Abstract
Trichothecenes are sesquiterpenoid toxins produced by diverse ascomycetes, including Fusarium. The trichothecene analog deoxynivalenol (DON) produced by the Fusarium head blight (FHB) pathogen Fusarium graminearum is a virulence factor on wheat and a major food and feed safety concern. In Fusarium, the trichothecene biosynthetic gene (TRI) cluster consists of 7–14 genes. Most TRI cluster genes are conserved and their specific roles in trichothecene biosynthesis have been determined. An exception is TRI14, which is not required for DON synthesis in vitro but is required for spread of F. graminearum in wheat heads. In the current study, gene expression analyses revealed that TRI14 was highly induced in infected wheat heads. We demonstrated that TRI14 was not only required for F. graminearum spread but also important for initial infection in wheat. Although a prior study did not detect DON in infected seeds, our analyses showed significantly less DON and fungal biomass in TRI14-mutant (designated ∆tri14)-inoculated heads than wild-type-inoculated heads. Gene expression comparison showed that the level of expression of TRI genes was similar in the wheat tissues infected with ∆tri14 or the wild type, indicating the reduced toxin levels caused by ∆tri14 may be due to less fungal growth. ∆tri14 also caused less lesion and grew less in wheat coleoptiles than the wild type. The growth of ∆tri14 in carboxymethylcellulose medium was more sensitive to hydrogen peroxide than the wild type. The data suggest that TRI14 plays a critical role in F. graminearum growth, and potentially protects the fungus from plant defense compounds.
1. Introduction
Trichothecenes are toxic sesquiterpenoid metabolites produced by diverse ascomycetous fungi including some species of Fusarium, Aspergillus, Myrothecium, Stachybotrys, Trichothecium and Trichoderma [1]. Trichothecenes produced by plant pathogenic species of Fusarium are a particular concern because of their frequent occurrence in multiple food and feed crops, where they pose health risks to humans and other mammals [2]. Fusarium diseases and trichothecene contamination of crops cause billion dollar losses to agriculture every year [3]. In addition, some of the Fusarium trichothecene analogs contribute to pathogenesis on plants. For example, production of the trichothecene deoxynivalenol (DON) by F. graminearum is required for the fungus to spread within wheat heads and, therefore, to cause Fusarium head blight (FHB) epidemics in wheat fields [4,5].
In fungi, trichothecene production is conferred by 7–14 genes that tend to be located adjacent to one another in a biosynthetic gene cluster. Orthologs of the trichothecene biosynthetic gene (TRI) cluster have been identified in all trichothecene-producing fungi that have been examined as well as in several fungi that have not yet been shown to produce trichothecenes [6]. These fungi include species Aspergillus, Myrothecium, Stachybotrys, Trichothecium and Trichoderma as well as members of three multispecies lineages of Fusarium [7,8]. It is presumed that fungi with a TRI cluster but that have not been reported to produce trichothecenes can produce them under yet-to-be-identified environmental conditions [6]. Although the gene content of TRI cluster orthologs varies markedly among known and presumed trichothecene-producing fungi, all orthologs characterized to date have three genes in common: TRI3, TRI5 and TRI14 [1]. Roles for TRI3 and TRI5 in trichothecene biosynthesis have been determined. TRI5 encodes a terpene synthase that catalyzes conversion of the primary metabolite farnesyl diphosphate to trichodiene, the first committed intermediate in the trichothecene biosynthetic pathway. TRI3 encodes an acetyltransferase that catalyzes the O-acetylation of trichothecenes at carbon atom 15 (C15) or 4 (C4) depending on the fungal species [6,9,10]. In contrast, BLAST analysis indicates that the putative protein encoded by TRI14 shares little homology to previously characterized proteins, and deletion of the gene in F. graminearum did not impact trichothecene production in culture [11]. However, F. graminearum TRI14 deletion mutants are markedly less virulent on wheat than their wild-type progenitor strain and the complemented mutants restores full virulence of the TRI14 deletion mutant [11].
Wheat resistance to F. graminearum has been classified as Type I or II. Type I resistance is the ability of wheat to resist initial infection of the fungus. This type of resistance can be assessed by spray or dip inoculation of an entire wheat head followed by monitoring of FHB symptoms on the head [12]. Information on how F. graminearum initiates infection and how wheat responds to the infection is limited. Type II resistance is the ability of wheat to inhibit spread of F. graminearum from an infected spikelet to neighboring spikelets. Type II resistance can be assessed by point inoculation of one spikelet on a head followed by monitoring of spread of FHB symptoms to other spikelets on the same head. DON and other tricoherences appear to suppress Type II resistance [4,13,14]. That is, wild-type, DON-producing strains of F. graminearum can spread from an infected spikelet to adjacent uninfected spikelets via the node connecting the infected spikelet to the central axis (rachis) of the wheat head. By contrast, DON-nonproducing mutants, such as TRI5 deletion mutants, can infect a spikelet but cannot pass through the rachis node and, therefore, cannot spread to other spikelets [5,13].
The previous analyses indicate that TRI14, despite being present in all TRI clusters examined, is not required for trichothecene production in culture, but is required by F. graminearum to spread in wheat heads. In the current study, we investigated the role of TRI14 during F. graminearum spread and the initial infection of wheat. We compared fungal growth, toxin production and TRI gene expression in wheat tissues infected by a TRI14 deletion mutant (∆tri14) and its wildtype parent. We also explored the effect of TRI14 deletion on infection of wheat coleoptiles and sensitivity to the reactive oxygen species (ROS) hydrogen peroxide (H2O2).
2. Materials and Methods
2.1. Strains and Plant Cultivation
F. graminearum strain GZ3639 was isolated from seeds harvested from FHB symptomatic wheat grown in Kansas [15] and served as the parent strain of the TRI14 deletion mutants FgΔTri14-27 and -50. Replacement of TRI14 with a hygromycin cassette was confirmed by Southern analysis and complementation experiments [11]. F. graminearum cultures were maintained on V8® juice agar culture plates [16]. Macroconidia for wheat FHB virulence assays were prepared from strains grown in mung bean media at 28 °C with shaking at 200 rpm for four days [17].
Spring wheat cultivar Norm was used in FHB virulence assays. Five wheat plants were grown per 5-gallon bucket containing SunShine Mix (Sun Gro Horticulture, Agawam, MA, USA) supplemented with Osmocote and Micromax (TerraLink, Lynden, WA, USA) and maintained in controlled chambers under 14:10 h light/dark cycle at 23/20 °C with 50% relative humidity.
2.2. Identification of TRI Gene Orthologs in Fungi
We used BLASTn analysis to determine the presence and absence of TRI genes in filamentous fungal genome sequences. TRI genes in Fusarium scirpi and Trichoderma arundinaceum served as query sequences. Together, these two species include all 21 previously characterized TRI genes [1,18]. The databases used in BLAST analyses were (1) the non-redundant fungal database at the National Center for Biotechnology Information (NCBI) and (2) an in-house database of fungal genome sequences that have been reported to produce trichothecenes or have multiple adjacent TRI genes. Percent identities of deduced amino acid sequences of TRI gene orthologs were determined using the “Align two or more sequences” option in BLASTp analysis at NCBI.
2.3. FHB Virulence Assays
The macroconidia inoculum for the wheat infection assays was collected from mung bean cultures after passage through a 40 µm cell strainer (Biologix, Jinan, Shandong, China) and centrifugation for 10 min at 3000 rpm. The pellet was suspended in water and adjusted to a concentration of 105 conidia/mL. For evaluation of disease spread at anthesis, one floret in the fifth spikelet from the top of the wheat head was injected with 10 µL conidial suspension of GZ3639 or ∆tri14. Inoculated wheat heads were covered with a plastic bag for three days to maintain high humidity. FHB progress was evaluated 7, 14, and 21 days post inoculation (dpi). At 21 dpi, the inoculated spikelets were harvested, and placed in 1 mL vials for toxin extraction and measurement. Twenty-four wheat spikelets were inoculated for each strain.
For evaluation of initial infection, whole wheat heads were dip-inoculated as described [12]. In brief, a conidial suspension was prepared as above and adjusted to 5 × 104 conidia/mL suspension in 0.02% Tween 20. Wheat heads at mid-anthesis were dipped into the conidial suspension. Inoculated wheat heads were covered with a plastic bag for three days. Disease was scored at 5 dpi. At 5 dpi, the inoculated heads were harvested and three heads as a group were ground for toxin extraction and measurement. Twenty-two wheat heads were inoculated for each strain.
Coleoptile assays were conducted as described [19]. Briefly, the tips (2–3 mm) of coleoptiles from 3-day-old seedlings were removed, and a 1.5 μL conidial suspension (106 conidia/mL) was placed on the top of coleoptiles. About 20 seedlings were inoculated with each strain. Water-inoculated coleoptiles served as controls. The inoculated seedlings were maintained in a growth chamber at 23 °C with 95% humidity. Lesions were measured at 7 dpi, photographed, and collected for DNA isolation and fungal biomass determination.
2.4. Gene Expression Analyses by Quantitative PCR (qPCR)
To examine the expression of TRI14 and TRI5 during wheat head infection, wheat heads were inoculated by dipping in 0.02% Tween 20 water containing 105 conidia/mL and covered with a plastic bag to maintain high humidity. Wheat heads were collected daily for 7 days post inoculation. Each day, six heads were collected and divided into 3 groups for RNA preparation.
For comparison of gene expression of GZ3636 or ∆tri14, wheat heads were dip-inoculated in a 105 conidia/mL suspension of GZ3636 or ∆tri14 spores containing 0.02% Tween 20, as described above. Nine wheat heads were treated per strain. Wheat heads were collected at 3 dpi, divided into three groups for each treatment, frozen in liquid nitrogen and stored at −80 °C.
RNA was extracted from lyophilized and pulverized tissues using Trizol combined with column purification and on column DNA digestion according to manufacture instructions (Thermo Fisher Scientific, Waltham, MA, USA). No DNA contamination in the samples was confirmed by qPCR. First-strand cDNA was synthesized, and RT-qPCR was conducted in a CFX96 Real time PCR Detection system (Bio-Rad, Hercules, CA, USA). Gene expression analyses were conducted with the 2−∆∆Ct method [20]. Expression of β-tubulin served as a reference control for normalization of gene expression values. TRI14 and β-tubulin primers are listed in Table S1.
2.5. Fungal Biomass Quantification
To assess the effect of TRI14 deletion on fungal growth in wheat, we quantified fungal DNA in inoculated wheat spikelets at 21 dpi. Genomic DNA was isolated from 70 mg grounded tissue for fungal biomass quantification [12]. Relative biomass of F. graminearum in the infected tissue was quantified by qPCR using TRI6 gene-specific primers and wheat GAPDH primers (Table S2). The relative amount of fungal DNA to wheat DNA (relative biomass) per sample was estimated using the Ct value of TRI6 relative to the corresponding Ct values of the wheat gene GAPDH, and using the 2−ΔΔCt method [20]. Three technical replicates for each sample were performed. Means from biological replicates were compared using one-way analysis of variance (ANOVA) (n = 8).
2.6. Trichothecene Production in Liquid Culture and in Planta
The trichothecene analog 15-ADON was measured after growth of GZ3639 and the ∆tri14 mutant in liquid agmatine media [21]. Cultures were grown in 20 mL for 7 days on a rotary shaker at 200 rpm in the dark at 28 °C and then extracted with 8 mL of ethyl acetate. Extracts were analyzed by gas chromatography–mass spectrometry (GC-MS) with a Hewlett Packard 6890 gas chromatograph fitted with an HP-5 MS column (30 m; 0.25 mm, 0.25 µm) and a 5973 mass detector. The carrier gas was helium with a 20:1 split ratio and a 20 mL/min split flow. The column was held at 150 °C for one minute following injection, heated to 280 °C at 30 °C/min and held for 3.7 min. Quantification of DON was performed using GC-MS methods [22].
The trichothecene DON was measured in wheat spikelets collected at 21 dpi from point inoculation and in wheat heads collected at 7 dpi from dip inoculation. Point-inoculated spikelets and dip inoculated heads were freeze-dried and extracted with acetonitrile/water (86:14). Extracts were then purified with Romer MycoSep 225 Trich cartridges and dried under a stream of nitrogen. TMS derivatives were prepared by adding 100 µL of a 100:1 freshly prepared mixture of N-trimethylsilylimadazole/trimethylchlorosilane to the dried extracts. Trimethylsilyl (TMS) derivatives of purified DON were prepared in the same way. DON concentrations were determined with GC-MS using the same temperature conditions as above, but with a splitless inlet and selective ion monitoring for the DON triTMS derivative.
2.7. ROS Assays
ROS assays were performed as described [23]. Briefly, wheat coleoptiles from 5-day-old wheat seedlings were cut into 2–3 mm fragments using a razorblade. A total of twelve pieces were treated with different elicitors on one plate. Each piece was placed in an individual well of a clear 96-well plate with 200 mL of water per well at room temperature and covered with aluminum foil. After an overnight period, the water was removed, and ROS production was detected in a solution containing 100 µg/mL crab chitin, 3 mg/mL laminarin or the combination the two elicitors, 20 mM L012, and 1 µg/mL of horseradish peroxidase (Sigma, St. Louis, MO, USA). The plate was run with twelve samples for each treatment. Luminescence was measured over a period of 120 min using the Synergy HT and Gen5 software (BioTek Instruments Inc. Winooski, VT, USA). Means from twelve readings for each treatment were calculated and compared. The assays were repeated at least three times with similar results.
2.8. Effect of Plant Defense Compounds on GZ3639 and ∆tri14 Growth
The effect of H2O2 and 2-benzoxazolinone (BOA) on the radial growth of GZ3639 and ∆tri14 was assessed on PDA plates containing 25 mM H2O2 or 0.5 mg/mL BOA. Stock solution of BOA (100 mg/mL, Sigma Chemical Co., St. Louis, MO, USA) was prepared in ethanol. Plates without H2O2 or BOA were used as controls. Each plate was inoculated with a plug from a V8 plate.
Inoculum plugs were taken from the advancing margins of growth on a V8 agar plate and placed at the center of a PDA plate. Plates inoculated with either GZ3639 or ∆tri14 were incubated in the dark at room temperature. At 5 days post inoculation, radial measurements were taken outward from the center of the plate in four directions along the lines from the edge of the inoculum to the advancing margin of the colony. Significant differences in mean radial growth were statistically assessed by one-way ANOVA in JMP.
The effect of H2O2 and BOA on the conidiation and growth of GZ3639 and ∆tri14 was evaluated in 96-well plates containing CMC liquid medium with or without either 1 mM or 2 mM H2O2 or 0.5 mg/mL BOA. Twelve wells per strain were inoculated with 1 × 105 macroconidia in 200 µL CMC and incubated in the dark at 23 °C. OD600 was measured every 2 h for 72 h using the Synergy HT plate reader and Gen5.304 software (BioTek Instruments, Inc. Winooski, VT, USA). At the end of 72 h, macroconidia in CMC medium were counted. Significant differences in OD600 and conidiation were statistically assessed by one-way ANOVA in JMP.
2.9. Tri14 Three-Dimensional (3D) Structure Prediction
The 3D protein structures of FgTri14 and its homologs from selected fungi were predicted using AlphaFold 2 installed on the SCINet Ceres high-performance computing cluster, essentially as described [24,25]. To identify proteins with a similar 3D structure, the predicted FgTri14 3D structure was used as query files to compare against all structures in NCBI’s Molecular Modeling Database using VAST (Vector Alignment Search Tool) [26].
2.10. Statistical Analysis
One-way ANOVA and Tukey–Kramer HSD tests were conducted using JMP Statistical Discovery Software version 15 (Cary, NC, USA).
3. Results
3.1. TRI14 Is Conserved among Trichothecene-Producing Fungi
We used BLAST analysis to assess the distribution of TRI genes in fungi. This analysis detected TRI cluster orthologs in species from 13 fungal genera in three fungal classes: Dothideomycetes, Eurotiomycetes, and Sordariomycetes (Figure 1A). Although cluster orthologs in species from 11 of these genera have been reported previously [1], as far as we are aware, the cluster orthologs in Calonectria aciculate, C. fujianensis and Endocarpon pusillum have not been reported previously. In species of all 13 genera, TRI cluster orthologs consisted of 2–15 known TRI genes, but species with clusters consisting of 2–3 genes had a second locus with one or more additional TRI genes. Several species had a TRI gene that was not clustered with other TRI genes. This included the E. pusillum TRI3 ortholog and, as previously reported, the F. graminearum TRI1 and TRI101 orthologs and the Trichoderma TRI5 orthologs [6]. In comparisons of deduced amino acid sequences of TRI gene orthologs from F. graminearum and non-Fusarium species shown in Figure 1, TRI3 (42–73%) and TRI6 (41–68%) orthologs tended to have the lowest percent identities, whereas TRI4 (56–86%), TRI11 (67–85%), and TRI14 (55–83%) orthologs tended to have the highest percent identities (Table S1). TRI3, TRI5 and TRI14 were the only TRI genes common to all species with a TRI cluster.
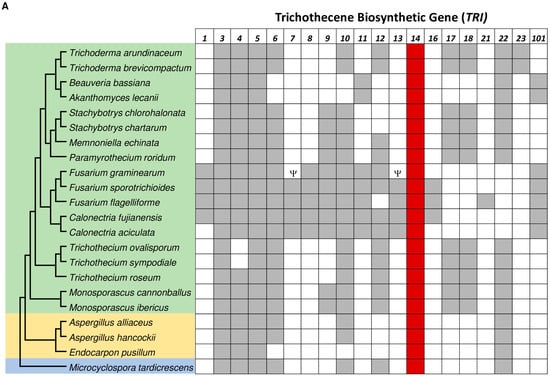
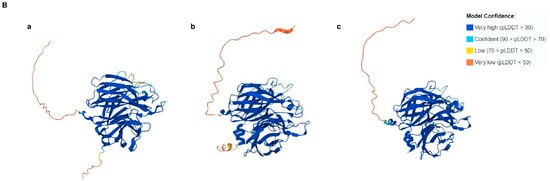
Figure 1.
(A) Distribution of trichothecene biosynthetic (TRI) genes in representative species of 13 fungal genera. The tree to the left was inferred from six housekeeping genes (DPA1, FAS1, RPB1, RPB2, TEF1, and TUB2). The colored shading indicates the fungal class to which the species belong: blue indicates Dothideomycetes; orange indicates Eurotiomycetes; and green indicates Sordariomycetes. The grid to the right indicates whether an ortholog of the 21 known TRI genes were detected by BLASTn analysis of a genome sequence for each fungal species in the tree. Tri14 is shaded in red. The Greek letter Psi (ψ) indicates the corresponding gene can be nonfunctional. In some previous studies on TRI genes, the genus names Cordyceps, Myrothecium, or Spicellum were used instead of Akanthomyces, Paramyrothecium or Trichothecium, respectively [1,6,18]. (B) Three-dimensional structures of Tri14 proteins predicted by AlphaFold. (a) FgTri14; (b) F. sporotrichioides; (c) Trichoderma arundinaceum.
The critical roles of TRI3 and TRI5 in trichothecene biosynthesis provide an explanation for why they are conserved in known and presumed trichothecene-producing fungi. Given that TRI14 is not required for trichothecene biosynthesis in F. graminearum and F. sporotrichioides cultures, the reason for its conservation is not clear. To begin to address this issue, we searched for TRI14 orthologs located outside of a TRI cluster. To do this, we did BLASTp analysis in which we used the predicted amino acid sequences of the F. graminearum and T. arundinaceum TRI14-encoded proteins (Tri14) to query the NCBI fungal protein database, but we excluded Tri14 proteins encoded by genes located in a TRI cluster. This analysis revealed only one gene that was closely related to TRI14 but not in a TRI cluster. The gene (GenBank accession PGH23486) was from Polytolypa hystricis and shared 53% and 59% amino acid sequence identity with the F. graminearum and T. arundinaceum Tri14 orthologs. The function of this gene has not been described, and it does not appear to be located within another secondary metabolite biosynthetic gene cluster in that is not located within 30 kb of genes encoding a terpene synthase, polyketide synthase or nonribosomal peptide synthase gene. The highest percent identities of the other 50 highest scoring hits in the BLASTp analysis shared only 32–38% amino acid sequence identity with the F. graminearum and T. arundinaceum Tri14 orthologs.
3.2. Tri14 Orthologs Share Conserved Three-Dimensional Structures
To gain further insight of the function of TRI14, we analyzed the predicted three-dimensional (3D) structures of selected Tri14 orthologs from trichothecene-producing fungi, including the F. graminearum FgTri14, using the program AlphaFold (Figure 1B). BLASTp analysis of the FgTri14 protein against the NCBI protein database did not identify any known functional domains. In contrast, a VAST search of the predicted FgTri14 3D protein structure against the solved 3D structures in the NCBI Molecular Modeling Database identified sialidases (Protein Data Base (PDB ID: 2XZJ) [27] and arabinofuranosidases (PDB ID: 2W5N) that cleave terminal glycoside residues of arabinosides [28] as structure neighbors. Sialidases, a member of the glycosyl hydrolase (GH) family 3 (GH3), 34, 58, 83, and 156, cleave the terminal sialic acid residues in sugars, proteins, and lipids. Bacterial sialidases have been reported to be involved in pathogenesis and nutrition [29]. FgTri14 is also a structural neighbor of the major allergen Mala s 1 of the yeast Malassezia sympodial (PDB ID: 2P9W) [30]. AlphaFold prediction and VAST search of the other Tri14 proteins yielded similar results. The similar 3D structures predicted by AlphaFold indicate that Tri14 proteins from different fungi share a conserved function.
3.3. TRI14 Deletion Does Not Affect DON Production of F. graminearum in Liquid Cultures
In a previous study, TRI14 deletion mutants of F. graminearum produced trichothecenes on a complex substrate, i.e., cracked maize kernel medium [11]. Here, we examined the effect of TRI14 deletion on trichothecene production in agmatine medium, a defined liquid medium that induces trichothecene production in Fusarium species [21]. We found that two previously generated TRI14 deletion mutants (∆tri14-27 and ∆tri14-50) and their wild-type progenitor strain, GZ3639 (hereafter wild type), produced similar levels of the trichothecene analog 15-acetyldeoxynivalenol (15-ADON) in agmatine medium (Figure 2), providing additional evidence that TRI14 is not required for trichothecene production in culture.
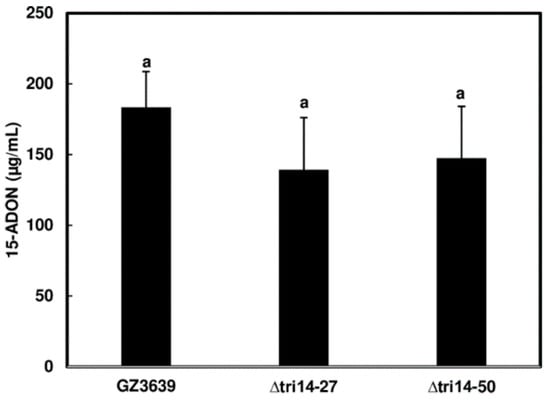
Figure 2.
Trichothecene produced by TRI14 deletion mutants (∆tri14-27 and -50) and wild-type parent strain (GZ3639). Three replicate cultures of each strain were grown for 7 days in liquid agmatine medium. Culture extracts were examined by GC-MS for 15-acetyldeoxynivalenol (15-ADON), and the resulting data were analyzed by one-way ANOVA and Tukey–Kramer HSD tests using JMP (n = 3).
3.4. TRI14 Expression Is Highly Induced during Wheat Head Infection
To assess the role of TRI14 during wheat infection, we examined its expression in F. graminearum-inoculated wheat heads over seven days. Heads of cultivar Norm were inoculated by dipping the whole head at anthesis in a macroconidia suspension of the wild-type F. graminearum strain. The heads were collected daily from 1 to 7 days after inoculation for gene expression analyses. We found that the expression of TRI14 was much higher than TRI5 from day 2 to 7 and peaked at day 3 (Figure 3). The high levels of TRI14 expression observed here indicates that TRI14 plays an important role during F. graminearum infection of wheat.
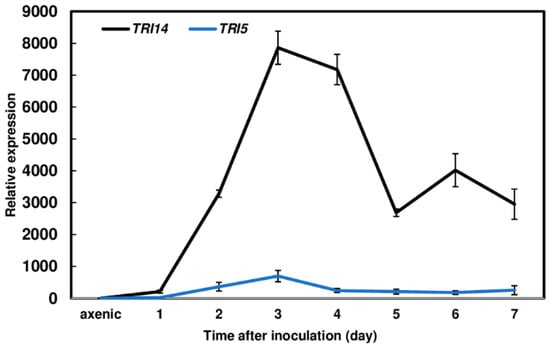
Figure 3.
Induction of TRI14 in F. graminearum-inoculated wheat heads. Heads of wheat cultivar Norm were inoculated by immersion in a macroconidial suspension of wild-type F. graminearum (105 conidia/mL in 0.02% Tween 20). Heads were collected daily for 7 days post inoculation. Three biological replicates, with two heads per replicate, were collected at each time point for RNA isolation. Gene expression was determined by RT-qPCR. β-tubulin expression served as an internal control to normalize gene expression. Fold changes in gene expression were relative to expression levels determined for a 7-day axenic F. graminearum wild-type strain grown on V8 Juice agar, which was set as one. Three technical replicates were conducted for each sample from three biological replicates at each time points.
3.5. ∆tri14 Has Restricted FHB Symptoms, Less Growth and DON Production in the Inoculated Spikelets
Dyer et al. [11] found that although TRI14 deletion did not affect DON production in cracked maize kernel cultures, the ∆tri14-27 and -50 deletion mutants caused little FHB spread on susceptible wheat cultivars Wheaton and Norm. In addition, Dyer et al. did not detect DON in seeds from inoculated heads. In the current study, we reevaluated the role of TRI14 in wheat cultivar Norm using mutant strain ∆tri14-50 (hereafter ∆tri14) by inoculating the fifth spikelet from the top of the wheat head. In addition to assessing FHB symptoms and DON contamination, we examined F. graminearum biomass in inoculated spikelets. FHB symptoms on ∆tri14-inoculated heads were restricted to the inoculated spikelets, whereas symptoms on wild-type-inoculated heads spread from the inoculated spikelet to most other spikelets (Figure 4A,B). Analysis of F. graminearum biomass in inoculated spikelets revealed significantly less biomass in ∆tri14-inoculated spikelets compared to wild-type-inoculated spikelets (Figure 4C). GC-MS analysis revealed that the amount of DON was significantly less in Δtri14-inoculated than wild-type-inoculated spikelets (Figure 4D). These data indicate that TRI14 is required for F. graminearum spread and growth in wheat head. Although DON levels were lower in ∆tri14-inoculated than wild-type-inoculated spikelets in the current study, these contrast the results of Dyer et al. [11], who did not detect DON in seeds from ∆tri14-inoculated heads.
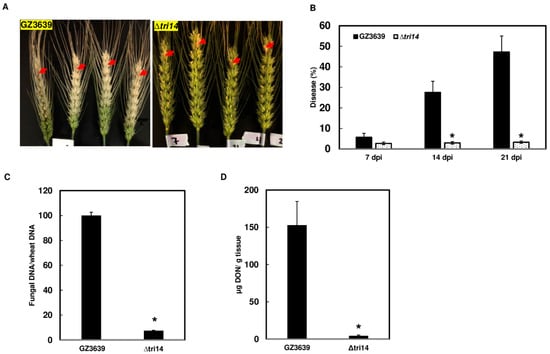
Figure 4.
Deletion of TRI14 restricts FHB spread and reduces fungal biomass and DON contents in inoculated spikelets. (A) Spikelets of wheat cultivar Norm were point inoculated with conidia suspensions of ∆tri14 or the wild-type parent strain GZ3639 (10 µL, 105 conidia/mL). Photographs were taken at 21 days post inoculation (dpi). Red arrows mark the inoculated spikelets, which were collected for DON and fungal biomass quantification. (B) The percentage of diseased spikelets at 7, 14, and 21 dpi. (C) Fungal biomass in inoculated wheat spikelets at 21 dpi. Fungal biomass was quantified by qPCR by calculating TRI6 Ct value relative to wheat TaGAPDH Ct value. Bars represents percentage of fungal biomass in heads inoculated with ∆tri14 relative to those inoculated with GZ3639. (D) DON content in inoculated wheat spikelets at 21 dpi. Asterisk (*) indicates significant difference at the p < 0.05 confidence level. One-way ANOVA and Tukey–Kramer HSD (n = 24 for disease, and n = 8 for biomass and toxin) tests were conducted using JMP.
3.6. ∆tri14 Has Reduced Growth and DON Production in Dip-Inoculated Wheat Heads
DON is not required for the initial infection process [13]. To assess whether TRI14 affects initial infection, a dip-inoculation assay was performed on whole wheat heads and FHB levels were compared at 5 dpi. This time point was selected because FHB severity may be potentially caused by disease spread instead of initial infection, which are difficult to discern at a later time point. FHB severity was not significantly reduced in Δtri14-inoculated heads (29.3%) compared to wild-type-inoculated heads (39.9%). However, there was significantly less fungal biomass (p < 0.001) and DON (p < 0.001) in wheat heads inoculated with the Δtri14 compared to the wild type (Figure 5). These results indicate that TRI14 contributes to invasive growth of F. graminearum during initial infection, which may indirectly result in less DON contamination.
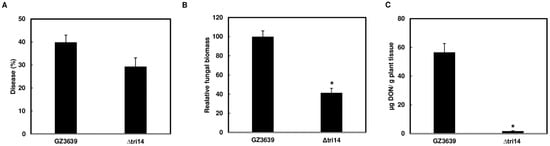
Figure 5.
Deletion of TRI14 reduces fungal biomass and DON in dip-inoculated wheat heads. Dip inoculations were performed by immersing whole heads of wheat cv. Norm in suspensions of ∆tri14 or the wild-type parent strain GZ3639 (5 × 104 conidia/mL). (A) FHB was scored as the percentage of spikelets with visual symptoms at 5 days post inoculation (dpi). (B) Fungal biomass in inoculated wheat heads at 5 dpi. Fungal biomass was quantified by qPCR by calculating TRI6 Ct value relative to wheat TaGAPDH Ct value. Bars represents percentage of fungal biomass in heads inoculated with ∆tri14 relative to those inoculated with GZ3639. (C) DON content in inoculated wheat heads at 5 dpi. Asterisk (*) indicates significant difference at the p < 0.05 confidence level. One-way ANOVA and Tukey–Kramer HSD (n = 22 for disease, and n = 7 for biomass and DON) tests were conducted using JMP.
3.7. Deletion of TRI14 Does Not Affect TRI Cluster Gene Expression
To determine that the lower amount of DON in Δtri14-inoculated versus wild-type-inoculated heads was due to less growth or TRI gene expression of F. graminearum in planta, we examined the expression of nine TRI genes, including TRI14, in wheat heads three days after dip inoculation with ∆tri14 or the wild type. The 3-dpi time point was selected for gene expression comparison because a prior study reported that the induction of TRI genes peaked 2–4 days after wheat head infection [31], and we also found TRI14 was highly induced at 3 dpi. As expected, TRI14 transcripts were not detected in wheat heads inoculated with Δtri14. In contrast, TRI14 was highly expressed in wild-type-inoculated heads (Figure 6A). The expression of the eight other TRI genes was similar in Δtri14- and wild type-inoculated heads (Figure 6B). These data indicate that the reduced DON production observed in Δtri14-infected wheat heads does not directly result from altered TRI gene expression, but rather is a consequence of less fungal growth, as indicated above.
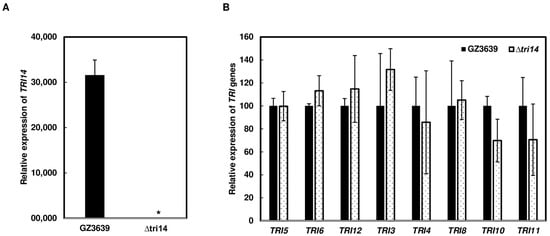
Figure 6.
Comparison of TRI gene expression in wheat heads inoculated with GZ3639 and ∆tri14. (A) TRI14. (B) TRI genes (eight): TRI5, TRI6, TRI12, TRI3, TRI4, TRI8, TRI10 and TRI11. Heads of wheat cultivar Norm were inoculated by immersing the entire head in a macroconidia suspension of GZ3639 or ∆tri14 (105 conidia/mL in 0.02% Tween 20). Three biological replicates, each replicate with three heads, were collected 3 days post inoculation (dpi) and used for RNA isolation. Gene expression was determined by RT-qPCR. Fungal β-tubulin was used as an internal control for transcript normalization. Relative gene expression was calculated using the 2−ΔΔCt method by setting the expression in inoculated heads as base (A) and the expression level in GZ3639 inoculated as base (B). Three technical replicates were conducted for each biological replicates. Asterisk (*) indicates significant difference at the p < 0.05 confidence level. One-way ANOVA and Tukey–Kramer HSD tests (n = 3) were conducted using JMP.
3.8. Deletion of TRI14 Reduces Fungal Growth and Lesions in Wheat Coleoptiles
To assess whether TRI14 affects the F. graminearum infection of wheat coleoptiles, we conducted coleoptile infection assays, essentially as previously described [19]. We found that, on average, ∆tri14 caused significantly smaller lesions on inoculated coleoptiles than the wild type (Figure 7A,B). In addition, there was significantly less F. graminearum biomass in ∆tri14-inoculated coleoptiles than in wild-type-inoculated coleoptiles (Figure 7C). These results indicate that TRI14 is required by F. graminearum to cause wild-type levels of disease and growth in wheat coleoptiles.
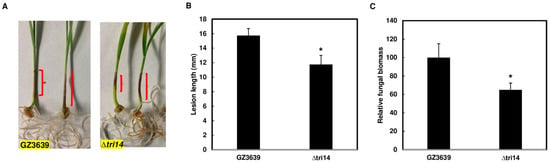
Figure 7.
TRI14 deletion mutant reduces lesion and fungal biomass in infected wheat coleoptiles. (A) Lesions on coleoptiles at 7 dpi infected by wild type (GZ3639) and ∆tri14. Red parentheses indicate lesion lengths on individual coleoptiles. (B) Average lesion length on coleoptiles caused by the wild-type and ∆tri14 strains. (C) Fungal biomass in coleoptiles inoculated with the ∆tri14 strain and wild-type strains. Bars represents percentage of fungal biomass in heads inoculated with ∆tri14 relative to those inoculated with GZ3639. Histograms in (B,C) indicate mean values from 20 coleoptiles. Error bars indicate standard error. Asterisks (*) denote significant difference at the confidence level (p < 0.05). One-way ANOVA and Tukey–Kramer HSD (n = 20) tests were conducted in JMP.
3.9. TRI14 Mutant Is More Sensitive to H2O2 under Certain Growth Conditions
Because TRI14 deletion resulted in the impaired growth of F. graminearum in three different wheat tissues—spikelets, heads and coleoptiles—we speculated that the Tri14 protein may be involved in interactions with plant defense responses such as reactive oxygen species (ROS). Our prior studies showed that chitin induced ROS in wheat head tissues, especially rachis and rachis nodes, that are critical for FHB spread [23,32]. Therefore, we examined whether chitin and laminarin induced a similar ROS production pattern in coleoptiles. A small ROS peak was induced by chitin in wheat coleoptiles whereas a higher ROS peak was induced by either laminarin or chitin plus laminarin in wheat coleoptiles (Figure S1). This suggests that wheat coleoptiles have a similar ROS response pattern to wheat heads when presented with these molecules. Therefore, we hypothesize that ∆tri14 may be more sensitive to wheat defense compounds such as hydrogen peroxide (H2O2), a key component of ROS. To support our hypothesis, we conducted studies to examine ∆tri14 sensitivity to H2O2. In the absence of H2O2, growth of Δtri14 and the wild type was similar on the PDA medium. Growth of Δtri14 in the presence of 25 mM H2O2 on solid PDA medium was reduced but was not significantly different from the wild type (Figure 8A,B). Growth of Δtri14 and the wild type was also tested in the presence of 5 and 10 mM H2O2 on PDA, but no significant difference was observed. In contrast, in CMC medium with 2 mM H2O2, Δtri14 grew significantly less than the wild type (Figure 8C). Despite this reduced growth, Δtri14 as well as the wild type produced similar abundant macroconidia in CMC medium with 2 mM H2O2 (Figure 8D). These observations indicate that TRI14 may provide protection from some plant defense compounds, such as exogenous ROS, under certain environmental or nutritional conditions.
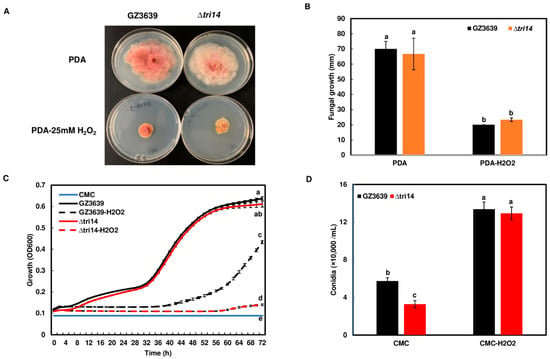
Figure 8.
H2O2 affects growth of ∆tri14 mutant. (A) Growth comparison of wild type and ∆tri14 on PDA plates containing 25 mM H2O2 at five days post inoculation. (B) H2O2 similarly inhibited growth of wild type and ∆tri14. Values are averages of four biological replicates. (C) Comparison of growth of wild type and ∆tri14 in CMC medium containing 2 mM H2O2. (D) Comparison of conidiation of wild type and ∆tri14 in CMC medium containing 2 mM H2O2 after 72 h incubation. Values are averages of 12 biological replicates. One-way ANOVA and Tukey–Kramer HSD tests of means were conducted in JMP. Different letters indicate significant difference with p < 0.05 confidence.
In addition to ROS, the antifungal benzoxazinoid compounds (e.g., 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one (DIMBOA) and 2-benzoxazolinone (BOA) were reported to inhibit fungal growth [33,34]. Therefore, we also examined the growth of F. graminearum on PDA and in CMC in the presence of antifungal compound BOA, which is produced by wheat and some other cereal crops [35]. Growth of Δtri14 and the wild type were inhibited to similar extents on PDA or in CMC media containing 0.5 mg/mL BOA (Figure S2), indicating that TRI14 does not confer protection from BOA.
4. Discussion
F. graminearum primarily infects wheat head through floral tissues during flowering. After infection, F. graminearum grows epiphytically on wheat plant surfaces, forming specialized unbranched hyphae (runner hyphae). The runner hyphae develop multicellular complex appressoria called infection cushions, which generate multiple penetration sites and assist the fungus to enter the plant cuticle [36]. In addition, F. graminearum directly penetrates stomata and underlying parenchyma, the opening of anthers and the openings between the lemma [37]. Once established, F. graminearum can spread to other spikelet on the same head by producing trichothecenes, including DON and NX3.
Previous studies have indicated that TRI14 has contradictory characteristics. On the one hand, it occurs widely in TRI cluster orthologs [6,8], suggesting an important and conserved role for this gene in the fungi with a TRI cluster (Figure 1A), but on the other hand, it is not required for trichothecene production in cultures of F. graminearum or F. sporotrichioides [11]. Furthermore, TRI14 mutants of F. graminearum were markedly reduced in their ability to cause FHB spread on wheat heads and they did not produce detectable levels of DON in seeds harvested from wheat heads inoculated with the mutants [11]. Therefore, we initiated the current study to address some of the knowledge gaps left by the previously reported analyses of TRI14. First, we reassessed the distribution of TRI14 among fungi with a TRI cluster. We examined the gene content of TRI loci in 22 species representing three fungal classes, including species in which a TRI cluster has not been described previously. Second, we investigated the effect of TRI14 on the ability of F. graminearum to cause FHB of wheat by conducting dip inoculation to assess initial infection and conducting point inoculation to assess the spikelet-to-spikelet spread of F. graminearum. This comparison was facilitated by measuring the FHB symptoms, DON content, fungal biomass, and TRI gene expression in wheat heads inoculated with either the F. graminearum ∆tri14 or its wild-type parent strain.
FHB symptoms caused by F. graminearum mutants, such as TRI5 mutants that lack the ability to synthesize DON, are restricted to inoculated wheat spikelets [4,5]. DON enables the fungus to pass through the wheat rachis node and spread within a wheat head, likely because DON inhibits eukaryotic protein synthesis [2]. Like TRI5 mutants, ∆tri14 caused restricted FHB symptoms in inoculated Norm spikelets (Figure 3). These observations led us to speculate that TRI14 may interfere with DON biosynthesis in planta only. Indeed, in the current study, we detected low levels of DON in ∆tri14 inoculated wheat heads by dip or point inoculations. However, we also found that ∆tri14 had reduced fungal growth in different wheat tissues including spikelets, heads, and coleoptiles, regardless of the role DON plays during F. graminearum infecting these tissues. Multiple studies have demonstrated DON is not involved in initial infection when inoculated by whole head dip inoculation or coleoptile infection [13,19]. Therefore, we suspect that the decrease in FHB and DON in ∆tri14-infected wheat is primarily due to slow or limited fungal growth in planta. However, we could not rule out that less ∆tri14 growth may be due to less DON production, which plays a complicated role during F. graminearum and wheat interactions. At low concentrations, DON elicits H2O2 generation, cell death and defense gene activation when infiltrated in wheat leaves [38]. However, at high concentrations, DON might assist F. graminearum growth during the necrotrophic stage [38]. Further investigations are needed to illustrate the mode of action of Tri14 in Fusarium pathogenesis.
Based on the finding that seeds from ∆tri14 mutant-inoculated heads lacked detectable levels of DON, Dyer et al. [11] speculated that TRI14 might regulate trichothecene production in planta. The results of the current study indicate that although DON levels in the ∆tri14-inoculated wheat heads or spikelets were markedly reduced compared to the wild type, the TRI gene cluster was expressed similarly in wheat heads inoculated with ∆tri14 or the wild type (Figure 6), indicating that TRI14 did not regulate the other TRI genes at the transcriptional level as suggested. The expression of TRI14 and other genes within the TRI cluster is regulated by TRI6 in infected wheat heads [39]. However, it is possible that Tri14 protein could interact with other proteins encoded by TRI genes or virulence genes. Proteomics comparison in wheat infected with ∆tri14 or the wild type will assist in shedding light on the function of Tri14 in F. graminearum and wheat interactions.
Our gene expression analysis showed that TRI14 was highly induced in infected wheat head (Figure 2). It is worth noting that TRI14 is one of the TRI genes that is not expressed in dead wheat heads inoculated with F. graminearum [40]. Therefore, we suspect that the expression of TRI14 is induced under certain conditions, such as biotic or abiotic stress. Many studies have shown that ROS play important roles during pathogen and plant interactions. In addition to direct suppression of pathogen growth, ROS could function as cellular signaling molecules to trigger plant defense responses, such as cell wall strengthening, hormone synthesis and programmed cell death [41]. We demonstrated that chitin, a key component of fungal cell walls, triggers tissue-specific ROS responses in wheat tissues, especially in the rachis and rachis nodes, the tissues critical for F. graminearum spread within a wheat head [23]. Our recent study showed that the combination of chitin and laminarin induce stronger ROS responses in wheat head tissues [32]. Similar to the restricted FHB symptoms in ∆tri14-inoculated wheat heads, ∆tri14 caused significantly smaller lesions in wheat coleoptiles. Here, we found that chitin and laminarin synergistically induced high ROS responses in wheat coleoptiles; a similar response was observed in previous studies in wheat head tissues (Figure S1). This indicates that TRI14 may protect F. graminearum in wheat by mitigating plant ROS. In support of this hypothesis, we found that H2O2 inhibited ∆tri14 growth in CMC medium significantly more than that of the wild type (Figure 8C). The key role of ROS in F. graminearum–wheat interactions was recently highlighted in studies of the ROS-generating enzyme NoxD (NADPH-dependent oxidase) [42]. NoxD is required for virulence both in coleoptiles and wheat heads, which appears partially due to less DON production in the presence of H2O2 [42]. A role for ROS in F. graminearum pathogenesis is also supported by the presence of over 30 putative peroxidase genes in F. graminearum, of which at least four peroxidase mutants increase sensitivity to extracellular H2O2 [43]. Loss of TRI14 increased H2O2 sensitivity in CMC medium (Figure 8), suggesting that TRI14 is involved in protecting F. graminearum growth from oxidative stress. However, H2O2 increased the conidiation of both ∆tri14 and the wild type without a significant difference between them (Figure 8D). It is intriguing that ∆tri14 growth was only inhibited by H2O2 in CMC media but not in PDA. Less ∆tri14 growth in wheat may be partially due to increased sensitivity to extracellular H2O2 pressure. Further investigations are needed to confirm the hypothesis.
We speculate that TRI14 could support H2O2 tolerance by scavenging external ROS, either nonenzymatically via small molecules, or enzymatically, thereby protecting the fungus from the plant defensive oxidative burst [44]. AlphaFold revealed that Tri14 shares a six-bladed β-propeller structure also found in sialidases that cleave sialic acid from glycan including glycoproteins and glycolipids and play an important role in some bacterial pathogenesis [29]. Products released from plants by Tri14 in a similar fashion could directly affect ROS production or serve as secondary messengers to affect plant ROS burst or defense responses. Transcriptome studies of F. graminearum infected coleoptiles recorded an increased expression of fungal genes encoding extracellular ROS scavenging enzymes. Alternatively, the loss of TRI14 in the presence of increased extracellular ROS could cause intracellular levels of ROS to rise and negatively impact fungal growth. For example, TRI14 could influence the expression or function of fungal enzymes that help mitigate changes in external and/or internal levels of ROS. Since AlphaFold predicted that Tri14 proteins from different fungi share similar 3D structures, it will be interesting to determine if Tri14 is involved with in-planta growth in other fungi containing a TRI cluster.
In conclusion, our findings demonstrate that TRI14 positively impacts the pathogenesis of F. graminearum in wheat heads and coleoptiles. By contrast, TRI14 does not appear to impact trichothecene production in culture or in planta, although assessments of production in planta are complicated by the severely restricted growth of ∆tri14 in wheat. The reduced growth in wheat and increased sensitivity to H2O2 of Δtri14 indicate that TRI14 may protect the fungus from plant defense responses, and thereby enhance the aggressiveness of the fungus in planta. Further examination of the role of Tri14 orthologs in the biology of trichothecene-producing fungi from other genera may provide additional insight into the function of the gene.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/applmicrobiol4020058/s1, Figure S1: Reactive oxygen species (ROS) induced in wheat coleoptiles treated with chitin, laminarin (Lam) and chitin plus laminarin; Figure S2: BOA has no effect on conidiation and growth of ∆tri14 mutant; Table S1: Percent identity of TRI genes in F. graminearum to their orthologs in other selected fungi with TRI clusters; Table S2: Primers used in this study.
Author Contributions
Conceptualization, G.H. and R.H.P.; methodology, G.H., R.H.P., N.A.R. and S.P.M.; software, G.H., R.H.P. and H.K.; validation, G.H. and N.A.R.; formal analysis, G.H. and N.A.R.; investigation, G.H. and N.A.R.; resources, G.H., R.H.P., T.A.N. and S.G.; data curation, G.H. and N.A.R.; writing—original draft preparation, G.H., R.H.P. and D.W.B.; writing—review and editing, G.H., R.H.P., N.A.R., D.W.B. and S.P.M.; visualization, G.H., R.H.P. and H.K.; supervision, G.H.; project administration, G.H.; funding acquisition, G.H. and R.H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the U.S. Department of Agriculture, Agricultural Research Service National Program for Food Safety, ARS project number 5010-11420-001-000D. This research used resources provided by the SCINet project of the USDA Agricultural Research Service, ARS project number 0500-00093-001-00-D.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank Helene Tiley, Thomas Usgaard, Jackson Edwards, Stephanie Folmar, and Christine Poppe for their excellent technical assistance. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Proctor, R.H.; McCormick, S.P.; Gutierrez, S. Genetic bases for variation in structure and biological activity of trichothecene toxins produced by diverse fungi. Appl. Microbiol. Biot. 2020, 104, 5185–5199. [Google Scholar] [CrossRef]
- Desjardins, A.E.; McCormick, S.P.; Appell, M. Structure-activity relationships of trichothecene toxins in an Arabidopsis thaliana leaf assay. J. Agric. Food Chem. 2007, 55, 6487–6492. [Google Scholar] [CrossRef]
- Wilson, W.; Dahl, B.; Nganje, W. Economic costs of Fusarium head blight, scab and deoxynivalenol. World Mycotoxin J. 2018, 11, 291–302. [Google Scholar] [CrossRef]
- Bai, G.H.; Desjardins, A.E.; Plattner, R.D. Deoxynivalenol-nonproducing fusarium graminearum causes initial infection, but does not cause disease spread in wheat spikes. Mycopathologia 2002, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef]
- Proctor, R.H.; McCormick, S.P.; Kim, H.S.; Cardoza, R.E.; Stanley, A.M.; Lindo, L.; Kelly, A.; Brown, D.W.; Lee, T.; Vaughan, M.M.; et al. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog. 2018, 14, e1006946. [Google Scholar] [CrossRef]
- Proctor, R.H.; Hao, G.; Kim, H.S.; Whitaker, B.K.; Laraba, I.; Vaughan, M.M.; McCormick, S.P. A Novel Trichothecene Toxin Phenotype Associated with Horizontal Gene Transfer and a Change in Gene Function in Fusarium. Toxins 2022, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Proctor, R.H.; Kim, H.S.; Brown, D.W.; Logrieco, A.F.; Amatulli, M.T.; Moretti, A.; Susca, A. Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genom. 2019, 20, 314. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Hohn, T.M.; Desjardins, A.E. Isolation and characterization of Tri3, a gene encoding 15-O- acetyltransferase from Fusarium sporotrichioides. Appl. Environ. Microbiol. 1996, 62, 353–359. [Google Scholar] [CrossRef]
- Alexander, N.J.; Proctor, R.H.; McCormick, S.P. Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 2009, 24, 198–215. [Google Scholar] [CrossRef]
- Dyer, R.B.; Plattner, R.D.; Kendra, D.F.; Brown, D.W. Fusarium graminearum TRI14 is required for high virulence and DON production on wheat but not for DON synthesis in vitro. J. Agric. Food Chem. 2005, 53, 9281–9287. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; McCormick, S.; Usgaard, T.; Tiley, H.; Vaughan, M.M. Characterization of three Fusarium graminearum effectors and their roles during Fusarium Head Blight. Front. Plant Sci. 2020, 11, 579553. [Google Scholar] [CrossRef]
- Jansen, C.; von Wettstein, D.; Schafer, W.; Kogel, K.H.; Felk, A.; Maier, F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef]
- Hao, G.; McCormick, S.; Tiley, H.; Gutierrez, S.; Yulfo-Soto, G.; Vaughan, M.M.; Ward, T.J. NX Trichothecenes Are Required for Fusarium graminearum Infection of Wheat. Mol. Plant Microbe Interact. 2023, 36, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Bowden, R.L.; Leslie, J.F. Nitrate non-utilizing mutants of Gibberella zeae and their use in determining vegetative compatibility. Exp. Mycol. 1992, 16, 308–315. [Google Scholar] [CrossRef]
- Tuite, J. Plant Pathological Methods: Fungi and Bacteria; Burgess Publishing Company: Minneapolis, MN, USA, 1969; 239p. [Google Scholar]
- Bai, G.-H.; Shaner, G. Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis. 1996, 80, 975–979. [Google Scholar] [CrossRef]
- Gutierrez, S.; McCormick, S.P.; Cardoza, R.E.; Kim, H.S.; Yugueros, L.L.; Vaughan, M.M.; Carro-Huerga, G.; Busman, M.; Saenz de Miera, L.E.; Jaklitsch, W.M.; et al. Distribution, function, and evolution of a gene essential for trichothecene toxin biosynthesis in Trichoderma. Front. Microbiol. 2021, 12, 791641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Jia, L.J.; Zhang, Y.; Jiang, G.; Li, X.; Zhang, D.; Tang, W.H. In planta stage-specific fungal gene profiling elucidates the molecular strategies of Fusarium graminearum growing inside wheat coleoptiles. Plant Cell 2012, 24, 5159–5176. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gardiner, D.M.; Kazan, K.; Manners, J.M. Novel genes of Fusarium graminearum that negatively regulate deoxynivalenol production and virulence. Mol. Plant-Microbe Interact. 2009, 22, 1588–1600. [Google Scholar] [CrossRef]
- Hao, G.; McCormick, S.; Vaughan, M.M.; Naumann, T.A.; Kim, H.S.; Proctor, R.; Kelly, A.; Ward, T.J. Fusarium graminearum arabinanase (Arb93B) enhances wheat head blight susceptibility by suppressing plant immunity. Mol. Plant Microbe Interact. 2019, 32, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Tiley, H.; McCormick, S. Chitin triggers tissue-specific immunity in wheat associated with Fusarium Head Blight. Front. Plant Sci. 2022, 13, 832502. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Naumann, T.A.; Chen, H.; Bai, G.; McCormick, S.; Kim, H.; Tian, B.; Trick, H.N.; Naldrett, M.J.; Proctor, R. Fusarium graminearum effector FgNls1 targets plant nuclei to induce wheat head blight. Mol. Plant Microbe Interact. 2023, 36, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Gibrat, J.F.; Madej, T.; Bryant, S.H. Surprising similarities in structure comparison. Curr. Opin. Struct. Biol. 1996, 6, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Telford, J.C.; Yeung, J.H.; Xu, G.; Kiefel, M.J.; Watts, A.G.; Hader, S.; Chan, J.; Bennet, A.J.; Moore, M.M.; Taylor, G.L. The Aspergillus fumigatus sialidase is a 3-deoxy-D-glycero-D-galacto-2-nonulosonic acid hydrolase (KDNase): Structural and mechanistic insights. J. Biol. Chem. 2011, 286, 10783–10792. [Google Scholar] [CrossRef] [PubMed]
- Carapito, R.; Imberty, A.; Jeltsch, J.M.; Byrns, S.C.; Tam, P.H.; Lowary, T.L.; Varrot, A.; Phalip, V. Molecular basis of arabinobio-hydrolase activity in phytopathogenic fungi: Crystal structure and catalytic mechanism of Fusarium graminearum GH93 exo-alpha-L-arabinanase. J. Biol. Chem. 2009, 284, 12285–12296. [Google Scholar] [CrossRef] [PubMed]
- Corfield, T. Bacterial sialidases--roles in pathogenicity and nutrition. Glycobiology 1992, 2, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmsson, M.; Zargari, A.; Crameri, R.; Rasool, O.; Achour, A.; Scheynius, A.; Hallberg, B.M. Crystal structure of the major Malassezia sympodialis allergen Mala s 1 reveals a beta-propeller fold: A novel fold among allergens. J. Mol. Biol. 2007, 369, 1079–1086. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Fernando, W.G. Comparative Analysis of Deoxynivalenol Biosynthesis Related Gene Expression among Different Chemotypes of Fusarium graminearum in Spring Wheat. Front. Microbiol. 2016, 7, 1229. [Google Scholar] [CrossRef]
- Hao, G.; Rhoades, N.A.; McCormick, S. Chitin and laminarin additively trigger wheat reactive oxygen species but not resistance to Fusarium head blight. Plant Direct 2023, 7, e538. [Google Scholar] [CrossRef]
- Walter, S.; Nicholson, P.; Doohan, F.M. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2010, 185, 54–66. [Google Scholar] [CrossRef]
- Glenn, A.E.; Hinton, D.M.; Yates, I.E.; Bacon, C.W. Detoxification of corn antimicrobial compounds as the basis for isolating Fusarium verticillioides and some other Fusarium species from corn. Appl. Environ. Microbiol. 2001, 67, 2973–2981. [Google Scholar] [CrossRef]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassao, D.G. Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef]
- Boenisch, M.J.; Schafer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Pritsch, C.; Muehlbauer, G.J.; Bushnell, W.R.; Somers, D.A.; Vance, C.P. Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum. Mol. Plant Microbe Interact. 2000, 13, 159–169. [Google Scholar] [CrossRef]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef]
- Seong, K.Y.; Pasquali, M.; Zhou, X.; Song, J.; Hilburn, K.; McCormick, S.; Dong, Y.; Xu, J.R.; Kistler, H.C. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 2009, 72, 354–367. [Google Scholar] [CrossRef]
- Boedi, S.; Berger, H.; Sieber, C.; Munsterkotter, M.; Maloku, I.; Warth, B.; Sulyok, M.; Lemmens, M.; Schuhmacher, R.; Guldener, U.; et al. Comparison of Fusarium graminearum Transcriptomes on Living or Dead Wheat Differentiates Substrate-Responsive and Defense-Responsive Genes. Front. Microbiol. 2016, 7, 1113. [Google Scholar] [CrossRef]
- Grant, J.J.; Loake, G.J. Role of reactive oxygen intermediates and cognate redox signaling in disease resistance. Plant Physiol. 2000, 124, 21–29. [Google Scholar] [CrossRef]
- Li, T.; Kim, D.; Lee, J. NADPH oxidase gene, FgNoxD, plays a critical role in development and virulence in Fusarium graminearum. Front. Microbiol. 2022, 13, 822682. [Google Scholar] [CrossRef]
- Lee, Y.; Son, H.; Shin, J.Y.; Choi, G.J.; Lee, Y.W. Genome-wide functional characterization of putative peroxidases in the head blight fungus Fusarium graminearum. Mol. Plant Pathol. 2018, 19, 715–730. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).