Soil Symphony: A Comprehensive Overview of Plant–Microbe Interactions in Agricultural Systems
Abstract
1. Introduction
2. Plant–Microbe Interactions: Nature’s Secret Superheroes
2.1. Diversity of Plant–Microbe Interaction
2.2. Positive Interactions
2.3. Negative Interactions
3. Mechanisms of Plant–Microbe Interactions
3.1. Plant-Released Signals
3.2. Microbial Signalling Molecules in Plant–Microbe Interactions
3.3. Physiological Mechanisms of Plant Growth Promotion by Beneficial Microbiome
4. Applications of Plant–Microbe Interactions in Agriculture and Environmental Sustainability
4.1. Plant Health and Productivity in Agriculture
4.2. Enhanced Nutrient Acquisition
4.3. Enhanced Nutrient Cycling and Soil Health
4.4. Soil Erosion Control and Phytoremediation
4.5. Improvement of Stress Tolerance
4.6. Protection Against Pathogens
4.7. Environmental Sustainability and Bioremediation
5. Future Directions and Challenges
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, Q.; Zhang, Z.; Peijnenburg, W.J.G.M.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere microbiome assembly and its impact on plant growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- Priya, P.; Aneesh, B.; Harikrishnan, K. Genomics as a potential tool to unravel the rhizosphere microbiome interactions on plant health. J. Microbiol. Methods 2021, 185, 106215. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. In Phyto-Microbiome in Stress Regulation; Springer: Singapore, 2020; pp. 147–203. [Google Scholar]
- Kumawat, K.C.; Razdan, N.; Saharan, K. Rhizospheric microbiome: Bio-based emerging strategies for sustainable agriculture development and future perspectives. Microbiol. Res. 2022, 254, 126901. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D.; et al. Linking soil microbial diversity to modern agriculture practices: A review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: A review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Wiesmann, C.L.; Wang, N.R.; Zhang, Y.; Liu, Z.; Haney, C.H. Origins of symbiosis: Shared mechanisms underlying microbial pathogenesis, commensalism and mutualism of plants and animals. FEMS Microbiol. Rev. 2023, 47, fuac048. [Google Scholar] [CrossRef]
- Iqbal, B.; Li, G.; Alabbosh, K.F.; Hussain, H.; Khan, I.; Tariq, M.; Javed, Q.; Naeem, M.; Ahmad, N. Advancing environmental sustainability through microbial reprogramming in growth improvement, stress alleviation, and phytoremediation. Plant Stress 2023, 10, 100283. [Google Scholar] [CrossRef]
- Soto, M.J.; Domínguez-Ferreras, A.; Pérez-Mendoza, D.; Sanjuán, J.; Olivares, J. Mutualism versus pathogenesis: The give-and-take in plant–bacteria interactions. Cell. Microbiol. 2009, 11, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 2021, 12, 669404. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.A. On classifying interactions between populations. Oecologia 1987, 73, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, K.C.; Nagpal, S.; Sharma, P. Present scenario of bio-fertilizer production and marketing around the globe. In Biofertilizers; Woodhead Publishing: Cambridge, UK, 2021; pp. 389–413. [Google Scholar]
- Spribille, T.; Resl, P.; Stanton, D.E.; Tagirdzhanova, G. Evolutionary biology of lichen symbioses. New Phytol. 2022, 234, 1566–1582. [Google Scholar] [CrossRef]
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities: Positive interactions play a critical, but underappreciated, role in ecological communities by reducing physical or biotic stresses in existing habitats and by creating new habitats on which many species depend. Bioscience 2001, 51, 235–246. [Google Scholar]
- Chaudhry, V.; Runge, P.; Sengupta, P.; Doehlemann, G.; Parker, J.E.; Kemen, E. Shaping the leaf microbiota: Plant–microbe–microbe interactions. J. Exp. Bot. 2021, 72, 36–56. [Google Scholar] [CrossRef]
- Morris, B.E.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar] [CrossRef]
- Kirchman, D.L. Degradation of organic material. In Processes in Microbial Ecology; Oxford University Press Inc: New York, NY, USA, 2012; pp. 79–98. [Google Scholar]
- Mutungwazi, A.; Ijoma, G.N.; Matambo, T.S. The significance of microbial community functions and symbiosis in enhancing methane production during anaerobic digestion: A review. Symbiosis 2021, 83, 1–24. [Google Scholar] [CrossRef]
- Mathis, K.A.; Bronstein, J.L. Our current understanding of commensalism. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 167–189. [Google Scholar] [CrossRef]
- Sahu, P.K.; Singh, D.P.; Prabha, R.; Meena, K.K.; Abhilash, P.C. Connecting microbial capabilities with the soil and plant health: Options for agricultural sustainability. Ecol. Indic. 2019, 105, 601–612. [Google Scholar] [CrossRef]
- Nadarajah, K.; Abdul Rahman, N.S.N. Plant–microbe interaction: Aboveground to belowground, from the good to the bad. Int. J. Mol. Sci. 2021, 22, 10388. [Google Scholar] [CrossRef] [PubMed]
- Schirawski, J.; Perlin, M.H. Plant–microbe interaction 2017—The good, the bad and the diverse. Int. J. Mol. Sci. 2018, 19, 1374. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M. Plant–herbivore interactions. In Evolutionary Ecology: Concepts and Case Studies; Oxford University Press: Oxford, UK, 2001; pp. 303–314. [Google Scholar]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma–chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Wu, R. Dynamic behaviors of a nonlinear amensalism model. Adv. Differ. Equ. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Ehrmann, J.; Ritz, K. Plant: Soil interactions in temperate multi-cropping production systems. Plant and Soil 2014, 376, 1–29. [Google Scholar] [CrossRef]
- Casper, B.B.; Jackson, R.B. Plant competition underground. Annu. Rev. Ecol. Syst. 1997, 28, 545–570. [Google Scholar] [CrossRef]
- Koo, B.J.; Adriano, D.C.; Bolan, N.S.; Barton, C.D. Root exudates and microorganisms. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands; Hanley and Belfus Inc.: Philadelphia, PA, USA, 2004; pp. 421–428. [Google Scholar]
- Hodge, A.; Fitter, A.H. Microbial mediation of plant competition and community structure. Funct. Ecol. 2013, 27, 865–875. [Google Scholar] [CrossRef]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2, 100. [Google Scholar] [CrossRef]
- Mitiku, M. Plant-parasitic nematodes and their management: A review. Agric. Res. Technol. 2018, 8, 30–38. [Google Scholar] [CrossRef]
- Afridi, M.S.; Fakhar, A.; Kumar, A.; Ali, S.; Medeiros, F.H.; Muneer, M.A.; Ali, H.; Saleem, M. Harnessing microbial multitrophic interactions for rhizosphere microbiome engineering. Microbiol. Res. 2022, 265, 127199. [Google Scholar] [CrossRef]
- Lambers, H.; Mougel, C.; Jaillard, B.; Hinsinger, P. Plant-microbe-soil interactions in the rhizosphere: An evolutionary perspective. Plant Soil 2009, 321, 83–115. [Google Scholar] [CrossRef]
- Schiessl, K.; Lilley, J.L.; Lee, T.; Tamvakis, I.; Kohlen, W.; Bailey, P.C.; Thomas, A.; Luptak, J.; Ramakrishnan, K.; Carpenter, M.D.; et al. NODULE INCEPTION recruits the lateral root developmental program for symbiotic nodule organogenesis in Medicago truncatula. Curr. Biol. 2019, 29, 3657–3668. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Djebaili, R.; Pellegrini, M.; Mahakur, B.; Sarker, A.; Chaudhary, P.; Khoshru, B.; Gallo, M.D.; Kitouni, M.; Barik, D.P.; et al. Arbuscular mycorrhizal symbiosis: Plant growth improvement and induction of resistance under stressful conditions. J. Plant Nutr. 2021, 44, 1993–2028. [Google Scholar] [CrossRef]
- Sellstedt, A.; Richau, K.H. Aspects of nitrogen-fixing Actinobacteria, in particular free-living and symbiotic Frankia. FEMS Microbiol. Lett. 2013, 342, 179–186. [Google Scholar] [CrossRef]
- Kabiraj, A.; Majhi, K.; Halder, U.; Let, M.; Bandopadhyay, R. Role of Plant Growth-Promoting Rhizobacteria (PGPR) for crop stress management. In Sustainable Agriculture in the Era of Climate Change; Springer: Cham, Switzerland, 2020; pp. 367–389. [Google Scholar]
- Jacott, C.N.; Murray, J.D.; Ridout, C.J. Trade-offs in arbuscular mycorrhizal symbiosis: Disease resistance, growth responses and perspectives for crop breeding. Agronomy 2017, 7, 75. [Google Scholar] [CrossRef]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B.; et al. The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef]
- de la Fuente Cantó, C.; Simonin, M.; King, E.; Moulin, L.; Bennett, M.J.; Castrillo, G.; Laplaze, L. An extended root phenotype: The rhizosphere, its formation and impacts on plant fitness. Plant J. 2020, 103, 951–964. [Google Scholar] [CrossRef]
- Rai, A.N.; Singh, A.K.; Syiem, M.B. Plant growth-promoting abilities in cyanobacteria. In Cyanobacteria; Academic Press: Cambridge, MA, USA, 2019; pp. 459–476. [Google Scholar]
- Lin, Z.; Muhammad, U.K.; Fang, C.; Lin, W. Crop allelopathy types: Current research status and prospects in China. Chin. J. Eco-Agric. 2022, 30, 343–355. [Google Scholar]
- Essarioui, A.; LeBlanc, N.; Kistler, H.C.; Kinkel, L.L. Plant community richness mediates inhibitory interactions and resource competition between Streptomyces and Fusarium populations in the rhizosphere. Microb. Ecol. 2017, 74, 157–167. [Google Scholar] [CrossRef]
- Akram, M.S.; Shahid, M.; Tahir, M.; Mehmood, F.; Ijaz, M. Plant-microbe interactions: Current perspectives of mechanisms behind symbiotic and pathogenic associations. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 1: Fundamental Mechanisms, Methods and Functions; Springer: Singapore, 2017; pp. 97–126. [Google Scholar]
- Chandra, N.; Kumar, S. Antibiotics producing soil microorganisms. In Antibiotics and Antibiotics Resistance Genes in Soils: Monitoring, Toxicity, Risk Assessment and Management; Springer: Cham, Switzerland, 2017; pp. 1–18. [Google Scholar]
- Nguyen, S.D.; Trinh, T.H.T.; Tran, T.D.; Nguyen, T.V.; Chuyen, H.V.; Ngo, V.A.; Nguyen, A.D. Combined application of rhizosphere bacteria with endophytic bacteria suppresses root diseases and increases productivity of black pepper (Piper nigrum L.). Agriculture 2020, 11, 15. [Google Scholar] [CrossRef]
- Cipollini, D.; Rigsby, C.M.; Barto, E.K. Microbes as targets and mediators of allelopathy in plants. J. Chem. Ecol. 2012, 38, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.A.; Rothballer, M.; Chowdhury, S.P.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Castro, R.; Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; López-Bucio, J. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G.E. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef]
- Rosier, A.; Bishnoi, U.; Lakshmanan, V.; Sherrier, D.J.; Bais, H.P. A perspective on inter-kingdom signaling in plant–beneficial microbe interactions. Plant Mol. Biol. 2016, 90, 537–548. [Google Scholar] [CrossRef]
- Williams, P.; Winzer, K.; Chan, W.C.; Camara, M. Look who’s talking: Communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1119–1134. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.P.; Vierheilig, H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Dundek, P.; Holík, L.; Rohlík, T.; Hromádko, L.; Vranová, V.; Rejšek, K.; Formánek, P. Methods of plant root exudates analysis: A review. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2011, 59, 241–246. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.R.; Borriss, R.; von Wirén, N. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 2011, 174, 3–11. [Google Scholar] [CrossRef]
- Werner, S.; Polle, A.; Brinkmann, N. Belowground communication: Impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl. Microbiol. Biotechnol. 2016, 100, 8651–8665. [Google Scholar] [CrossRef] [PubMed]
- Srikamwang, C.; Onsa, N.E.; Sunanta, P.; Sangta, J.; Chanway, C.P.; Thanakkasaranee, S.; Sommano, S.R. Role of microbial volatile organic compounds in promoting plant growth and disease resistance in horticultural production. Plant Signal. Behav. 2023, 18, 2227440. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Zaman, M.; Pharis, R.P. Phytohormonal basis for the plant growth promoting action of naturally occurring biostimulators. J. Sci. Food Agric. 2014, 94, 1715–1722. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Koprivova, A.; Kopriva, S. Pinpointing secondary metabolites that shape the composition and function of the plant microbiome. J. Exp. Bot. 2021, 72, 57–69. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Stojanovic-Radic, Z.Z.; Stojanovic, N.M. Antimicrobial plant metabolites: Structural diversity and mechanism of action. Curr. Med. Chem. 2013, 20, 932–952. [Google Scholar]
- Swift, S.; Downie, J.A.; Whitehead, N.A.; Barnard, A.M.; Salmond, G.P.; Williams, P. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 2001, 45, 199–270. [Google Scholar]
- Fuqua, C.; Parsek, M.R.; Greenberg, E.P. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001, 35, 439–468. [Google Scholar] [CrossRef]
- Čáp, M.; Váchová, L.; Palková, Z. Reactive oxygen species in the signaling and adaptation of multicellular microbial communities. Oxidative Med. Cell. Longev. 2012, 2012, 976753. [Google Scholar] [CrossRef]
- Nath, M.; Bhatt, D.; Prasad, R.; Tuteja, N. Reactive oxygen species (ROS) metabolism and signaling in plant-mycorrhizal association under biotic and abiotic stress conditions. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Springer: Cham, Switzerland, 2017; pp. 223–232. [Google Scholar]
- Macabuhay, A.; Arsova, B.; Walker, R.; Johnson, A.; Watt, M.; Roessner, U. Modulators or facilitators? Roles of lipids in plant root–microbe interactions. Trends Plant Sci. 2022, 27, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.; Brands, M.; Wewer, V.; Duan, Y.; Hölzl, G.; Dörmann, P. Lipids in plant–microbe interactions. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1379–1395. [Google Scholar] [CrossRef] [PubMed]
- Daayf, F.; El Hadrami, A.; El-Bebany, A.F.; Henriquez, M.A.; Yao, Z.; Derksen, H.; El-Hadrami, I.; Adam, L.R. Phenolic compounds in plant defense and pathogen counter-defense mechanisms. Recent Adv. Polyphen. Res. 2012, 3, 191–208. [Google Scholar]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; De Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef]
- Weisskopf, L.; Schulz, S.; Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 2021, 19, 391–404. [Google Scholar] [CrossRef]
- Lyapina, I.; Filippova, A.; Fesenko, I. The role of peptide signals hidden in the structure of functional proteins in plant immune responses. Int. J. Mol. Sci. 2019, 20, 4343. [Google Scholar] [CrossRef]
- Nandi, A.K. Application of antimicrobial proteins and peptides in developing disease-resistant plants. In Plant Pathogen Resistance Biotechnology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 51–70. [Google Scholar]
- Shastri, B.; Kumar, R. Microbial secondary metabolites and plant–microbe communications in the rhizosphere. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–111. [Google Scholar]
- Jamil, F.; Mukhtar, H.; Fouillaud, M.; Dufossé, L. Rhizosphere signaling: Insights into plant–rhizomicrobiome interactions for sustainable agronomy. Microorganisms 2022, 10, 899. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Ramamurthy, P.C.; Singh, J. Molecular mechanism and signaling pathways interplay between plant hormones during plant-microbe crosstalk. In Microbial Management of Plant Stresses; Woodhead Publishing: Cambridge, UK, 2021; pp. 93–105. [Google Scholar]
- Majdura, J.; Jankiewicz, U.; Gałązka, A.; Orzechowski, S. The role of quorum sensing molecules in bacterial–plant interactions. Metabolites 2023, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tian, X. Quorum sensing and bacterial social interactions in biofilms. Sensors 2012, 12, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biology and fertility of soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Altomare, C.; Tringovska, I. Beneficial soil microorganisms, an ecological alternative for soil fertility management. In Genetics, Biofuels and Local Farming Systems; Springer: Dordrecht, The Netherlands, 2011; pp. 161–214. [Google Scholar]
- Choudhary, D.K.; Kasotia, A.; Jain, S.; Vaishnav, A.; Kumari, S.; Sharma, K.P.; Varma, A. Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J. Plant Growth Regul. 2016, 35, 276–300. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic acquired resistance (SAR) and induced systemic resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Rosier, A.; Medeiros, F.H.; Bais, H.P. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 2018, 428, 35–55. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Balasubramanian, V.K.; Jansson, C.; Baker, S.E.; Ahkami, A.H. Molecular mechanisms of plant–microbe interactions in the rhizosphere as targets for improving plant productivity. In Rhizosphere Biology: Interactions Between Microbes and Plants; Springer: Singapore, 2021; pp. 295–338. [Google Scholar]
- Bücking, H.; Kafle, A. Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: Current knowledge and research gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Ahmed, A.; Hasnain, S. Auxins as one of the factors of plant growth improvement by plant growth promoting rhizobacteria. Pol. J. Microbiol. 2014, 63, 261. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Sharif Ahmed, S.; Rehman, A.; et al. Phytohormones as growth regulators during abiotic stress tolerance in plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Khashi u Rahman, M.; Wu, F. The impact of root exudates, volatile organic compounds, and common mycorrhizal networks on root system architecture in root-root interactions. J. Plant Interact. 2022, 17, 685–694. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Naveed, M.; Zahir, Z.A.; Asghar, H.N. Plant–microbe interactions for sustainable agriculture: Fundamentals and recent advances. In Plant Microbe Symbiosis: Fundamentals and Advances; Springer: New Delhi, India, 2013; pp. 51–103. [Google Scholar]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Kumar, V.; Joshi, S.; Pant, N.C.; Sangwan, P.; Yadav, A.N.; Saxena, A.; Singh, D. Molecular approaches for combating multiple abiotic stresses in crops of arid and semi-arid region. In Molecular Approaches in Plant Biology and Environmental Challenges; Springer: Singapore, 2019; pp. 149–170. [Google Scholar]
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE. 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Roat, C.; Saraf, M. Unravelling the interaction of plant and their phyllosphere microbiome. In Understanding Host-Microbiome Interactions-An Omics Approach: Omics of Host-Microbiome Association; Springer: Singapore, 2017; pp. 157–172. [Google Scholar]
- Shukla, A.K.; Behera, S.K.; Chaudhari, S.K.; Singh, G. Fertilizer use in Indian agriculture and its impact on human health and environment. Indian J. Fertil. 2022, 18, 218–237. [Google Scholar]
- Malgioglio, G.; Rizzo, G.F.; Nigro, S.; Lefebvre du Prey, V.; Herforth-Rahmé, J.; Catara, V.; Branca, F. Plant-microbe interaction in sustainable agriculture: The factors that may influence the efficacy of PGPM application. Sustainability 2022, 14, 2253. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. In PGPR Amelioration in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–157. [Google Scholar]
- Dobbelaere, W.; De Graaf, D.C.; Reybroeck, W.; Desmedt, E.; Peeters, J.E.; Jacobs, F.J. Disinfection of wooden structures contaminated with Paenibacillus larvae subsp. larvae spores. J. Appl. Microbiol. 2001, 91, 212–216. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.D.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tailor, S. Plant-microbe interactions in photosynthesis, nutrient acquisition, and plant growth. In Plant-Microbe Interaction-Recent Advances in Molecular and Biochemical Approaches; Academic Press: Cambridge, MA, USA, 2023; pp. 421–434. [Google Scholar]
- Bhantana, P.; Rana, M.S.; Sun, X.-C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Alam Bhat, M.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Nitrogen fixation in perspective: An overview of research and extension needs. Field Crops Res. 2000, 65, 93–106. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. The role of microbes to improve crop productivity and soil health. In Ecological Wisdom Inspired Restoration Engineering; Springer: Singapore, 2019; pp. 249–265. [Google Scholar]
- Prasad, S.; Malav, L.C.; Choudhary, J.; Kannojiya, S.; Kundu, M.; Kumar, S.; Yadav, A.N. Soil microbiomes for healthy nutrient recycling. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Springer: Singapore, 2021; pp. 1–21. [Google Scholar]
- Wilkes, T.I. Arbuscular mycorrhizal fungi in agriculture. Encyclopedia 2021, 1, 1132–1154. [Google Scholar] [CrossRef]
- Crecchio, C.; Mimmo, T.; Bulgarelli, D.; Pertot, I.; Pii, Y.; Perazzolli, M.; Scagliola, M.; Cesco, S. Beneficial soil microbiome for sustainable agriculture production. In Sustainable Agriculture Reviews 31: Biocontrol; Springer: Cham, Switzerland, 2018; pp. 443–481. [Google Scholar]
- Upadhyay, N.; Vishwakarma, K.; Singh, J.; Verma, R.K.; Prakash, V.; Jain, S.; Kumar, V.; Rani, R.; Tripathi, D.K.; Sharma, S. Plant-Microbe-Soil Interactions for Reclamation of Degraded Soils: Potential and Challenges. In Phyto and Rhizo Remediation; Springer: Singapore, 2019; pp. 147–173. [Google Scholar]
- Zhang, L.; Xiao, T.; Liu, H.; Ge, P.; Xia, J.; Dai, C.; Zhang, W.; Zhao, X. Effects of AM fungi and grass strips on soil erosion characteristics in red sandstone erosion areas in Southern China. Forests 2022, 13, 1351. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’ resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef]
- Subiramani, S.; Ramalingam, S.; Muthu, T.; Nile, S.H.; Venkidasamy, B. Development of abiotic stress tolerance in crops by plant growth-promoting rhizobacteria (PGPR). In Phyto-Microbiome in Stress Regulation; Springer: Singapore, 2020; pp. 125–145. [Google Scholar]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, A.; Keswani, C.; Saxena, A.; Sarma, B.K.; Singh, H.B. Harnessing plant-microbe interactions for enhanced protection against phytopathogens. In Plant Microbes Symbiosis: Applied Facets; Springer: New Delhi, India, 2015; pp. 111–125. [Google Scholar]
- Al-Ani, L.K.T. PGPR: A good step to control several of plant pathogens. In Advances in PGPR Research; CABI: Wallingford, UK, 2017; pp. 398–410. [Google Scholar]
- Divya, B.; Deepak Kumar, M. Plant–microbe interaction with enhanced bioremediation. Res. J. Biotechnol. 2011, 6, 4. [Google Scholar]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent strategies for bioremediation of emerging pollutants: A review for a green and sustainable environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef]
- Gkorezis, P.; Daghio, M.; Franzetti, A.; Van Hamme, J.D.; Sillen, W.; Vangronsveld, J. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 2016, 7, 1836. [Google Scholar] [CrossRef]
- Basu, S.; Banerjee, P.; Banerjee, S.; Ghosh, B.; Bhattacharjee, A.; Roy, D.; Singh, P.; Kumar, A. Bioremediation strategies to overcome heavy metals and radionuclides from the environment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 287–302. [Google Scholar]
- Chiquito-Contreras, C.J.; Meza-Menchaca, T.; Guzmán-López, O.; Vásquez, E.C.; Ricaño-Rodríguez, J. Molecular Insights into Plant–Microbe Interactions: A Comprehensive Review of Key Mechanisms. Front. Biosci. 2024, 16, 9. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Yadav, A.N.; Santoyo, G.; Babalola, O.O. Understanding the plant-microbe interactions in environments exposed to abiotic stresses: An overview. Microbiol. Res. 2023, 271, 127368. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Verma, J.P.; Belwal, T.; Pereira, A.P.D.A.; Ade, A.B. Microbial co-cultures: A new era of synthetic biology and metabolic engineering. Front. Microbiol. 2023, 14, 1235565. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action mechanisms of effectors in plant-pathogen interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.S.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.-M.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 2020, 11, 514909. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Kumar, K.; Rani, A.; Mishra, V. Signaling pathways and downstream effectors of host innate immunity in plants. Int. J. Mol. Sci. 2021, 22, 9022. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control 2020, 140, 104095. [Google Scholar] [CrossRef]
- van Bruggen, A.H.; Gamliel, A.; Finckh, M.R. Plant disease management in organic farming systems. Pest Manag. Sci. 2016, 72, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Scortichini, M. Sustainable Management of Diseases in Horticulture: Conventional and New Options. Horticulturae 2022, 8, 517. [Google Scholar] [CrossRef]
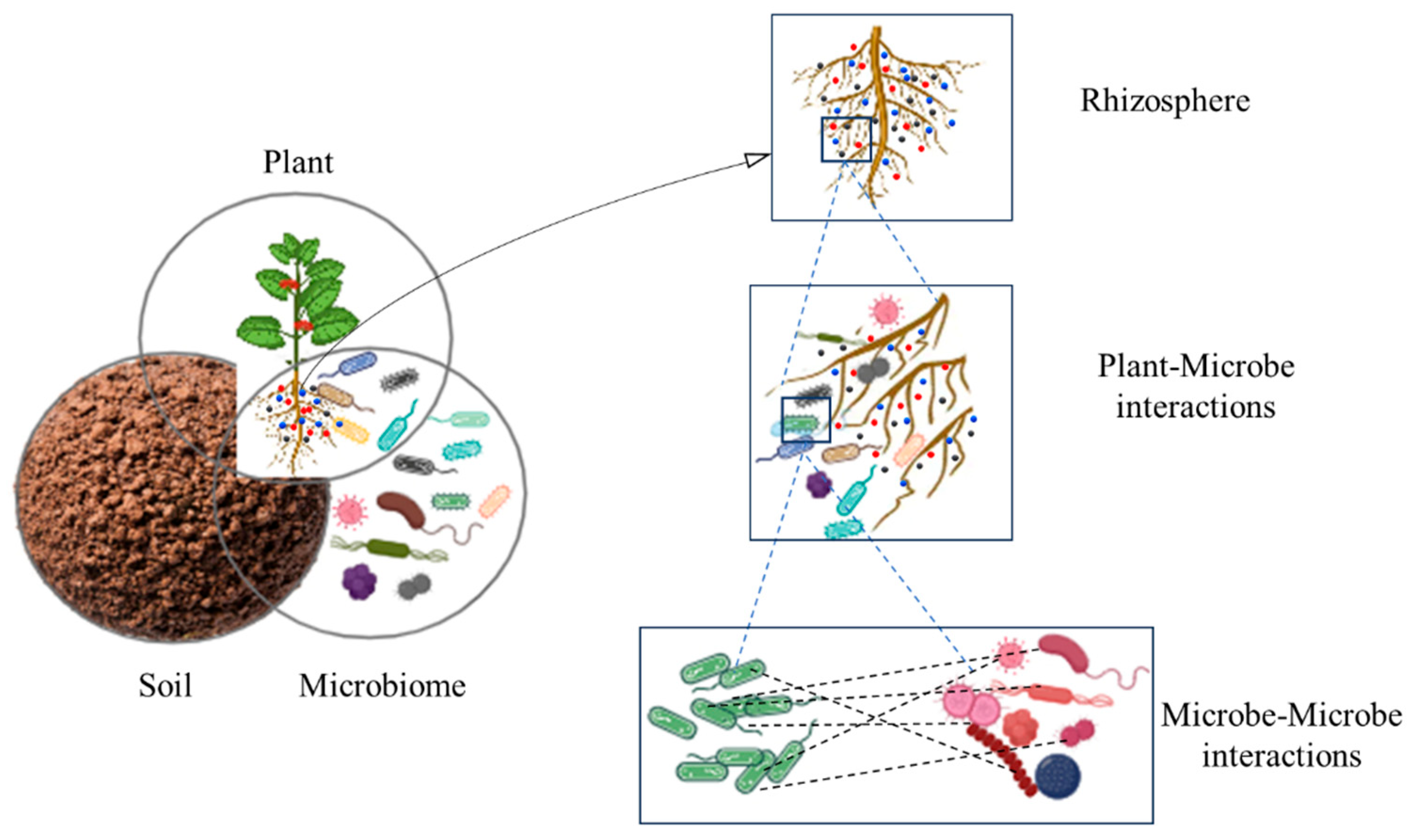
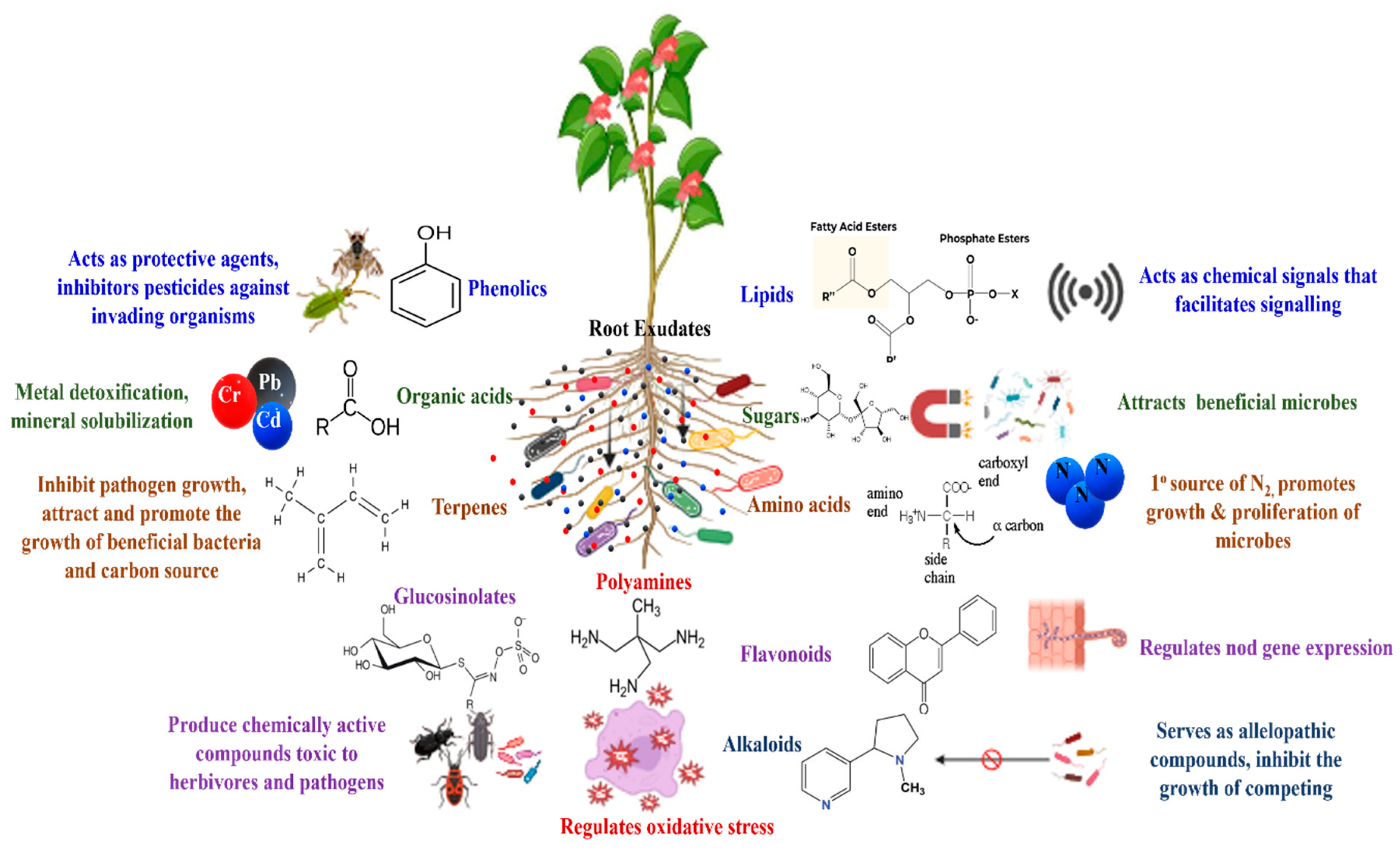
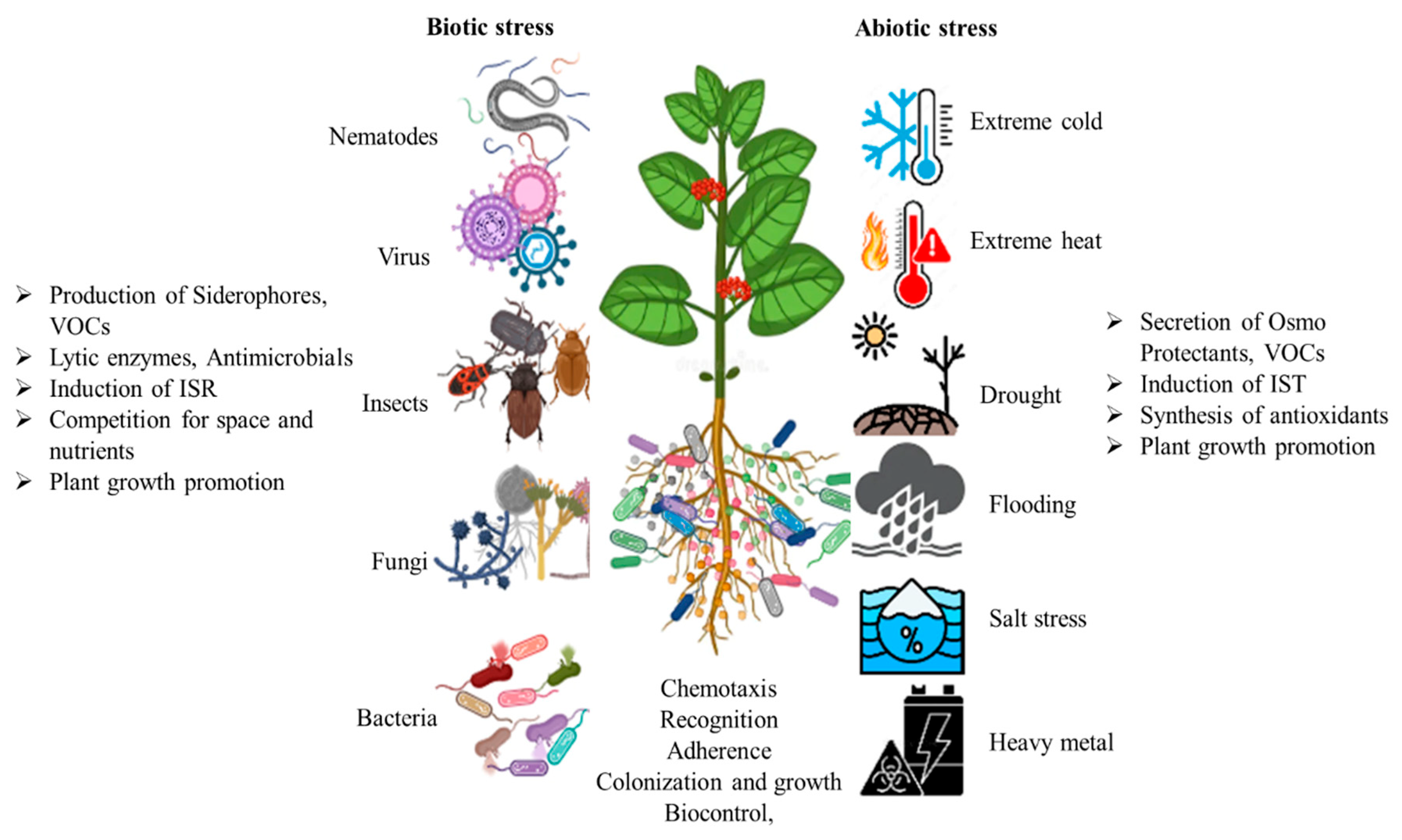
| Interaction | Sp. A | Sp. B | |
|---|---|---|---|
| Positive Interactions | Protocooperation | + | + |
| Syntrophism | |||
| Mutualism | |||
| Facilitation | |||
| Commensalism | + | 0 | |
| Neutralism | 0 | 0 | |
| Negative Interactions | Amensalism (Antagonism) | 0 | - |
| Competition | - | - | |
| Parasitism | + | - | |
| Predation | + | - | |
| Interaction Type | Microbial Group | Plant Response | Example | Reference |
|---|---|---|---|---|
| Symbiosis | Rhizobium | Nitrogen fixation | Legume-rhizobia symbiosis | Schiessl et al. 2019 [37] |
| AMF | Nutrient uptake, Stress tolerance | Mycorrhizal symbiosis | Mitra et al. 2021 [38] | |
| Frankia | Nitrogen fixation | Actinorrhizal symbiosis | Sellstedt and Richau, 2013 [39] | |
| Mutualism | Plant growth-promoting rhizobacteria (PGPR) | Growth promotion, stress resistance | Rhizobacteria-plant symbiosis | Kabiraj etal. 2020 [40] |
| Mycorrhizal fungi | Nutrient acquisition, disease resistance | Mycorrhizal symbiosis | Jacott et al. 2017 [41] | |
| Endophytic bacteria | Disease resistance, growth promotion | Endophytic bacteria-plant symbiosis | Vandana et al. 2020 [42] | |
| Commensalism | Plant growth-promoting fungi | Altered root exudation | Bacterial colonization of the rhizosphere | de la Fuente Cantó et al. 2020 [43] |
| Nitrogen-fixing cyanobacteria | Nitrogen fixation, growth promotion | Cyanobacterial-plant symbiosis | Rai et al. 2019 [44] | |
| Amensalism | Allelopathic microorganisms | Inhibition of competing plants | Allelopathy in soil microbial communities | [45] |
| Competition | Soil bacteria and fungi | Nutrient competition, antibiotic production | Fungal colonization of the rhizosphere | Essarioui et al. 2017 [46] |
| Root pathogens | Disease development, reduced growth | Pathogen-plant interactions in the rhizosphere | Akram et al. 2017 [47] | |
| Antibiosis | Antibiotic-producing bacteria | Suppression of pathogens, pests | Antibiosis in soil microbiome | Chandra, and Kumar, 2017 [48] |
| Antifungal-producing fungi | Suppression of fungal pathogens | Fungal-plant interactions | Nguyen et al. 2020 [49] | |
| Allelopathic plants | Inhibition of microbial growth | Plant–microbe interactions in allelopathic systems | Cipollini et al. 2012 [50] |
| Signal Type | Description | Reference |
|---|---|---|
| Root Exudates | Organic compounds released by roots into the rhizosphere, including sugars, root colonization amino acids and organic acids. | [60,61] |
| Volatile Organic Compounds (VOCs) | Gaseous compounds emitted by plants that can attract or repel microorganisms, affect microbial growth and behaviour. Ex. Terpenes (limonene, pinene), Alcohols (ethanol), Aldehydes (hexanal). | [62,63] |
| Phytohormones | Plant hormones like auxins, gibberellins, and cytokinin that regulate plant growth and development, and can also influence microbial activities | [64,65] |
| Secondary Metabolites | Chemical compounds (Alkaloids (nicotine, caffeine), Flavonoids (quercetin, kaempferol), Saponins) produced by plants that can have antimicrobial properties, influence microbial community composition, or modulate microbial activities | [66,67] |
| Quorum Sensing Molecules | Signaling molecules (Acyl-homoserine lactones (AHLs), Pheromones (cis-2-dodecenoic acid) produced by plants and microorganisms to communicate and regulate gene expression in response to population density. | [68,69] |
| Reactive Oxygen Species (ROS) | ROS (such as, Hydrogen peroxide (H2O2), Superoxide anion (O2−), Hydroxyl radical (•HO) produced by plants as signaling molecules in response to microbial colonization or stress. | [70,71] |
| Lipids and Fatty Acids | Lipids and fatty acids released by plants that can influence microbial colonization and activity in the rhizosphere. | [72,73] |
| Phenolic Compounds | Phenolic compounds such as phenolic acids (ferulic acid, caffeic acid), tannins, flavonoids produced by plants with antimicrobial properties, involved in defense against pathogens and modulation of microbial communities | [74,75] |
| Volatile Terpenes | Terpenes (monoterpenes (limonene, pinene), sesquiterpenes (farnesene, caryophyllene) (released by plants with diverse biological activities, including antimicrobial properties and modulation of microbial communities. | [76,77] |
| Peptides and Proteins | Peptides and proteins released by plants that can act as signaling molecules or antimicrobial agents against pathogens. | [78,79] |
| Microbial Metabolites | Metabolites like antibiotics (penicillin, streptomycin), exopolysaccharides, volatile fatty acids produced by microorganisms in response to plant signals, influencing plant–microbe interactions and rhizosphere ecology. | [80] |
| Physiological Response | Plant–Microbe Interaction | Example | Reference |
|---|---|---|---|
| Nutrient Uptake | Mycorrhizal Symbiosis | AMF facilitate nutrient uptake in plants by extending their hyphae into the soil to access nutrients like phosphorus and nitrogen. | [94] |
| Growth Promotion | PGPR | Rhizobacteria in the rhizosphere produce plant growth-promoting constituents such as auxins and cytokinin, stimulating root and shoot growth in plants. | [95] |
| Stress Tolerance | ISR | Beneficial microorganisms like Bacillus spp. and Trichoderma spp. stimulate systemic resistance in plants, enhancing their tolerance to environmental stresses such as drought, salinity, and pathogens. | [96] |
| Disease Resistance | Antagonistic Interactions | Certain microorganisms such as Streptomyces spp. and Bacillus cereus in the rhizosphere produce antimicrobial composites that hinder the growth of pathogens, providing disease resistance to the host plant. | [97] |
| Hormonal Regulation | Phytohormone Production | Microorganisms like Azospirillum brasilense and Rhizobium leguminosarum produce phytohormones like auxins, cytokinin, and gibberellins, which regulate various physiological processes in plants such as growth and development | [98] |
| Root Architecture Modification | Indirect Effects on Soil Microbiome | Plant–microbe interactions influence root architecture, with some microorganisms promoting lateral root formation and others inhibiting primary root growth, thereby affecting nutrient uptake and soil structure. | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tharanath, A.C.; Upendra, R.S.; Rajendra, K. Soil Symphony: A Comprehensive Overview of Plant–Microbe Interactions in Agricultural Systems. Appl. Microbiol. 2024, 4, 1549-1567. https://doi.org/10.3390/applmicrobiol4040106
Tharanath AC, Upendra RS, Rajendra K. Soil Symphony: A Comprehensive Overview of Plant–Microbe Interactions in Agricultural Systems. Applied Microbiology. 2024; 4(4):1549-1567. https://doi.org/10.3390/applmicrobiol4040106
Chicago/Turabian StyleTharanath, Arpitha Chatchatnahalli, Raje Siddiraju Upendra, and Karthik Rajendra. 2024. "Soil Symphony: A Comprehensive Overview of Plant–Microbe Interactions in Agricultural Systems" Applied Microbiology 4, no. 4: 1549-1567. https://doi.org/10.3390/applmicrobiol4040106
APA StyleTharanath, A. C., Upendra, R. S., & Rajendra, K. (2024). Soil Symphony: A Comprehensive Overview of Plant–Microbe Interactions in Agricultural Systems. Applied Microbiology, 4(4), 1549-1567. https://doi.org/10.3390/applmicrobiol4040106






