From species evolution to industrial applications. Appl. Microbiol. Biotechnol. 2021, 105, 7095–7113. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Yadav, A.; Prasad, R.; Kalra, S.J.S.; Singh, A.; Bhardwaj, N.; Gupta, K.K. Fungal endophytes from medicinal plants acting as natural therapeutic reservoir. Microbe 2024, 3, 73–80. [Google Scholar] [CrossRef]
- Szilagyi-Zecchin, V.J.; Adamoski, D.; Gomes, R.R.; Hungria, M.; Ikeda, A.C.; Kava-Cordeiro, V.; Galli-Terasawa, L.V. Composition of endophytic fungal community associated with leaves of maize cultivated in south Brazilian field. Acta Microbiol. Immunol. Hung. 2016, 63, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Doohan, F.M.; Hodkinson, T.R. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis 2014, 62, 29–39. [Google Scholar] [CrossRef]
- Suman, A.; Yadav, A.N.; Verma, P. Endophytic microbes in crops: Diversity and beneficial impact for sustainable agriculture. Microb. Inoculants Sustain. Agric. Product. 2016, 33, 117–143. [Google Scholar]
- Rana, K.L.; Kour, D.; Sheikh, I.; Yadav, N.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Leeuwenhoek 2020, 113, 1075–1107. [Google Scholar] [CrossRef] [PubMed]
- Kousar, R.; Naeem, M.; Jamaludin, M.I.; Arshad, A.; Shamsuri, A.N.; Ansari, N.; Al-Harrasi, A. Exploring the anticancer activities of novel bioactive compounds derived from endophytic fungi: Mechanisms of action, current challenges and future perspectives. Am. J. Cancer Res. 2022, 12, 2897. [Google Scholar] [PubMed]
- Li, Q.; Shi, J.; Huang, C.; Guo, J.; He, K.; Wang, Z. Asian corn borer (Ostrinia furnacalis) infestation increases Fusarium verticillioides infection and fumonisin contamination in maize and reduces the yield. Plant Dis. 2023, 107, 1557–1564. [Google Scholar] [CrossRef]
- Shan, J.; Peng, F.; Yu, J.; Li, Q. Identification and characterization of a plant endophytic fungus Paraphaosphaeria sp. JRF11 and its growth-promoting effects. J. Fungi 2024, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Zimowska, B. Endophytic fungi of hazelnut (Corylus avellana). Plant Prot. Sci. 2023, 59, 107–123. [Google Scholar] [CrossRef]
- Rai, N.; Gupta, P.; Keshri, P.K.; Verma, A.; Mishra, P.; Kumar, D.; Kumar, A.; Singh, S.K.; Gautam, V. Fungal endophytes: An accessible source of bioactive compounds with potential anticancer activity. Appl. Biochem. Biotechnol. 2022, 194, 3296–3319. [Google Scholar] [CrossRef] [PubMed]
- Ujam, N.T.; Eze, P.M.; Chukwunwejim, C.R.; Okoye, F.B.; Esimone, C.O. Antimicrobial and immunomodulatory activities of secondary metabolites of an endophytic fungus isolated from Ageratum conyzoides. Curr. Life Sci. 2024, 5, 19–27. [Google Scholar]
- Gurgel, R.S.; de Melo Pereira, D.Í.; Garcia, A.V.F.; Fernandes de Souza, A.T.; Mendes da Silva, T.; de Andrade, C.P.; Lima da Silva, W.; Nunez, C.V.; Fantin, C.; de Lima Procópio, R.E.; et al. Antimicrobial and antioxidant activities of endophytic fungi associated with Arrabidaea chica (Bignoniaceae). J. Fungi 2023, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Selim, K.A.; Elkhateeb, W.A.; Tawila, A.M.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I.; Ahmed, E.F. Antiviral and antioxidant potential of fungal endophytes of Egyptian medicinal plants. Fermentation 2018, 4, 49. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, T.; Wang, L.; Zhao, Y.; Huang, H.; Zhang, D.; Han, J. Bioprospecting of novel and bioactive metabolites from endophytic fungi isolated from rubber tree Ficus elastica leaves. J. Microbiol. Biotechnol. 2019, 29, 731–738. [Google Scholar] [CrossRef]
- Satheesan, J.; Sabu, K.K. Endophytic fungi for a sustainable production of major plant bioactive compounds. In Plant-Derived Bioactives; Springer: Berlin/Heidelberg, Germany, 2020; pp. 195–207. [Google Scholar]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Pervez, R.; Romman, M.; Khan, N. Isolation of endophytic fungi from halophytic plants and their identification and screening for auxin production and other plant growth promoting traits. J. Plant Growth Regul. 2023, 42, 4707–4723. [Google Scholar] [CrossRef]
- Shastri, B.; Kumar, R.; Lal, R.J. Isolation, characterization and identification of indigenous endophytic bacteria exhibiting PGP and antifungal traits from the internal tissue of sugarcane crop. Int. J. Sugar Crops Relat. Ind. 2020, 22, 563–573. [Google Scholar] [CrossRef]
- De Lima, J.D.; de Souza, A.J.; Nunes, A.L.P.; Rivadavea, W.R.; Zaro, G.C.; da Silva, G.J. Expanding agricultural potential through biological nitrogen fixation: Recent advances and diversity of diazotrophic bacteria. Aust. J. Crop Sci. 2024, 18, 324–333. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Xu, Y.; Wang, W.; Liu, Y. Functional activity of endophytic bacteria G9H01 with high salt tolerance and anti-Magnaporthe oryzae isolated from saline-alkali-tolerant rice. Sci. Total Environ. 2024, 9, 171822. [Google Scholar] [CrossRef]
- Semenzato, G.; Fani, R. Endophytic bacteria: A sustainable strategy for enhancing medicinal plant cultivation and preserving microbial diversity. Front. Microbiol. 2024, 15, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Kim, T.-M.; Choi, B.; Kim, Y.; Kim, E. Invasive Lactuca serriola seeds contain endophytic bacteria that contribute to drought tolerance. Sci. Rep. 2021, 11, 133–143. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.M.; Marina, M.; Pieckenstain, F.L. The communities of tomato (Solanum lycopersicum L.) leaf endophytic bacteria, analyzed by 16S-ribosomal RNA gene pyrosequencing. FEMS Microbiol. Lett. 2014, 351, 187–194. [Google Scholar] [CrossRef]
- Oukala, N.; Aissat, K.; Pastor, V. Bacterial endophytes: The hidden actor in plant immune responses against biotic stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Drozdzyński, P.; Rutkowska, N.; Rodziewicz, M.; Marchut-Mikołajczyk, O. Bioactive compounds produced by endophytic bacteria and their plant hosts—An insight into the world of chosen herbaceous ruderal plants in Central Europe. Molecules 2024, 29, 4456. [Google Scholar] [CrossRef]
- Murugesan, R.; Ulagan, M.P.; Stephen, D.N.; Vairakannu, T.; Gurusamy, M.; Govindarajan, S. Biotreatment of Chromium Enriched Electroplating Effluent Using Bacterial Consortium. Int. J. Pharm. Res. Allied Sci. 2024, 13, 9–18. [Google Scholar] [CrossRef]
- Dogan, G.; Taskin, B. Hydrolytic enzymes producing bacterial endophytes of some Poaceae plants. Pol. J. Microbiol. 2021, 70, 297–304. [Google Scholar] [CrossRef]
- Glamazdin, I.G.; Medvedev, I.N.; Kutuzov, D.D.; Fayzullina, I.I.; Nazarova, S.V.; Sysoeva, N.Y.; Tarasova, V.V. Main Parasitic Infestations of Wild Ungulates Used for Food. J. Biochem. Technol. 2024, 15, 59–63. [Google Scholar] [CrossRef]
- Muthu, M.; Ahmad, N.; Shivanand, P.; Metali, F. The role of endophytes in combating fungal- and bacterial-induced stress in plants. Molecules 2022, 27, 6549. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.C.; Watt, M.; Roessner, U. Alleviation of salinity stress in plants by endophytic plant-fungal symbiosis: Current knowledge, perspectives, and future directions. Plant Soil 2020, 2, 22–43. [Google Scholar] [CrossRef]
- James, E.K. Nitrogen fixation in endophytic and associative symbiosis. Field Crops Res. 2000, 65, 197–209. [Google Scholar] [CrossRef]
- Narayanan, Z.; Glick, B.R. Secondary metabolites produced by plant growth-promoting bacterial endophytes. Microorganisms 2022, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.K.; Mandal, A.; Sinha, N.K.; Singh, A.B.; Jayaraman, S.; Shirale, A.O. Culturable diversity of endophytic and rhizoplane colonizing bacteria of Indian mustard (Brassica juncea (L.) Czern and Coss), affected by soil types and assessment of plant growth promoting attributes. Geomicrobiol. J. 2024, 41, 568–576. [Google Scholar] [CrossRef]
- Vardharajula, S.; SkZ, A.; Shiva Krishna Prasad Vurukonda, S.; Shrivastava, M. Plant growth promoting endophytes and their interaction with plants to alleviate abiotic stress. Curr. Biotechnol. 2017, 6, 252–263. [Google Scholar] [CrossRef]
- Kumar, A.; Radhakrishnan, E.K. (Eds.) Microbial Endophytes: Functional Biology and Applications; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A promising source of plant growth-promoting molecules and their non-legume interactions: Examining applications and mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Lucy, M.; Reed, E.; Glick, B.R. Applications of free living plant growth-promoting rhizobacteria. Antonie Leeuwenhoek 2004, 86, 1–25. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Zhang, W. Biofertilizers and biopesticides: The role of endophytes in sustainable agriculture. Plant Sci. Rev. 2021, 32, 450–467. [Google Scholar]
- Anjum, R.; Afzal, M.; Baber, R.; Khan, M.A.J.; Kanwal, W.; Sajid, W.; Raheel, A. Endophytes: As potential biocontrol agent—Review and future prospects. J. Agric. Sci. 2019, 11, 113–222. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Kumar, A.; Dayanandan, S.; Khan, M.L. Plants endophytes: Unveiling hidden agenda for bioprospecting toward sustainable agriculture. Crit. Rev. Biotechnol. 2020, 40, 1210–1231. [Google Scholar] [CrossRef] [PubMed]
- Grabka, R.; d’Entremont, T.W.; Adams, S.J.; Walker, A.K.; Tanney, J.B.; Abbasi, P.A.; Ali, S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Agric. J. 2023, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, J.; Chen, C.; Mo, X.; Tan, Q.; He, Y.; Wang, Z.; Yin, J.; Zhou, G. The multifunctions and future prospects of endophytes and their metabolites in plant disease management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, A.A. Endophytes: Colonization, behaviour, and their role in defense mechanism. Int. J. Microbiol. 2020, 69, 8–22. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Kailoo, S.; Khan, R.T.; Khan, S.S.; Rasool, S. Harnessing fungal endophytes for natural management: A biocontrol perspective. Front. Microbiol. 2023, 14, 128–158. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- El-Komy, M.H.; Hassouna, M.G.; Abou-Taleb, E.M.; Al-Sarar, A.S.; Abobakr, Y. A mixture of Azotobacter, Azospirillum, and Klebsiella strains improves root-rot disease complex management and promotes growth in sunflowers in calcareous soil. Eur. J. Plant Pathol. 2020, 156, 713–726. [Google Scholar] [CrossRef]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 1179, 101754. [Google Scholar] [CrossRef]
- Natsiopoulos, D.; Tziolias, A.; Lagogiannis, I.; Mantzoukas, S.; Eliopoulos, P.A. Growth-promoting and protective effect of Trichoderma atrobrunneum and T. simmonsii on tomato against soil-borne fungal pathogens. Crops 2022, 2, 202–217. [Google Scholar] [CrossRef]
- Kumari, P.; Deepa, N.; Trivedi, P.K.; Singh, B.K.; Srivastava, V.; Singh, A. Plants and endophytes interaction: A “secret wedlock” for sustainable biosynthesis of pharmaceutically important secondary metabolites. Microb. Cell Factories 2023, 22, 226–334. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.-Z.; Bastías, D.A.; Christensen, M.J.; Zhong, R.; Nan, Z.-B.; Zhang, X.-X. The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an Epichloë endophyte. J. Fungi 2021, 7, 633. [Google Scholar] [CrossRef]
- Schilirò, E.; Ferrara, M.; Nigro, F.; Mercado-Blanco, J. Genetic responses induced in olive roots upon colonization by the biocontrol endophytic bacterium Pseudomonas fluorescens PICF7. Public Libr. Sci. 2012, 7, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Okubara, P.A.; Dickman, M.B.; Blechl, A.E. Molecular and genetic aspects of controlling the soilborne necrotrophic pathogens Rhizoctonia and Pythium. Plant Sci. 2014, 228, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, V.N.; do Nascimento Silva, R.; Steindorff, A.S.; Costa, F.T.; Noronha, E.F.; Ricart CA, O.; Ulhoa, C.J. New insights in Trichoderma harzianum antagonism of fungal plant pathogens by secreted protein analysis. Curr. Microbiol. 2010, 61, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Nwobodo, D.C.; Ugwu, M.C.; Egbujor, C.M.; Okoye, F.B.C.; Esimone, C.O. Antimicrobial and Antioxidant Compounds Produced by Fungal Endophytes Isolated from Selected Nigerian Medicinal Plants. Int. J. Pharm. Phytopharm. Res. 2023, 13, 6–15. [Google Scholar] [CrossRef]
- Ahmed, W.; Yang, J.; Tan, Y.; Munir, S.; Liu, Q.; Zhang, J.; Zhao, Z. Ralstonia solanacearum, a deadly pathogen: Revisiting the bacterial wilt biocontrol practices in tobacco and other Solanaceae. Rhizosphere 2022, 21, 47–69. [Google Scholar] [CrossRef]
- Zheng, H.; Qiao, M.; Lv, Y.; Du, X.; Zhang, K.-Q.; Yu, Z. New Species of Trichoderma Isolated as Endophytes and Saprobes from Southwest China. J. Fungi 2021, 7, 467. [Google Scholar] [CrossRef]
- Jisha, S.; Sabu, K.; Manjula, S. Multifunctional aspects of Piriformospora indica in plant endosymbiosis. Mycology 2019, 10, 182–190. [Google Scholar]
- Card, S.D.; Bastías, D.A.; Caradus, J.R. Antagonism to plant pathogens by Epichloë fungal endophytes—A review. Plants 2021, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhang, T.; Tang, S.; Zhou, L.; Li, K.; Fu, X.; Yu, S. Biocontrol of citrus canker with endophyte Bacillus amyloliquefaciens QC-Y. Plant Prot. Sci. 2021, 57, 1–13. [Google Scholar] [CrossRef]
- Ambreetha, S.; Marimuthu, P.; Mathee, K.; Balachandar, D. Rhizospheric and endophytic Pseudomonas aeruginosa in edible vegetable plants share molecular and metabolic traits with clinical isolates. J. Appl. Microbiol. 2022, 132, 3226–3248. [Google Scholar] [CrossRef]
- Sivalingam, P.; Easwaran, M.; Ganapathy, D.; Basha, S.F.; Poté, J. Endophytic Streptomyces: An underexplored source with potential for novel natural drug discovery and development. Front. Microbiol. 2024, 206, 442–460. [Google Scholar] [CrossRef]
- Karuppiah, V.; Zhixiang, L.; Liu, H.; Vallikkannu, M.; Chen, J. Co-culture of Vel1-over- expressed Trichoderma asperellum and Bacillus amyloliquefaciens: An ecofriendly strategy to hydrolyze the lignocellulose biomass in soil to enrich the soil fertility, plant growth and disease resistance. Microbiology 2021, 20, 5766. [Google Scholar]
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023, 14, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Wippel, K. Plant and microbial features governing an endophytic lifestyle. Curr. Opin. Plant Biol. 2023, 76, 102483. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Dhingra, N.; Gacem, A.; Yadav, V.K.; Verma, R.K.; Choudhary, M.; Jeon, B.H. Towards further understanding the applications of endophytes: Enriched source of bioactive compounds and bio factories for nanoparticles. Front. Plant Sci. 2023, 14, 119–130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najjar, A.A. Therapeutic Potential of Endophytic Microbes: Emphasizing Both Fungal and Bacterial Endophytes. Appl. Microbiol. 2025, 5, 5. https://doi.org/10.3390/applmicrobiol5010005
Najjar AA. Therapeutic Potential of Endophytic Microbes: Emphasizing Both Fungal and Bacterial Endophytes. Applied Microbiology. 2025; 5(1):5. https://doi.org/10.3390/applmicrobiol5010005
Chicago/Turabian StyleNajjar, Azhar Abdullah. 2025. "Therapeutic Potential of Endophytic Microbes: Emphasizing Both Fungal and Bacterial Endophytes" Applied Microbiology 5, no. 1: 5. https://doi.org/10.3390/applmicrobiol5010005
APA StyleNajjar, A. A. (2025). Therapeutic Potential of Endophytic Microbes: Emphasizing Both Fungal and Bacterial Endophytes. Applied Microbiology, 5(1), 5. https://doi.org/10.3390/applmicrobiol5010005




