Abstract
Diarrhoea is a serious cause of mortality worldwide that can lead to dehydration, gut barrier function impairment, nutrient malabsorption, and alterations of the gut microbiota (dysbiosis). The current solutions for its management, such as oral rehydration salts (ORS), inhibitors of gut motility, antibiotics, and living probiotics, only partially counteract the mechanisms of the disease and do not provide a full coverage of the problem. The potential risks of the use of living probiotic strains, particularly in immunocompromised patients, can be eliminated with the use of tyndallized (heat-killed) postbiotic bacteria and yeast. ABB C22® is a postbiotic combination of three tyndallized yeasts, namely Saccharomyces boulardii, Saccharomyces cerevisiae, and Kluyveromyces marxianus. To assess the action of the postbiotic combination on diarrhoea, immune and gut epithelial cell signalling assays, the gut barrier formation assay, and the rotavirus gene expression assay were performed. ABB C22® showed a strong anti-inflammatory effect, an induction of the build-up of the gut epithelium, and a degree of protection against rotavirus infection. These experimental studies support the use of the postbiotic ABB C22® as a solution for the management of diarrhoea and gastrointestinal conditions, alone or in combination with existing but incomplete treatments.
1. Introduction
Diarrhoeal disease is a major social concern, especially in developing countries. It can affect adults and children, but the youngest are particularly vulnerable to its harsh effects and mortality. Diarrhoea accounts for one in nine children’s deaths worldwide, making it the second leading cause of mortality among children less than 5 years of age [1,2]. Data of the Centres for Disease Control (CDC) indicate that diarrhoea kills 2195 children every day, which is more than AIDS, malaria, and measles combined [2]. Diarrhoea is both preventable and treatable [1]. Acute diarrhoeal episodes are typically caused by infections, mainly the rotavirus [3]. Chronic diarrhoea can have diverse causes, such as food allergies and intolerances, infections, inflammatory bowel disease (IBD), and functional gastrointestinal disorders [4,5]. The pathogenesis of diarrhoea is heterogenous, and may involve decreased water and electrolyte absorption, increased water secretion by the intestinal mucosa, increased luminal osmotic load, and/or inflammation of the mucosa and increased permeability. Independently of the aetiology of diarrhoea, a strong alteration of the gut epithelial barrier and an acceleration of the intestinal transit lead to severe complications, such as dehydration [6], gut barrier function impairment with a risk of septicaemia, nutrient malabsorption, and disturbances and loss of gut microbial communities (dysbiosis) [7,8]. Diarrhoea is a recognized cause of dysbiosis, leading to changes in the diversity of gut bacterial and fungal (mycobiota) populations [9]. Healthy microbiota and mycobiota lead to gut homeostasis and drive a strong gut barrier function. Microbiota and mycobiota dysbiosis compromise this equilibrium and drive a pro-inflammatory status in the lumen that leads to an altered immune response and to the disruption of the gut epithelium. This increases the susceptibility to pathogens favouring diarrhoea, and their toxins further affect gut microbiota disorders [8,10].
Current solutions for the management of diarrhoea, such as oral rehydration salts (ORSs), inhibitors of gut motility, antibiotics, and living probiotics, only partially counteract the pathogenic mechanisms of the disease, and do not provide a full coverage of the problem [1,11,12,13,14,15,16,17]. Antibiotics eliminate the bacterial-associated gastrointestinal infection causing diarrhoea but are not effective in cases of resistant bacteria, infections caused by viruses or parasites, or in cases of underlying diseases or food intolerance [15]. Moreover, antibiotics may cause antibiotic-associated diarrhoea due to dysbiosis [18]. Its use is a major disruptor of gut microbiota with negative effects including the reduction of species diversity and allowing the overgrowth of pathogens (i.e., Clostridium difficile) [18]. The indiscriminate use of antibiotics for the management of diarrhoea not only increases antibiotic resistance but impairs the homeostasis of the gut microbiome and may result in worsening diarrhoeal conditions or even to the establishment of chronic transit disturbances due to gut dysbiosis [19]. In this sense, there is an increasing focus on the development of nutritional supplements that show effects, help reduce the use of antibiotics and, at the same time, are safe and suitable for all populations.
Microbial interventions such as faecal microbiota transplantation (FMT) and the use of prebiotics, probiotics, and postbiotics are gaining focus as strategies to regulate the composition of the intestinal flora for the prevention and treatment of diarrhoeal episodes. The anti-diarrhoeal mechanism of probiotics mainly relies on the regulation and improvement of the balance of gut microbiota, production of antibacterial compounds, and improvement of the intestinal defence barrier and immunity [10,20].
More than 50 clinical trials (more than 8000 subjects involved in total) have been conducted to date regarding the efficacy of probiotic yeasts. The results point to a major advantage over bacterial probiotics in the prevention and treatment of many gastrointestinal conditions [21]. Cell wall components in yeasts, such as β-glucans, act as antigens recognized by receptors in the host’s immune cells and can therefore exert immunomodulating effects. Although yeasts represent only a minority of the organisms composing the intestinal flora, their cell size is 10 times larger than that of bacteria and they could represent a significant stearic hindrance for bacteria. In addition, bacterial probiotics only modulate the bacterial microbiota but do not influence the gut mycobiota, which has a relevant role in maintaining gut homeostasis. Furthermore, yeasts are not affected by antibiotics, so yeast can be co-administered to exert a concomitant/immediate effect without compromising the efficacy or safety of the antibiotic treatment. Finally, yeast probiotics can better withstand the extreme environments of the stomach.
However, the safety of the use of live probiotics has been a matter of concern. Risks associated with the use of live microorganisms have been reported, including systemic infection (septicaemia) caused by translocation, especially in paediatric populations and vulnerable patients, the acquisition of antimicrobial resistant genes, and interference with gut colonization in neonates [22]. The potential risks of the use of living probiotic strains, particularly in immunocompromised patients, has been eliminated with the use of tyndallized (heat-killed) probiotic bacteria and yeast, named postbiotics. After inactivation, dead cells can release bacterial or yeast components with key immunomodulating effects and with antagonizing properties against pathogens [22,23]. At the clinical level, products containing tyndallized probiotic strains have showed a role in gastrointestinal diseases, including diarrhoea.
ABB C22® is a postbiotic combination of three fractions of the yeast groups of Saccharomyces boulardii, Saccharomyces cerevisiae, and Kluyveromyces marxianus. The product exhibits complementary mechanisms for the protection of the integrity and function of enterocytes. ABB C22® exerts microbiome-modulating properties due to the intrinsic nature of the properties of its consortia of yeasts strains, which confer protection to the gut epithelium from the attachment of pathogens and can effectively compensate diarrhoea-related dysbiosis.
The aim of this study is to assess the synergistic mechanism of action by which ABB C22® exerts its benefits on different levels influencing diarrhoeal episodes: anti-inflammatory effects, protection of the gut barrier function, and protection from rotavirus infection. The confirmation of the favourable effects of ABB C22® in these experimental studies may further support its use as a solution for improving diarrhoeal episodes and support gut health.
2. Materials and Methods
2.1. Investigational Product and Strains of Study
The following strains from AB Biotek Human Nutrition & Health’s yeast strain collection were studied in the in vitro tests: Saccharomyces boulardii ABB S3, a tyndallized yeast strain with broad scientific evidence as a probiotic; zinc-enriched Saccharomyces cerevisiae ABB S6, a tyndallized yeast strain enriched with zinc salts that has proved to increase the bioavailability of this mineral; and Kluyveromyces marxianus ABB S8, a tyndallized yeast with anti-inflammatory and antioxidant properties. The investigation product (ABB C22®, AB Biotek Human Nutrition & Health, Peterborough, UK) is composed of a synergistic combination the aforementioned heat-treated postbiotic yeasts Saccharomyces boulardii, zinc-enriched Saccharomyces cerevisiae, and Kluyveromyces marxianus. The yeast strains were tested at a concentration of 107 cells/mL in all experiments.
2.2. Immune Cell Signalling Assay
The in vitro immune-modulating activity of ABB C22® and individual yeasts was studied through an analysis of the cytokine production by macrophages (human THP-1 cell line). The THP-1 cell line was cultured (1 × 105 cells/well) in 96-well plates in the presence of 100 nM phorbol 12-myristate 13-acetate (PMA, Sigma, Kawasaki, Japan) and incubated for 48 h to induce differentiation of the THP-1 monocytes into macrophages. Cells were washed and incubated for another 72 h in culture medium. After this, cells were incubated for 1 h with the test components (individual yeasts strains or ABB C22®). Cells were then incubated for another 16 h with and without LPS (100 ng/mL, Sigma) in the presence of the test components. All conditions were tested in triplicate. Supernatants were collected after stimulation and stored at −20 °C. ELISA assays (IL-10 Human Uncoated ELISA Kit, TNF-alpha Human Uncoated ELISA Kit, Life Technologies, Carlsbad, CA, USA) were used to measure the tumour necrosis factor alpha (TNF-α) and interleukin 10 (IL-10) levels, according to the manufacturers protocol. Levels of the individual cytokines TNF-α and IL-10 were standardized against the effect of the negative control. The TNF-α/IL-10 ratio was used as a marker of pro-inflammatory status and was also standardized against the effect of the negative control.
The metabolic activity of the cells for testing cytotoxicity of the test compounds was analysed using the WST-1 assay (Roche, Basel, Switzerland) according to the manufacturers protocol after collecting the culture supernatant of the immune cell signalling assay.
2.3. Gut Epithelial Cell Signalling Assay
The impact of ABB C22® and three individual yeast strains on the inflammatory state of intestinal cells was investigated through cytokine production by Caco-2 cells in the presence or absence of a pro-inflammatory trigger. Caco-2 cells were grown in 96-well plates with modified Eagle’s medium (MEM) supplemented with foetal bovine serum (FBS), non-essential amino acids (NEAA), Glutamax™, sodium pyruvate, and optionally penicillin-streptomycin and gentamicin (Invitrogen, Breda, The Netherlands). After washing with antibiotic-free medium, cells were exposed to the test components (yeast strains or ABB C22®) for 1 h at 37 °C, followed by a 24 h incubation with the test components and gentamicin, with or without a mix of recombinant tumour necrosis factor alpha (TNF-α) (10 ng/mL) and recombinant interferon (IFN-γ) (5 ng/mL) as a pro-inflammatory stimulus. After 24 h, supernatants were collected, stored at 20 °C, and analysed for IFN-γ-induced protein-10 (IP-10), interleukin 8 (IL-8), and monocyte chemoattractant protein-1 (MCP-1) levels using a Bio-Plex Multiplex Immunoassay System according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). The results were standardized against the negative control, and all experiments were conducted in triplicate. The metabolic activity of the cells was assessed for cytotoxicity using the WST-1 assay after collecting the culture supernatant from the epithelial signalling assay.
2.4. Gut Barrier Integrity Assay
The impact of ABB C22® and the three distinct yeasts on the integrity and functionality of the gut barrier was examined using transepithelial electric resistance (TEER) across a monolayer of gut cells. TEER was utilized to monitor the natural development of the gut epithelium over time. Caco-2 cells were cultured in MEM medium under the same conditions as the previous experiment. Subsequently, the cells were seeded (2 × 104 cells/cm2) on Transwell polycarbonate cell culture inserts with a mean pore size of 0.4 µm and a diameter of 0.33 cm2 until complete differentiation (±1000 ohms [Ω]) (Greiner Bio-one, Alphen aan de Rijn, The Netherlands). As an indicator of barrier integrity, TEER was measured using an EVOM2 Epithelial Volt/Ohm Meter (World Precision Instruments, Sarasota, FL, USA). At the time of the experiment, cells were washed and incubated for 1 h at 37 °C with antibiotic- and serum-free medium containing the test components (individual yeast strains, ABB C22® and a commercial control). Subsequently, the medium containing the metabolites of the test components was refreshed weekly and changed every 2 days. TEER measurements were taken prior to the start of the experiment (t = −1) every 2 days for a total of 22 days. The TEER values of the individual conditions were compared to their respective TEER value at t = 0 and expressed as ∆TEER in relative values. A negative control without the study product and a commercial control, Saccharomyces boulardii CNCM I-745®, which had previously demonstrated a significant effect on gut barrier formation, were included. The commercial control was added at a concentration of 107 CFU/mL using the same procedure as the rest of the test components. The experiment was conducted in triplicate.
2.5. Rotavirus Gene Expression Assay
To assess the in vitro protective effects of ABB C22® and the individual yeasts on intestinal epithelial cells subjected to infection with rotavirus (RV) supernatants, gene expression assays were performed in MA104 Monkey African Green kidney cells subjected to rotavirus infection. For the generation of viral supernatants, MA104 cells were planted in a 75 cm2 culture flask at an 80% confluence 24 h prior to infection. Once attached to the culture plastic, DMEM + 10% FBS was removed and replaced with serum-free EMEM, and cells were incubated overnight in serum starving conditions to promote viral infection. The next day, a new vial of viral supernatant was thawed at room temperature and diluted up to 3 mL with serum-free EMEM and incubated in the presence of trypsin-EDTA 2 µg/mL for 30 min at 37 °C. After the incubation period, medium was removed from the culture flask and replaced by the viral supernatant. MA104 cells were incubated in the presence of viral supernatant for 4 h at 37 °C 5% CO2. After the incubation period, 7 mL of serum-free EMEM containing trypsin-EDTA 2 µg/mL was added to the culture flask and cells were incubated for 7 days to allow the accumulation of viral particles in the culture supernatant. From this culture, virus aliquots were prepared as follows: the flask containing the infected cells was subjected to three consecutive freeze–thaw cycles at −80 °C to allow the lysis of cells and subsequent release of viral particles. Then, medium was harvested and centrifuged at 2000 rpm for 5 min to separate cell debris from the liquid fraction. This supernatant was recovered and stored in aliquots for further infection assays at −80 °C. Confluent MA104 cells were detached after incubation with 0.5% Trypsin for 1 min at 37 °C and further inactivation with DMEM containing 10% FBS. Then, cells were seeded in 96-well plates at a density of 104 cells/well and incubated overnight at 37 °C. Culture medium was then replaced by the test components (individual yeast strains, their paired combination, or the combination of the three strains) suspended in serum-free EMEM. After incubation of MA104 cells in the presence of the test components for 24 h at 37 °C, cell culture supernatants were removed and washed three times with 200 μL PBS per well. Parallelly, viral aliquots were thawed and incubated for 30 min at 37 °C in the presence of trypsin/EDTA 2 µg/mL. Then, 25 µL of the trypsin-activated viral supernatants were added to each well and incubated with cells for 4 h at 37 °C and 5% CO2. After this period, 75 µL of serum-free EMEM containing trypsin/EDTA 2 µg/mL was added per well and cells were incubated for additional 72 h to allow viral expansion. The isolation of viral RNA and the rotavirus gene amplification assay were conducted as follows: After infection with rotavirus supernatants, 100 µL of RLT Plus buffer were added per well and mixed thoroughly with culture medium by repeated pipetting. Then, plates were frozen at −80 °C to allow cell lysis and release of intracellular material. For RNA isolation, the whole volume of each well was incubated with 0.4 mg Dynabeads suspended in 80 µL ethanol 100% for 5 min at room temperature. After this period, Dynabeads were decanted with a DynaMag™-96 Side Magnet. The liquid fraction was removed, and the Dynabeads-bound RNA was washed twice with 100 µL ethanol 80%. After the second wash step, ethanol was allowed to evaporate for 3 min at room temperature and RNA was eluted in 25 µL of RNase-free H2O. RNA was reverse transcribed to copy DNA (cDNA) with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA) from 206 ng of starting material. From the reverse transcription products, 45 ng was used as an input for qPCR reactions in the presence of fluorescent probe assays designed to amplify the rotavirus genes NSP3 [24] and VP7. Beta actin (ACTB) gene was used as housekeeping gene. Relative gene expression was calculated with the Pfaffl method [25].
2.6. Statistical Analysis
For all the experiments, quantitative data are expressed as mean and standard deviation (±SD). Student’s t-test (two-sided) or one-way analysis of variance (ANOVA) with Dunnett’s procedure was used for the comparison of data according to conditions of application.
For the viral gene expression assays, one biological replicate with five technical replicates per condition was set. Outliers were identified using the Rout method (Q = 5%) and excluded from the analysis. Cleaned data were statistically analysed through one-way ANOVA and Dunnett’s post hoc multiple comparisons test.
Statistical significance was declared at p < 0.05, 95% of confidence. Bars in the charts represent the mean value for each condition and error bars indicate the standard error of the mean (SEM) for each group of values.
3. Results
3.1. Immune-Modulating Activity of ABB C22® by Immune Cell Signalling Assay
The in vitro immune-modulating activity of ABB C22® and individual yeast strains was studied through an analysis of TNF-α and IL-10 cytokine production by macrophages (THP-1 cell line). The cytotoxicity of individual yeasts and ABB C22® was discarded using a WST-1 assay before assessing the anti-inflammatory effect on immune cells. When we focus on the individual levels of TNF-α and IL-10 cytokines, the combination of the three heat-treated strains shows an effect in keeping TNF-α levels under control (Figure 1A) while strongly enhancing IL-10 levels (Figure 1C); therefore, decreasing the TNF-α/IL-10 ratio in basal conditions is not a challenge (Figure 1E). Interestingly, when the cells are subjected to LPS challenge to mirror an infection process, the combined strains are able to slightly increase pro-inflammatory cytokine TNF-α levels (Figure 1B) while maintaining IL-10 levels at bay (Figure 1D); overall, the TNF-α/IL-10 ratio is maintained in a way that is similar to the negative control. In other words, ABB C22® caused a 71% reduction of the TNF-α/IL-10 ratio, a marker of pro-inflammatory status, compared to the negative control in basal conditions (Figure 1E), and the TNF-α/IL-10 ratio was unaffected in lipopolysaccharide (LPS)-challenged macrophages supplemented with ABB C22® (Figure 1F). Interestingly, none of the individual yeast strains had a regulatory effect when tested singularly (Figure 1E).
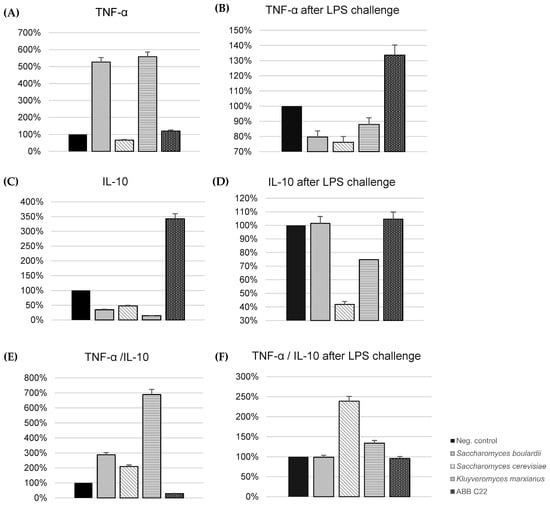
Figure 1.
In vitro modulation of TNF-α and IL-10 levels and TNF-α/IL-10 ratio in human THP-1 cell line (macrophages). (A) TNF-α levels in the absence of pro-inflammatory stimulus. (B) TNF-α levels in the presence of a pro-inflammatory stimulus (LPS challenge) simulating an infection process. (C) IL-10 levels in the absence of pro-inflammatory stimulus. (D) IL-10 levels in the presence of a pro-inflammatory stimulus (LPS challenge) simulating an infection process. (E) TNF-α/IL-10 ratio in the absence of pro-inflammatory stimulus. (F) TNF-α/IL-10 ratio in the presence of a pro-inflammatory stimulus (LPS challenge) simulating an infection process. All results are expressed as the standardization against the effect of the negative control.
3.2. Anti-Inflammatory Effect of ABB C22® on Intestinal Cells by Gut Epithelial Cell Signalling Assay
The in vitro immune-modulating activity of individual yeasts and ABB C22® in the gut was studied using cytokine production by Caco-2 cells in the presence and absence of a pro-inflammatory challenge with TNF-α/IFN-γ. The cytotoxicity of individual yeasts and ABB C22® was discarded using a WST-1 assay before assessing the anti-inflammatory effect on intestinal cells. ABB C22® showed statistically significant reductions in IP-10, IL-8, and MCP-1 levels in the presence and absence of a pro-inflammatory challenge compared to the negative control and to the single yeast strains (Figure 2A–F). In the absence of a pro-inflammatory stimulus, IP-10 cytokine production by Caco-2 cells showed a 26% and 23% decrease after incubation with K. marxianus alone and ABB C22®, respectively (Figure 2A). A synergistic reduction of the pro-inflammatory cytokines IL-8 and MCP-1 in gut epithelial cells was particularly observed for ABB C22® compared to individual yeasts in the absence of a challenge (41% and 36%, respectively) (Figure 2C,E). On the other hand, after a pro-inflammatory challenge, ABB C22® synergistically reduced IP-10 and MCP-1 levels in gut epithelial cells (37% and 50%, respectively) (Figure 2B,F), and had an effect on reducing IL-8 levels by 22% (Figure 2D).
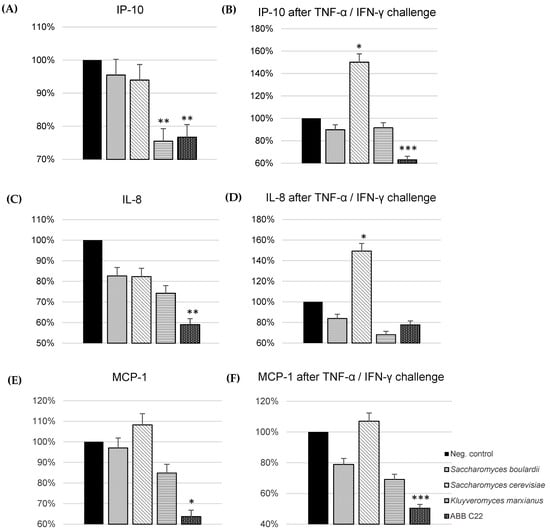
Figure 2.
Production of pro-inflammatory cytokines by Caco-2 cells after incubation with ABB C22® and three yeasts. *, ** and *** represent statistical significance with p-value < 0.05, 0.01, and 0.001, respectively, for between-group comparisons (one-way analysis of variance, ANOVA, and Dunnett’s post hoc test). (A) IP-10 cytokine production in the absence of pro-inflammatory stimulus. (B) IP-10 cytokine production in the presence of a pro-inflammatory stimulus (TNF-α/IFN-γ) simulating an inflamed gut epithelium. (C) IL-8 cytokine production in the absence of pro-inflammatory stimulus. (D) IL-8 cytokine production in the presence of a pro-inflammatory stimulus (TNF-α/IFN-γ) simulating an inflamed gut epithelium. (E) MCP-1 cytokine production in the absence of pro-inflammatory stimulus. (F) MCP-1 cytokine production in the presence of a pro-inflammatory stimulus (TNF-α/IFN-γ) simulating an inflamed gut epithelium. The results of each cytokine are expressed as the standardization against the effect of the negative control.
3.3. Gut Barrier Integrity Assay
The capacity of ABB C22® to induce the spontaneous formation of the gut epithelium over time was evaluated through TEER in a Caco-2 cell monolayer. ABB C22® shows the ability to increase TEER until day 22 when compared to a negative control and a commercial control of a live S. boulardii CNCM I-745® (Figure 3).
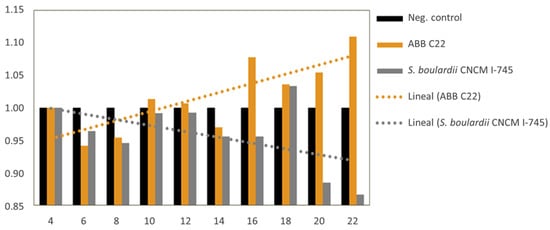
Figure 3.
Bars and slopes of trendlines for TEER increases associated with incubation of Caco-2 monolayer cells with a negative control, ABB C22®, and a commercial control of live S. boulardii CNCM I-745®. The comparison of ∆TEER values (Y axis) over the course of 22 days (X axis) indicates a higher spontaneous build-up of the epithelium monolayer for the ABB C22® condition versus the controls.
3.4. Rotavirus Antagonism
The results from the gene expression assay revealed a statistically significant decrease in the relative expression of NSP3 after pre-treatment with all test components compared to the untreated cells (p < 0.0001 for all treatments). A significant decrease in NSP3 was also observed for single inactivated Saccharomyces boulardii, single inactivated Kluyveromyces marxianus, and ABB C22®, but no significant effect was observed for gene VP7 for these strains. Moreover, pre-treatment with single live Saccharomyces boulardii (positive control), single inactivated Saccharomyces cerevisiae, inactivated Saccharomyces boulardii + inactivated Saccharomyces cerevisiae, and inactivated Saccharomyces cerevisiae + inactivated Kluyveromyces marxianus triggered a significant relative decrease in VP7 gene expression as well. In other words, all the test components had the ability to reduce the relative expression of one or the two rotavirus genes, and the test components, including the single live Saccharomyces boulardii, single inactivated Saccharomyces cerevisiae, inactivated Saccharomyces boulardii + inactivated Saccharomyces cerevisiae, and inactivated Saccharomyces cerevisiae + inactivated Kluyveromyces marxianus had a remarkable protective effect against rotavirus infection, which was reflected in a significant relative reduction of both RV genes (Figure 4).
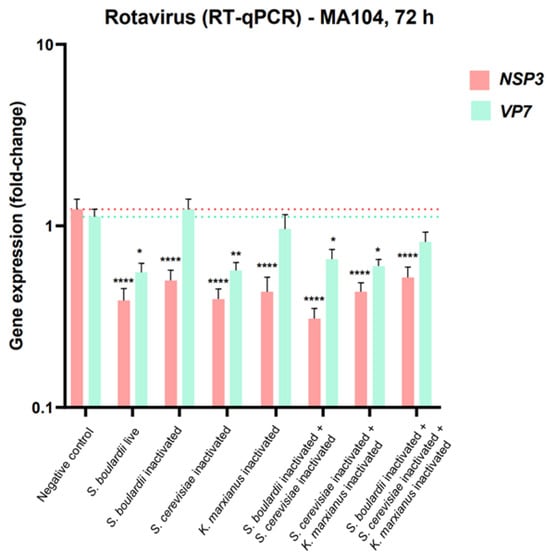
Figure 4.
Bar diagram showing relative NSP3 and VP7 gene expression in RNA extracts from MA104 cells subjected to a 24 h pre-treatment with the selected combinations of yeast strains and to infection with rotavirus for 72 h. *, ** and **** represent statistical significance with p-value < 0.05, 0.01, and 0.0001, respectively.
4. Discussion
Inflammatory diarrhoea is typically caused by cytotoxin-producing and invasive pathogens (e.g., enteroaggregative E. coli, enterohaemorrhagic E. coli, C. difficile) [26]. These organisms cause disease either by secreting noxious cytotoxins intraluminally or by invading the intestinal epithelium, resulting in an acute inflammatory reaction in the mucosa with a disruption of the epithelium [27]. By contrast, non-inflammatory diarrhoeas are usually caused by pathogens (e.g., rotavirus) that adhere to the small intestine mucosa, disrupting the absorptive and/or secretory process of the enterocyte [28]. The aim of this study was to assess the synergistic mechanism of action by which ABB C22® exerts its benefits on different levels, influencing both inflammatory and non-inflammatory diarrhoeal episodes: anti-inflammatory effects, protection of the gut barrier function, and protection from rotavirus infection.
In this study, ABB C22® has demonstrated a markedly beneficial anti-inflammatory effect both on gut epithelial cells and on immune cells. These in vitro experiments have shown that ABB C22® is able to reduce the TNFα/IL-10 ratio, a parameter used to assess immune homeostasis maintenance, in macrophages. The excessive production of TNF-α induces an exacerbated inflammatory response and tissue damage. In physiological conditions, an increase in TNF-α levels is counterbalanced with the synthesis of IL-10, an anti-inflammatory cytokine which suppresses the production of many activating and regulatory inflammatory mediators. The balance between the two molecules prevents exacerbated reactions of the inflammatory response and manages immune homeostasis. Our results indicate that ABB C22® suppresses TNFα release while stimulating IL-10 production, contributing to immune homeostasis. Interestingly, the TNFα/IL-10 ratio remains unaffected after ABB C22® supplementation under lipopolysaccharide (LPS) challenge (simulating infection). This is due to the capacity of LPS-activated macrophages to promote a strong pro-inflammatory state to fight the LPS-secreting agent. In this case, the overwhelming expression of IL-10 is undesirable since it may contribute to an impaired immune reaction to infection [29]. If the macrophage response under these conditions was to increase the presence of IL-10, the effect of TNFα would be counteracted by IL-10 and the macrophage-induced inflammatory response would not be effective against the LPS-secreting agent. Therefore, ABB C22® prevents an overwhelming expression of IL-10 after LPS challenge, enabling macrophages to effectively exert their pro-inflammatory properties against the infection. Very importantly, this effect was not seen when the strains were tested singularly, proving that the modulatory power relies on the synergy of the combined components in ABB C22®. These findings show the strong effects of ABB C22® in modulating the inflammatory response to counteract infection while preventing exacerbated immune responses in patients suffering from inflammatory diarrhoeal episodes.
The evidence from various studies indicates the significant role of IP-10, IL-8, and MCP-1 cytokines in inflammatory processes that participate in diarrhoea. Research has shown the increased expression of these cytokines in conditions like ulcerative colitis (UC), where they are involved in the recruitment and activation of inflammatory cells, contributing to tissue damage. Furthermore, IL-8 has been detected in samples from UC patients with inflammation, highlighting its role in inflammatory processes [30]. ABB C22® has been shown to exhibit further anti-inflammatory effects on gut epithelium cells, by decreasing the release of the pro-inflammatory cytokines IP-10, IL-8, and MCP-1 in the gut epithelium cells in a healthy state (26%, 41% and 36% reduction of IP-10, IL-8, and MCP-1, respectively) and in an inflamed epithelium model challenged with TNF-α/IFN-γ (37%, 22%, and 50% reduction, respectively). Particularly, ABB C22® had a synergistic anti-inflammatory effect on IL-8 and MCP-1 compared to the single strains. ABB C22®’s synergistic power was also observed on IP-10 and MCP-1 after a pro-inflammatory challenge. These findings demonstrate the crucial power of the combination of the three strains, as contained in ABB C22®, to counteract inflammation in the gut, which is a critical factor in inflammatory diarrhoeas.
Data of the in vitro assays on TEER have shown that ABB C22® exhibits a superior effect in stimulating the build-up of the digestive epithelium compared to Saccharomyces boulardii CNCM I-745® (a commonly studied yeast probiotic in gut health that had previously shown a strong protective effect on the gut barrier [31,32]). The effect of ABB C22® was stronger and lasted for a longer time. Gut microbiota modifications, mucus layer alterations, and epithelial damage can alter the intestinal permeability of the gut barrier function, worsening diarrhoeal episodes [33]. ABB C22® has been demonstrated to enhance and protect the gut barrier function and integrity, contributing to the recovery of microbiota balance and gut homeostasis during diarrhoeal episodes.
The goal of the rotavirus gene expression assay was to assess the in vitro protective effects of the proposed combinations of yeast strains and ABB C22® on intestinal epithelial cells subjected to infection with RV supernatants. The results showed that all the test components had the ability to reduce the relative expression of one or the two rotavirus genes. Moreover, the test components, including the single inactivated Saccharomyces cerevisiae, inactivated Saccharomyces boulardii + inactivated Saccharomyces cerevisiae, and inactivated Saccharomyces cerevisiae + inactivated Kluyveromyces marxianus showed a remarkable protective effect against rotavirus infection, which was reflected in a significant relative reduction in the expression of both RV genes. This highlights the synergistic effect of the paired combination of S. boulardii with S. cerevisiae and K. marxianus strains. These results are remarkable evidence for the combination of these yeast strains to counteract rotavirus infection, and therefore prevent or manage non-inflammatory diarrhoea. The putative mechanisms that mediate the antiviral effects of these strains could be further explored through mechanistic experiments, since the strains assessed in this study have shown anti-inflammatory effects through the increase in IL-10 and reduction in TNF-α. This could promote the production of anti-rotavirus IgA by the intestinal mucosa and guide the gut immune response to infective agents [34].
In the current literature, individual yeasts components of ABB C22® have shown different protective mechanisms of action against diarrhoea. S. boulardii has been long studied as a treatment for diarrhoea and the evidence of its effectiveness is wide. It has shown potent antisecretory properties versus water and electrolyte secretion in a rat colon model with castor oil-induced diarrhoea [35]. In a model of cholera toxin-induced secretion in rat jejunum cells, S. boulardii inhibited Cl secretion through both cAMP- and Ca2+-mediated signalling pathways [36]. In a model of pig intact jejunal epithelia, S. boulardii showed specific duration–response effects after stimulation with the secretagogue theophylline [37]. S. boulardii exerts anti-inflammatory action and reduces chloride secretion, decreases IL-8 production via the inhibition of the activation of MAP kinases ErkI/2 and JNK/SAPK, and prevents oxidative stress via the inhibition of reactive oxygen species (ROS) formation and the restoration of the balance of the glutathione-dependent redox system [38,39]. In an adult mice model, S. cerevisiae extracts containing rotavirus-like particles were successfully used for producing an immunological response capable of reducing the replication of the rotavirus after viral shedding infection [40]. The third component of ABB C22®, K. marxianus, has shown the control of intestinal inflammatory pathways and reduction of ROS in different murine and colitis-induced models [41].
Some limitations in this study should be considered. The assessment of rotavirus supernatant gene expression in kidney cells could be assessed on other cell types, such as intestinal epithelial cells, to constitute a system closer to the in vivo conditions. Furthermore, the rotavirus assay should include a positive control for added meaningful comparisons, such as known inhibitors of rotavirus viral replication (e.g., calcium chelators like EGTA [42]). The in vitro evidence should be carefully extrapolated to clinical situations, and in vivo or ex vivo studies are needed to further support and confirm the findings presented in this paper.
Overall, with the experiments exposed in this paper, we have proven evidence that the combination of these three yeast strains, each with their own independent evidence in the management of diarrhoea, provides added value for the management of diarrhoea compared to the use of the single strains alone. This study further supports the concept that natural products, such as nutraceuticals, are a useful therapeutic tool not only for treating infectious diarrhoea but also for other conditions in which an inflamed, leaky gut plays a pathogenic role [43].
5. Conclusions
ABB C22® is a postbiotic combination of the tyndallized yeasts S. boulardii ABB S3, S. cerevisiae ABB S6, and K. marxianus ABB S8. In this study, the synergistic combination of ABB C22® has demonstrated a marked upregulation of anti-inflammatory cytokines and a downregulation of pro-inflammatory cytokines on gut epithelial cells and on immune cells. These findings suggest an effect of ABB C22® in modulating the inflammatory response both in the absence and presence of an infection. Very importantly, this effect was not seen when the strains were tested singularly, suggesting that the modulatory power relies on the synergy of the combined components in ABB C22®. Moreover, ABB C22® has been demonstrated to enhance the gut barrier function and integrity. Finally, the paired combination of S. boulardii with S. cerevisiae and single K. marxianus yeast strains have shown an effect in reducing RV supernatant gene expression.
Showing an effect in different underlying mechanisms involved in gastrointestinal homeostasis, the postbiotic ABB C22® could be considered for its potential use in the management of conditions in which an inflamed, leaky gut plays a pathogenic role, such as infectious diarrhoea and other gastrointestinal conditions.
6. Patents
Three patent applications have resulted from the work reported in this manuscript: WO2023275325: Physiologically acceptable yeast compositions and uses thereof; WO2023275293: Physiologically acceptable yeast compositions and uses thereof; WO2023275323A9: Physiologically acceptable yeast compositions for use in the treatment of a gastrointestinal disorder.
Author Contributions
Conceptualization, J.C.C., C.d.L. and M.T.G.; data curation, L.C.M. and M.T.G.; formal analysis, L.C.M.; funding acquisition, C.d.L.; investigation, L.C.M.; methodology, L.C.M., J.C.C. and M.T.G.; resources, C.d.L.; software, L.C.M. and M.T.G.; supervision, C.d.L.; validation, J.C.C., C.d.L. and M.T.G.; visualization, M.T.G.; writing—original draft, L.C.M.; writing—review and editing, J.C.C., L.M.M. and M.T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article. Study data are available from the authors upon request.
Conflicts of Interest
Lydia Carrera Marcolin, Jordi Cuñé Castellana, Laia Martí Melero, Carlos de Lecea, and Maria Tintoré Gazulla are full-time employees of AB Biotek Human Nutrition and Health. They declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- World Health Organization (WHO). Diarrhoeal Disease. Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 10 September 2021).
- CDC. Centers for Disease Control and Prevention. U.S. Department of Health and Human Services. Diarrhea: Common Illness, Global Killer. Available online: https://www.cdc.gov/healthywater/pdf/global/programs/global-diarrhea508c.pdf (accessed on 10 September 2021).
- Parashar, U.D.; Gibson, C.J.; Bresee, J.S.; Glass, R.I. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 2006, 12, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rosenbaum, J. Chronic diarrhoea in children: A practical algorithm-based approach. J. Paediatr. Child. Health 2020, 56, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.R.; Lima, N.L.; Soares, A.M.; Oriá, R.B.; Pinkerton, R.C.; Barrett, L.J.; Guerrant, R.L.; Lima, A.A. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology 2010, 139, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.M.; Avva, U. Pediatric Dehydration. In StatPearls. 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK436022/ (accessed on 10 September 2021).
- Gorkiewicz, G.; Thallinger, G.G.; Trajanoski, S.; Lackner, S.; Stocker, G.; Hinterleitner, T.; Gülly, C.; Högenauer, C. Alterations in the colonic microbiota in response to osmotic diarrhea. PLoS ONE 2013, 8, e55817. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut microbiota and diarrhea: An updated review. Front. Cell Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, P.; Izquierdo, M.; Vidal, R.M.; Soto, F.; Ossa, J.C.; Farfan, M.J. Gut Microbiota-metabolome changes in children with diarrhea by diarrheagenic E. coli. Front. Cell Infect. Microbiol. 2020, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Sendid, B.; Hoarau, G.; Colombel, J.F.; Poulain, D.; Ghannoum, M.A. Mycobiota in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 77–87. [Google Scholar] [CrossRef] [PubMed]
- White, C.M. Loperamide: A readily available but dangerous opioid substitute. J. Clin. Pharmacol. 2019, 59, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, W.; Andresen, V.; Eberlin, M.; Mueck, T.; Layer, P. A comprehensive comparison of the efficacy and tolerability of racecadotril with other treatments of acute diarrhea in adults. Front. Med. 2016, 3, 44. [Google Scholar] [CrossRef]
- Buccigrossi, V.; Russo, C.; Guarino, A.; de Freitas, M.B.; Guarino, A. Mechanisms of antidiarrhoeal effects by diosmectite in human intestinal cells. Gut Pathog. 2017, 9, 23. [Google Scholar] [CrossRef][Green Version]
- Das, R.R.; Sankar, J.; Naik, S.S. Efficacy and safety of diosmectite in acute childhood diarrhoea: A meta-analysis. Arch. Dis. Child. 2015, 100, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.R. Antibiotic therapy for acute watery diarrhea and dysentery. Mil. Med. 2017, 182, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Guandalini, S.; Lo Vecchio, A. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2015, 49 (Suppl. S1), S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Antibiotic-associated diarrhea. Clin. Infect. Dis. 1992, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E. Probiotics: Definition, sources, selection, and uses. Clin. Infect. Dis. 2008, 46 (Suppl. S2), S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics–Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health benefits of heat-killed (tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- Tyndall, J. On heat as a germicide when discontinuously applied. Proc. R. Soc. Lond. 1877, 25, 569–570. [Google Scholar]
- Lee, D.Y.; Leung, K.T.; Lee, H.; Habash, M.B. Simultaneous Detection of Selected Enteric Viruses in Water Samples by Multiplex Quantitative PCR. Water Air Soil. Pollut. 2016, 227, 107. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Giannella, R.A. Pathogenesis of acute bacterial diarrheal disorders. Annu. Rev. Med. 1981, 32, 341–357. [Google Scholar] [CrossRef]
- Stephen, J. Pathogenesis of infectious diarrhea. Can. J. Gastroenterol. 2001, 15, 669–683. [Google Scholar] [CrossRef][Green Version]
- Navaneethan, U.; Giannella, R.A. Mechanisms of infectious diarrhea. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Shmarina, G.V.; Pukhalsky, A.L.; Kokarovtseva, S.N.; Pukhalskaya, D.A.; Shabalova, L.A.; Kapranov, N.I.; Kashirskaja, N.J. Tumor necrosis factor-α/interleukin-10 balance in normal and cystic fibrosis children. Med. Inflamm. 2001, 10, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Uguccioni, M.; Gionchetti, P.; Robbiani, D.F.; Rizzello, F.; Peruzzo, S.; Campieri, M.; Baggiolini, M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am. J. Pathol. 1999, 155, 331–336. [Google Scholar]
- Terciolo, C.; Dapoigny, M.; Andre, F. Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical disorders associated with intestinal barrier disruption. Clin. Exp. Gastroenterol. 2019, 12, 67–82. [Google Scholar] [CrossRef]
- Generoso, S.V.; Viana, M.L.; Santos, R.G.; Arantes, R.M.; Martins, F.S.; Nicoli, J.R.; Machado, J.A.; Correia, M.I.; Cardoso, V.N. Protection against increased intestinal permeability and bacterial translocation induced by intestinal obstruction in mice treated with viable and heat-killed Saccharomyces boulardii. Eur. J. Nutr. 2011, 50, 261–269. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Fu, H.; Li, J.; Xu, X.; Xia, C.; Pan, Y. Effectiveness and Safety of Saccharomyces Boulardii for the Treatment of Acute Gastroenteritis in the Pediatric Population: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Comput. Math. Methods Med. 2022, 2022, 6234858. [Google Scholar] [CrossRef]
- Girard, P.; Pansart, Y.; Coppe, M.C.; Gillardin, J.M. Saccharomyces boulardii inhibits water and electrolytes changes induced by castor oil in the rat colon. Dig. Dis. Sci. 2005, 50, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Rampal, P. Effect of Saccharomyces boulardii on cAMP- and Ca2+ -dependent Cl- secretion in T84 cells. Dig. Dis. Sci. 1999, 44, 2359–2368. [Google Scholar] [CrossRef]
- Schroeder, B.; Winckler, C.; Failing, K.; Breves, G. Studies on the time course of the effects of the probiotic yeast Saccharomyces boulardii on electrolyte transport in pig jejunum. Dig. Dis. Sci. 2004, 49, 1311–1317. [Google Scholar] [CrossRef]
- Stier, H.; Bischoff, S.C. Influence of Saccharomyces boulardii CNCM I-745 on the gut-associated immune system. Clin. Exp. Gastroenterol. 2016, 9, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Buccigrossi, V.; Laudiero, G.; Russo, C.; Miele, E.; Sofia, M.; Monini, M.; Ruggeri, F.M.; Guarino, A. Chloride secretion induced by rotavirus is oxidative stress-dependent and inhibited by Saccharomyces boulardii in human enterocytes. PLoS ONE 2014, 9, e99830. [Google Scholar] [CrossRef]
- Rodríguez-Limas, W.A.; Pastor, A.R.; Esquivel-Soto, E.; Esquivel-Guadarrama, F.; Ramírez, O.T.; Palomares, L.A. Immunogenicity and protective efficacy of yeast extracts containing rotavirus-like particles: A potential veterinary vaccine. Vaccine 2014, 32, 2794–2798. [Google Scholar]
- Romanin, D.E.; Llopis, S.; Genovés, S.; Martorell, P.; Ramón, V.D.; Garrote, G.L.; Rumbo, M. Probiotic yeast Kluyveromyces marxianus CIDCA 8154 shows anti-inflammatory and anti-oxidative stress properties in in vivo models. Benef. Microbes 2016, 7, 83–93. [Google Scholar] [CrossRef]
- Dormitzer, P.R.; Greenberg, H.B. Calcium chelation induces a conformational change in recombinant herpes simplex virus-1-expressed rotavirus VP7. Virology 1992, 189, 828–832. [Google Scholar] [PubMed]
- Romano, L.; Napolitano, L.; Crocetto, F.; Sciorio, C.; Priadko, K.; Fonticelli, M.; Federico, A.; Romano, M.; Gravina, A.G. The potential therapeutic role of Hericium erinaceus extract in pathologic conditions involving the urogenital-gut axis: Insights into the involved mechanisms and mediators. J. Physiol. Pharmacol. 2024, 75, 3–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).