Effects of Vacuum Pasteurization on the Nutritional, Sensory and Microbiological Properties of Orange (Citrus × sinensis) and Carrot (Daucus carota L.) Nectar
Abstract
1. Introduction
2. Materials and Methods
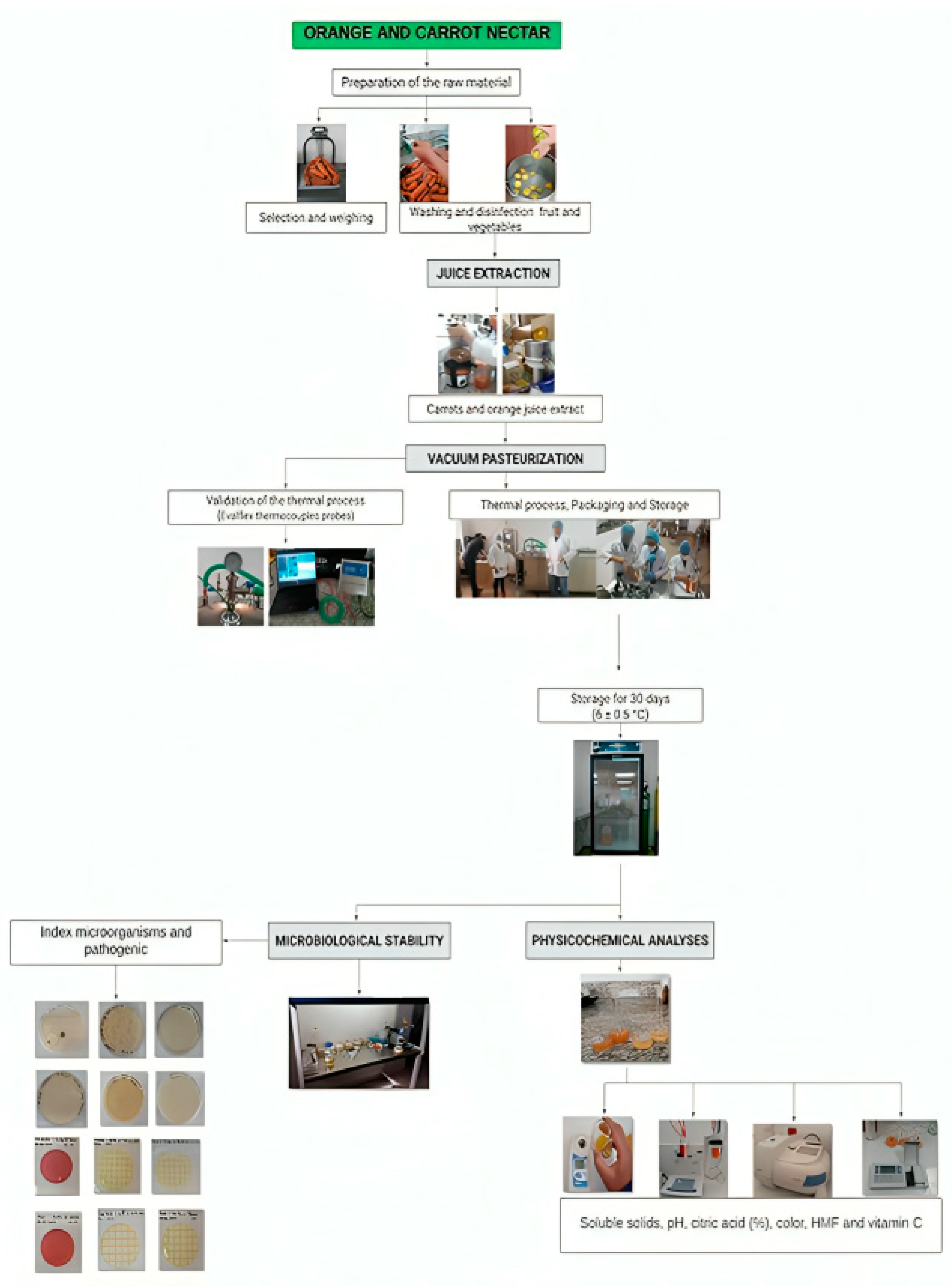
2.1. Orange and Carrot Nectar Preparation
2.2. Physicochemical Analysis
2.3. Vitamin C
2.4. Hydroxymethylfurfural
2.5. Microbiologic Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. pH
3.2. Titratable Acidity
3.3. Total Soluble Solids
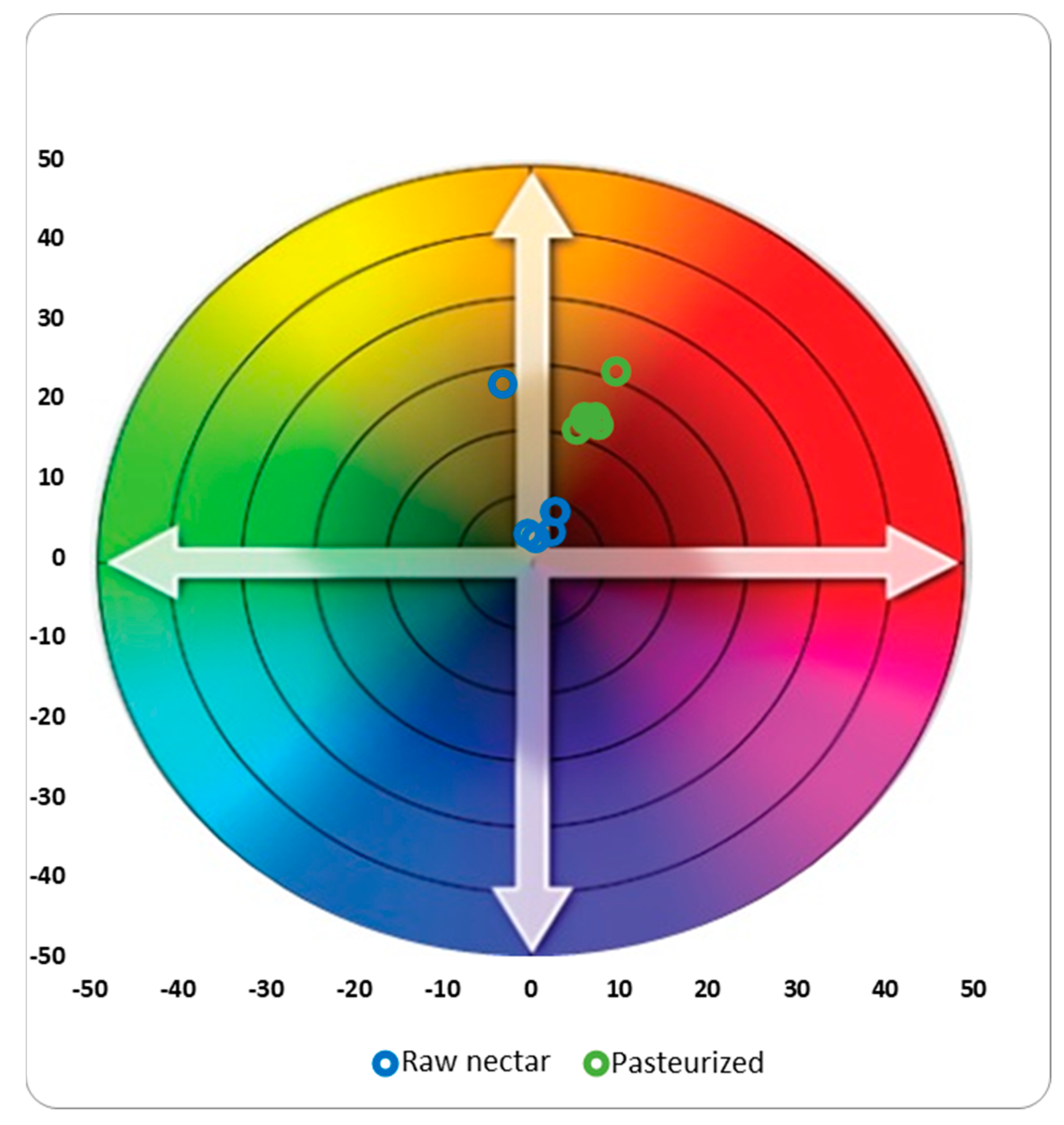
3.4. Color
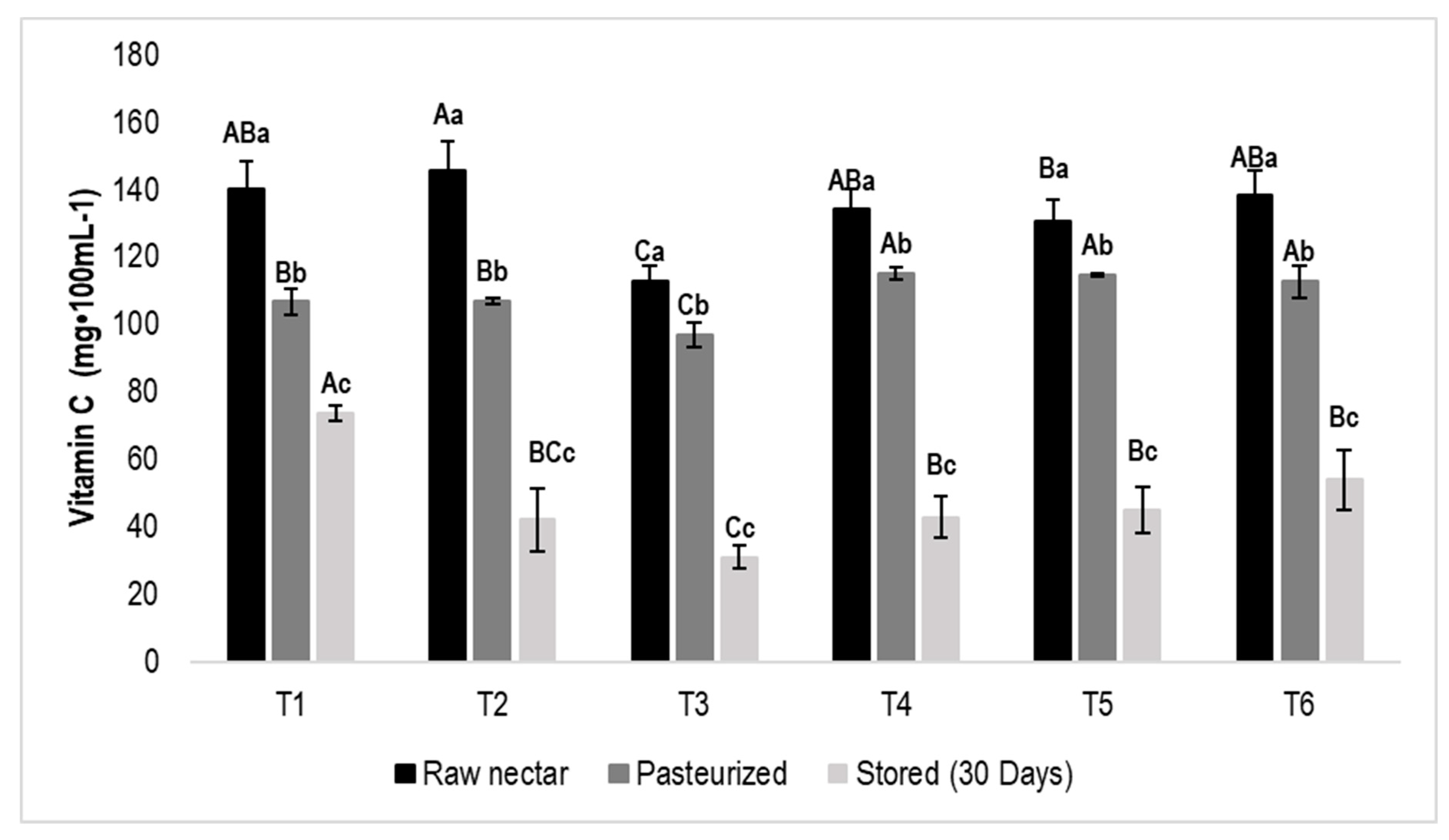
3.5. Vitamin C
3.6. Determination of 5-Hydroxymethylfurfural
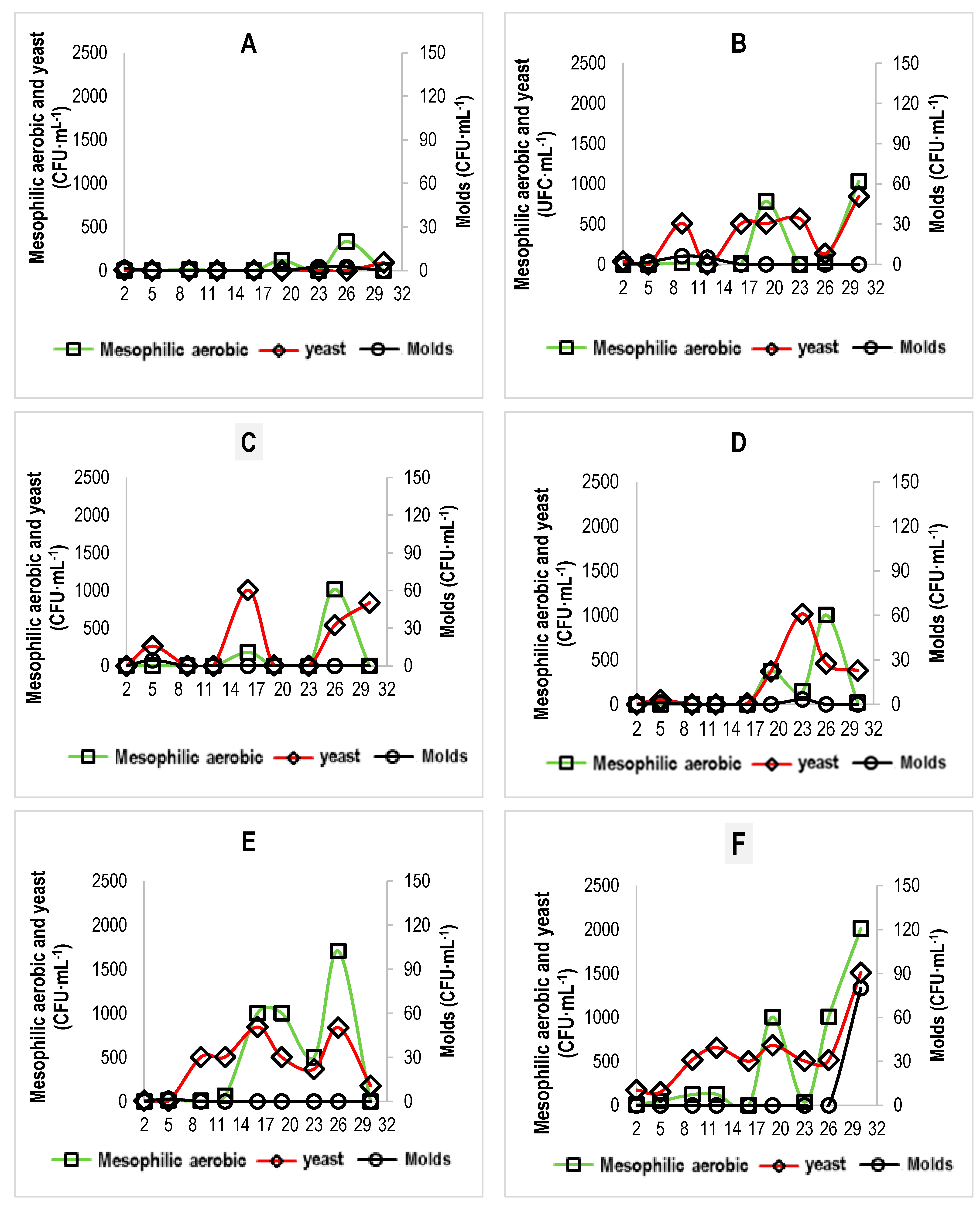
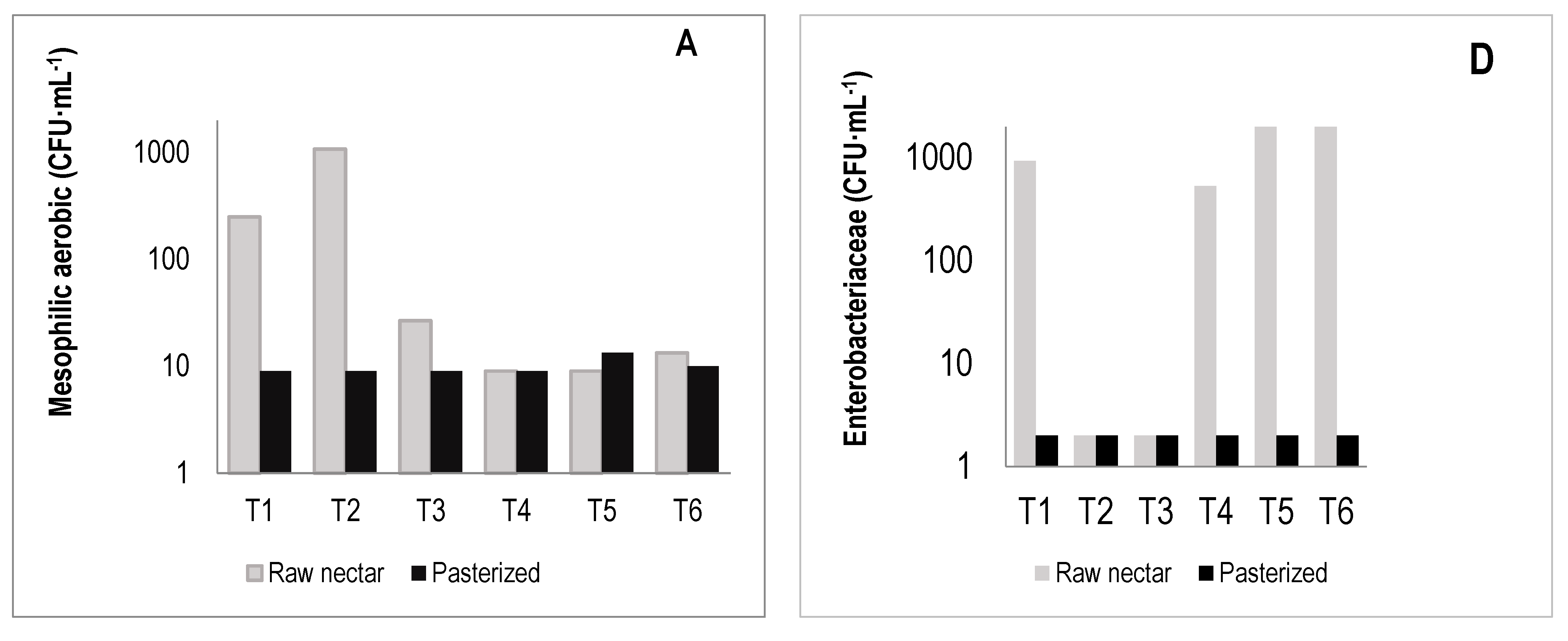
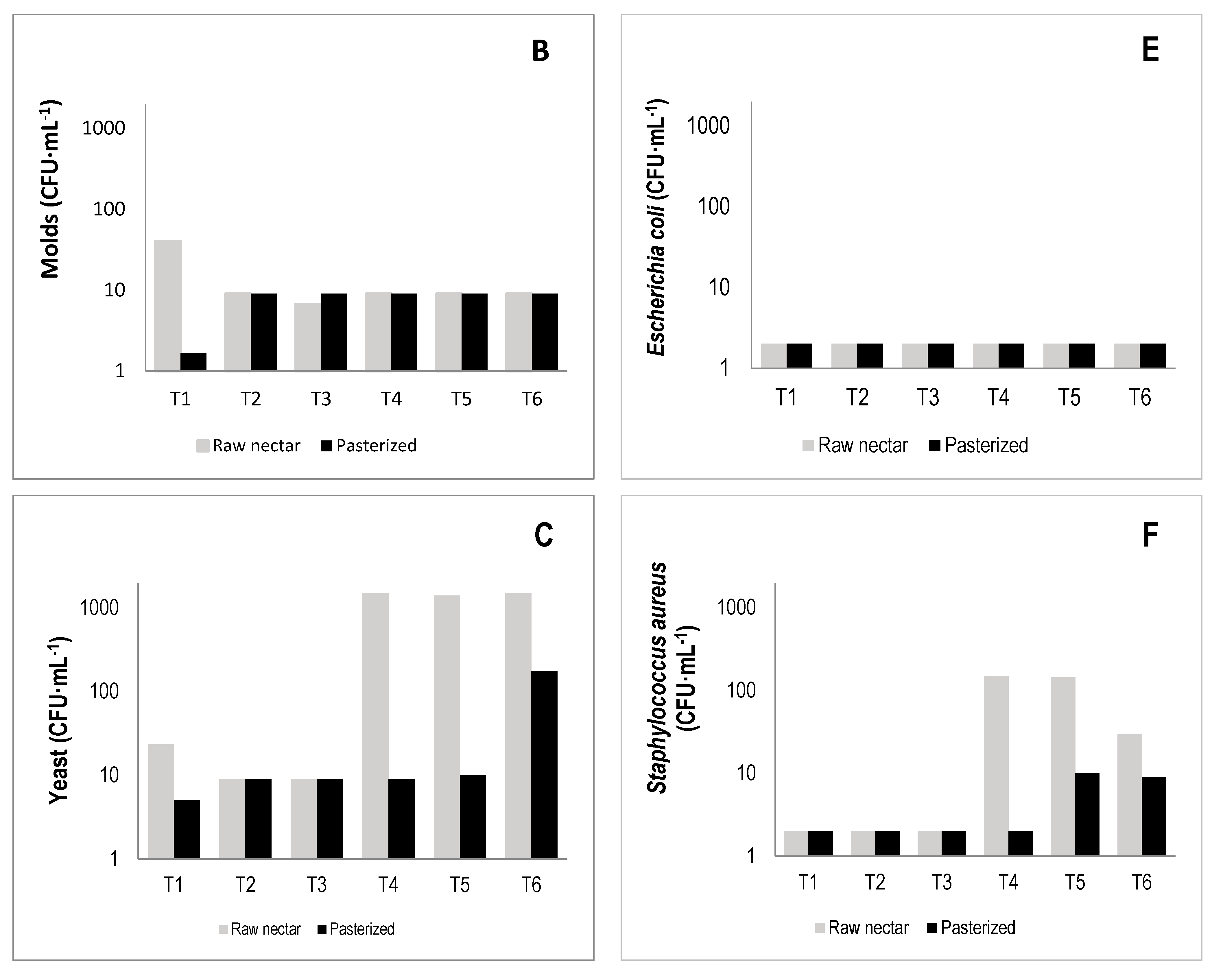
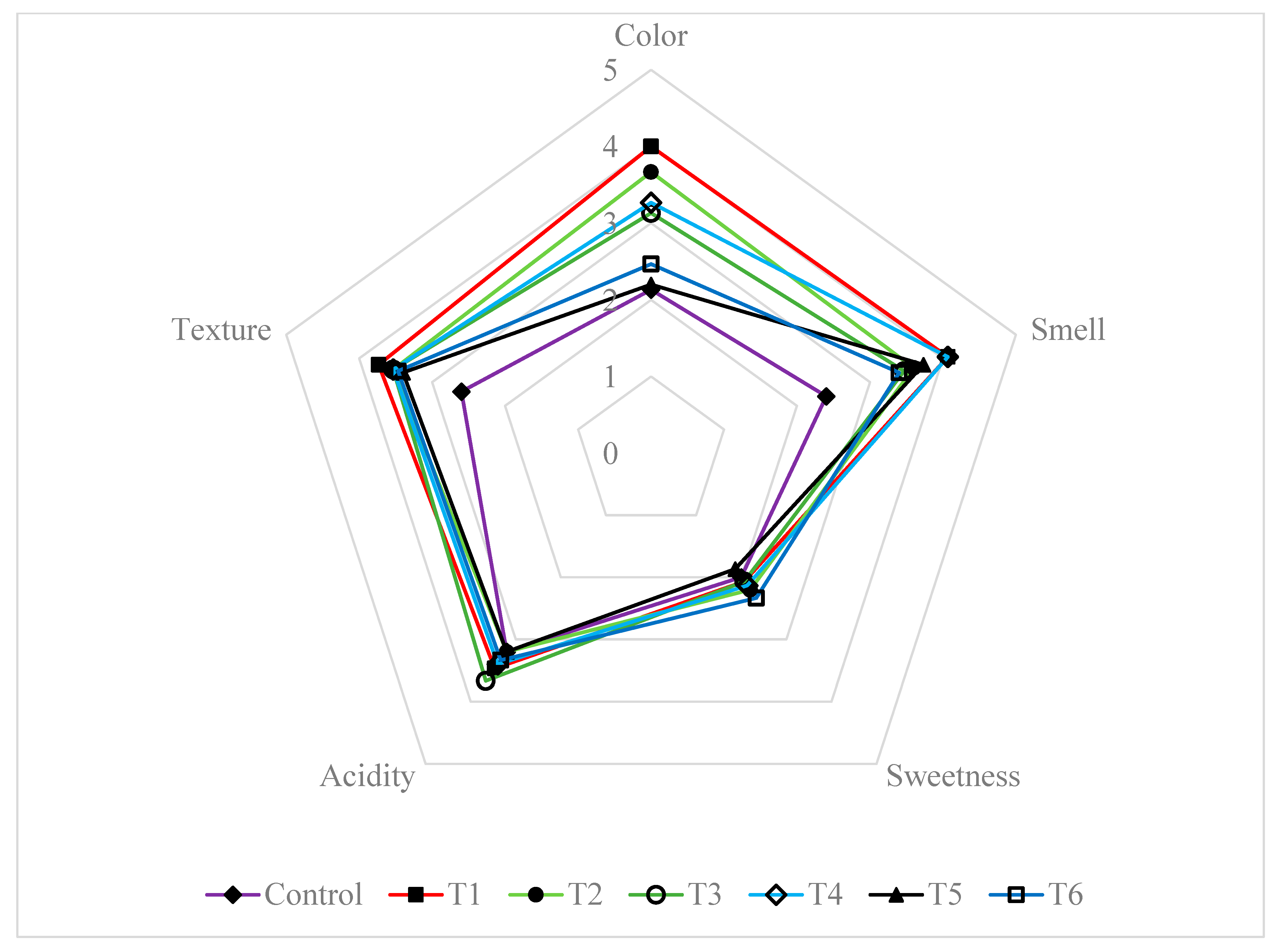
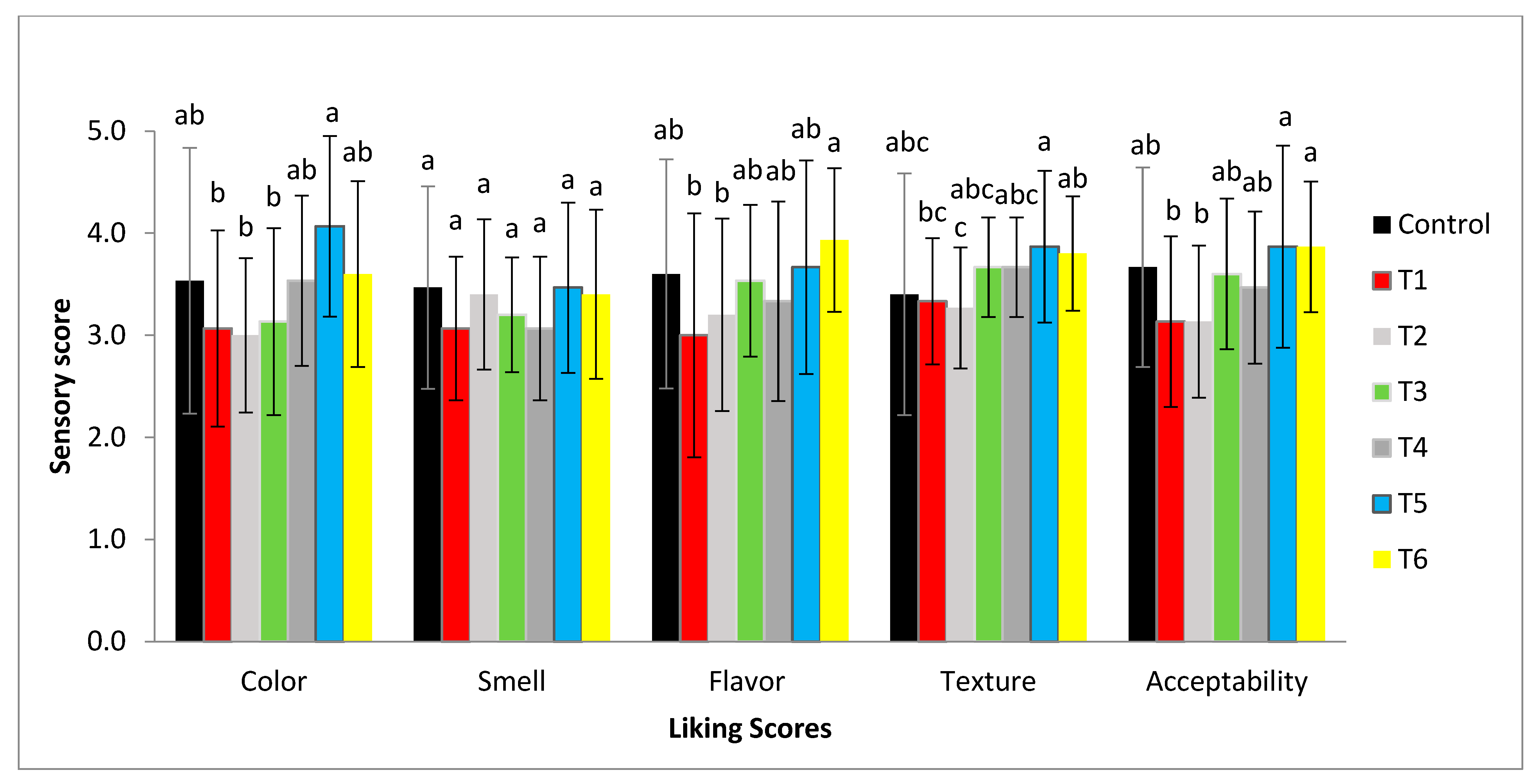
3.7. Microbiological and Sensory Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seifter, J.L. Potassium Disorders Goldman’s Cecil Medicine, 24th ed.; Elsevier: Philadelphia, PA, USA, 2008; pp. 734–752. [Google Scholar]
- Sadecka, J.; Polovka, M.; Kolek, E.; Belajova, E.; Tobolkova, B.; DAŠKO, L.; Durec, J.Á.N. Orange juice with pulp: Impact of pasteurization and storage on flavour, polyphenols, ascorbic acid and antioxidant activity. J. Food Nutr. Res. 2014, 53, 371–388. [Google Scholar]
- Nadulski, R.; Grochowicz, J.; Sobczak, P.; Kobus, Z.; Panasiewicz, M.; Zawiślak, K.; Mazur, J.; Starek, A.; Żukiewicz-Sobczak, W. Application of Freezing and Thawing to Carrot (Daucus carota L.) Juice Extraction. Food Bioprocess Technol. 2014, 8, 218–227. [Google Scholar] [CrossRef]
- Gabriel, A.A.; Albura, M.P.; Faustino, K.C. Thermal death times of acid-habituated Escherichia coli and Salmonella enterica in selected fruit bev-erages. Food Control. 2015, 55, 236–241. [Google Scholar] [CrossRef]
- Wibowo, S.; Grauwet, T.; Santiago, J.S.; Tomic, J.; Vervoort, L.; Hendrickx, M.; Van Loey, A. Quality changes of pasteurised orange juice during storage: A kinetic study of specific parameters and their relation to colour instability. Food Chem. 2015, 187, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Kalia, P.K.M. Pectin Methylesterases: A Review. J. Bioprocess. Biotech. 2015, 5, 1000227. [Google Scholar] [CrossRef]
- Tchango, J.T.; Tailliez, R.; Eb, P.; Njine, T.; Hornez, J. Heat resistance of the spoilage yeastsCandida pelliculosaandKloeckera apisand pasteurization values for some tropical fruit juices and nectars. Food Microbiol. 1997, 14, 93–99. [Google Scholar] [CrossRef]
- Salomão, B.; Slongo, A.; Aragão, G. Heat resistance of Neosartorya fischeri in various juices. LWT Food Sci. Technol. 2007, 40, 676–680. [Google Scholar] [CrossRef]
- Hommel, R.K. Gluconobacter. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 1, p. 994. [Google Scholar]
- Juvonen, R.; Virkajärvi, V.; Priha, O.; Laitila, A. Microbiological spoilage and safety risks in non-beer beverages. VTT Tied. Res. Notes 2011, 2599, 107. [Google Scholar]
- Nielsen, P.V.; Beuchat, L.R.; Frisvad, J.C. Growth of and fumitremorgin production by Neosartorya fischeri as affected by temperature, light, and water activity. Appl. Environ. Microbiol. 1988, 54, 1504–1510. [Google Scholar] [CrossRef]
- Palaniappan, S.; Sastry, S.K.; Richter, E.R. Effects of Electroconductive Heat Treatment and Electrical Pretreatment on Thermal Death Kinetics of Selected Microorganisms. Biotechnol. Bioeng. 1992, 39, 225–232. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT Food Sci. Technol. 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- IDFA. Pasteurization. Consulted on 09/27/2016. 2016. Available online: https://www.idfa.org/news-views/media-kits/milk/pasteurization (accessed on 15 March 2024).
- Burbano, J. Influence of Open and Vacuum Pasteurization on the Physicochemical Properties and Acceptability of Pineapple (Ananas comosus L.), Naranjilla (Solanum quitoense Lam.) and Borojo (Borojoa patinoi Cuatrec.) Nectar. Master’s Thesis, Technical University of Ambato, Ambato, Ecuador, 2015. [Google Scholar]
- NTE INEN-ISO 2337; Juices, Pulps, Concentrates, Nectars, Fruit and Vegetable Drinks. INEN, Ecuadorian Technical Standard: Quito, Ecuador, 2008; Requirements. p. 15.
- Rajashekhara, E.; Suresh, E.; Ethiraj, S. Note: Influence of different heating media on thermal resistance of Neosartorya fischeri isolated from papaya fruit. J. Appl. Bacteriol. 1996, 81, 337–340. [Google Scholar] [CrossRef]
- NTE INEN-ISO 1842; Vegetable and Fruit Products. Determination of pH (IDT). INEN, Ecuadorian Technical Standard: Quito, Ecuador, 2013; p. 4.
- Aleman, R.S.; Cedillos, R.; Page, R.; Olson, D.; Aryana, K. Physico-chemical, microbiological, and sensory characteristics of yogurt as affected by ingredients that help treat leaky gut. J. Dairy Sci. 2023, 106, 3868–3883. [Google Scholar] [CrossRef] [PubMed]
- NTE INEN-ISO 2173; Vegetable and Fruit Products. Determination of Soluble Solids. Refractometric Method (IDT). INEN, Ecuadorian Technical Standard: Quito, Ecuador, 2013; p. 9.
- Fuentes, J.A.M.; Gil, M.D.J.; Fernández, H.Z.; Montero-Fernández, I.; Martín-Vertedor, D.; Yadav, A.; Aleman, R.S. Impact of 5’ Adenosine Monophosphate, Potassium Chloride, and Glycine on the Physicochemical and Sensory Characteristics of Sodium-Reduced Chicken. Dietetics 2024, 3, 87–97. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Sabatino, L.; D’Anna, F.; Moncada, A. Influence of Preharvest Gibberellic Acid Treatments on Postharvest Quality of Minimally Processed Leaf Lettuce and Rocket. Horticulturae 2019, 5, 63. [Google Scholar] [CrossRef]
- He, O.; Zhang, Y.; Wang, P.; Liu, L.; Wang, Q.; Yang, N.; Li, W.; Champagne, P.; Yu, H. Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose. Catalysts 2021, 11, 11. [Google Scholar] [CrossRef]
- NTE INEN 1529-5; Microbiological Control of Food. Determination of the Amount of Mesophilic Aerobic Microorganisms. INEN, Ecuadorian Technical Standard: Quito, Ecuador, 2006; REP. p. 6.
- NTE INEN 1529-10; Microbiological Control of Food. Molds and Viable Yeasts. Plate Count by Deep Seeding. INEN, Ecuadorian Technical Standard: Quito, Ecuador, 2013; p. 6.
- 3M Petrifilm Interpretation Guides. 2016. Available online: https://solutions.3m.co.uk/wps/portal/3M/en_GB/FoodSafetyEU/FoodSafety/EducationTraining/BrochuresDownloads/PetrifilmInterpretationGuides/ (accessed on 7 March 2024).
- AOAC. AOAC Official Method 2003.07 for Counting Staphylococcus aureus in Prepared Processed Foods; AOAC International: Rockville, MD, USA, 2003. [Google Scholar]
- AOAC. AOAC RI 0401101 Official Method for Counting Listeria Monocytogenes; AOAC International: Rockville, MD, USA, 2002. [Google Scholar]
- AOAC. AOAC Official Method 991.14 for Counting E. coli/Coliforms; AOAC International: Rockville, MD, USA, 2002. [Google Scholar]
- Aguilar-Rosas, S.; Ballinas-Casarrubias, M.; Nevarez-Moorillon, G.; Martin-Belloso, O.; Ortega-Rivas, E. Thermal and pulsed electric fields pasteurization of apple juice: Effects on physicochemical properties and flavour compounds. J. Food Eng. 2007, 83, 41–46. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, S.; Liu, F.; Dong, P.; Huang, W.; Xiong, L.; Liao, X. Comparing the effects of high hydrostatic pressure and thermal pasteurization combined with nisin on the quality of cucumber juice drinks. Innov. Food Sci. Emerg. Technol. 2013, 17, 27–36. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.; Martínez-Navarrete, N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Finlayson, G.D.; Schaefer, G. Hue that is invariant to brightness and gamma. In Proceedings of the 12th British Machine Vision Conference, Manchester, UK, 10–13 September 2001. [Google Scholar]
- Jin, Z.; Min, S.; Yeom, H.; Zhang, Q. Commercial-Scale Pulsed Electric Field Processing of Orange Juice. J. Food Sci. 2003, 68, 1265–1271. [Google Scholar] [CrossRef]
- Choi, M.; Kim, G.; Lee, H. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int. 2002, 35, 753–759. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Effect of thermal pasteurization on Valencia orange juice color and pigments. LWT Food Sci. Technol. 2003, 36, 153–156. [Google Scholar] [CrossRef]
- Silva, F.M.; Silva, C.L.M. Colour changes in thermally processed cupuaçu (Theobroma grandiflorum) puree: Critical times and kinetics modelling. Int. J. Food Sci. Technol. 1999, 34, 87–94. [Google Scholar] [CrossRef]
- Cortés, C.; Esteve, M.J.; Frígola, A. Color of orange juice treated by High Intensity Pulsed Electric Fields during refrigerated storage and comparison with pasteurized juice. Food Control. 2008, 19, 151–158. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Aarnisalo, K.; Tallavaara, K.; Wirtanen, G.; Maijala, R.; Raaska, L. The hygienic working practices of maintenance personnel and equipment hygiene in the Finnish food industry. Food Control. 2006, 17, 1001–1011. [Google Scholar] [CrossRef]
| Parameters | Sample | Thermal Treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 (92 °C/3.3 min) | T2 (90 °C/10.3 min) | T3 (88 °C/32.7 min) | T4 (70 °C/2.3 min) | T5 (65 °C/11.4 min) | T6 (60 °C/56.6 min) | ||||||||
| L* | Raw nectar | 33.41 ± 1.13 | Bd | 52.42 ± 11.16 | Abc | 57.76 ± 3.99 | Aab | 48.60 ± 12.66 | Aa | 33.87 ± 4.66 | Ad | 47.7 ± 10.59 | Acd |
| Pasteurized | 42.05 ± 2.55 | Ac | 61.07 ± 4.36 | Aab | 61.85 ± 19.37 | Aa | 65.60 ± 10.22 | Aa | 34.02 ± 7.62 | Ac | 43.89 ± 3.80 | Abc | |
| a* | Raw nectar | 10.47 ± 0.46 | Ba | 7.91 ± 1.42 | Ab | 6.41 ± 0.85 | Abc | 7.77 ± 1.86 | Ac | 8.72 ± 0.45 | Ab | 7.54 ± 1.38 | Ab |
| Pasteurized | 9.65 ± 0.03 | Aa | 5.97 ± 0.46 | Ab | 6.23 ± 3.04 | Ab | 5.23 ± 1.96 | Ab | 7.68 ± 0.14 | Aab | 7.26 ± 0.51 | Aab | |
| b* | Raw nectar | 18.23 ± 0.43 | Ba | 18.29 ± 1.26 | Aa | 16.71 ± 1.85 | Aa | 18.02 ± 1.24 | Aa | 16.43 ± 1.56 | Aa | 16.30 ± 1.35 | Aa |
| Pasteurized | 23.29 ± 0.02 | Aa | 17.71 ± 0.34 | Ab | 17.34 ± 5.22 | Ab | 16.12 ± 2.79 | Ab | 16.61 ± 0.64 | Ab | 17.55 ± 0.52 | Ab | |
| Chroma (C*) | Raw nectar | 21.02 ± 0.59 | Ba | 19.94 ± 1.57 | Aab | 17.90 ± 2.01 | Ab | 19.65 ± 1.84 | Ab | 18.61 ± 1.58 | Aab | 17.97 ± 1.79 | Aab |
| Pasteurized | 25.21 ± 0.01 | Aa | 18.73 ± 0.12 | Ab | 18.45 ± 5.92 | Ab | 16.97 ± 3.23 | Ab | 18.30 ± 0.53 | Ab | 18.99 ± 0.60 | Ab | |
| Hue (°h) | Raw nectar | 12.29 ± 0.28 | Bc | 16.34 ± 2.18 | Bbc | 18.11 ± 0.80 | Aab | 16.64 ± 3.19 | Aa | 13.24 ± 0.62 | Abc | 15.31 ± 1.69 | Ab |
| Pasteurized | 16.82 ± 0.07 | Abc | 20.76 ± 1.13 | Aab | 20.70 ± 4.92 | Aab | 22.25 ± 5.35 | Aa | 15.14 ± 0.79 | Ac | 16.86 ± 0.99 | Aabc | |
| Dark index (DI) | Raw nectar | 82.88 ± 5.01 | Ba | 45.83 ± 17.38 | Acd | 33.64 ± 6.23 | Acd | 50.51 ± 20.16 | Ad | 64.43 ± 4.55 | Aab | 46.17 ± 15.28 | Abc |
| Pasteurized | 71.77 ± 0.67 | Aa | 33.50 ± 5.42 | Ab | 37.25 ± 24.30 | Ab | 27.49 ± 10.38 | Ab | 68.78 ± 16.46 | Aa | 50.27 ± 6.95 | Aab | |
| Total soluble solids (°Brix) | Raw nectar | 14.71 ± 0.12 | Aab | 15.68 ± 0.84 | Aa | 14.05 ± 1.26 | Ab | 15.12 ± 1.23 | Aab | 15.06 + 0.42 | Aab | 14.71 ± 0.30 | Aab |
| Pasteurized | 16.67 ± 1.49 | Aa | 15.96 ± 1.40 | Aa | 14.72 ± 0.17 | Aa | 16.41 ± 2.43 | Aa | 14.71 ± 0.55 | Aa | 14.67 ± 0.78 | Aa | |
| Citric acid (%) | Raw nectar | 0.23 ± 0.04 | Ad | 0.14 ± 0.01 | Aa | 0.18 ± 0.01 | Ab | 0.22 ± 0.01 | Acd | 0.19 ± 0.01 | Abc | 0.23 ± 0.01 | Ad |
| Pasteurized | 0.24 ± 0.01 | Ad | 0.14 ± 0.01 | Aa | 0.18 ± 0.01 | Ab | 0.20 ± 0.02 | Abc | 0.19 ± 0.01 | Ab | 0.22 ± 0.02 | Acd | |
| pH | Raw nectar | 4.09 ± 0.10 | Ac | 4.33 ± 0.03 | Ab | 4.45 ± 0.04 | Aa | 4.32 ± 0.03 | Ab | 4.37 ± 0.02 | Aab | 4.39 ± 0.01 | Aab |
| Pasteurized | 4.21 ± 0.08 | Ac | 4.33 ± 0.03 | Ab | 4.45 ± 0.04 | Aa | 4.34 ± 0.03 | Ab | 4.42 ± 0.03 | Aa | 4.40 ± 0.001 | Aab | |
| 5-HMF | Raw nectar | *ND | 1.37 ± 0.15 | A | *ND | *ND | *ND | *ND | |||||
| Pasteurized | 1.05 ± 0.05 | a | 1.30 ± 0.20 | Aa | 1.45 ± 0.45 | a | *ND | *ND | *ND | ||||
| Thermal Treatments | Inhibiting Listeria monocytogenes | |
|---|---|---|
| Raw Nectar | Pasteurized | |
| T1 (92 °C/3.3min) | Absence | Absence |
| T2 (90 °C/10.3min) | Absence | Absence |
| T3 (88 °C/32.7min) | Absence | Absence |
| T4 (70 °C/2.3min) | Absence | Absence |
| T5 (65 °C/11.4min) | Absence | Absence |
| T6 (60 °C/56.6 min) | Absence | Absence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilma, L.-S.; Burgos, J.; Ortiz, J.; Samaniego, I.; Marcia, J.; José, M.; Vallejo, C.; Angós, I.; Yaday, A.; Alemán, R.S. Effects of Vacuum Pasteurization on the Nutritional, Sensory and Microbiological Properties of Orange (Citrus × sinensis) and Carrot (Daucus carota L.) Nectar. Appl. Microbiol. 2024, 4, 731-744. https://doi.org/10.3390/applmicrobiol4020050
Wilma L-S, Burgos J, Ortiz J, Samaniego I, Marcia J, José M, Vallejo C, Angós I, Yaday A, Alemán RS. Effects of Vacuum Pasteurization on the Nutritional, Sensory and Microbiological Properties of Orange (Citrus × sinensis) and Carrot (Daucus carota L.) Nectar. Applied Microbiology. 2024; 4(2):731-744. https://doi.org/10.3390/applmicrobiol4020050
Chicago/Turabian StyleWilma, Llerena-Silva, José Burgos, Jacqueline Ortiz, Iván Samaniego, Jhunior Marcia, Molina José, Christian Vallejo, Ignacio Angós, Ajitesh Yaday, and Ricardo Santos Alemán. 2024. "Effects of Vacuum Pasteurization on the Nutritional, Sensory and Microbiological Properties of Orange (Citrus × sinensis) and Carrot (Daucus carota L.) Nectar" Applied Microbiology 4, no. 2: 731-744. https://doi.org/10.3390/applmicrobiol4020050
APA StyleWilma, L.-S., Burgos, J., Ortiz, J., Samaniego, I., Marcia, J., José, M., Vallejo, C., Angós, I., Yaday, A., & Alemán, R. S. (2024). Effects of Vacuum Pasteurization on the Nutritional, Sensory and Microbiological Properties of Orange (Citrus × sinensis) and Carrot (Daucus carota L.) Nectar. Applied Microbiology, 4(2), 731-744. https://doi.org/10.3390/applmicrobiol4020050








