A Sublethal Concentration of Chlorine Induces Antibiotic Resistance in Salmonella via Production of Reactive Oxygen Species
Abstract
1. Introduction
2. Material and Methods
2.1. Microorganisms, Media, and Growth Conditions
2.2. Rifampicin-Based Selection Assay
2.3. Development of Antibiotic Resistance
2.4. Statistical Analysis
3. Results
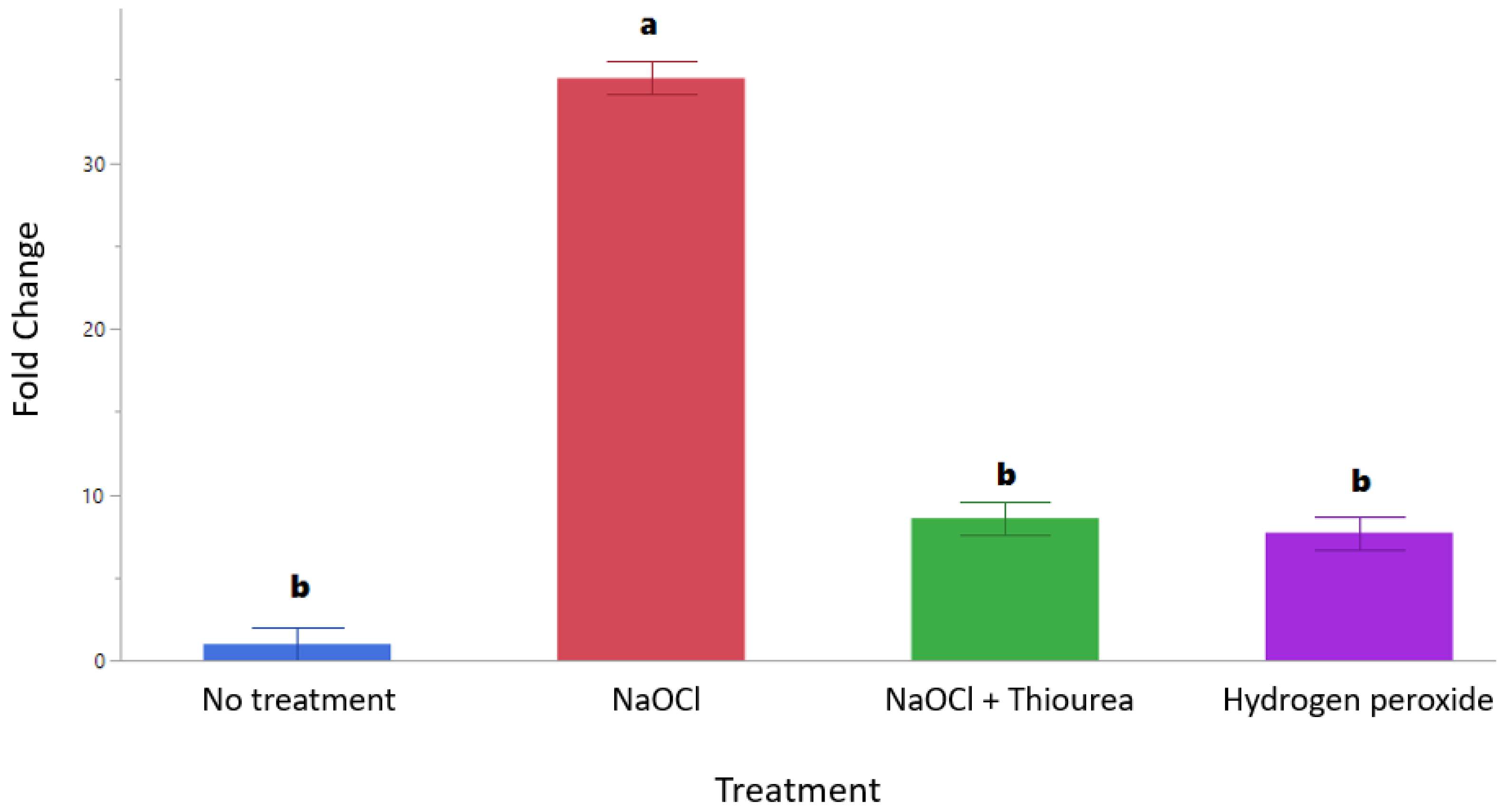
3.1. NaOCl Exposure and Mutation Rates
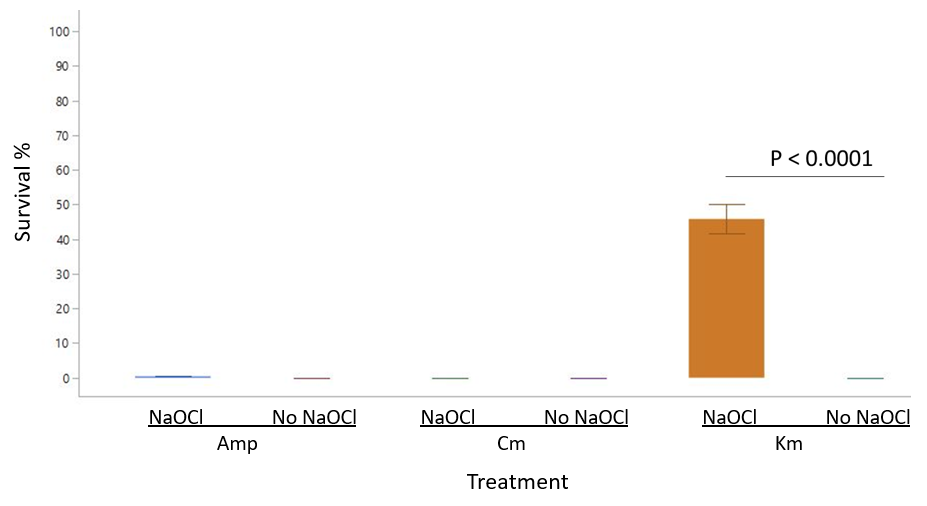
3.2. NaOCl Exposure and Resistance to Other Antibiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knodler, L.A.; Elfenbein, J.R. Salmonella enterica. Trends Microbiol. 2019, 27, 964–965. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.M.P.; Ferrari, R.G.; Conte-Junior, C.A. Virulence factors in Salmonella Typhimurium: The sagacity of a bacterium. Curr. Microbiol. 2019, 76, 762–773. [Google Scholar] [CrossRef]
- Fàbrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 102622. [Google Scholar] [CrossRef]
- Monack, D.M. Salmonella persistence and transmission strategies. Curr. Opin. Microbiol. 2012, 15, 100–107. [Google Scholar] [CrossRef]
- Bell, R.L.; Jarvis, K.G.; Ottesen, A.R.; McFarland, M.A.; Brown, E.W. Recent and emerging innovations in Salmonella detection: A food and environmental perspective. Microb. Biotechnol. 2016, 9, 279–292. [Google Scholar] [CrossRef]
- Lamas, A.; Miranda, J.M.; Regal, P.; Vázquez, B.; Franco, C.M.; Cepeda, A.A. comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol. Res. 2018, 206, 60–73. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Ricke, S.C.; Marcy, J.A. Public health impact of Salmonella spp. on raw poultry: Current concepts and future prospects in the United States. Food Control 2022, 132, 108539. [Google Scholar] [CrossRef]
- Logue, C.; Sherwood, J.; Olah, P.; Elijah, L.; Dockter, M. The incidence of antimicrobial-resistant Salmonella spp. on freshly processed poultry from US Midwestern processing plants. J. Appl. Microbiol. 2003, 94, 16–24. [Google Scholar] [CrossRef]
- Krüger, G.I.; Pardo-Esté, C.; Zepeda, P.; Olivares-Pacheco, J.; Galleguillos, N.; Suarez, M.; Castro-Severyn, J.; Alvarez-Thon, L.; Tello, M.; Valdes, J.H.; et al. Mobile genetic elements drive the multidrug resistance and spread of Salmonella serotypes along a poultry meat production line. Front. Microbiol. 2023, 14, 1072793. [Google Scholar] [CrossRef]
- McMillan, E.A.; Gupta, S.K.; Williams, L.E.; Jové, T.; Hiott, L.M.; Woodley, T.A.; Barrett, J.B.; Jackson, C.R.; Wasilenko, J.L.; Simmons, M.; et al. Antimicrobial resistance genes, cassettes, and plasmids present in Salmonella enterica associated with United States food animals. Front. Microbiol. 2019, 10, 440139. [Google Scholar] [CrossRef] [PubMed]
- McMillan, E.A.; Jackson, C.R.; Frye, J.G. Transferable plasmids of Salmonella enterica associated with antibiotic resistance genes. Front. Microbiol. 2020, 11, 562181. [Google Scholar] [CrossRef]
- Algarni, S.; Ricke, S.C.; Foley, S.L.; Han, J. The dynamics of the antimicrobial resistance mobilome of Salmonella enterica and related enteric bacteria. Front. Microbiol. 2022, 13, 859854. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef]
- Imlay, J.A. Where in the world do bacteria experience oxidative stress? Environ. Microbiol. 2019, 21, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef]
- Li, X.; Imlay, J.A. Improved measurements of scant hydrogen peroxide enable experiments that define its threshold of toxicity for Escherichia coli. Free Radic. Biol. Med. 2018, 120, 217–227. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Kohanski, M.A.; Collins, J.J. Role of reactive oxygen species in antibiotic action and resistance. Curr. Opin. Microbiol. 2009, 12, 482–489. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Wiegmann, K.; Farid, A.; Wolf, A.; Utermöhlen, O.; Krut, O.; Krönke, M.; Schramm, M. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci. Signal. 2019, 12, eaar5926. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Tsourouktsoglou, T.-D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 2017, 46, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Kohanski, M.A.; Hayete, B.; Collins, J.J. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 2007, 3, 91. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Reniere, M.L. Reduce, induce, thrive: Bacterial redox sensing during pathogenesis. J. Bacteriol. 2018, 200, e00128-18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.; Matic, I.; Tenaillon, O.; Clara, A.; Radman, M.; Fons, M.; Taddei, F. Costs and benefits of high mutation rates: Adaptive evolution of bacteria in the mouse gut. Science 2001, 291, 2606–2608. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Glandorf, D.C.M.; Brand, I.; Bakker, P.A.H.M.; Schippers, B. Stability of rifampicin resistance as a marker for root colonization studies of Pseudomonas putida in the field. Plant Soil 1992, 147, 135–142. [Google Scholar] [CrossRef]
- Novogrodsky, A.; Ravid, A.; Rubin, A.L.; Stenzel, K.H. Hydroxyl radical scavengers inhibit lymphocyte mitogenesis. Proc. Natl. Acad. Sci. USA 1982, 79, 1171–1174. [Google Scholar] [CrossRef]
- Repine, J.E.; Fox, R.B.; Berger, E.M. Hydrogen peroxide kills Staphylococcus aureus by reacting with Staphylococcal iron to form hydroxyl radical. J. Biol. Chem. 1981, 256, 7084–7096. [Google Scholar] [CrossRef]
- Touati, D.; Jacques, M.; Tardat, B.; Bouchard, L.; Despied, S. Lethal oxidative damage and mutagenensis are generated by iron in ∆fur mutants of superoxide dismutase. J. Bacteriol. 1995, 177, 2305–2314. [Google Scholar] [CrossRef]
- Dewachter, L.; Herpels, P.; Verstraeten, N.; Fauvart, M.; Michiels, J. Reactive oxygen species do not contribute to ObgE*-mediated programmed cell death. Sci. Rep. 2016, 6, 33723. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.; Soni, V.; Rhee, K.Y.; Helmann, J.D. Mutations in rpoB that confer rifampicin resistance can alter levels of peptidoglycan precursors and affect β-lactam susceptibility. mBio 2023, 14, e03168-22. [Google Scholar] [CrossRef] [PubMed]
- Karash, S.; Kwon, Y.M. Iron-dependent essential genes in Salmonella Typhimurium. BMC Genom. 2018, 19, 610. [Google Scholar] [CrossRef] [PubMed]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Pribis, J.P.; García-Villada, L.; Zhai, Y.; Lewin-Epstein, O.; Wang, A.Z.; Liu, J.; Xia, J.; Mei, Q.; Fitzgerald, D.M.; Bos, J. Gamblers: An antibiotic-induced evolvable cell subpopulation differentiated by reactive-oxygen-induced general stress response. Mol. Cell 2019, 74, 785–800.e7. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Jonker, M.J.; de Leeuw, W.; Brul, S.; Ter Kuile, B.H. Reactive oxygen species accelerate de novo acquisition of antibiotic resistance in E. coli. iScience 2023, 26, 108373. [Google Scholar] [CrossRef]
- Cahill, S.M.; Desmarchelier, P.; Fattori, V.; Bruno, A.; Cannavan, A. Global perspectives on antimicrobial resistance in the food chain. Food Prot. Trends 2017, 37, 353–360. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljuwayd, M.; Malli, I.A.; Ricke, S.C.; Kwon, Y.M. A Sublethal Concentration of Chlorine Induces Antibiotic Resistance in Salmonella via Production of Reactive Oxygen Species. Appl. Microbiol. 2024, 4, 745-752. https://doi.org/10.3390/applmicrobiol4020051
Aljuwayd M, Malli IA, Ricke SC, Kwon YM. A Sublethal Concentration of Chlorine Induces Antibiotic Resistance in Salmonella via Production of Reactive Oxygen Species. Applied Microbiology. 2024; 4(2):745-752. https://doi.org/10.3390/applmicrobiol4020051
Chicago/Turabian StyleAljuwayd, Mohammed, Israa Abdullah Malli, Steven C. Ricke, and Young Min Kwon. 2024. "A Sublethal Concentration of Chlorine Induces Antibiotic Resistance in Salmonella via Production of Reactive Oxygen Species" Applied Microbiology 4, no. 2: 745-752. https://doi.org/10.3390/applmicrobiol4020051
APA StyleAljuwayd, M., Malli, I. A., Ricke, S. C., & Kwon, Y. M. (2024). A Sublethal Concentration of Chlorine Induces Antibiotic Resistance in Salmonella via Production of Reactive Oxygen Species. Applied Microbiology, 4(2), 745-752. https://doi.org/10.3390/applmicrobiol4020051





