Diversity of Microbial Communities in Trade Wastes—Implications for Treatments and Operations
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Physicochemical Analyses of Wastes
2.3. Biomethane Potential Assessment
2.4. Targeted 16S-rRNA Sequencing
2.5. Data Analysis and Visualisation
3. Results and Discussion
3.1. Wastewater Properties
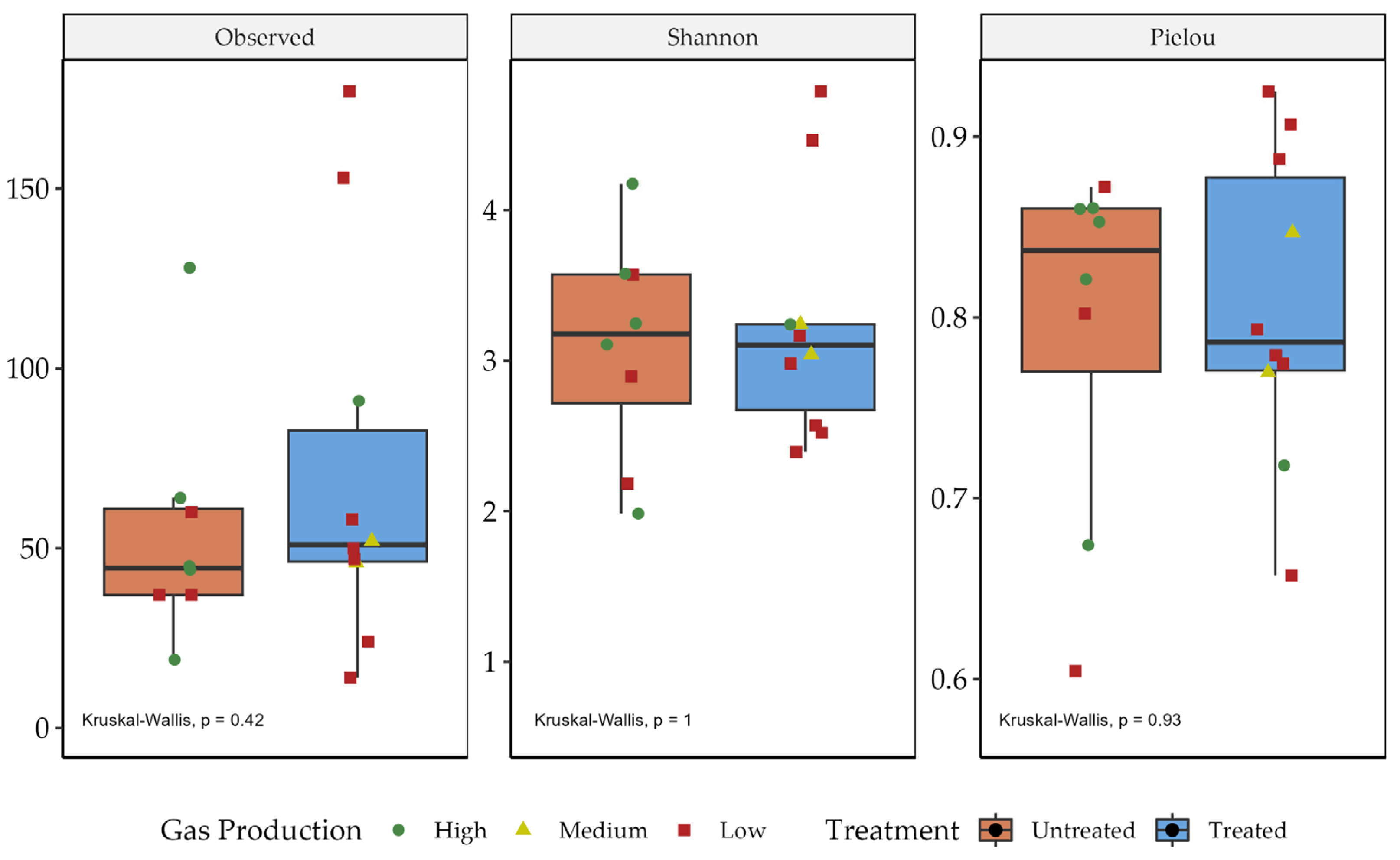
3.2. Richness and Diversity Assessments of Microbial Communities among Treated and Untreated Trade Wastes
3.3. Microbial Community Analysis of Microbial Communities among Trade Wastes at Different Levels of Treatment or BMP Yields
3.4. Taxonomic Abundances—Overall Trends
3.5. Microbial Community Phylogenetic Analysis—Individual Sites
3.5.1. Group A, Site 1—Edible Fats
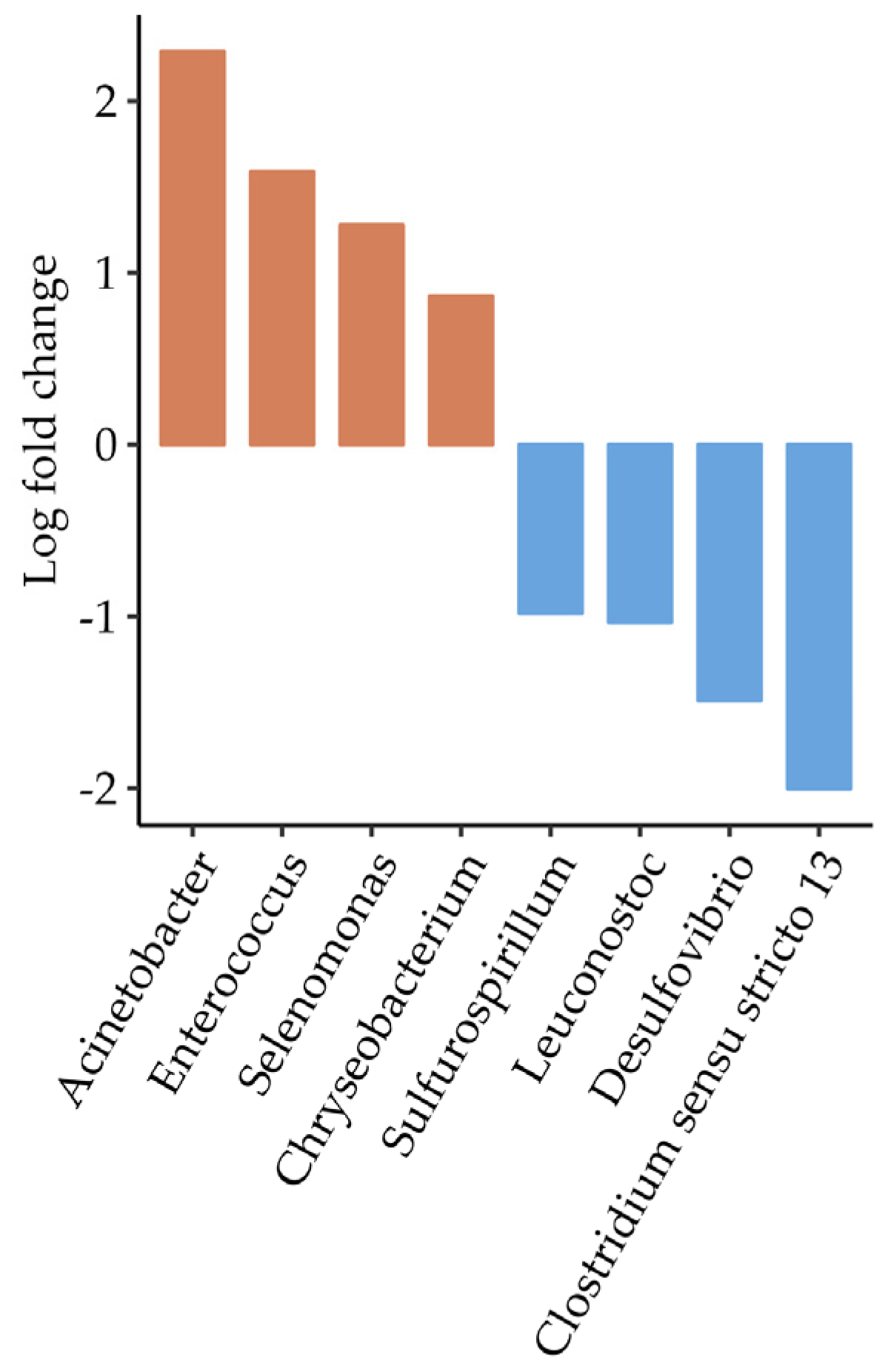
3.5.2. Group A, Site 5—Foods
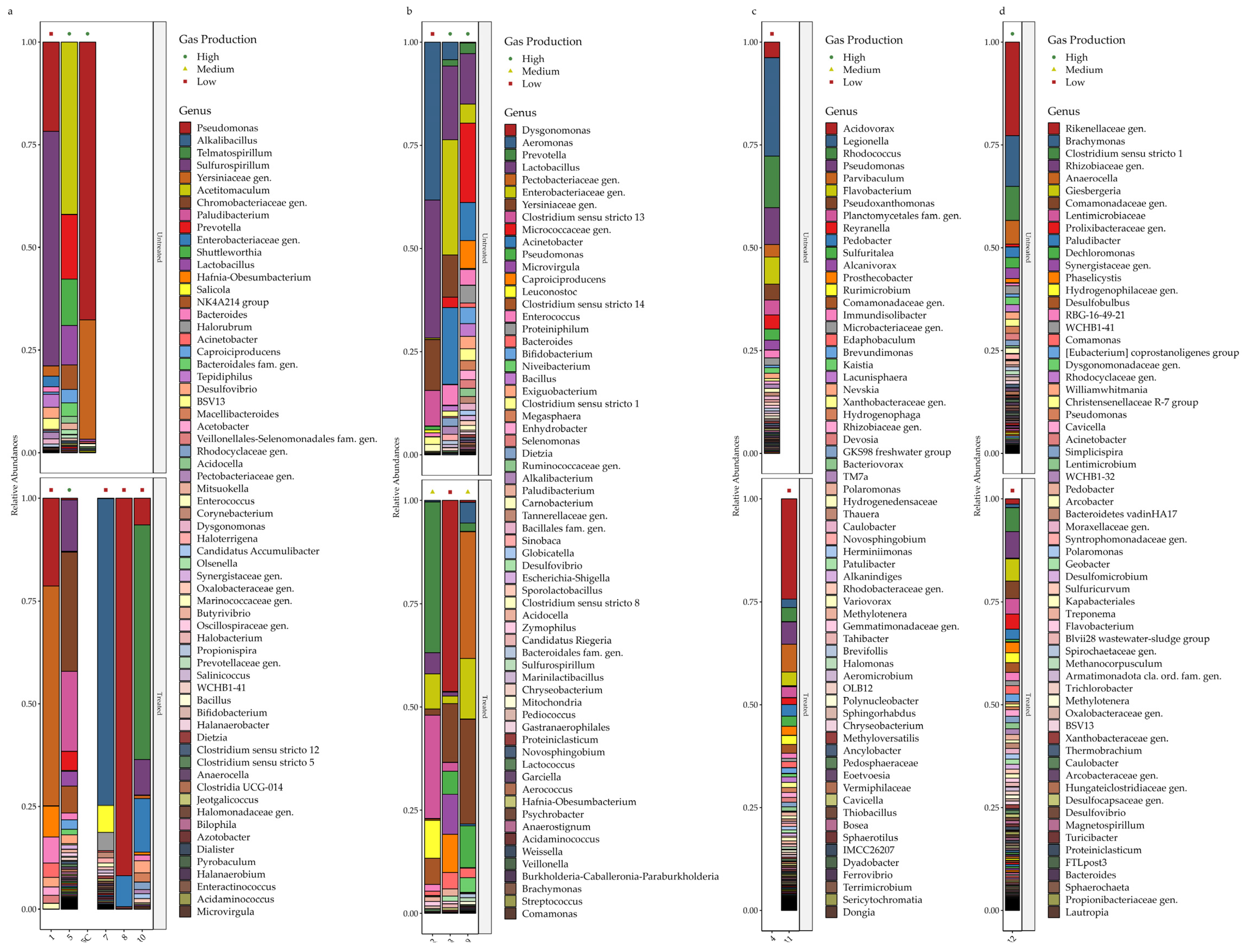
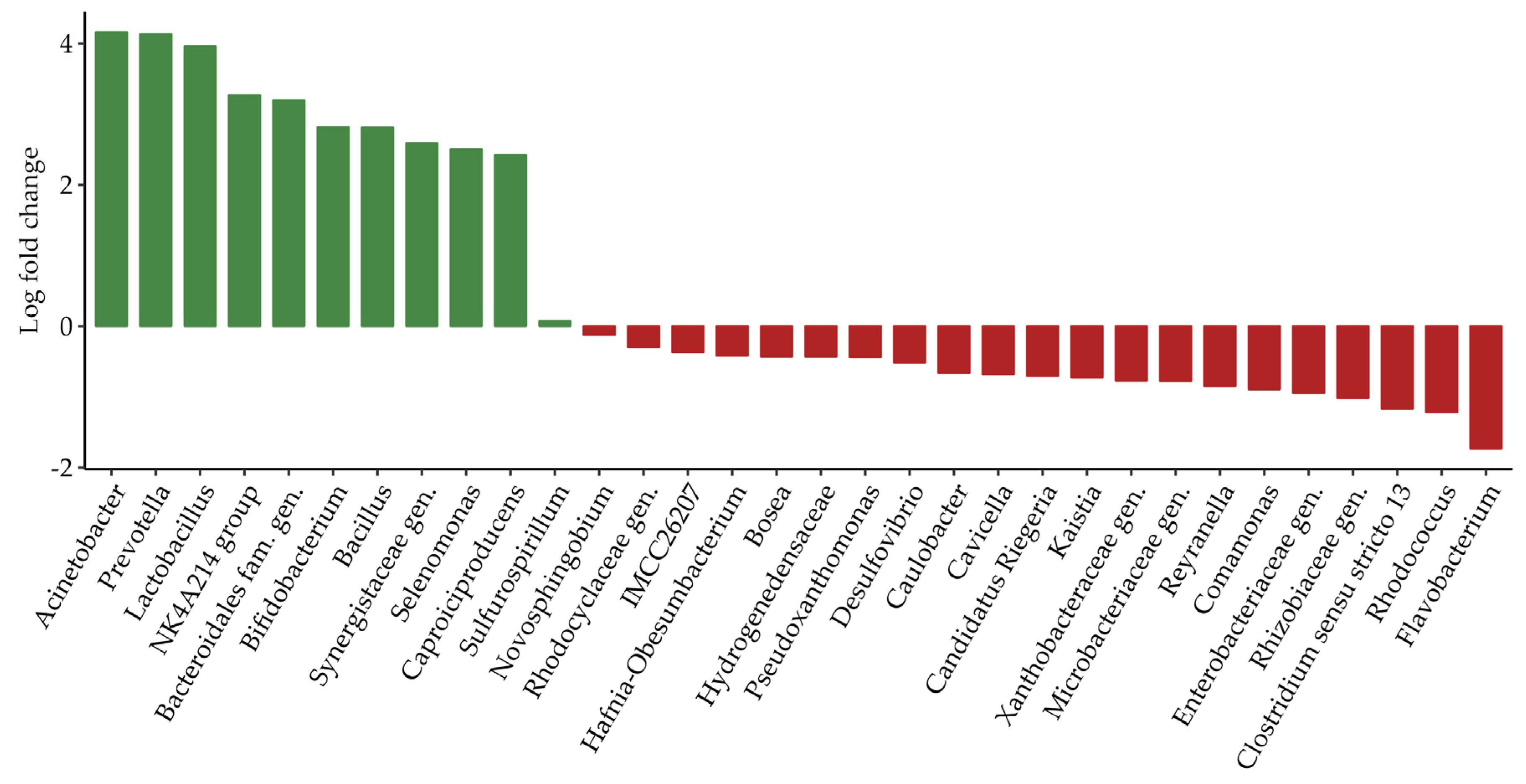
3.5.3. Group A, Site 7—Animal Products
3.5.4. Group A, Site 8—Chemicals
3.5.5. Group A, Site 10—Chemicals
3.5.6. Group B, Site 2—Brewery
3.5.7. Group B, Site 3—Waste Management/Logistics
3.5.8. Group B, Site 9—Waste Management/Logistics
3.5.9. Group C, Site 4—Chemicals
3.5.10. Group C, Site 11—Chemicals
3.5.11. Group D, Site 12—Animal Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fanchiang, J.M.; Tseng, D.H.; Guo, G.L.; Chen, H.J. Ozonation of complex industrial park wastewater: Effects on the change of wastewater characteristics. J. Chem. Technol. Biotechnol. 2009, 84, 1007–1014. [Google Scholar] [CrossRef]
- Gutierrez-Sarabia, A.; Fernandez-Villagomez, G.; Martinez-Pereda, P.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. Slaughterhouse wastewater treatment in a full-scale system with constructed wetlands. Water Environ. Res. 2004, 76, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, L.; Deeb, R.; Sawaya, C.; El Khoury, C.; Wazne, M.; Harb, M. Anaerobic membrane bioreactor-based treatment of poultry slaughterhouse wastewater: Microbial community adaptation and antibiotic resistance gene profiles. Biochem. Eng. J. 2023, 192, 108847. [Google Scholar] [CrossRef]
- Parker, C.D. Species of sulphur bacteria associated with the corrosion of concrete. Nature 1947, 159, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Anandkumar, B.; George, R.P.; Maruthamuthu, S.; Palaniswamy, N.; Dayal, R.K. Corrosion behavior of srb desulfobulbus propionicus isolated from an Indian petroleum refinery on mild steel. Mater. Corros. 2011, 63, 355–362. [Google Scholar] [CrossRef]
- Murat, J.B.; Grenouillet, F.; Reboux, G.; Penven, E.; Batchili, A.; Dalphin, J.C.; Thaon, I.; Millon, L. Factors influencing the microbial composition of metalworking fluids and potential implications for machine operator’s lung. Appl. Environ. Microbiol. 2012, 78, 34–41. [Google Scholar] [CrossRef]
- Bonetta, S.; Bonetta, S. Editorial comments to the special issue: “Legionella contamination in water environment”. Pathogens 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Bu, Y.; Zhang, X.X.; Huang, K.; He, X.; Ye, L.; Shan, Z.; Ren, H. Metagenomic analysis of bacterial community composition and antibiotic resistance genes in a wastewater treatment plant and its receiving surface water. Ecotoxicol. Environ. Saf. 2016, 132, 260–269. [Google Scholar] [CrossRef]
- Haenelt, S.; Richnow, H.H.; Muller, J.A.; Musat, N. Antibiotic resistance indicator genes in biofilm and planktonic microbial communities after wastewater discharge. Front. Microbiol. 2023, 14, 1252870. [Google Scholar] [CrossRef]
- Xu, R.; Fan, F.; Lin, Q.; Yuan, S.; Meng, F. Overlooked ecological roles of influent wastewater microflora in improving biological phosphorus removal in an anoxic/aerobic MBR process. Environ. Sci. Technol. 2021, 55, 6270–6280. [Google Scholar] [CrossRef]
- Yu, L.; Li, R.; Delatolla, R.; Zhang, R.; Yang, X.; Peng, D. Natural continuous influent nitrifier immigration effects on nitrification and the microbial community of activated sludge systems. J. Environ. Sci. 2018, 74, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, A.; Jordaan, E.M.; Flores-Orozco, D.; Khafipour, E.; Levin, D.B.; Sparling, R.; Cicek, N. Response of microbial community to induced failure of anaerobic digesters through overloading with propionic acid followed by process recovery. Front. Bioeng. Biotechnol. 2020, 8, 604838. [Google Scholar] [CrossRef] [PubMed]
- Leta, S.; Assefa, F.; Dalhammar, G. Enhancing biological nitrogen removal from tannery effluent by using the efficient Brachymonas denitrificans in pilot plant operations. World J. Microbiol. Biotechnol. 2005, 21, 545–552. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Gibson, C.; Frigon, D. Wastewater microbial community structure and functional traits change over short timescales. Sci. Total Environ. 2019, 662, 779–785. [Google Scholar] [CrossRef]
- Martinez-Santos, M.; Lanzen, A.; Unda-Calvo, J.; Martin, I.; Garbisu, C.; Ruiz-Romera, E. Treated and untreated wastewater effluents alter river sediment bacterial communities involved in nitrogen and sulphur cycling. Sci. Total Environ. 2018, 633, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A.K.; Ball, A.S.; Shah, K. Investigations into valorisation of trade wastewater for biomethane production. Heliyon 2023, 9, e13309. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Hach Company. Oxygen Demand, Chemical. 2021. Available online: https://au.hach.com/asset-get.download.jsa?id=7639983816 (accessed on 13 December 2021).
- Hach Company. Nitrogen, Total, Method 10071, doc316.53.01086. 2014. Available online: https://au.hach.com/asset-get.download.jsa?id=763998380410 (accessed on 13 December 2021).
- Hach Company. Nitrate, HR, Method 10020, doc316.53.01068. 2015. Available online: https://au.hach.com/asset-get.download.jsa?id=763998373810 (accessed on 13 December 2021).
- Hach Company. Nitrite, Method 10019, doc316.53.01073. 2015. Available online: https://sea.hach.com/asset-get.download.jsa?id=763998374210 (accessed on 13 December 2021).
- Hach Company. Nitrogen, Ammonia, Method 10031, doc316.53.01079. 2015. Available online: https://au.hach.com/asset-get.download.jsa?id=763998374710 (accessed on 13 December 2021).
- Hach Company. Phosphorus, Total. 2017. Available online: https://au.hach.com/asset-get.download.jsa?id=7639983838 (accessed on 13 December 2021).
- Anaero Technology. Available online: https://www.anaerotech.com/ (accessed on 13 December 2021).
- Qiagen. Dneasy® Powersoil® Pro Kit Handbook; Qiagen: Hilden, Germany, 2023. [Google Scholar]
- Illumina. 16S Metagenomic Sequencing Library Preparation; (15044223 b); Illumina: San Diego, CA, USA, 2013. [Google Scholar]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Mazzoli, L.; Munz, G.; Lotti, T.; Ramazzotti, M. A novel universal primer pair for prokaryotes with improved performances for anammox containing communities. Sci. Rep. 2020, 10, 15648. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using qiime 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with qiime 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.K.D.; Nierychlo, M.; Andersen, K.S.; Rudkjobing, V.; Knutsson, S.; MiDAS Global Consortium; Albertsen, M.; Nielsen, P.H. Midas 4: A global catalogue of full-length 16S rrna gene sequences and taxonomy for studies of bacterial communities in wastewater treatment plants. Nat. Commun. 2022, 13, 1908. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.K.D.; Andersen, K.S.; Petersen, A.-K.C.; Rudkjøbing, V.; Nielsen, P.H. MiDAS 5: Global diversity of bacteria and archaea in anaerobic digesters. bioRxiv 2023. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L.; Shetty, S. Microbiome R Package. 2012–2019. Available online: https://www.bioconductor.org/packages/release/bioc/html/microbiome.html (accessed on 11 December 2022).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2022. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 11 December 2022).
- Lin, H.; Eggesbo, M.; Peddada, S.D. Linear and nonlinear correlation estimators unveil undescribed taxa interactions in microbiome data. Nat. Commun. 2022, 13, 4946. [Google Scholar] [CrossRef]
- Eurofins. Trade Waste Testing, 7th ed.; Available online: https://cdnmedia.eurofins.com/apac/media/610106/trade-waste-testing-v7-sept-21.pdf (accessed on 21 February 2024).
- Wang, L.K.; Hung, Y.-T.; Lo, H.H.; Yapijakis, C. Waste Treatment in the Food Processing Industry; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- El-Abbassi, A.; Hafidi, A.; García-Payo, M.; Khayet, M. Concentration of olive mill wastewater by membrane distillation for polyphenols recovery. Desalination 2009, 245, 670–674. [Google Scholar] [CrossRef]
- Krishnan, S.; Md Din, M.F.; Taib, S.M.; Nasrullah, M.; Sakinah, M.; Wahid, Z.A.; Kamyab, H.; Chelliapan, S.; Rezania, S.; Singh, L. Accelerated two-stage bioprocess for hydrogen and methane production from palm oil mill effluent using continuous stirred tank reactor and microbial electrolysis cell. J. Clean. Prod. 2019, 229, 84–93. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- City West Water. Approved Acceptance Criteria for Discharge to the Sewerage System; City West Water: Melbourne, Australia, 2017. [Google Scholar]
- Zeng, T.; Hu, Q.; Rene, E.R.; Lens, P.N.L. Microbial community and extracellular polymeric substances analysis of anaerobic granular sludge exposed to selenate, cadmium and zinc. Microb. Biotechnol. 2023, 16, 463–473. [Google Scholar] [CrossRef]
- Burdon, F.J.; Bai, Y.; Reyes, M.; Tamminen, M.; Staudacher, P.; Mangold, S.; Singer, H.; Rasanen, K.; Joss, A.; Tiegs, S.D.; et al. Stream microbial communities and ecosystem functioning show complex responses to multiple stressors in wastewater. Glob. Chang. Biol. 2020, 26, 6363–6382. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Isa, M.H.; Ghaleb, A.A.S.; Lawal, I.M.; Usman, A.K.; Birniwa, A.H.; Noor, A.; Abubakar, S.; Umaru, I.; et al. Toxic effects of xenobiotic compounds on the microbial community of activated sludge. ChemBioEng Rev. 2022, 9, 497–535. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Jensen, P.D.; Dennis, P.G.; Hugenholtz, P.; Rabaey, K.; Tyson, G.W. Deterministic processes guide long-term synchronised population dynamics in replicate anaerobic digesters. ISME J. 2014, 8, 2015–2028. [Google Scholar] [CrossRef]
- Tong, T.; Tong, J.; Xue, K.; Li, Y.; Yu, J.; Wei, Y. Microbial community structure and functional prediction in five full-scale industrial park wastewater treatment plants. Sci. Total Environ. 2023, 904, 166529. [Google Scholar] [CrossRef]
- Rodriguez Lopez, J.; Grande, M.J.; Perez-Pulido, R.; Galvez, A.; Lucas, R. Impact of high-hydrostatic pressure treatments applied singly or in combination with moderate heat on the microbial load, antimicrobial resistance, and bacterial diversity of guacamole. Microorganisms 2020, 8, 909. [Google Scholar] [CrossRef]
- Ferguson, R.M.W.; Coulon, F.; Villa, R. Understanding microbial ecology can help improve biogas production in AD. Sci. Total Environ. 2018, 642, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Vanwonterghem, I.; Rinke, C.; Parks, D.H.; Zhang, Y.; Takai, K.; Sievert, S.M.; Simon, J.; Campbell, B.J.; Hanson, T.E.; et al. Erratum: Addendum: Comparative genomic analysis of the class epsilonproteobacteria and proposed reclassification to epsilonbacteraeota (phyl. nov.). Front. Microbiol. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, M.; Yang, S.; Gong, H.; Ma, J.; Li, C.; Wang, K. Performance and microbial community evaluation of full-scale two-phase anaerobic digestion of waste activated sludge. Sci. Total Environ. 2022, 814, 152525. [Google Scholar] [CrossRef]
- Boone, D.R.; Castenholz, R.W. The Archaea and the Deeply Branching and Phototrophic Bacteria, 2nd ed.; Springer: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T. The Proteobacteria: Part C: The Alpha-, Beta-, Delta-, and Epsilonproteobacteria, 2nd ed.; Springer: New York, NY, USA, 2005; Volume 2C. [Google Scholar]
- Bonk, F.; Popp, D.; Weinrich, S.; Strauber, H.; Kleinsteuber, S.; Harms, H.; Centler, F. Intermittent fasting for microbes: How discontinuous feeding increases functional stability in anaerobic digestion. Biotechnol. Biofuels 2018, 11, 274. [Google Scholar] [CrossRef]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T. The Proteobacteria: Part B: The Gammaproteobacteria, 2nd ed.; Springer: New York, NY, USA, 2005; Volume 2B. [Google Scholar]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. Divided into the families Enterobacteriaceae, Ewiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar]
- Boulton, C. Encyclopaedia of Brewing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Chen, H.L.; Zhao, X.Y.; Zhao, G.X.; Huang, H.B.; Li, H.R.; Shi, C.W.; Yang, W.T.; Jiang, Y.L.; Wang, J.Z.; Ye, L.P.; et al. Dissection of the cecal microbial community in chickens after Eimeria tenella infection. Parasit Vectors 2020, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Guo, C.; Gong, Y.; Sun, X.; Wang, W.; Wang, Y.; Yang, H.; Cao, Z.; Li, S. Rumen fermentation, digestive enzyme activity, and bacteria composition between pre-weaning and post-weaning dairy calves. Animals 2021, 11, 2527. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Petri, R.M.; Schwaiger, T.; Penner, G.B.; Beauchemin, K.A.; Forster, R.J.; McKinnon, J.J.; McAllister, T.A. Characterization of the core rumen microbiome in cattle during transition from forage to concentrate as well as during and after an acidotic challenge. PLoS ONE 2013, 8, e83424. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.W.; Kim, B.Y.; Kim, W.G.; Yoo, K.H.; Yoo, S.H.; Son, J.A.; Weon, H.Y. Paludibacterium yongneupense gen. nov., sp. Nov., isolated from a wetland, yongneup, in korea. Int. J. Syst. Evol. Microbiol. 2008, 58, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rudra, B.; Gupta, R.S. Phylogenomics and molecular signatures support division of the order Neisseriales into emended families Neisseriaceae and Chromobacteriaceae and three new families Aquaspirillaceae fam. nov., Chitinibacteraceae fam. nov., and Leeiaceae fam. nov. Syst. Appl. Microbiol. 2021, 44, 126251. [Google Scholar] [CrossRef] [PubMed]
- Edzwald, J.K. Dissolved air flotation and me. Water Res. 2010, 44, 2077–2106. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.L.; Sueitt, A.P.E.; Dos Santos, P.R.; Leite, L.S.; Daniel, L.A. Removal of protozoan (oo)cysts and bacteria during microalgae harvesting: Outcomes from a lab-scale experiment. Chemosphere 2022, 286, 131767. [Google Scholar] [CrossRef]
- Huber, R.; Kristjansson, J.K.; Stetter, K.O. Pyrobaculum gen. nov., a new genus of neutrophilic, rod-shaped archaebacteria from continental solfataras growing optimally at 100 °C. Arch. Microbiol. 1987, 149, 95–101. [Google Scholar] [CrossRef]
- Nazina, T.N.; Tourova, T.P.; Poltaraus, A.B.; Novikova, E.V.; Grigoryan, A.A.; Ivanova, A.E.; Lysenko, A.M.; Petrunyaka, V.V.; Osipov, G.A.; Belyaev, S.S.; et al. Taxonomic study of aerobic thermophilic bacilli: Descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. From petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermo-catenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermoglucosidasius and Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. thermocatenulatus, G. thermoleovorans, G. kaustophilus, G. thermoglucosidasius and G. thermodenitrificans. Int. J. Syst. Evol. Microbiol. 2001, 51, 433–446. [Google Scholar]
- Hai, D.; Jiang, H.; Meng, Z.; Qiao, M.; Xu, T.; Song, L.; Huang, X. The impact of high temperature on microbial communities in pork and duck skin. Microorganisms 2023, 11, 2869. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, W.; Jones, D.; Rainey, F.A.; Whitman, W.B.; De Vos, P.; Krieg, N.R.; Garrity, G.M.; Schleifer, K.-H. The Firmicutes, 2nd ed.; Springer: New York, NY, USA, 2009; Volume 3. [Google Scholar]
- Maturrano, L.; Valens-Vadell, M.; Rossello-Mora, R.; Anton, J. Salicola marasensis gen. nov., sp. nov., an extremely halophilic bacterium isolated from the Maras solar salterns in Peru. Int. J. Syst. Evol. Microbiol. 2006, 56, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes: Other Major Lineages of Bacteria and the Archaea, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Wang, Y.; Cao, L.L.; Tang, S.K.; Lou, K.; Mao, P.H.; Jin, X.; Jiang, C.L.; Xu, L.H.; Li, W.J. Marinococcus luteus sp. nov., a halotolerant bacterium isolated from a salt lake, and emended description of the genus Marinococcus. Int. J. Syst. Evol. Microbiol. 2009, 59, 2875–2879. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Xu, D.; Bi, X.; Ng, H.Y.; Shi, X. Intertidal wetland sediment as a novel inoculation source for developing aerobic granular sludge in membrane bioreactor treating high-salinity antibiotic manufacturing wastewater. Bioresour. Technol. 2020, 314, 123715. [Google Scholar] [CrossRef]
- Yoon, J.H.; Lee, K.C.; Weiss, N.; Kang, K.H.; Park, Y.H. Jeotgalicoccus halotolerans gen. nov., sp. nov. and Jeotgalicoccus psychrophilus sp. nov., isolated from the traditional Korean fermented seafood jeotgal. Int. J. Syst. Evol. Microbiol. 2003, 53, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.E.; Ludwig, W.; Suzuki, K.-I. The Actinobacteria, 2nd ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Liaw, H.J.; Mah, R.A. Isolation and characterization of haloanaerobacter Chitinovorans gen. nov., sp. nov., a halophilic, anaerobic, chitinolytic bacterium from a solar saltern. Appl. Environ. Microbiol. 1992, 58, 260–266. [Google Scholar] [CrossRef]
- Korshunova, T.; Kuzina, E.; Mukhamatdyarova, S.; Sharipova, Y.; Iskuzhina, M. Promising strains of hydrocarbon-oxidizing pseudomonads with herbicide resistance and plant growth-stimulating properties for bioremediation of oil-contaminated agricultural soils. Agriculture 2023, 13, 1111. [Google Scholar] [CrossRef]
- Peng, X.; Pan, X.; Wang, X.; Li, D.; Huang, P.; Qiu, G.; Shan, K.; Chu, X. Accelerated removal of high concentration p-chloronitrobenzene using bioelectrocatalysis process and its microbial communities analysis. Bioresour. Technol. 2018, 249, 844–850. [Google Scholar] [CrossRef]
- Yuan, K.; Li, S.; Zhong, F. Treatment of coking wastewater in biofilm-based bioaugmentation process: Biofilm formation and microbial community analysis. J. Hazard. Mater. 2020, 400, 123117. [Google Scholar] [CrossRef]
- Luijten, M.L.; Weelink, S.A.; Godschalk, B.; Langenhoff, A.A.; van Eekert, M.H.; Schraa, G.; Stams, A.J. Anaerobic reduction and oxidation of quinone moieties and the reduction of oxidized metals by halorespiring and related organisms. FEMS Microbiol. Ecol. 2004, 49, 145–150. [Google Scholar] [CrossRef]
- Hubert, C.; Voordouw, G. Oil field souring control by nitrate-reducing Sulfurospirillum spp. that outcompete sulfate-reducing bacteria for organic electron donors. Appl. Environ. Microbiol. 2007, 73, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, E.; Beuerle, J.; Sulger, K.; Kroneck, P.M.H.; Schumacher, W. Lithotrophic growth of Sulfurospirillum deleyianum with sulfide as electron donor coupled to respiratory reduction of nitrate to ammonia. Arch. Microbiol. 1995, 164, 180–185. [Google Scholar] [CrossRef]
- Petriglieri, F.; Singleton, C.M.; Kondrotaite, Z.; Dueholm, M.K.D.; McDaniel, E.A.; McMahon, K.D.; Nielsen, P.H.; McGrath, J. Reevaluation of the phylogenetic diversity and global distribution of the genus “Candidatus accumulibacter”. mSystems 2022, 7, e00016-22. [Google Scholar] [CrossRef] [PubMed]
- Malfliet, S.; Juste, A.; Crauwels, S.; Willems, K.; De Cooman, L.; Lievens, B.; Aerts, G. Assessing the xylanolytic bacterial diversity during the malting process. Food Microbiol. 2013, 36, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Dilek, F.B.; Anderson, G.K.; Bloor, J. Investigation into the microbiology of a high rate jet-loop activated sludge reactor treating brewery wastewater. Water Sci. Technol. 1996, 34, 107–112. [Google Scholar] [CrossRef]
- Geissler, A.J.; Behr, J.; von Kamp, K.; Vogel, R.F. Metabolic strategies of beer spoilage lactic acid bacteria in beer. Int. J. Food Microbiol. 2016, 216, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Cocuzza, S.; Biendl, M.; Peifer, F.; Hans, S.; Methner, Y.; Pehl, F.; Back, W.; Jacob, F.; Hutzler, M. The impact of different hop compounds on the growth of selected beer spoilage bacteria in beer. J. Inst. Brew. 2020, 126, 354–361. [Google Scholar]
- Ueki, A.; Akasaka, H.; Satoh, A.; Suzuki, D.; Ueki, K. Prevotella paludivivens sp. nov., a novel strictly anaerobic, gram-negative, hemicellulose-decomposing bacterium isolated from plant residue and rice roots in irrigated rice-field soil. Int. J. Syst. Evol. Microbiol. 2007, 57, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Hattori, S.; Ueki, K. Xylanibacter oryzae gen. nov., sp. nov., a novel strictly anaerobic, gram-negative, xylanolytic bacterium isolated from rice-plant residue in flooded rice-field soil in japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 2215–2221. [Google Scholar] [CrossRef][Green Version]
- Keyser, M.; Britz, T.J.; Witthuhn, R.C. Fingerprinting and identification of bacteria present in UASB granules used to treat winery, brewery, distillery or peach-lye canning wastewater. S. Afr. J. Enol. Vitic. 2007, 28, 69–79. [Google Scholar] [CrossRef]
- Wright, M.S.; Haft, D.H.; Harkins, D.M.; Perez, F.; Hujer, K.M.; Bajaksouzian, S.; Benard, M.F.; Jacobs, M.R.; Bonomo, R.A.; Adams, M.D. New insights into dissemination and variation of the health care-associated pathogen Acinetobacter baumannii from genomic analysis. mBio 2014, 5, e00963-13. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Alonso, S.; Top, J.; McNally, A.; Puranen, S.; Pesonen, M.; Pensar, J.; Marttinen, P.; Braat, J.C.; Rogers, M.R.C.; van Schaik, W.; et al. Plasmids shaped the recent emergence of the major nosocomial pathogen Enterococcus faecium. mBio 2020, 11, e03284-19. [Google Scholar] [CrossRef] [PubMed]
- Antoun, M.; Hattab, Y.; Akhrass, F.A.; Hamilton, L.D. Uncommon pathogen, Lactobacillus, causing infective endocarditis: Case report and review. Case Rep. Infect. Dis. 2020, 2020, 8833948. [Google Scholar] [CrossRef] [PubMed]
- Chery, J.; Dvoskin, D.; Morato, F.P.; Fahoum, B. Lactobacillus fermentum, a pathogen in documented cholecystitis. Int. J. Surg. Case Rep. 2013, 4, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Kita, A.; Miura, T.; Okamura, Y.; Aki, T.; Matsumura, Y.; Tajima, T.; Kato, J.; Nakashimada, Y. Dysgonomonas alginatilytica sp. nov., an alginate-degrading bacterium isolated from a microbial consortium. Int. J. Syst. Evol. Microbiol. 2015, 65, 3570–3575. [Google Scholar] [CrossRef] [PubMed]
- Efsa Panel on Additives and Products or Substances Used in Animal Feed; Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Safety and efficacy of sodium and potassium alginate for pets, other non food-producing animals and fish. EFSA J. 2017, 15, e04945. [Google Scholar] [PubMed]
- Hay, I.D.; Ur Rehman, Z.; Ghafoor, A.; Rehm, B.H.A. Bacterial biosynthesis of alginates. J. Chem. Technol. Biotechnol. 2010, 85, 752–759. [Google Scholar] [CrossRef]
- Bouchez, T.; Patureau, D.; Delgenes, J.P.; Moletta, R. Successful bacterial incorporation into activated sludge flocs using alginate. Bioresour. Technol. 2009, 100, 1031–1032. [Google Scholar] [CrossRef]
- Kim, B.C.; Seung Jeon, B.; Kim, S.; Kim, H.; Um, Y.; Sang, B.I. Caproiciproducens galactitolivorans gen. nov., sp. nov., a bacterium capable of producing caproic acid from galactitol, isolated from a wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2015, 65, 4902–4908. [Google Scholar] [CrossRef]
- Tang, J.; Dai, K.; Wang, Q.T.; Zheng, S.J.; Hong, S.D.; Jianxiong Zeng, R.; Zhang, F. Caproate production from xylose via the fatty acid biosynthesis pathway by genus Caproiciproducens dominated mixed culture fermentation. Bioresour. Technol. 2022, 351, 126978. [Google Scholar] [CrossRef]
- Nishiyama, T.; Ueki, A.; Kaku, N.; Watanabe, K.; Ueki, K. Bacteroides graminisolvens sp. nov., a xylanolytic anaerobe isolated from a methanogenic reactor treating cattle waste. Int. J. Syst. Evol. Microbiol. 2009, 59, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.M.; Schmidt, T.; Lv, Z.; Liebetrau, J.; Richnow, H.H.; Kleinsteuber, S.; Nikolausz, M. Reduction of the hydraulic retention time at constant high organic loading rate to reach the microbial limits of anaerobic digestion in various reactor systems. Bioresour. Technol. 2016, 217, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Pagnier, I.; Raoult, D.; La Scola, B. Isolation and characterization of Reyranella massiliensis gen. nov., sp. nov. from freshwater samples by using an amoeba co-culture procedure. Int. J. Syst. Evol. Microbiol. 2011, 61, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, D.; Veenendaal, H.R.; Italiaander, R.; van der Mark, E.J.; Dignum, M. Primary colonizing Betaproteobacteriales play a key role in the growth of Legionella pneumophila in biofilms on surfaces exposed to drinking water treated by slow sand filtration. Appl. Environ. Microbiol. 2018, 84, e01732-18. [Google Scholar] [CrossRef] [PubMed]
- Frayne, C. (Ed.) Chemical treatments and programs for cooling water. In Cooling Water Treatment—Principles and Practice; Chemical Publishing Company: Revere, MA, USA, 1999. [Google Scholar]
- de Carvalho, C.C.; da Fonseca, M.M. The remarkable Rhodococcus erythropolis. Appl. Microbiol. Biotechnol. 2005, 67, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Ryu, S.H.; Hwang, H.W.; Kim, Y.J.; Park, M.; Lee, J.R.; Lee, S.S.; Jeon, C.O. Pseudoxanthomonas sacheonensis sp. nov., isolated from btex-contaminated soil in Korea, transfer of Stenotrophomonas dokdonensis Yoon et al. 2006 to the genus Pseudoxanthomonas as Pseudoxanthomonas dokdonensis comb. nov. and emended description of the genus Pseudoxanthomonas. Int. J. Syst. Evol. Microbiol. 2008, 58, 2235–2240. [Google Scholar] [PubMed]
- Bedics, A.; Tancsics, A.; Banerjee, S.; Toth, E.; Harkai, P.; Gottschall, G.G.; Boka, K.; Kriszt, B. Acidovorax benzenivorans sp. nov., a novel aromatic hydrocarbon-degrading bacterium isolated from a xylene-degrading enrichment culture. Int. J. Syst. Evol. Microbiol. 2024, 74, 006219. [Google Scholar] [CrossRef]
- Corteselli, E.M.; Aitken, M.D.; Singleton, D.R. Description of Immundisolibacter cernigliae gen. nov., sp. nov., a high-molecular-weight polycyclic aromatic hydrocarbon-degrading bacterium within the class Gammaproteobacteria, and proposal of Immundisolibacterales ord. nov. and Immundisolibacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 925–931. [Google Scholar] [PubMed]
- Schleheck, D.; Tindall, B.J.; Rossello-Mora, R.; Cook, A.M. Parvibaculum lavamentivorans gen. nov., sp. nov., a novel heterotroph that initiates catabolism of linear alkylbenzenesulfonate. Int. J. Syst. Evol. Microbiol. 2004, 54, 1489–1497. [Google Scholar] [CrossRef]
- Sperfeld, M.; Diekert, G.; Studenik, S. Anaerobic aromatic compound degradation in Sulfuritalea hydrogenivorans sk43h. FEMS Microbiol. Ecol. 2019, 95, fiy199. [Google Scholar] [CrossRef]
- Park, H.; Shah, S.S.A.; Korshin, G.; Angelidaki, I.; Choo, K.-H. The impact of sunlight on fouling behaviors and microbial communities in membrane bioreactors. J. Membr. Sci. 2023, 672, 121443. [Google Scholar] [CrossRef]
- Petriglieri, F.; Singleton, C.; Peces, M.; Petersen, J.F.; Nierychlo, M.; Nielsen, P.H. “Candidatus dechloromonas phosphoritropha” and “Ca. D. Phosphorivorans”, novel polyphosphate accumulating organisms abundant in wastewater treatment systems. ISME J. 2021, 15, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Shin, Y.K.; Sugiyama, J. Brachymonas denitrificans gen. nov., sp. nov., an aerobic chemoorganotrophic bacterium which contains rhodoquinones, and evolutionary relationships of rhodoquinone producers to bacterial species with various quinone classes. J. Gen. Appl. Microbiol. 1995, 41, 99–117. [Google Scholar] [CrossRef]
- Halpern, M.; Shaked, T.; Schumann, P. Brachymonas chironomi sp. nov., isolated from a chironomid egg mass, and emended description of the genus Brachymonas. Int. J. Syst. Evol. Microbiol. 2009, 59, 3025–3029. [Google Scholar] [CrossRef] [PubMed]
- Volmer, J.G.; Soo, R.M.; Evans, P.N.; Hoedt, E.C.; Astorga Alsina, A.L.; Woodcroft, B.J.; Tyson, G.W.; Hugenholtz, P.; Morrison, M. Isolation and characterisation of novel Methanocorpusculum species indicates the genus is ancestrally host-associated. BMC Biol. 2023, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Browne, P.; Tamaki, H.; Kyrpides, N.; Woyke, T.; Goodwin, L.; Imachi, H.; Brauer, S.; Yavitt, J.B.; Liu, W.T.; Zinder, S.; et al. Genomic composition and dynamics among Methanomicrobiales predict adaptation to contrasting environments. ISME J. 2017, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Ueki, A.; Ohtaki, Y.; Kaku, N.; Watanabe, K.; Ueki, K. Anaerocella delicata gen. nov., sp. nov., a strictly anaerobic bacterium in the phylum Bacteroidetes isolated from a methanogenic reactor of cattle farms. J. Gen. Appl. Microbiol. 2012, 58, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Jinno, C.; Wong, B.; Klunemann, M.; Htoo, J.; Li, X.; Liu, Y. Effects of supplementation of Bacillus amyloliquefaciens on performance, systemic immunity, and intestinal microbiota of weaned pigs experimentally infected with a pathogenic enterotoxigenic E. coli f18. Front. Microbiol. 2023, 14, 1101457. [Google Scholar] [CrossRef]
- Ramees, T.P.; Dhama, K.; Karthik, K.; Rathore, R.S.; Kumar, A.; Saminathan, M.; Tiwari, R.; Malik, Y.S.; Singh, R.K. Arcobacter: An emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control—A comprehensive review. Vet. Q. 2017, 37, 136–161. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Lyu, Z.; Hoover, R.B.; Liu, Y.; Patel, N.B.; Busse, H.J.; Lawson, P.A. Williamwhitmania taraxaci gen. nov., sp. nov., a proteolytic anaerobe with a novel type of cytology from Lake Untersee in Antarctica, description of Williamwhitmaniaceae fam. nov., and emendation of the order Bacteroidales krieg 2012. Int. J. Syst. Evol. Microbiol. 2017, 67, 4132–4145. [Google Scholar] [CrossRef]
- Singh, S.; Keating, C.; Ijaz, U.Z.; Hassard, F. Molecular insights informing factors affecting low temperature anaerobic applications: Diversity, collated core microbiomes and complexity stability relationships in LCFA-fed systems. Sci. Total Environ. 2023, 874, 162420. [Google Scholar] [CrossRef] [PubMed]
- Engle, M.; Li, Y.; Rainey, F.; Deblois, S.; Mai, V.; Reichert, A.; Mayer, F.; Messner, P.; Wiegel, J. Thermobrachium celere gen. nov., sp. nov., a rapidly growing thermophilic, alkalitolerant, and proteolytic obligate anaerobe. Int. J. Syst. Evol. Microbiol. 1996, 46, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović, N.; Fagorzi, C.; Mengoni, A.; Lassalle, F.; Dicenzo, G.C. Taxonomy of Rhizobiaceae revisited: Proposal of a new framework for genus delimitation. Int. J. Syst. Evol. Microbiol. 2022, 72, 005243. [Google Scholar] [CrossRef] [PubMed]
- Riisgaard-Jensen, M.; Dottorini, G.; Nierychlo, M.; Nielsen, P.H. Primary settling changes the microbial community of influent wastewater to wastewater treatment plants. Water Res. 2023, 244, 120495. [Google Scholar] [CrossRef] [PubMed]
- Grabovich, M.; Gavrish, E.; Kuever, J.; Lysenko, A.M.; Podkopaeva, D.; Dubinina, G. Proposal of Giesbergeria voronezhensis gen. nov., sp. nov. and G. kuznetsovii sp. nov. and reclassification of [Aquaspirillum] anulus, [A.] sinuosum and [A.] giesbergeri as Giesbergeria anulus comb. nov., G. sinuosa comb. nov. and G. giesbergeri comb. nov., and [Aquaspirillum] metamorphum and [A.] psychrophilum as Simplicispira metamorpha gen. nov., comb. nov. and S. psychrophila comb. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 569–576. [Google Scholar] [PubMed]
- Sun, L.; Toyonaga, M.; Ohashi, A.; Tourlousse, D.M.; Matsuura, N.; Meng, X.Y.; Tamaki, H.; Hanada, S.; Cruz, R.; Yamaguchi, T.; et al. Lentimicrobium saccharophilum gen. nov., sp. nov., a strictly anaerobic bacterium representing a new family in the phylum Bacteroidetes, and proposal of Lentimicrobiaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 2635–2642. [Google Scholar] [CrossRef]
- Garcia, R.O.; Reichenbach, H.; Ring, M.W.; Muller, R. Phaselicystis flava gen. nov., sp. nov., an arachidonic acid-containing soil myxobacterium, and the description of Phaselicystidaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 1524–1530. [Google Scholar] [CrossRef]



| Sample Name | Site | Treatment Level | Industry | COD (mg/L) | VS (mg/L) | PO4 (mg/L) | pH | NO2 (mg/L) | NO3 (mg/L) | NH3 (mg/L) | TotN (mg/L) | Na (mg/L) | Gas Yield | Observed Taxa | Shannon Diversity | Pielou’s Evenness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1U | 1 | Untreated | Edible Fats | 44,900 | 7430 | 272 | 6.02 | 0.7 | 5 | 0 | 11 | 2.1 | Low | 37 | 2.182 | 0.604 |
| 1T | 1 | Treated | Edible Fats | 1086 | 614 | 170 | 6.33 | 0 | 10 | 0 | 8 | 2.1 | Low | 14 | 2.393 | 0.907 |
| 2U | 2 | Untreated | Brewery | 13,800 | 3778 | 3.47 | 3.89 | 0.17 | 2.8 | 0 | 93 | 0.9 | Low | 37 | 2.896 | 0.802 |
| 2T | 2 | Treated | Brewery | 5530 | 1864 | 63 | 4.02 | 0.4 | 7 | 0 | 49 | 0.9 | Medium | 46 | 3.243 | 0.847 |
| 3U | 3 | Untreated | Logistics | 9710 | 5556 | 72 | 12.29 | 0.8 | 9 | 0 | 83 | 1.5 | High | 45 | 3.247 | 0.853 |
| 3T | 3 | Treated | Logistics | 2880 | 874 | 53 | 6.72 | 0.6 | 7 | 20 | 65 | 1.7 | Low | 58 | 3.164 | 0.779 |
| 4U | 4 | Untreated | Chemicals | 20 | 84 | 10 | 6.89 | 0.02 | 8 | 0 | 4 | 0.2 | Low | 60 | 3.57 | 0.872 |
| 5U | 5 | Untreated | Foods | 22,200 | 6982 | 263 | 5.39 | 0 | 12 | 20 | 239 | 1.8 | High | 44 | 3.107 | 0.821 |
| 5CU * | 5 | Untreated * | Foods | 1,466,300 | 279,271 | 1090 | 4.86 | 0.62 | 8 | 210 | 7500 | 3.7 | High | 19 | 1.984 | 0.674 |
| 5T | 5 | Treated | Foods | 9210 | 3332 | 170 | 6.32 | 0.5 | 9 | 0 | 103 | 1.5 | High | 91 | 3.24 | 0.718 |
| 7T | 7 | Treated | Animal Products | >100,000 | 19,460 | 3.06 | 6.77 | 2.3 | 8 | 100 | 870 | 310 | Low | 50 | 2.571 | 0.657 |
| 8T | 8 | Treated | Chemicals | 10,000 | 1326 | 10 | 6.50 | 0.15 | 11 | 0 | 0 | 0.4 | Low | 24 | 2.521 | 0.793 |
| 9U | 9 | Untreated | Logistics | 17,700 | 9992 | 3.34 | 12.16 | 0 | 6.2 | 0 | 19 | 2.4 | High | 64 | 3.577 | 0.86 |
| 9T | 9 | Treated | Logistics | 3650 | 1173 | 2.46 | 5.63 | 0.04 | 1.4 | 0 | 50 | 1.2 | Medium | 52 | 3.041 | 0.77 |
| 10T | 10 | Treated | Chemicals | 2560 | 615 | 235 | 6.21 | 0.09 | 1 | 0 | 8 | 0.6 | Low | 47 | 2.981 | 0.774 |
| 11T | 11 | Treated | Chemicals | 140 | 443 | 1.36 | 7.91 | 4.9 | 10.3 | 0 | 9 | 0.6 | Low | 153 | 4.465 | 0.888 |
| 12U | 12 | Untreated | Animal Products | 2920 | 1109 | 95 | 6.72 | 0.03 | 2 | 270 | 254 | 1.4 | High | 128 | 4.175 | 0.86 |
| 12T | 12 | Treated | Animal Products | 2000 | 211 | 16 | 6.96 | 0.04 | 0.7 | 120 | 96 | 1 | Low | 177 | 4.788 | 0.925 |
| Site | Industry | Straining | Settling | Mixing | Oil Interception | Neutralisation | Cooling | Screening | Ferric Sulphate | Polymer | DAF * | Sludge Removal | Sulphide Control | Aeration | Separation | pH Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Edible Fats | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ |
| 2 | Foods | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 3 | Logistics | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 4 | Chemicals | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 5 | Foods | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 7 | Animal Products | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 8 | Chemicals | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| 9 | Logistics | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 10 | Chemicals | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ |
| 11 | Chemicals | ✗ | ✓ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ |
| 12 | Animal Products | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, J.A.K.; Krohn, C.; Ball, A.S. Diversity of Microbial Communities in Trade Wastes—Implications for Treatments and Operations. Appl. Microbiol. 2024, 4, 682-703. https://doi.org/10.3390/applmicrobiol4020047
Elliott JAK, Krohn C, Ball AS. Diversity of Microbial Communities in Trade Wastes—Implications for Treatments and Operations. Applied Microbiology. 2024; 4(2):682-703. https://doi.org/10.3390/applmicrobiol4020047
Chicago/Turabian StyleElliott, Jake A. K., Christian Krohn, and Andrew S. Ball. 2024. "Diversity of Microbial Communities in Trade Wastes—Implications for Treatments and Operations" Applied Microbiology 4, no. 2: 682-703. https://doi.org/10.3390/applmicrobiol4020047
APA StyleElliott, J. A. K., Krohn, C., & Ball, A. S. (2024). Diversity of Microbial Communities in Trade Wastes—Implications for Treatments and Operations. Applied Microbiology, 4(2), 682-703. https://doi.org/10.3390/applmicrobiol4020047






