Reassessing Gout Management through the Lens of Gut Microbiota
Abstract
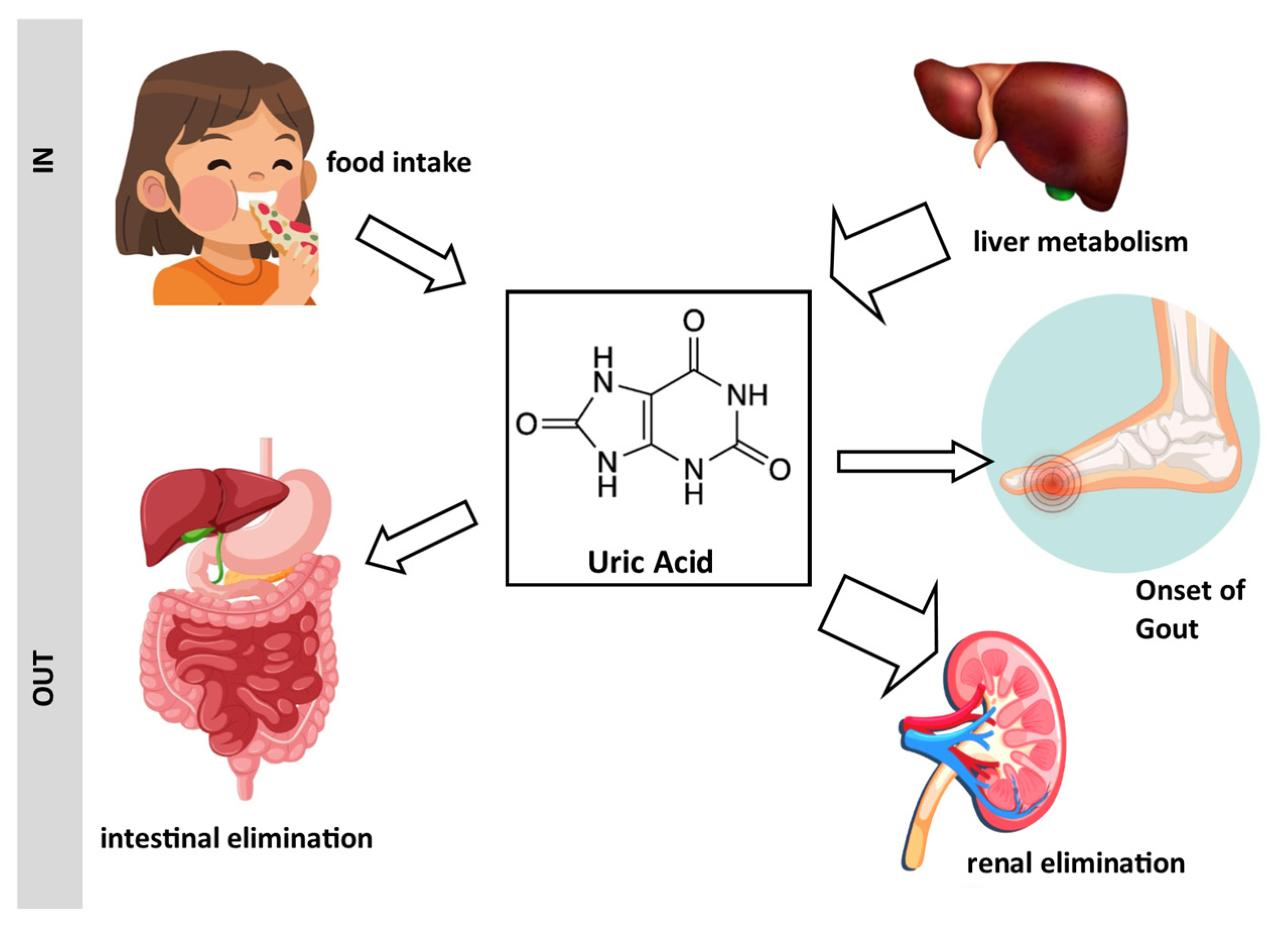
1. Introduction
- Firmicutes: Firmicutes are among the dominant bacterial phyla in the human gut. They include various genera, such as Clostridium, Lactobacillus, and Ruminococcus.
- Bacteroidetes: Bacteroidetes are another major bacterial phylum in the gut microbiota. Bacteroides is a prominent genus within this phylum.
- Actinobacteria: This phylum includes genera like Bifidobacterium, which are known for their beneficial roles in the gut, such as the fermentation of dietary fibers.
- Proteobacteria: This phylum consists of a diverse group of bacteria, including Escherichia coli (E. coli) and Helicobacter pylori.
- Verrucomicrobia: Although less abundant than Firmicutes and Bacteroidetes, Verrucomicrobia includes the genus Akkermansia, which has been associated with a healthy gut environment.
- Fusobacteria: This phylum is present in lower abundance and includes various species like Fusobacterium.
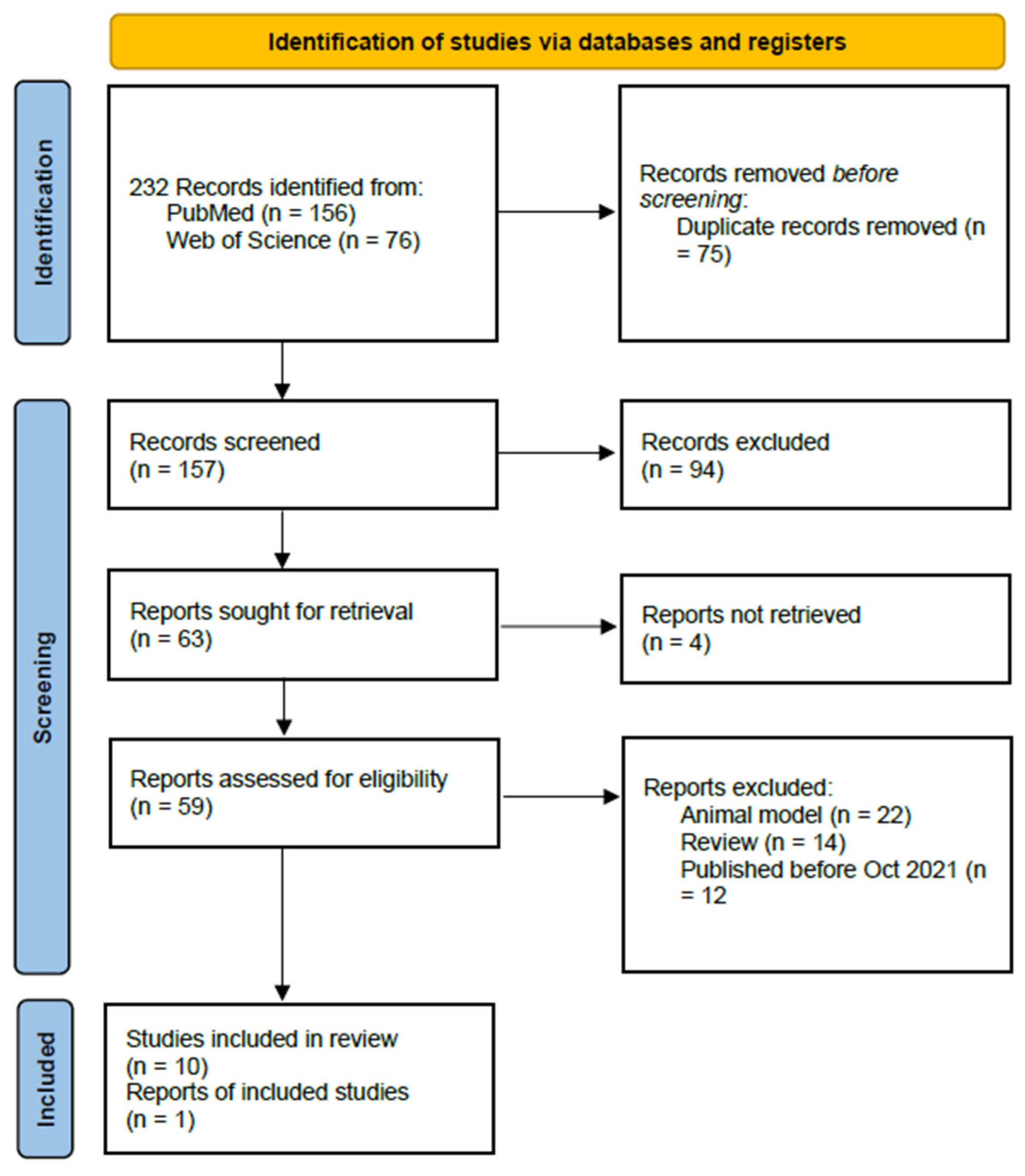
1.1. Database Search
1.2. Snowballing
2. Identification of Links between Gout and the Gut Microbiota
2.1. Analysis of Information Available until 2021
2.2. Update since 2021
2.2.1. Dysbiosis and Gout
2.2.2. Clinical Studies
2.2.3. FMT and Gout
3. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dehlin, M.; Jacobsson, L.; Roddy, E. Global epidemiology of gout: Prevalence, incidence, treatment patterns and risk factors. Nat. Rev. Rheumatol. 2020, 16, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.A.; Tan, S.; Parks, D.A. Uric Acid Metabolism. In Encyclopedia of Life Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Demarquoy, J.; Fairand, A.; Gautier, C.; Vaillant, R. Regulation of argininosuccinate synthetase level by corticosteroid and pancreatic hormones during perinatal period. Mol. Cell Biochem. 1995, 143, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M. New insights into the epidemiology of gout. Rheumatology 2009, 48 (Suppl. S2), ii2–ii8. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Aoyagi, Y.; Fukuuchi, T.; Inazawa, K.; Yamaoka, N. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biol. Pharm. Bull. 2014, 37, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Takayanagi, F.; Fukuuchi, T.; Yamaoka, N.; Yasuda, M.; Mawatari, K.I.; Fujimori, S. Determination of total purine and purine base content of 80 food products to aid nutritional therapy for gout and hyperuricemia. Nucleosides Nucleotides Nucleic Acids 2020, 39, 1449–1457. [Google Scholar] [CrossRef]
- Flores, N.M.; Nuevo, J.; Klein, A.B.; Baumgartner, S.; Morlock, R. The economic burden of uncontrolled gout: How controlling gout reduces cost. J. Med. Econ. 2019, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Helget, L.N.; Mikuls, T.R. Health disparities in gout. Curr. Opin. Rheumatol. 2024, 36, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; You, C. The biomarkers discovery of hyperuricemia and gout: Proteomics and metabolomics. PeerJ 2023, 11, e14554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jarman, J.B.; Low, Y.S.; Augustijn, H.E.; Huang, S.; Chen, H.; DeFeo, M.E.; Sekiba, K.; Hou, B.H.; Meng, X.; et al. A widely distributed gene cluster compensates for uricase loss in hominids. Cell 2023, 186, 4472–4473. [Google Scholar] [CrossRef]
- Tong, S.; Zhang, P.; Cheng, Q.; Chen, M.; Chen, X.; Wang, Z.; Lu, X.; Wu, H. The role of gut microbiota in gout: Is gut microbiota a potential target for gout treatment. Front. Cell Infect. Microbiol. 2022, 12, 1051682. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Asquith, M.J. The Microbiome: A Revolution in Treatment for Rheumatic Diseases? Curr. Rheumatol. Rep. 2016, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Sun, S.; Huang, Y.; Gao, Q.; Xie, X.; Wang, P.; Li, J.; Liang, L.; He, X.; Jiang, Y.; et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. NPJ Biofilms Microbiomes 2021, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Luna, A.J.; Carlson, T.J.; Garey, K.W. Gut microbiota changes associated with. Gut Microbes 2023, 15, 2223345. [Google Scholar] [CrossRef] [PubMed]
- Demarquoy, J.; Othman, H.; Demarquoy, C. Modify gut microbiome in autism: A promising strategy? Explor. Neurosci. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef] [PubMed]
- Pianta, A.; Arvikar, S.; Strle, K.; Drouin, E.E.; Wang, Q.; Costello, C.E.; Steere, A.C. Evidence of the Immune Relevance of Prevotella copri, a Gut Microbe, in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2017, 69, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Klinger, C.N.; Felix, K.M.; Bradley, C.P.; Wu, E.; Tran, N.L.; Umesaki, Y.; Wu, H.J. Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity 2016, 44, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yan, Y.; Webb, R.J.; Li, Y.; Mehrabani, S.; Xin, B.; Sun, X.; Wang, Y.; Mazidi, M. Psychological Stress and Gut Microbiota Composition: A Systematic Review of Human Studies. Neuropsychobiology 2023, 82, 247–262. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Shirvani-Rad, S.; Khatibzade-Nasari, N.; Ejtahed, H.S.; Larijani, B. Exploring the role of gut microbiota dysbiosis in gout pathogenesis: A systematic review. Front. Med. 2023, 10, 1163778. [Google Scholar] [CrossRef]
- Wohlin, C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering (EASE’14), London, UK, 12–14 May 2014; Association for Computing Machinery: New York, NY, USA, 2014; pp. 1–10. [Google Scholar]
- Wei, J.; Zhang, Y.; Dalbeth, N.; Terkeltaub, R.; Yang, T.; Wang, Y.; Yang, Z.; Li, J.; Wu, Z.; Zeng, C.; et al. Association Between Gut Microbiota and Elevated Serum Urate in Two Independent Cohorts. Arthritis Rheumatol. 2022, 74, 682–691. [Google Scholar] [CrossRef]
- Cao, C.; Jin, X.; Ding, Q.; Zhu, J.; Yang, D.; Fan, B. The altered composition of gut microbiota and biochemical features as well as dietary patterns in a southern Chinese population with recurrent renal calcium oxalate stones. Urolithiasis 2023, 51, 95. [Google Scholar] [CrossRef]
- Wang, M.; Fan, J.; Huang, Z.; Zhou, D.; Wang, X. Causal Relationship between Gut Microbiota and Gout: A Two-Sample Mendelian Randomization Study. Nutrients 2023, 15, 4260. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Garranzo, M.; Segura, J.; Orgaz, B.; Arroyo, R.; Alba, C.; Beltrán, D.; Fernández, L. A randomized pilot trial assessing the reduction of gout episodes in hyperuricemic patients by oral administration of. Front. Microbiol. 2023, 14, 1111652. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Yao, X.; Yan, Q.; Li, S.; Zhong, Q.; Liu, Z.; Tang, F.; Liu, C.; Li, H.; et al. Characterizations of the multi-kingdom gut microbiota in Chinese patients with gouty arthritis. BMC Microbiol. 2023, 23, 363. [Google Scholar] [CrossRef]
- Kim, H.W.; Yoon, E.J.; Jeong, S.H.; Park, M.C. Distinct Gut Microbiota in Patients with Asymptomatic Hyperuricemia: A Potential Protector against Gout Development. Yonsei Med. J. 2022, 63, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Dai, H.; Wang, Q.; Hou, Y.; Zhang, X.; Lin, H.; Wang, S.; Li, M.; Zhao, Z.; Lu, J.; et al. Dissecting the causal effect between gut microbiota, DHA, and urate metabolism: A large-scale bidirectional Mendelian randomization. Front. Immunol. 2023, 14, 1148591. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Nava, G.A.; Méndez-Salazar, E.O.; Vázquez-Mellado, J.; Zamudio-Cuevas, Y.; Francisco-Balderas, A.; Martínez-Flores, K.; Fernández-Torres, J.; Lozada-Pérez, C.; Guido-Gómora, D.L.; Martínez-Gómez, L.E.; et al. The impact of short-chain fatty acid-producing bacteria of the gut microbiota in hyperuricemia and gout diagnosis. Clin. Rheumatol. 2023, 42, 203–214. [Google Scholar] [CrossRef]
- Ul-Haq, A.; Lee, K.A.; Seo, H.; Kim, S.; Jo, S.; Ko, K.M.; Moon, S.J.; Kim, Y.S.; Choi, J.R.; Song, H.Y.; et al. Characteristic alterations of gut microbiota in uncontrolled gout. J. Microbiol. 2022, 60, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, J.; Wang, Z.; Ang, K.Y.; Huang, S.; Hou, Q.; Su, X.; Qiao, J.; Zheng, Y.; Wang, L.; et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci. Rep. 2016, 6, 20602. [Google Scholar] [CrossRef] [PubMed]
- Airola, C.; Severino, A.; Porcari, S.; Fusco, W.; Mullish, B.H.; Gasbarrini, A.; Cammarota, G.; Ponziani, F.R.; Ianiro, G. Future Modulation of Gut Microbiota: From Eubiotics to FMT, Engineered Bacteria, and Phage Therapy. Antibiotics 2023, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Servetas, S.L.; Daschner, P.J.; Guyard, C.; Thomas, V.; Affagard, H.; Sergaki, C.; Sokol, H.; Wargo, J.A.; Wu, G.D.; Sabot, P. Evolution of FMT—From early clinical to standardized treatments. Biologicals 2022, 76, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Tang, S.; Han, J.; Fan, S.; Huang, Y.; Zhang, Z.; Zhou, J.; Ming, T.; Li, Y.; Su, X. Apostichopus japonicus Oligopeptide Induced Heterogeneity in the Gastrointestinal Tract Microbiota and Alleviated Hyperuricemia in a Microbiota-Dependent Manner. Mol. Nutr. Food Res. 2021, 65, e2100147. [Google Scholar] [CrossRef]
- Vendrik, K.E.; Chernova, V.O.; Kuijper, E.J.; Terveer, E.M.; van Hilten, J.J.; Contarino, M.F.; FMT4PD study group. Safety and feasibility of faecal microbiota transplantation for patients with Parkinson’s disease: A protocol for a self-controlled interventional donor-FMT pilot study. BMJ Open 2023, 13, e071766. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.; Kocabas, Ş. The effect of probiotic, prebiotic and gut microbiota on ASD: A review and future perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.R.; Yang, X.Y.; Deng, Z.H.; Zheng, Y.M.; Zhang, R.; Wu, L.H.; Cai, J.Y.; Kong, L.P.; Xia, H.H.; He, X.X. Effects of Washed Microbiota Transplantation on Serum Uric Acid Levels, Symptoms, and Intestinal Barrier Function in Patients with Acute and Recurrent Gout: A Pilot Study. Dig. Dis. 2022, 40, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Schucht, J.E.; Miller, K.R.; McClave, S.A. Technical Aspects of Fecal Microbial Transplantation (FMT). Curr. Gastroenterol. Rep. 2018, 20, 30. [Google Scholar] [CrossRef]
- Qu, Z.; Tian, P.; Yang, B.; Zhao, J.; Wang, G.; Chen, W. Fecal microbiota transplantation for diseases: Therapeutic potential, methodology, risk management in clinical practice. Life Sci. 2022, 304, 120719. [Google Scholar] [CrossRef]

| Foodstuff | Total Purine Content | Uric Acid Correspondence | |
|---|---|---|---|
| Cereals | Buckwheat flour | 75.9 | 89.1 |
| Rice (unpolished) | 37.4 | 43.7 | |
| Beans | Peanut | 49.1 | 57.1 |
| Almond | 31.4 | 37 | |
| Dried seaweed | Kombu | 46.4 | 54.5 |
| Nori | 591.7 | 695.6 | |
| Wakame | 262.4 | 306.5 | |
| Eggs | Chicken | 0 | 0 |
| Dairy products | Milk | 0 | 0 |
| Yogurt | 5.2 | 6.2 | |
| Fruit | Banana | 3.0 | 3.5 |
| Strawberry | 2.1 | 2.4 | |
| Vegetables | Broccoli | 70.0 | 81.8 |
| Carrot | 2.2 | 3.5 | |
| Eggplant | 50.7 | 58.7 | |
| Onion | 23 | 2.7 | |
| Potato | 6.5 | 7.5 | |
| Zucchini | 13.1 | 15.3 | |
| Beef | Liver | 219.8 | 255.2 |
| Ribs | 77.4 | 95.5 | |
| Tongue | 90.4 | 109.3 | |
| Chicken | Breast | 141.2 | 171.8 |
| Gizzard | 142.9 | 169.8 | |
| Heart | 125.4 | 150.0 | |
| Liver | 312.2 | 363.1 | |
| Wing | 137.5 | 168.1 | |
| Pork | Heart | 119.2 | 144.6 |
| Liver | 284.8 | 331.2 | |
| Ribs | 75.8 | 92.5 | |
| Tenderloin | 119.7 | 146.2 | |
| Fish | Carp | 103.2 | 126.1 |
| Herring | 139.6 | 169.8 | |
| Salmon | 119.3 | 146.2 | |
| Tuna | 157.4 | 193.3 | |
| Other | Beer yeast | 2995.7 | 3561.5 |
| Author (First), Year | Population | Type of Study | Main Findings | Refs. |
|---|---|---|---|---|
| Shirvani-Rad et al., 2022 | 15 studies (10 in humans) | Various | This thorough systematic review underscores the intricate influence of intestinal dysbiosis on gout, with a particular focus on the variability in alpha diversity and richness indices observed across various studies. The inconsistent results regarding richness levels among gout patients may stem from differences in the demographics of the study populations or the disease phases being examined. | [19] |
| Wei et al., 2022 | Over 1000 individuals | Microbiota analysis | Beneficial effects of Coprococcus. | [21] |
| Cao et al., 2022 | Over 300 individuals | Microbiota analysis | The abundance of Bacteroides and Fusobacterium was significantly positively correlated with the serum uric acid levels of UAS patients. | [22] |
| Wang et al., 2023 | Over 50000 individuals | Mendelian randomization | The phylum Actinobacteria exhibited a negative correlation with serum uric acid levels. However, elevated serum uric acid levels may increase the abundance of the Actinobacteria phylum. | [23] |
| Rodriguez et al., 2022 | 30 hyperuricemic patients | Clinical study | L. salivarius CECT 30632 effectively lowered serum urate levels and reduced the frequency of gout episodes. | [24] |
| Chen et al., 2022 | A total of 54 individuals | Clinical study | Significant alterations were observed in the intestinal bacteriome, mycobiome, and virome of gout patients. | [25] |
| Kim et al., 2022 | A total of 38 individuals | Clinical study | The microbiota of gout patients exhibited a decreased Firmicutes/Bacteroidetes ratio and, conversely, an increased Prevotella/Bacteroides ratio. | [26] |
| Hou et al., 2023 | Over 1 million individuals | Mendelian randomization | Critical role of crosstalk between the host and microbiota, notably in hyperuricemic patients. | [27] |
| Martinez-Nava et al., 2023 | 162 individuals | Clinical study | Richness was significantly lower in individuals with asymptomatic hyperuricemia compared to both controls and gout patients. | [28] |
| Ul-Haq et al., 2022 | 65 individuals in total | Clinical study | The presence of P. copri seemed to be a marker for gout. | [29] |
| Xie et al., 2022 | WMT in 11 individuals | FMT | WMT induced the reduction in serum uric acid levels and improved gout symptoms in individuals with acute and recurrent gout. | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demarquoy, J.; Dehmej, O. Reassessing Gout Management through the Lens of Gut Microbiota. Appl. Microbiol. 2024, 4, 824-838. https://doi.org/10.3390/applmicrobiol4020057
Demarquoy J, Dehmej O. Reassessing Gout Management through the Lens of Gut Microbiota. Applied Microbiology. 2024; 4(2):824-838. https://doi.org/10.3390/applmicrobiol4020057
Chicago/Turabian StyleDemarquoy, Jean, and Oumaima Dehmej. 2024. "Reassessing Gout Management through the Lens of Gut Microbiota" Applied Microbiology 4, no. 2: 824-838. https://doi.org/10.3390/applmicrobiol4020057
APA StyleDemarquoy, J., & Dehmej, O. (2024). Reassessing Gout Management through the Lens of Gut Microbiota. Applied Microbiology, 4(2), 824-838. https://doi.org/10.3390/applmicrobiol4020057






