The Rumen and Gastrointestinal Microbial Environment and Its Association with Feed Efficiency and Pregnancy in Female Beef Cattle
Abstract
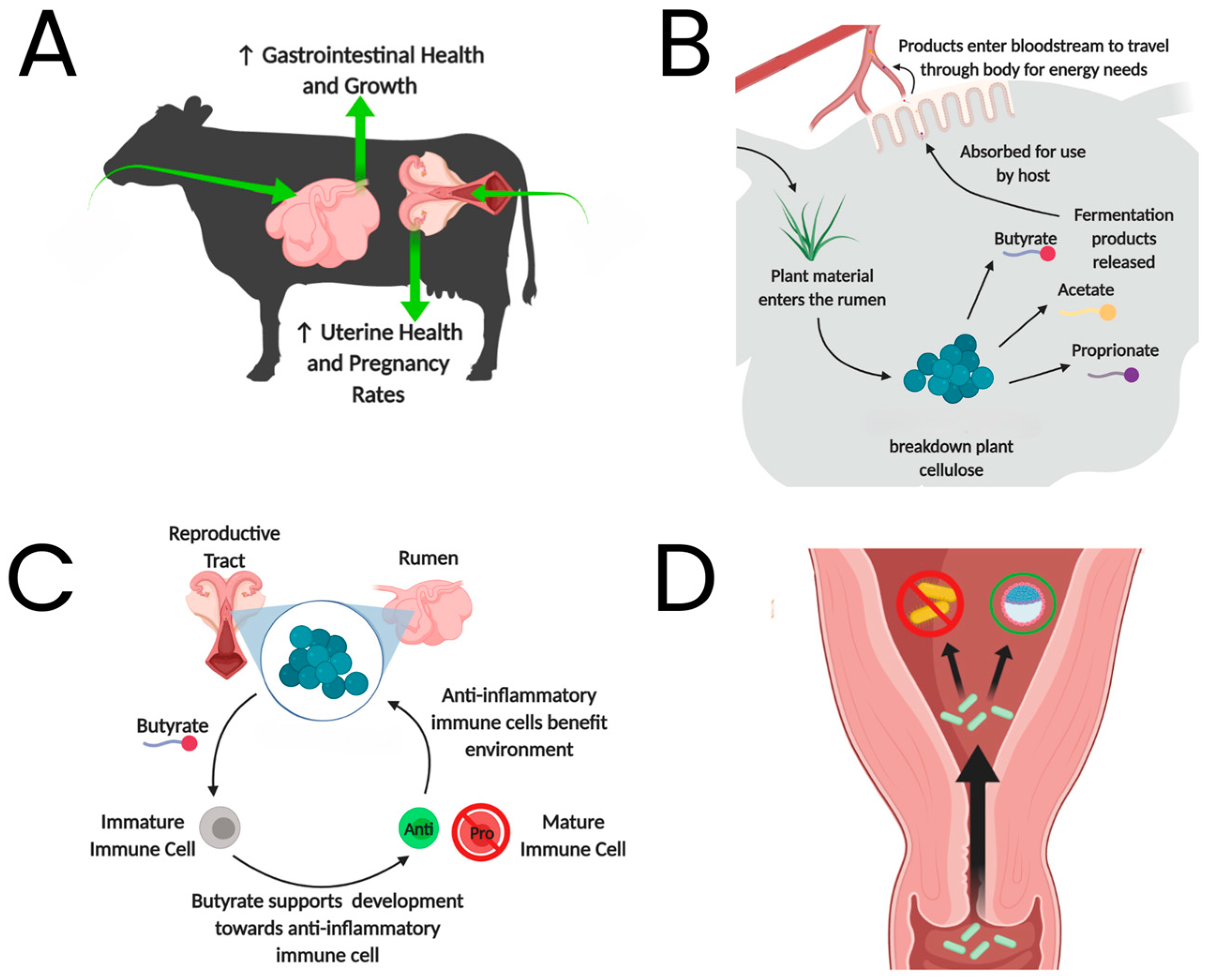
:1. Introduction
2. Feed Efficiency, Diet, and the Rumen Microbiome of Fed Cattle Systems
3. Pregnancy Effects on Gut Microbes
4. Pregnancy Effects on Rumen Microbiome and Metabolic Status in Cattle
5. Next Generation and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- World Population Prospects-Population Division-United Nations. Available online: https://population.un.org/wpp/ (accessed on 30 August 2024).
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Bayer, E.A. Plant Cell Wall Breakdown by Anaerobic Microorganisms from the Mammalian Digestive Tract. Ann. N. Y. Acad. Sci. 2008, 1125, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Seymour, W.M.; Campbell, D.R.; Johnson, Z.B. Relationships between rumen volatile fatty acid concentrations and milk production in dairy cows: A literature study. Anim. Feed. Sci. Technol. 2005, 119, 155–169. [Google Scholar] [CrossRef]
- Li, F.; Hitch, T.C.A.; Chen, Y.; Creevey, C.J.; Guan, L.L. Comparative metagenomic and metatranscriptomic analyses reveal the breed effect on the rumen microbiome and its associations with feed efficiency in beef cattle. Microbiome 2019, 7, 6. [Google Scholar] [CrossRef]
- Myer, P.R.; Smith, T.P.L.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen Microbiome from Steers Differing in Feed Efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef]
- McCann, J.C.; Luan, S.; Cardoso, F.C.; Derakhshani, H.; Khafipour, E.; Loor, J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front. Microbiol. 2016, 7, 701. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; Rooke, J.A.; McKain, N.; Duthie, C.-A.; Hyslop, J.J.; Ross, D.W.; Waterhouse, A.; Watson, M.; Roehe, R. The rumen microbial metagenome associated with high methane production in cattle. BMC Genom. 2015, 16, 839. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Macías, B.; Pinloche, E.; Newbold, C.J. The persistence of bacterial and methanogenic archaeal communities residing in the rumen of young lambs. FEMS Microbiol. Ecol. 2010, 72, 272–278. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census Collaborators; Abecia, L.; Angarita, E.; Aravena, P.; Nora Arenas, G.; et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The rumen microbiome: Balancing food security and environmental impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef]
- Weimer, P.J. Redundancy, resilience, and host specificity of the ruminal microbiota: Implications for engineering improved ruminal fermentations. Front. Microbiol. 2015, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Peng, Y.-J.; Chen, Y.; Klinger, C.M.; Oba, M.; Liu, J.-X.; Guan, L.L. Assessment of microbiome changes after rumen transfaunation: Implications on improving feed efficiency in beef cattle. Microbiome 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Loerch, S.C. Effects of feeding growing cattle high-concentrate diets at a restricted intake on feedlot performance. J. Anim. Sci. 1990, 68, 3086–3095. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, M.; Vaarst, M. Shaping cow-calf contact systems: Farmers’ motivations and considerations behind a range of different cow-calf contact systems. J. Dairy Sci. 2023, 106, 7769–7785. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, J.; Li, S.; Ji, S.; Cao, Z.; Zhang, H.; Wang, Y. Effects of a wide range of dietary forage-to-concentrate ratios on nutrient utilization and hepatic transcriptional profiles in limit-fed Holstein heifers. BMC Genom. 2018, 19, 148. [Google Scholar] [CrossRef]
- Dixon, R.M.; Stockdale, C.R. Associative effects between forages and grains: Consequences for feed utilisation. Aust. J. Agric. Res. 1999, 50, 757–774. [Google Scholar] [CrossRef]
- Owens, F.N.; Basalan, M. Ruminal Fermentation. In Rumenology; Millen, D.D., De Beni Arrigoni, M., Lauritano Pacheco, R.D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 63–102. ISBN 978-3-319-30533-2. [Google Scholar]
- Allen, M.S. Relationship Between Fermentation Acid Production in the Rumen and the Requirement for Physically Effective Fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Beef Cattle. Nutrient Requirements of Beef Cattle: Eighth Revised Edition; National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-31702-3. [Google Scholar]
- Auffret, M.D.; Stewart, R.D.; Dewhurst, R.J.; Duthie, C.-A.; Watson, M.; Roehe, R. Identification of Microbial Genetic Capacities and Potential Mechanisms within the Rumen Microbiome Explaining Differences in Beef Cattle Feed Efficiency. Front. Microbiol. 2020, 11, 1229. [Google Scholar] [CrossRef]
- Ogunade, I.; Pech-Cervantes, A.; Schweickart, H. Metatranscriptomic Analysis of Sub-Acute Ruminal Acidosis in Beef Cattle. Animals 2019, 9, 232. [Google Scholar] [CrossRef]
- Martin, M.G.; McLean, K.J.; Voy, B.H.; Myer, P.R. Pregnancy Influences on The Rumen Environment of Angus Heifers Differing in Feed Efficiency. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2023. Available online: https://trace.tennessee.edu/utk_gradthes/9961 (accessed on 23 September 2024).
- Cabrera, V.E. A simple formulation and solution to the replacement problem: A practical tool to assess the economic cow value, the value of a new pregnancy, and the cost of a pregnancy loss. J. Dairy Sci. 2012, 95, 4683–4698. [Google Scholar] [CrossRef]
- Pohler, K.G.; Franco, G.A.; Reese, S.T.; Smith, M.F. Chapter 3—Physiology and pregnancy of beef cattle. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 37–55. ISBN 978-0-12-817052-6. [Google Scholar]
- Jin, S.; Zhang, Z.; Zhang, G.; He, B.; Qin, Y.; Yang, B.; Yu, Z.; Wang, J. Maternal Rumen Bacteriota Shapes the Offspring Rumen Bacteriota, Affecting the Development of Young Ruminants. Microbiol. Spectr. 2023, 11, e03590-22. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, C.; Chen, Y.; Liu, J.; Zhang, C.; Irving, B.; Fitzsimmons, C.; Plastow, G.; Guan, L.L. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome 2019, 7, 92. [Google Scholar] [CrossRef]
- Koch, R.M.; Swiger, L.A.; Chambers, D.; Gregory, K.E. Efficiency of Feed Use in Beef Cattle. J. Anim. Sci. 1963, 22, 486–494. [Google Scholar] [CrossRef]
- Stewart, R.L. Cutting Costs, Not Corners: Managing Cattle in Tough Times; UGA Cooperative Extension: Athens, GA, USA, 2010. [Google Scholar]
- Becker, G.S. Livestock Feed Costs: Concerns and Options; Congressional Research Service, Library of Congress: Washington, DC, USA, 2008. [Google Scholar]
- Herd, R.M.; Archer, J.A.; Arthur, P.F. Reducing the cost of beef production through genetic improvement in residual feed intake: Opportunity and challenges to application1. J. Anim. Sci. 2003, 81, E9–E17. [Google Scholar] [CrossRef]
- Guan, L.L.; Nkrumah, J.D.; Basarab, J.A.; Moore, S.S. Linkage of microbial ecology to phenotype: Correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 2008, 288, 85–91. [Google Scholar] [CrossRef]
- Hernandez-Sanabria, E.; Goonewardene, L.A.; Wang, Z.; Durunna, O.N.; Moore, S.S.; Guan, L.L. Impact of Feed Efficiency and Diet on Adaptive Variations in the Bacterial Community in the Rumen Fluid of Cattle. Appl. Environ. Microbiol. 2012, 78, 1203–1214. [Google Scholar] [CrossRef]
- McLoughlin, S.; Spillane, C.; Claffey, N.; Smith, P.E.; O’Rourke, T.; Diskin, M.G.; Waters, S.M. Rumen Microbiome Composition Is Altered in Sheep Divergent in Feed Efficiency. Front. Microbiol. 2020, 11, 1981. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Chen, Y.; Wu, H.; Meng, Q.; Guan, L.L. Metatranscriptomic Profiling Reveals the Effect of Breed on Active Rumen Eukaryotic Composition in Beef Cattle with Varied Feed Efficiency. Front. Microbiol. 2020, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Arthur, P.F.; Archer, J.A.; Johnston, D.J.; Herd, R.M.; Richardson, E.C.; Parnell, P.F. Genetic and phenotypic variance and covariance components for feed intake, feed efficiency, and other postweaning traits in Angus cattle. J. Anim. Sci. 2001, 79, 2805–2811. [Google Scholar] [CrossRef]
- Sasson, G.; Kruger Ben-Shabat, S.; Seroussi, E.; Doron-Faigenboim, A.; Shterzer, N.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Halperin, E.; Mizrahi, I. Heritable Bovine Rumen Bacteria Are Phylogenetically Related and Correlated with the Cow’s Capacity to Harvest Energy from Its Feed. mBio 2017, 8, e00703-17. [Google Scholar] [CrossRef]
- Ellison, M.J.; Conant, G.C.; Lamberson, W.R.; Cockrum, R.R.; Austin, K.J.; Rule, D.C.; Cammack, K.M. Diet and feed efficiency status affect rumen microbial profiles of sheep. Small Rumin. Res. 2017, 156, 12–19. [Google Scholar] [CrossRef]
- Shabat, S.K.B.; Sasson, G.; Doron-Faigenboim, A.; Durman, T.; Yaacoby, S.; Berg Miller, M.E.; White, B.A.; Shterzer, N.; Mizrahi, I. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 2016, 10, 2958–2972. [Google Scholar] [CrossRef] [PubMed]
- Owens, F.N.; Secrist, D.S.; Hill, W.J.; Gill, D.R. The effect of grain source and grain processing on performance of feedlot cattle: A review. J. Anim. Sci. 1997, 75, 868–879. [Google Scholar] [CrossRef] [PubMed]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef]
- Elliott, C.L.; Edwards, J.E.; Wilkinson, T.J.; Allison, G.G.; McCaffrey, K.; Scott, M.B.; Rees-Stevens, P.; Kingston-Smith, A.H.; Huws, S.A. Using ‘Omic Approaches to Compare Temporal Bacterial Colonization of Lolium perenne, Lotus corniculatus, and Trifolium pratense in the Rumen. Front. Microbiol. 2018, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.C.; Purvis, H.T.; Najar, F.Z.; Sukharnikov, L.O.; Krehbiel, C.R.; Nagaraja, T.G.; Roe, B.A.; DeSilva, U. Rumen Microbial Population Dynamics during Adaptation to a High-Grain Diet. Appl. Environ. Microbiol. 2010, 76, 7482–7490. [Google Scholar] [CrossRef]
- Kittelmann, S.; Kirk, M.R.; Jonker, A.; McCulloch, A.; Janssen, P.H. Buccal Swabbing as a Noninvasive Method to Determine Bacterial, Archaeal, and Eukaryotic Microbial Community Structures in the Rumen. Appl. Environ. Microbiol. 2015, 81, 7470–7483. [Google Scholar] [CrossRef]
- Petri, R.M.; Schwaiger, T.; Penner, G.B.; Beauchemin, K.A.; Forster, R.J.; McKinnon, J.J.; McAllister, T.A. Characterization of the Core Rumen Microbiome in Cattle during Transition from Forage to Concentrate as Well as during and after an Acidotic Challenge. PLoS ONE 2013, 8, e83424. [Google Scholar] [CrossRef] [PubMed]
- Elam, C.J. Acidosis in Feedlot Cattle: Practical Observations. J. Anim. Sci. 1976, 43, 898–901. [Google Scholar] [CrossRef]
- Klieve, A.V.; Hennessy, D.; Ouwerkerk, D.; Forster, R.J.; Mackie, R.I.; Attwood, G.T. Establishing populations of Megasphaera elsdenii YE 34 and Butyrivibrio fibrisolvens YE 44 in the rumen of cattle fed high grain diets. J. Appl. Microbiol. 2003, 95, 621–630. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal Acidosis in Beef Cattle: The Current Microbiological and Nutritional Outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [PubMed]
- Petri, R.M.; Forster, R.J.; Yang, W.; McKinnon, J.J.; McAllister, T.A. Characterization of rumen bacterial diversity and fermentation parameters in concentrate fed cattle with and without forage. J. Appl. Microbiol. 2012, 112, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B. Rumen Microbiology and Its Role in Ruminant Nutrition; Department of Microbiology, Cornell University: Ithaca, NY, USA, 2002. [Google Scholar]
- Steele, M.A.; Croom, J.; Kahler, M.; AlZahal, O.; Hook, S.E.; Plaizier, K.; McBride, B.W. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 300, R1515–R1523. [Google Scholar] [CrossRef]
- Lamb, G.C.; Black, T.E.; Bischoff, K.M.; Mercadante, V.R.G. The Importance of Feed Efficiency in the Cow Herd; University of Florida: Marianna, FL, USA, 2013. [Google Scholar]
- Difford, G.F.; Plichta, D.R.; Løvendahl, P.; Lassen, J.; Noel, S.J.; Højberg, O.; Wright, A.-D.G.; Zhu, Z.; Kristensen, L.; Nielsen, H.B.; et al. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018, 14, e1007580. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Mesgaran, M.D.; Derakhshani, H.; Golder, H.; Khafipour, E.; Kleen, J.L.; Lean, I.; Loor, J.; Penner, G.; Zebeli, Q. Review: Enhancing gastrointestinal health in dairy cows. Animal 2018, 12, s399–s418. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L. Review: An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal 2021, 15, 100295. [Google Scholar] [CrossRef]
- Richards, M.W.; Spitzer, J.C.; Warner, M.B. Effect of Varying Levels of Postpartum Nutrition and Body Condition at Calving on Subsequent Reproductive Performance in Beef Cattle23. J. Anim. Sci. 1986, 62, 300–306. [Google Scholar] [CrossRef]
- Winters, L.M.; Green, W.W.; Comstock, R.E. Prenatal Development of the Bovine; University of Minnesota: Minneapolis, MN, USA, 1942; Available online: https://conservancy.umn.edu/bitstream/handle/11299/204085/mn1000_agexpstn_tb_151.pdf?sequence=1 (accessed on 23 September 2024).
- Swett, W.W.; Matthews, C.A.; Fohrman, M.H. Development of the Fetus in the Dairy Cow; University of Minnesota: Minneapolis, MN, USA, 1948; Available online: https://ageconsearch.umn.edu/record/170289/files/tb964.pdf (accessed on 23 September 2024).
- Lyne, A.G. Pre-natal growth of cattle. Proc. Aust. Soc. Anim. Prod. 1960, 3, 153–161. [Google Scholar]
- Ferrell, C.L.; Garrett, W.N.; Hinman, N. Growth, Development and Composition of the Udder and Gravid Uterus of Beef Heifers during Pregnancy. J. Anim. Sci. 1976, 42, 1477–1489. [Google Scholar] [CrossRef]
- Prior, R.L.; Laster, D.B. Development of the Bovine Fetus1. J. Anim. Sci. 1979, 48, 1546–1553. [Google Scholar] [CrossRef]
- Mao, W.H.; Albrecht, E.; Teuscher, F.; Yang, Q.; Zhao, R.Q.; Wegner, J. Growth- and Breed-related Changes of Fetal Development in Cattle. Asian-Australas. J. Anim. Sci. 2008, 21, 640–647. [Google Scholar] [CrossRef]
- Hall, J.B.; Seay, W.W.; Baker, S.M. Nutrition and Feeding of the Cow-Calf Herd: Production Cycle Nutrition and Nutrient Requirements of Cows, Pregnant Heifers and Bulls; Virginia Cooperative Extension: Blacksburg, VA, USA, 2001; Available online: https://vtechworks.lib.vt.edu/bitstreams/cc702ee1-e2de-47ae-ae40-04f70de23a14/download (accessed on 23 September 2024).
- Campos, V.; Teixeira, S.; Bianco, B.; Silva, F.A.; Chizzotti, M. Nutrient Requirements of Zebu and Crossbred Cattle-BR-CORTE; Universidade Federal de Viçosa: Viçosa, Brazil, 2023; ISBN 978-85-8179-194-4. [Google Scholar]
- Weller, M.M.D.C.A.; Fortes, M.R.S.; Marcondes, M.I.; Rotta, P.P.; Gionbeli, T.R.S.; Valadares Filho, S.C.; Campos, M.M.; Silva, F.F.; Silva, W.; Moore, S.; et al. Effect of maternal nutrition and days of gestation on pituitary gland and gonadal gene expression in cattle. J. Dairy Sci. 2016, 99, 3056–3071. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Wallace, J.M.; Spencer, T.E. BOARD-INVITED REVIEW: Intrauterine growth retardation: Implications for the animal sciences1. J. Anim. Sci. 2006, 84, 2316–2337. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, H.; Tun, H.M.; Cardoso, F.C.; Plaizier, J.C.; Khafipour, E.; Loor, J.J. Linking Peripartal Dynamics of Ruminal Microbiota to Dietary Changes and Production Parameters. Front. Microbiol. 2017, 7, 2143. [Google Scholar] [CrossRef]
- Lima, F.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.S.; Ganda, E.K.; de Oliveira Filho, J.C.; Lorenzo, G.; Trojacanec, P.; Bicalho, R.C. Prepartum and Postpartum Rumen Fluid Microbiomes: Characterization and Correlation with Production Traits in Dairy Cows. Appl. Environ. Microbiol. 2015, 81, 1327–1337. [Google Scholar] [CrossRef]
- Elderman, M.; Hugenholtz, F.; Belzer, C.; Boekschoten, M.; de Haan, B.; de Vos, P.; Faas, M. Changes in intestinal gene expression and microbiota composition during late pregnancy are mouse strain dependent. Sci. Rep. 2018, 8, 10001. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.D.; Phelan, G.; Dowd, S.E.; McDONOUGH, M.M.; Ferguson, A.W.; Delton Hanson, J.; Siles, L.; Ordóñez-Garza, N.; San Francisco, M.; Baker, R.J. Microbiome analysis among bats describes influences of host phylogeny, life history, physiology and geography. Mol. Ecol. 2012, 21, 2617–2627. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef]
- Castro, N.; Kawashima, C.; van Dorland, H.A.; Morel, I.; Miyamoto, A.; Bruckmaier, R.M. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J. Dairy Sci. 2012, 95, 5804–5812. [Google Scholar] [CrossRef]
- D’Occhio, M.J.; Baruselli, P.S.; Campanile, G. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology 2019, 125, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M. Functional Differences in the Growth Hormone and Insulin-like Growth Factor Axis in Cattle and Pigs: Implications for Post-partum Nutrition and Reproduction. Reprod. Domest. Anim. 2008, 43, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C. Regulation of Ovarian Follicular Growth by Somatotropin and Insulin-Like Growth Factors in Cattle1. J. Dairy Sci. 2000, 83, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C.; Butler, S.T.; Garverick, H.A. Endocrine and metabolic mechanisms linking postpartum glucose with early embryonic and foetal development in dairy cows. Animal 2014, 8, 82–90. [Google Scholar] [CrossRef]
- Roche, J.R.; Burke, C.R.; Meier, S.; Walker, C.G. Nutrition × reproduction interaction in pasture-based systems: Is nutrition a factor in reproductive failure? Anim. Prod. Sci. 2011, 51, 1045–1066. [Google Scholar] [CrossRef]
- Samadi, F.; Phillips, N.J.; Blache, D.; Martin, G.B.; D’Occhio, M.J. Interrelationships of nutrition, metabolic hormones and resumption of ovulation in multiparous suckled beef cows on subtropical pastures. Anim. Reprod. Sci. 2013, 137, 137–144. [Google Scholar] [CrossRef]
- Sartori, R.; Gimenes, L.U.; Monteiro, P.L.J.; Melo, L.F.; Baruselli, P.S.; Bastos, M.R. Metabolic and endocrine differences between Bos taurus and Bos indicus females that impact the interaction of nutrition with reproduction. Theriogenology 2016, 86, 32–40. [Google Scholar] [CrossRef]
- Silva, J.R.V.; Figueiredo, J.R.; van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Spicer, L.J.; Wathes, D.C. The role of endocrine insulin-like growth factor-I (IGF-I) in female bovine reproduction. Domest. Anim. Endocrinol. 2008, 35, 325–342. [Google Scholar] [CrossRef]
- Sartori, R.; Guardieiro, M.M.; Surjus, R.S.; Melo, L.F.; Prata, A.B.; Ishiguro, M.; Bastos, M.R.; Nascimento, A.B. Metabolic hormones and reproductive function in cattle. Anim. Reprod. 2018, 10, 199–205. [Google Scholar]
- LeBlanc, S. Monitoring programs for transition dairy cows. In Proceedings of the 26th World Biuatrics Congress, Nice, France, 15 October 2006. [Google Scholar]
- Smith, V.G.; Edgerton, L.A.; Hafs, H.D.; Convey, E.M. Bovine Serum Estrogens, Progestins and Glucocorticoids during Late Pregnancy, Parturition and Early Lactation. J. Anim. Sci. 1973, 36, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Journet, M.; Remond, B. Physiological factors affecting the voluntary intake of feed by cows: A review. Livest. Prod. Sci. 1976, 3, 129–146. [Google Scholar] [CrossRef]
- Beever, D.E. The impact of controlled nutrition during the dry period on dairy cow health, fertility and performance. Anim. Reprod. Sci. 2006, 96, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Etiology of Lipid-Related Metabolic Disorders in Periparturient Dairy Cows. J. Dairy Sci. 1993, 76, 3882–3896. [Google Scholar] [CrossRef]
- Spain, J.N.; Scheer, W.A. The 100-day contract with the dairy cow: 30 days prepartum to 70 days postpartum. In Proceedings of the Tri-State Dairy Nutrition Conference, Fort Wayne, IN, USA, 17–18 April 2001; Volume 414, p. 13. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=3d2aa04b7a561887ac2197f8730d7f4de3b92654#page=20 (accessed on 23 September 2024).
- Wathes, D. Mechanisms Linking Metabolic Status and Disease with Reproductive Outcome in the Dairy Cow. Reprod. Domest. Anim. 2012, 47, 304–312. [Google Scholar] [CrossRef]
- Mulak, A.; Taché, Y.; Larauche, M. Sex hormones in the modulation of irritable bowel syndrome. World J. Gastroenterol. WJG 2014, 20, 2433–2448. [Google Scholar] [CrossRef] [PubMed]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Martin, F.; Järvenpää, P.; Fotsis, T. Steroid absorption and enterohepatic recycling. Contraception 1979, 20, 201–223. [Google Scholar] [CrossRef]
- Henniger, M.T.; Wells, J.E.; Hales, K.E.; Lindholm-Perry, A.K.; Freetly, H.C.; Kuehn, L.A.; Schneider, L.G.; McLean, K.J.; Campagna, S.R.; Christopher, C.J.; et al. Effects of a Moderate or Aggressive Implant Strategy on the Rumen Microbiome and Metabolome in Steers. Front. Anim. Sci. 2022, 3, 889817. [Google Scholar] [CrossRef]
- Ault-Seay, T.B.; Brandt, K.J.; Henniger, M.T.; Payton, R.R.; Mathew, D.J.; Moorey, S.E.; Schrick, F.N.; Pohler, K.G.; Smith, T.P.L.; Rhinehart, J.D.; et al. Bacterial Communities of the Uterus and Rumen during Heifer Development with Protein Supplementation. Front. Anim. Sci. 2022, 3, 903909. [Google Scholar] [CrossRef]
- Laguardia-Nascimento, M.; Branco, K.M.G.R.; Gasparini, M.R.; Giannattasio-Ferraz, S.; Leite, L.R.; Araujo, F.M.G.; de Matos Salim, A.C.; Nicoli, J.R.; de Oliveira, G.C.; Barbosa-Stancioli, E.F. Vaginal Microbiome Characterization of Nellore Cattle Using Metagenomic Analysis. PLoS ONE 2015, 10, e0143294. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.B.; Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.L.; Edwards, J.L.; Myer, P.R.; Pohler, K.G. Bacterial taxonomic composition of the postpartum cow uterus and vagina prior to artificial insemination1. J. Anim. Sci. 2019, 97, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Myer, P.R.; Seay, T.B.; Rhinehart, J. Cattle Gut Microbe Series: Lactobacillus Species; W905. University of Tennessee: Knoxville, TN, USA, 2020. [Google Scholar]
- Myer, P.R.; Seay, T.B.; Rhinehart, J. Cattle Gut Microbe Series: Ruminococcus Species; W398. University of Tennessee: Knoxville, TN, USA, 2020. [Google Scholar]
- Zeineldin, M.; Barakat, R.; Elolimy, A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Monroy, J.C. Synergetic action between the rumen microbiota and bovine health. Microb. Pathog. 2018, 124, 106–115. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, M.G.; Cordero-Llarena, J.F.; Voy, B.H.; McLean, K.J.; Myer, P.R. The Rumen and Gastrointestinal Microbial Environment and Its Association with Feed Efficiency and Pregnancy in Female Beef Cattle. Appl. Microbiol. 2024, 4, 1422-1433. https://doi.org/10.3390/applmicrobiol4040098
Martin MG, Cordero-Llarena JF, Voy BH, McLean KJ, Myer PR. The Rumen and Gastrointestinal Microbial Environment and Its Association with Feed Efficiency and Pregnancy in Female Beef Cattle. Applied Microbiology. 2024; 4(4):1422-1433. https://doi.org/10.3390/applmicrobiol4040098
Chicago/Turabian StyleMartin, M. Gabbi, Juan F. Cordero-Llarena, Brynn H. Voy, Kyle J. McLean, and Phillip R. Myer. 2024. "The Rumen and Gastrointestinal Microbial Environment and Its Association with Feed Efficiency and Pregnancy in Female Beef Cattle" Applied Microbiology 4, no. 4: 1422-1433. https://doi.org/10.3390/applmicrobiol4040098
APA StyleMartin, M. G., Cordero-Llarena, J. F., Voy, B. H., McLean, K. J., & Myer, P. R. (2024). The Rumen and Gastrointestinal Microbial Environment and Its Association with Feed Efficiency and Pregnancy in Female Beef Cattle. Applied Microbiology, 4(4), 1422-1433. https://doi.org/10.3390/applmicrobiol4040098





